Abstract
Granulocyte–macrophage colony‐stimulating factor (GM‐CSF) is a well‐known stimulus for the activation, differentiation and survival of monocytes (MO). Up to now most investigations focused on the short‐term effects of GM‐CSF. In this study we investigated the effects of GM‐CSF on the long‐term differentiation of human MO in the presence of serum. We found that MO‐derived macrophages (Mφ) cultured with serum plus GM‐CSF (GM‐Mφ) were different from control Mφ (SER‐Mφ) in terms of lipopolysaccharide (LPS)‐stimulated cytokine release: GM‐Mφ showed an increased tumour necrosis factor‐α (TNF‐α) and interleukin‐6 (IL‐6) production, especially at lower LPS concentrations, but the secretion of IL‐10 was diminished. In addition, GM‐Mφ secreted TNF‐α but not IL‐6 and IL‐10, spontaneously. The spontaneous TNF‐α production was not due to LPS contamination as it could not be blocked by anti‐CD14 antibody. Flow cytometry revealed, however, that the receptor for LPS, CD14, was up‐regulated on GM‐Mφ and those Mφ released twice as much soluble CD14 into the supernatant as compared with SER‐Mφ. The higher CD14 expression also resulted in an enhanced LPS‐binding capacity of GM‐Mφ. Furthermore, the LPS‐response of GM‐Mφ could only be blocked by about fourfold higher concentration of anti‐CD14 antibody compared with SER‐Mφ. In summary, GM‐CSF promotes the generation of a pro‐inflammatory type of Mφ in two different ways: first, the down‐regulation of autocrine IL‐10 production increases the release of cytokines such as IL‐6 and TNF‐α and second, the up‐regulation of membrane and soluble CD14 expression leads to a higher sensitivity towards LPS‐stimulation.
Introduction
Macrophages (Mφ) are important effector cells of the immune system. They arise from circulating blood monocytes (MO) which migrate into the various tissues and body cavities where signals in the microenvironment induce the tissue‐specific differentiation of Mφ.1 In vitro maturation of blood MO in the presence of serum is a model system for this differentiation process.2,3 During this maturation step MO undergo characteristic changes in antigen phenotype4 and function.5 Most of the effector functions of Mφ, e.g. tumour cytotoxicity, accessory and microbicidal activity, are dependent on a special type of differentiation. Granulocyte–macrophage colony‐stimulating factor (GM‐CSF) has multiple effects on Mφ differentiation and modulates antigen phenotype, function and survival. GM‐CSF stimulates, for example, an increased expression of surface antigens like CD32,6 CD1a,b,c,7 CD11b,8 integrin avβ3/CD519 and human leucocyte antigen (HLA) ‐DR.10 Conflicting reports have been published on the influence on CD14 expression. Whereas some authors found a down‐regulation of CD14 after GM‐CSF treatment,11,12 or no effect,8,13,14 others report on an increased CD14 expression after GM‐CSF treatment.15 In addition, GM‐CSF has an impact on the functional activation of MO/Mφ. It stimulates the secretion of interleukin‐8 (IL‐8),16 G‐CSF17 and M‐CSF18 by blood MO and induces the mRNA for tumour necrosis factor‐α (TNF‐α).19,20 In combination with interferon‐γ (IFN‐γ) the release of TNF‐α21 and tissue‐type plasminogen‐activator is induced.22 In addition, MO are primed for an enhanced TNF‐α release after stimulation with lipopolysaccharide (LPS) and phorbol myristate acetate (PMA).13,19 Other functions, such as tumoricidal activity,23 killing of Leishmania donovani24 and accessory function25 are also enhanced by GM‐CSF. Little is known about the possible mechanisms involved in the regulation of MO/Mφ function by GM‐CSF. In this study we investigated the modulatory effect of GM‐CSF on serum‐induced MO differentiation. We found that GM‐CSF up‐regulated the expression of the LPS‐receptor CD14 on Mφ when added to serum‐containing cultures. This enhanced CD14 expression was accompanied by an increased secretion of TNF‐α and IL‐6 and diminished IL‐10 secretion, respectively, upon LPS‐stimulation. We conclude that GM‐CSF switches the differentiation towards a proinflammatory type of Mφ.
Materials and methods
Cell separation and culture
Peripheral blood mononuclear cells (PBMC) were obtained by leucapheresis of healthy donors, followed by density gradient centrifugation over Ficoll–Paque (Pharmacia, Freiburg, Germany). MO were isolated from PBMC by counter‐current elutriation (J6M‐E Beckmann centrifuge) using a large‐volume chamber (50 ml) and a JE‐5 rotor at 1100 g and a flow rate of 110 ml/min in Hanks' balanced salt solution supplemented with 8% autologous human plasma. Elutriated MO were > 90% pure as determined by morphology and antigen phenotype. Purified MO were cultured for 7 days on Teflon foils (Biofolie 25, Heraeus, Hanau, Germany) at a cell density of 106 cells/ml in RPMI‐1640 (Biochrom, Berlin, Germany) supplemented with antibiotics (50 U/ml penicillin and 50 mg/ml streptomycin, Gibco, Berlin, Germany), l‐glutamine (2 mm, Gibco) and 2% pooled human AB‐group serum (Sigma, Deisenhofen, Germany) with or without GM‐CSF (kindly provided by Sandoz, Nürnberg, Germany). After the 7‐day culture period cells were harvested and washed twice in RPMI‐1640.
Production of MO/Mφ supernatants
MO‐derived Mφ were seeded into six‐well microtitre plates (Falcon/Becton Dickinson, Heidelberg, Germany) at 106 cells/2 ml supplemented RPMI‐1640 with 2% pooled human AB‐group serum. Cells were stimulated for 24 hr with or without LPS at various concentrations. Supernatants were harvested, filtered through 0·22 mm filters (Millipore, Eschborn, Germany) and stored at – 20°. In selected experiments cells were preincubated for 30 min with various concentrations of the monoclonal anti‐CD14 (My4, Coulter, Krefeld, Germany) before LPS was added.
Detection of cytokines and soluble CD14
TNF‐α, IL‐6, IL‐10 and soluble CD14 were measured by commercially available sandwich‐enzyme‐linked immunosorbent assay (ELISA; TNF‐α and IL‐6, Biermann, Bad Nauheim, Germany; IL‐10, Coulter‐Immunotech, Hamburg, Germany; sCD14, IBL, Hamburg, Germany).
Fluorescence‐activated cell sorter (FACS) analysis
Mφ were washed twice with washing buffer [phosphate‐buffered saline (PBS), 1% Sandoglobin, 0·1% sodium azide] and then incubated at a cell density of 5 × 105 Mφ/ml for 30 min at 4° with anti‐CD14 (My4, Coulter). Polyclonal mouse immunoglobulins were used as negative control (Coulter). After this incubation step, cells were washed twice with washing buffer and incubated for another 30 min with a fluorescein isothiocyanate (FITC) ‐conjugated goat anti‐mouse antibody (Jackson Immuno Research, West Grove, PA). Then Mφ were washed again and fixed with 1% paraformaldehyde in PBS. Analysis was performed using a FACScan (Becton‐Dickinson, San Jose, CA).
For the determination of LPS‐binding, 5 × 105 Mφ/ml were incubated for 1 hr at 4° with LPS in the presence of 10% human serum. Cells were washed with washing buffer and then incubated for another 20 min with anti‐LPS antibody [S32‐32, immunoglobulin G2a (IgG2a), kindly provided by Dr L. Brade and Dr E. Grage‐Griebenow, Borstel, Germany]. Controls were incubated with IgG2a‐Isotype. After this incubation step, cells were washed again with washing buffer and incubated for another 20 min with FITC‐conjugated goat anti‐mouse antibody (Jackson Immuno Research). Analysis was performed using a FACScan (Becton‐Dickinson).
Results
Long‐term incubation with GM‐CSF modulates the LPS response of Mφ
MO to Mφ differentiation was induced by culturing MO for 7 days with 2% human serum (SER‐Mφ) or with 2% human serum in the presence of 100 ng/ml GM‐CSF (GM‐Mφ). To analyse the functional properties of the resulting Mφ populations, we investigated the LPS response of SER‐Mφ and GM‐Mφ. Cells were harvested and stimulated for another 24 hr with 100 ng/ml LPS. Supernatants were analysed for TNF‐α, IL‐6 and IL‐10. Table 1 shows that the release of TNF‐α and IL‐6 was enhanced after GM‐CSF treatment, whereas the IL‐10 production was diminished. The effect of GM‐CSF was dose dependent, with an optimum at 100 ng/ml GM‐CSF (data not shown). Next we investigated whether the change in LPS‐stimulated TNF‐α secretion would occur independent of the LPS dose and found that the effect was more pronounced at lower LPS concentrations (Fig. 1).
Table 1.
Spontaneous and LPS‐stimulated cytokine release after long‐term incubation wth GM‐CSF
| TNF‐α* | IL‐6 | IL‐10 | |
|---|---|---|---|
| SER‐Mφ† | |||
| Control | 31 ± 7‡ | 1·5 ± 0·8 | 5·5 ± 1·8 |
| LPS | 12 780 ± 2612 | 2832 ± 1193 | 980 ± 231 |
| GM‐Mφ | |||
| Control | 320 ± 83 | 23 ± 13 | 20·5 ± 9 |
| LPS | 33 460 ± 5377 | 8810 ± 1498 | 327 ± 45 |
Cytokines were measured by commercially available ELISA. Data are given as pg/ml.
MO were cultured for 7 days in serum alone (SER‐Mφ) or in serum with GM‐CSF (GM‐Mφ). Cells were harvested and cultured for another 24 hr with or without 100 ng/ml LPS.
Data represent the mean of at least three experiments with different donors. Spontaneous cytokine secretion was only statistically significantly different for TNF‐α (unpaired t‐test P < 0·002). For the LPS‐stimulated cytokine response all values were statistically significantly different (unpaired t‐test, P < 0·005 for TNF‐α and IL‐6; P < 0·0006 for IL‐10).
Figure 1.
Modulation of cytokine secretion by GM‐CSF is dependent on the LPS dose. MO were cultured for 7 days in serum alone (SER‐Mφ) or with serum plus GM‐CSF (GM‐Mφ). Cells were harvested and stimulated for 24 hr with various LPS concentrations in the presence of 2% serum. TNF‐α was measured by commercially available ELISA. One representative experiment out of three is shown.
Spontaneous release of cytokines after long‐term incubation with GM‐CSF
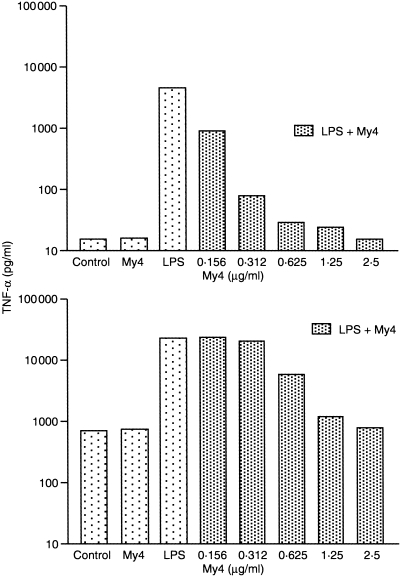
We were interested whether the increase in TNF‐α and IL‐6 secretion after long‐term incubation with GM‐CSF is due to a direct stimulation by GM‐CSF or reflects a priming of Mφ to a following LPS stimulus. Therefore we investigated the capacity of GM‐CSF to induce cytokines by itself after long‐term and short‐term exposure. MO were cultured for 7 days with serum with or without GM‐CSF. Mφ were harvested, cultured for another 24 hr and supernatants were analysed for TNF‐α, IL‐6 and IL‐10. Serum‐derived Mφ showed no spontaneous release of cytokines, whereas Mφ cultured for 7 days in the presence of serum plus GM‐CSF released TNF‐α spontaneously (Table 1). To examine whether spontaneous TNF‐α release would also be found after short‐time incubation with GM‐CSF, SER‐Mφ were stimulated for 24 hr with GM‐CSF. No cytokine release was seen under such conditions, indicating that the spontaneous TNF‐α release is not due to a short‐term stimulatory effect of GM‐CSF (data not shown). We next tried to block spontaneous cytokine secretion by anti‐CD14 antibody to exclude that a LPS‐contamination was responsible for the effect, yet TNF‐α secretion was not blocked by this treatment (Fig. 4b).
Figure 4.
Inhibition of TNF‐α release by CD14 antibody in SER‐Mφ (a) and GM‐Mφ (b). MO were cultured for 7 days in serum alone (SER‐Mφ) or in serum plus GM‐CSF (GM‐Mφ). Cells were harvested and preincubated for 30 min with anti‐CD14 antibody at the indicated concentrations in six‐well plates. Control cells were cultured without antibody. Then cells were incubated for another 24 hr with 50 pg/ml LPS. TNF‐α was measured by commercially available ELISA. One representative experiment out of three is shown.
The LPS‐receptor (CD14) is up‐regulated by GM‐CSF culture – correlation of CD14 expression and LPS binding
As CD14 is known as a receptor for LPS we analysed whether the increased sensitivity of Mφ to LPS stimulation after GM‐CSF culture may be due to an enhanced expression of CD14. Cells were cultured for 7 days with or without GM‐CSF and CD14 antigen expression was analysed by FACS. The percentage of CD14‐positive Mφ was comparable under both culture conditions, but the mean fluorescence intensity was always higher in GM‐Mφ (Fig. 2a). The increase in CD14 expression was not found after short‐term (24 hr) exposure to GM‐CSF, on the contrary, short‐term exposure to GM‐CSF even resulted in a slight decrease of CD14 expression (data not shown). In addition, we analysed whether the increase in CD14 expression would be paralled by an enhanced LPS binding of GM‐Mφ. Accordingly, GM‐Mφ showed an increase in LPS binding (Fig. 2b).
Figure 2.
FACS analysis of CD14 expression (a) and LPS binding (b). MO were cultured for 7 days in serum alone (SER‐Mφ) or with serum plus GM‐CSF (GM‐Mφ). Cells were harvested and FACS analysis for CD14 expression or LPS binding was performed according to the Materials and Methods. One representative experiment out of four is shown.
Secretion of soluble CD14 is increased by GM‐CSF
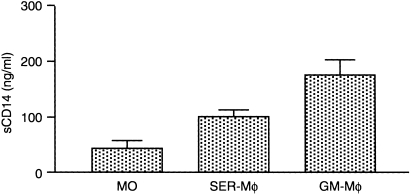
CD14 can also be cleaved from the cell membrane and is found in the supernatants of MO/Mφ. As GM‐Mφ showed a higher CD14 expression compared to control cultures, we analysed the sCD14 content of supernatants of SER‐Mφ and GM‐Mφ after 7 days of culture. We found that addition of GM‐CSF to the culture medium lead to a twofold increase in spontaneous sCD14 secretion as compared to control cultures without GM‐CSF (Fig. 3).
Figure 3.
Soluble CD14 release by SER‐Mφ and SER/GM‐Mφ. MO were cultured for 7 days in serum alone (SER‐Mφ) or with serum plus GM‐CSF (GM‐Mφ). Mφ were harvested and cultured for another 24 hr with 2% serum in six‐well plates. Soluble CD14 was measured by ELISA. Data represent the mean ± SEM of at least three experiments with different donors (The means of sCD14 secretion of SER‐Mφ versus GM‐Mφ are significantly different by unpaired t‐test P < 0·05).
Blocking of CD14 on SER‐Mφ and SER/GM‐Mφ
LPS stimulation with low LPS concentrations can be blocked by anti‐CD14 antibodies. As the amount of soluble and membrane‐bound CD14 was always higher after GM‐CSF culture, we were interested whether this would influence the capacity of anti‐CD14 antibodies to block the LPS‐response in those Mφ. Therefore we stimulated SER‐Mφ and GM‐Mφ after 7 days of culture for 24 hr with LPS and tried to block the cytokine response by preincubation with anti‐CD14 antibodies. We found that TNF‐α secretion by GM‐Mφ could only be blocked by about fourfold higher concentrations of CD14 antibody compared to SER‐Mφ (Fig. 4a,b).
Discussion
In this study we analysed the effect of GM‐CSF on the serum‐induced differentiation of MO into Mφ with respect to expression of surface antigens and function. We found a correlation between an increased expression of soluble and membrane‐bound CD14 and LPS‐binding. This was paralled by an up‐regulation of LPS‐stimulated TNF‐α and IL‐6 release of Mφ, especially after exposure to low LPS concentrations. As CD14 is known to be the most important receptor for LPS, the increase in TNF‐α and IL‐6 production could in part be due to the increased CD14 expression, leading to an enhanced sensitivity of Mφ to LPS‐stimulation. Accordingly, Martin et al. have shown that the CD14 expression on THP‐1 cells mediates the endotoxin responsiveness of those cells as measured by the induction of different cytokines, e.g. TNF‐α.26 In contrast, Gessani et al. reported that the increased cytokine secretion of human Mφ compared to MO is independent of the CD14 expression even though those Mφ showed an increased CD14 expression and LPS–FITC‐binding capacity.27 In our experiments, the increased TNF‐α and IL‐6 secretion can only partially be explained by an increased CD14 expression and LPS binding as the LPS‐response was differentially regulated by GM‐CSF. In contrast to TNF‐α and IL‐6, the LPS‐stimulated IL‐10 secretion was decreased after GM‐CSF treatment. IL‐10 is known as (autocrine) inhibitor of pro‐inflammatory cytokines like TNF‐α and IL‐6.28 Therefore, the down‐modulation of IL‐10 production by GM‐CSF in Mφ during differentiation may further enhance the up‐regulation of TNF‐α and IL‐6. Accordingly, the addition of an anti‐IL‐10 antibody led to an two‐ to threefold increase of the LPS‐stimulated TNF‐α secretion in SER‐Mφ (data not shown). To our knowledge, no in vitro data are available on the influence of GM‐CSF on IL‐10 secretion. Data from our laboratory show that, in whole blood samples of patients treated with GM‐CSF, the LPS‐stimulated release of TNF‐α and IL‐6 increased, whereas IL‐10 production decreased.29 In contrast to IL‐10, several investigations deal with the effect of GM‐CSF on TNF‐α regulation in MO/Mφ. Cannistra et al. showed that short‐term incubation with GM‐CSF for 18 hr primes human MO for an enhanced TNF‐α secretion after stimulation with LPS and PMA.19 Similar results have been obtained by Heidenreich et al. with murine Mφ.20 In accordance with our results, Hart et al. found that long‐term incubation with GM‐CSF primes human Mφ for an enhanced LPS‐stimulated TNF‐α secretion, however, they found no change in CD14 expression.13 The same results, regarding CD14 expression, were obtained by two other authors.8,14 Even a down‐regulation of membrane and soluble CD14 after short‐term exposure to GM‐CSF was reported by Hogasen et al. and Kruger et al.11,12 Only Dimri et al. found an increase in CD14 expression after 2 weeks of in vitro culture in the presence of GM‐CSF.15 These conflicting results can in part be explained by the various culture conditions and methods of CD14 determination used by the different authors. Short‐term exposure to GM‐CSF also resulted in a slight down‐regulation of CD14 expression in our experiments (data not shown). However, long‐term incubation always lead to an increase in CD14 expression, measured as an up‐regulation in the mean fluorescence intensity. No change was found based on the number of CD14‐positive cells which was determined by Geissler et al. and Eischen et al.8,14 In addition, in our studies 2% pooled human serum was used as medium supplement whereas most other investigators used 10% fetal calf serum,8,12,13 newborn bovine serum,15 or serum‐free conditions.11 In contrast to the conflicting results regarding the effect of GM‐CSF on CD14 expression, unanimity is found on the observation that GM‐CSF positively influences the survival of MO/Mφ,13–15 which was also true for our system (data not shown). In human MO it has been shown that down‐regulation or removal of the CD14 receptor expression triggers apoptosis whereas up‐regulation promotes cell survival.30 The increased CD14 expression of Mφ may therefore be one explanation for the higher percentage of Mφ survival after GM‐CSF treatment.
In summary, we have shown that GM‐CSF modulates the serum‐induced Mφ differentiation leading to an inflammatory type of Mφ with increased spontaneous and LPS‐stimulated TNF‐α and IL‐6 secretion and decreased production of IL‐10. In addition, the up‐regulation of soluble and membrane CD14 after GM‐CSF culture may lead to an enhanced sensitivity of Mφ, especially towards lower LPS concentrations. Further investigations will clarify the mechanisms involved in the inverse regulation of TNF‐α and IL‐10 after GM‐CSF treatment in human Mφ.
References
- 1.Rutherford MS, Witsell A, Schook LB. Mechanisms generating functionally heterogeneous macrophages: chaos revisited. J Leukoc Biol. 1993;53:602. doi: 10.1002/jlb.53.5.602. [DOI] [PubMed] [Google Scholar]
- 2.Johnson WD, Jr, Mei B, Cohn ZA. The separation, long‐term cultivation, and maturation of the human monocyte. J Exp Med. 1977;146:1613. doi: 10.1084/jem.146.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreesen R, Picht J, Lohr GW. Primary cultures of human blood‐born macrophages grown on hydrophobic teflon membranes. J Immunol Methods. 1983;56:295. doi: 10.1016/s0022-1759(83)80019-2. [DOI] [PubMed] [Google Scholar]
- 4.Andreesen R, Brugger W, Scheibenbogen C, et al. Surface phenotype analysis of human monocyte to macrophage maturation. J Leukoc Biol. 1990;47:490. doi: 10.1002/jlb.47.6.490. [DOI] [PubMed] [Google Scholar]
- 5.Scheibenbogen C, Andreesen R. Developmental regulation of the cytokine repertoire in human macrophages: IL‐1, IL‐6, TNF‐alpha, and M‐CSF. J Leukoc Biol. 1991;50:35. doi: 10.1002/jlb.50.1.35. [DOI] [PubMed] [Google Scholar]
- 6.Rossman MD, Ruiz P, Comber P, Gomez F, Rottem M, Schreiber AD. Modulation of macrophage Fc gamma receptors by rGM‐CSF. Exp Hematol. 1993;21:177. [PubMed] [Google Scholar]
- 7.Kasinrerk W, Baumruker T, Majdic O, Knapp W, Stockinger H. CD1 molecule expression on human monocytes induced by granulocyte‐macrophage colony‐stimulating factor. J Immunol. 1993;150:579. [PubMed] [Google Scholar]
- 8.Geissler K, Harrington M, Srivastava C, Leemhuis T, Tricot G, Broxmeyer HE. Effects of recombinant human colony stimulating factors (CSF) (granulocyte‐macrophage CSF, granulocyte CSF, and CSF‐1) on human monocyte/macrophage differentiation. J Immunol. 1989;143:140. [PubMed] [Google Scholar]
- 9.De Nichilo MO, Burns GF. Granulocyte‐macrophage and macrophage colony‐stimulating factors differentially regulate alpha v integrin expression on cultured human macrophages. Proc Natl Acad Sci USA. 1993;90:2517. doi: 10.1073/pnas.90.6.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falk LA, Wahl LM, Vogel SN. Analysis of Ia antigen expression in macrophages derived from bone marrow cells cultured in granulocyte‐macrophage colony‐stimulating factor or macrophage colony‐stimulating factor [published erratum appears in J Immunol 1988 Jul 15; 141 (2): 709] J Immunol. 1988;140:2652. [PubMed] [Google Scholar]
- 11.Hogasen AK, Hestdal K, Abrahamsen TG. Granulocyte‐macrophage colony‐stimulating factor, but not macrophage colony‐stimulating factor, suppresses basal and lipopolysaccharide‐stimulated complement factor production in human monocytes. J Immunol. 1993;151:3215. [PubMed] [Google Scholar]
- 12.Kruger M, Van de Winkel JG, De Wit TP, Coorevits L, Ceuppens JL. Granulocyte‐macrophage colony‐stimulating factor down‐regulates CD14 expression on monocytes. Immunology. 1996;89:89. doi: 10.1046/j.1365-2567.1996.d01-707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hart PH, Jones CA, Finlay Jones JJ. Monocytes cultured in cytokine‐defined environments differ from freshly isolated monocytes in their responses to IL‐4 and IL‐10. J Leukoc Biol. 1995;57:909. doi: 10.1002/jlb.57.6.909. [DOI] [PubMed] [Google Scholar]
- 14.Eischen A, Vincent F, Bergerat JP, et al. Long term cultures of human monocytes in vitro. Impact of GM‐CSF on survival and differentiation. J Immunol Methods. 1991;143:209. doi: 10.1016/0022-1759(91)90046-i. [DOI] [PubMed] [Google Scholar]
- 15.Dimri R, Nissimov L, Keisari Y. Effect of human recombinant granulocyte‐macrophage colony‐stimulating factor and IL‐3 on the expression of surface markers of human monocyte‐derived macrophages in long‐term cultures. Lymphokine Cytokine Res. 1994;13:239. [PubMed] [Google Scholar]
- 16.Takahashi GW, Andrews DF, Lilly MB, Singer JW, Alderson MR. Effect of granulocyte‐macrophage colony‐stimulating factor and interleukin‐3 on interleukin‐8 production by human neutrophils and monocytes. Blood. 1993;81:357. [PubMed] [Google Scholar]
- 17.Oster W, Lindemann A, Mertelsmann R, Herrmann F. Granulocyte‐macrophage colony‐stimulating factor (CSF) and multilineage CSF recruit human monocytes to express granulocyte CSF. Blood. 1989;73:64. [PubMed] [Google Scholar]
- 18.Horiguchi J, Warren MK, Kufe D. Expression of the macrophage‐specific colony‐stimulating factor in human monocytes treated with granulocyte‐macrophage colony‐stimulating factor. Blood. 1987;69:1259. [PubMed] [Google Scholar]
- 19.Cannistra SA, Rambaldi A, Spriggs DR, Herrmann F, Kufe D, Griffin JD. Human granulocyte‐macrophage colony‐stimulating factor induces expression of the tumor necrosis factor gene by the U937 cell line and by normal human monocytes. J Clin Invest. 1987;79:1720. doi: 10.1172/JCI113012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heidenreich S, Gong JH, Schmidt A, Nain M, Gemsa D. Macrophage activation by granulocyte/macrophage colony‐stimulating factor. Priming for enhanced release of tumor necrosis factor‐alpha and prostaglandin E2. J Immunol. 1989;143:1198. [PubMed] [Google Scholar]
- 21.Hart PH, Whitty GA, Piccoli DS, Hamilton JA. Synergistic activation of human monocytes by granulocyte‐macrophage colony‐stimulating factor and IFN‐gamma. Increased TNF‐alpha but not IL‐1 activity. J Immunol. 1988;141:1516. [PubMed] [Google Scholar]
- 22.Hart PH, Vitti GF, Burgess DR, Whitty GA, Royston K, Hamilton JA. Activation of human monocytes by granulocyte‐macrophage colony‐stimulating factor: increased urokinase‐type plasminogen activator activity. Blood. 1991;77:841. [PubMed] [Google Scholar]
- 23.Grabstein KH, Urdal DL, Tushinski RJ, et al. Induction of macrophage tumoricidal activity by granulocyte‐macrophage colony‐stimulating factor. Science. 1986;232:506. doi: 10.1126/science.3083507. [DOI] [PubMed] [Google Scholar]
- 24.Weiser WY, Van Niel A, Clark SC, David JR, Remold HG. Recombinant human granulocyte/macrophage colony‐stimulating factor activates intracellular killing of Leishmania donovani by human monocyte‐derived macrophages. J Exp Med. 1987;166:1436. doi: 10.1084/jem.166.5.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer HG, Frosch S, Reske K, Reske Kunz AB. Granulocyte‐macrophage colony‐stimulating factor activates macrophages derived from bone marrow cultures to synthesis of MHC class II molecules and to augmented antigen presentation function. J Immunol. 1988;141:3882. [PubMed] [Google Scholar]
- 26.Martin TR, Mongovin SM, Tobias PS, et al. The CD14 differentiation antigen mediates the development of endotoxin responsiveness during differentiation of mononuclear phagocytes. J Leukoc Biol. 1994;56:1. doi: 10.1002/jlb.56.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Gessani S, Testa U, Varano B, et al. Enhanced production of LPS‐induced cytokines during differentiation of human monocytes to macrophages. Role of LPS receptors. J Immunol. 1993;151:3758. [PubMed] [Google Scholar]
- 28.Moore KW, O'garra A, de Waal Malefyt R, Vieira P, Mosmann TR. Interleukin‐10. Annu Rev Immunol. 1993;11:165. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 29.Hennemann B, Kreutz M, Rehm A, Andreesen R. Effect of granulocyte‐macrophage colony‐stimulating factor treatment on phenotype, cytokine release and cytotoxicity of circulating blood monocytes and monocyte‐derived macrophages. Br J Haematol. 1998;102:1197. doi: 10.1046/j.1365-2141.1998.00922.x. [DOI] [PubMed] [Google Scholar]
- 30.Heidenreich S, Schmidt M, August C, Cullen P, Rademaekers A, Pauels HG. Regulation of human monocyte apoptosis by the CD14 molecule. J Immunol. 1997;159:3178. [PubMed] [Google Scholar]