Abstract
We compared nasal and vaginal immunizations using attenuated herpes simplex virus type‐2 (HSV‐2) for protection against vaginal infection with wild‐type HSV‐2. Mice were immunized once intranasally, intravaginally after progestin (DP) treatment, or intravaginally with scarification after oestradiol treatment. Compared with vaginal immunizations, nasal immunization did not increase immunoglobulin A (IgA) plasma cell numbers in the vagina or elicit a higher antiviral IgA titre in vaginal secretions. Both types of vaginal immunizations increased the number of immunoglobulin G (IgG) plasma cells in the vagina and the secretion/serum titre ratio of IgG antiviral antibody, indicating local production of virus‐specific IgG in these groups. Cell‐mediated immunity in the vagina, as indicated by memory T‐cell secretion of interferon‐γ (IFN‐γ) in situ 20 hr after HSV‐2 challenge, was essentially equivalent in the vaginally immunized groups but significantly lower in the nasal group, while lymphocyte recruitment to the vagina was similar in all three groups. All three immunizations protected all mice from neurological disease after challenge, but vaginal DP immunization induced the greatest immunity against reinfection of the vaginal epithelium.
Introduction
Vaginal immunization of mice with attenuated herpes simplex virus type‐2 (HSV‐2) elicits strong immunity against vaginal challenge infection by wild‐type HSV‐2. Arguably, this is the strongest immune protection yet observed against any infection of the female genital tract.1 The virus‐neutralizing antibody in vaginal secretions of immunized mice is mainly immunoglobulin G (IgG).2 This is different from the situation in the intestine and upper respiratory tract, where the main protective antibody is secretory immunoglobulin A (S‐IgA), but it is consistent with the theory that the mouse vagina is a poor inductive site for an IgA response because it lacks mucosal lymphoid nodules.3 The importance of S‐IgA for protective immunity in the intestine and upper respiratory tract has led to the hypothesis that a vigorous IgA response will be needed to achieve optimum immune protection in the female genital tract.4–6 It is thus of interest to investigate whether an immunization producing a stronger IgA response in the vagina would provide better immunity than that observed after vaginal immunization. Intranasal immunization elicits IgA responses at many mucosal sites, including the female genital tract, and in recent years the view has emerged that nasal immunization, more than immunization at any other IgA‐inductive site, has the potential to induce superior protection against genital tract infections because of its ability to induce IgA responses there.6 Thus, the purpose of the present study was to determine whether nasal immunization with attenuated HSV‐2 would induce a relatively strong IgA response in the vagina and give protection superior to that induced by vaginal immunization against vaginal challenge infection.
Materials and methods
Experimental design
Vaginal immunization in mice is strongly dependent on the hormonal status of the animals. We therefore wished to compare nasal immunization with two forms of vaginal immunization, using mice that were pretreated either with a progestin (DP; Upjohn Co., Kalamazoo, MI) or with oestradiol benzoate. Female BALB/c mice were purchased from Harlan/Sprague–Dawley (Indianapolis, IN) and were 12 weeks old at the start of treatment. Age‐matched mice (120 in total) were allocated to four groups of 30 mice each. Three groups were anaesthetized with tribromoethanol and immunized with attenuated HSV‐27 as follows: mice in one group were pretreated with 2·0 mg of DP and immunized 6 days later by intravaginal inoculation of 20 µl of virus at 1·5 × 106 plaque‐forming units (PFU)/ml (vaginal‐DP group). The vaginal epithelium of such mice is thin and mucified and is readily infected with HSV‐2, as it is during dioestrus and in early pregnancy, whereas the epithelium is thick and cornified and highly resistant to HSV‐2 infection during oestrus and after oestradiol treatment.8 Mice in the second group received 0·10 µg of oestradiol benzoate and 3 days later were immunized in the vagina with 20 µl of virus at 6·0 × 106 PFU/ml after scarification of the vaginal epithelium with a burred needle (vaginal‐E‐scar group). The vigorous immune responses observed in all mice in this group suggest that scarification breached a permeability barrier and allowed virus to enter and infect the thickened epithelial layer. However, the need for a higher dose of virus in this group suggests that scarification permeabilized the epithelium less effectively than progestin treatment. The third group was immunized intranasally using 20 µl of virus at 1·5 × 106 PFU/ml. These mice were not pretreated with steroids before immunization, but note below that they were later pretreated with DP before sample collection or vaginal challenge with wild‐type virus. The fourth group served as a non‐immunized control. The doses of attenuated virus used for immunization were selected because in preliminary studies they elicited similar mean titres of antiviral IgG antibody in serum of the three groups, thus facilitating comparison of the groups.
Eleven weeks after immunization, each group of 30 mice was subdivided into three groups of 10. One group of 10 mice was treated with DP as described above. Six days later, vaginal secretions and sera were collected for measurements of IgA and IgG anti‐HSV‐2 titres, and vaginae were collected for counts of plasma cells and lymphocytes. These measurements evaluated immunity at the time of vaginal challenge with wild‐type virus. The second group of 10 mice was examined 20 hr after vaginal challenge with 20 µl of wild‐type HSV‐2 at 1·0 × 107 PFU/ml.7 From these mice, vaginal secretions were collected for determination of interferon‐γ (IFN‐γ) levels and shed virus protein measurements, and vaginae were collected for measurements of per cent epithelial infection and for plasma cell and lymphocyte counts. The shed virus protein and per cent epithelial infection measurements indicated the magnitude of the challenge infections and thus the effectiveness of immunity in each group; IFN‐γ measurements indicated the in situ memory T‐cell response to the challenge antigen; and the lymphocyte counts evaluated lymphocyte recruitment to the vagina in response to the challenge antigen. From the remaining group, vaginal secretions were collected for shed virus protein measurements at 48 hr after challenge, and these mice were examined for signs of illness 8–14 days after vaginal challenge. Both measurements provided a further evaluation of the strength of immunity in each group. Mice to be challenged with HSV‐2 in the vagina were pretreated 6 days previously with DP, as described above.
Measurement of antibodies and IFN‐γ in vaginal secretions and sera
Immunoglobulins were extracted from vaginal secretions into 200 µl of Tris‐buffered saline, as previously described.2,9 Serum was obtained by standard methods. Titres of antiviral IgG and IgA antibody were measured by enzyme‐linked immunosorbent assay (ELISA) using minor modifications of previously described methods.9,10 Titre was defined as the reciprocal of the sample dilution at which the reaction had decreased to 10 times the mean value of non‐immune samples in the case of IgG, or to three standard deviations above the mean of non‐immune samples for IgA. Titres of IgG2a and IgG1 anti‐HSV‐2 were measured as described for IgG except that the antibodies were goat anti‐mouse IgG1 and goat anti‐mouse IgG2a (Southern Biotechnology Assoc. Inc., Birmingham, AL) and alkaline phosphatase donkey anti‐goat IgG (Jackson Immunoresearch Labs, West Grove, PA).
The IFN‐γ concentration in vaginal secretions was measured by standard methods using rat anti‐mouse IFN‐γ (clone R4‐6A2; Access Biomedical, San Diego, CA) as capture antibody, rabbit anti‐mouse IFN‐γ (Biosource International Inc., Camarillo, CA) as detection antibody, and alkaline phosphatase–goat anti‐rabbit IgG (Zymed, San Francisco, CA) as the enzyme conjugate. Recombinant dimeric mouse IFN‐γ (PharMingen, San Diego, CA) was used as the standard. The titres of shed virus proteins were measured as described previously.2,11 Titre was defined as the reciprocal of the dilution at which the reaction decreased to three standard derivations above the background reaction in samples from immune/non‐challenged mice. The resulting titres were correlated with the percentage of the vaginal epithelium that was infected with the virus, indicating that this method measures virus protein that is shed into the vaginal lumen from infected epithelial cells.7
Evaluation of vaginal tissues by immunofluorescence labelling
Quantification of epithelial infection was carried out as previously described.7,8 The lengths of HSV‐2‐stained segments and total lengths of vaginal epithelium were measured in histological sections sampled from four areas of each vagina. Immunolabelling of lymphocytes, plasma cells and major histocompatibility complex (MHC) class II was carried out as previously described.9 Lymphocytes were counted in four randomly selected high‐power fields (40×) from each of two separate regions of vagina. Plasma cells were counted in one complete cross‐section from each of four separate regions of vagina from each mouse. The staining of MHC class II antigens in the vaginal epithelium was evaluated in two coded sections from each mouse as: nil (0), weak (1), moderate (2), or bright (3), along with the proportion of epithelium with each kind of staining. The average staining in each mouse was obtained as half the sum of all the products of staining intensity multiplied by the proportion of epithelium showing that staining in the two sections. The group mean staining was the average score of the 10 mice in each group, and ranged from 0·0 for no epithelial staining in any mice to 3·0 for bright staining in all of the epithelium of all mice.
Results
Antibody responses
Immunization with attenuated HSV‐2 at both vaginal and nasal sites elicited virus‐specific IgG antibodies that were detected 11 weeks later in both sera and vaginal secretions (Table 1). Geometric mean IgG titres were essentially identical in the sera of mice that had been immunized in the vagina after progestin treatment or after oestradiol treatment and scarification, while the mean titre in the nasally immunized mice was lower by less than one twofold dilution. Our aim to elicit similar serum antibody titres in the three immunization groups was thus reasonably successful. Moreover, IgG2a/IgG1 subclass ratios in the three immunized groups were not significantly different, indicating that differences in immunity were probably not the result of selective induction of the highly protective IgG2a subclass.12 Antiviral IgG titres in vaginal secretions, expressed as a fraction of serum antibody titres, were significantly different in the three groups. The relatively high secretion/serum ratio in the vaginal‐DP group suggests local production of specific viral antibody in the vagina in this group and is consistent with results of a previous study.9 The mean antiviral IgA titre in vaginal secretions of the nasally immunized group was not higher than in either of the vaginally immunized groups.
Table 1.
Antibody responses to immunization with attenuated herpes simplex virus type‐2 (HSV‐2)
| Log2.5 GMT and GMT of IgG anti‐HSV‐2 in: | |||||
|---|---|---|---|---|---|
| Site of immunization | Serum* | Vagina | Log2.5 GMT ratio and GMT ratio of IgG2a/IgG1 anti‐HSV‐2 titres in serum† | Vaginal secretion/serum ratio of IgG anti‐HSV‐2 titres (10−3)‡ | Log3 GMT and GMT of S‐IgA anti‐HSV‐2 in the vagina§ |
| Vaginal‐DP | 11.5±0.16 | 4.8±0.23 | 0.50±0.30 | 2.5±0.49 | 4.0±0.24 |
| (38000) | (81) | (1.6) | (80) | ||
| Vaginal‐E‐scar | 11.5±0.17 | 4.1±0.25 | 1.3±0.23 | 1.8±0.73 | 3.1±0.30 |
| (38000) | (43) | (3.2) | (29) | ||
| Nasal | 10.8±0.13 | 2.9±0.21 | 0.59±0.33 | 0.80±0.12 | 3.0±0.30 |
| (21000) | (14) | (1.7) | (26) | ||
The mean serum immunoglobulin G (IgG) anti‐HSV‐2 titre in the nasal immunization group was significantly lower than in either vaginal immunization group (P = 0.0076; three‐group ANOVA).
The IgG2a and IgG1 anti‐HSV‐2 titres were measured, and their ratios were calculated for each mouse. For each group, the log2.5 geometric mean titer (GMT) ratio and its standard error was calculated and used for statistical comparison of groups. The GMT ratios are presented in parentheses. The IgG2a/IgG1 anti‐HSV‐2 titre ratios were not significantly different among the three immnized groups (P = 0.15; three‐group analysis of variance ANOVA).
The mean vaginal secretion/serum IgG anti‐HSV‐2 titre ratios were significantly different (P = 0.0079; Kruskal–Wallis non‐parametric ANOVA).
These titres cannot be directly compared to IgG titres because different tirre end‐points were used. S‐IgA, secretory immunoglobulin A.
Plasma cells in the vagina
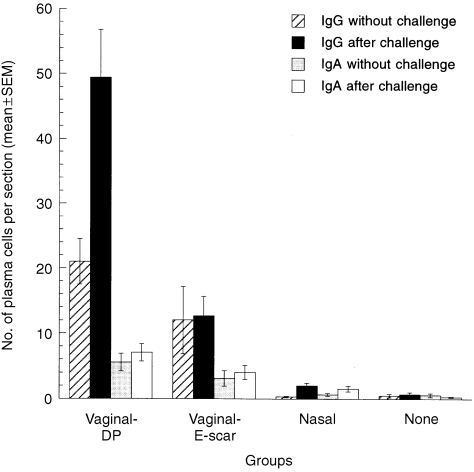
The numbers of IgG and IgA plasma cells in the vagina 11 weeks after immunization by the vaginal DP route were 42‐fold and 10‐fold higher, respectively, than in the non‐immunized group (Fig. 1), while the numbers of vaginal IgG and IgA cells in the intranasally immunized group were not significantly elevated. The numbers of both types of plasma cells in the vagina 20 hr after challenge were also much higher in the vaginal‐DP group than in non‐immunized or nasally immunized mice. The increased number of IgG plasma cells in the vagina of the vaginal‐DP group was correlated with an increased secretion/serum IgG titre ratio (Table 1), indicating that the vaginal plasma cells secreted antiviral antibody and raised the virus‐specific IgG titre in vaginal secretions. The numbers of vaginal IgG and IgA plasma cells were also higher in the vaginal‐E‐scar group both before and after challenge, but not as much as in the vaginal‐DP group.
Figure 1.
Vaginal plasma cells of immunized and non‐immunized mice before and 20 hr after vaginal challenge with wild‐type herpes simplex virus type‐2 (HSV‐2). The numbers of immunoglobulin G (IgG) and immunoglobulin A (IgA) plasma cells in the vagina were significantly higher in the vaginal‐DP group than in non‐immunized mice, with or without vaginal challenge (IgG: P < 0·0001 before challenge, P < 0·0001 after challenge; IgA: P = 0·0021 before challenge, P < 0·0001 after challenge; Mann–Whitney U‐test), and they were also significantly higher in the vaginal‐E‐scar group (IgG: P = 0·0089 before challenge, P < 0·0001 after challenge; IgA: P = 0·0011 before challenge, P = 0·0005 after challenge; Mann–Whitney U‐test). Plasma cell numbers in nasally immunized mice were not significantly different from those in non‐immunized mice without challenge (IgG: P = 0·68; IgA: P = 0·28; Mann–Whitney U‐test).
IFN‐γ in vaginal secretions
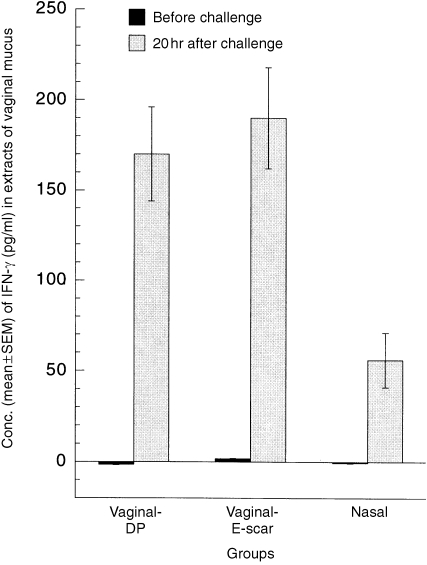
IFN‐γ was not detectable in vaginal secretions from any immunized mice before challenge, but it was readily detected in all three immunized groups 20 hr after the immune mice were challenged in the vagina with wild‐type HSV‐2 (Fig. 2). The mean IFN‐γ concentrations in the vaginal‐DP and vaginal‐E‐scar groups after challenge were essentially the same, at 170 pg/ml and 190 pg/ml, respectively, but the concentration was significantly lower in the nasally immunized group, at 56 pg/ml. MHC class II antigens were not detectable in the vaginal epithelial cells of any immunized mice before challenge or in non‐immunized mice after challenge, but their expression in the epithelium of immunized mice after challenge corresponded reasonably well to the IFN‐γ concentrations in vaginal secretions, being 2·3 ± 0·1 in the vaginal‐DP group, 1·8 ± 0·2 in the vaginal‐E‐scar group and 0·9 ± 0·2 in the nasal group.
Figure 2.
Concentrations of interferon‐γ (IFN‐γ) in extracts of vaginal mucus from immunized mice before and 20 hr after vaginal challenge with wild‐type herpes simplex virus type‐2 (HSV‐2). The mean IFN‐γ concentrations in vaginal secretions before challenge were not significantly different from 0·0 (P > 0·50 in each group, two‐tailed t‐tests). The IFN‐γ concentration in nasally immunized mice after challenge was significantly lower than in the two vaginally immunized groups (P = 0·0008, three‐group analysis of variance [anova]).
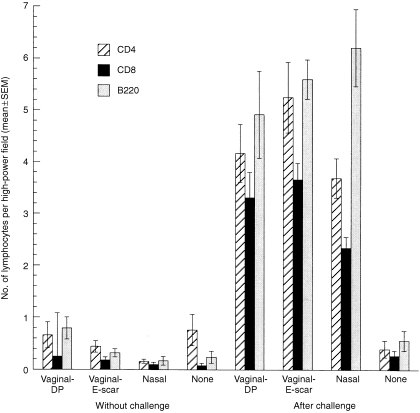
Lymphocytes in the vaginal mucosa
Few lymphocytes were present in the vaginal mucosa of immunized or non‐immunized mice without vaginal challenge or in non‐immunized mice after vaginal challenge (Fig. 3). A comparison of the lymphocyte counts in these five groups revealed no statistically significant differences in the CD4+ or CD8+ counts and only a marginally significant difference in the B220+‐cell counts. After vaginal challenge, the lymphocyte numbers were markedly increased in all three immunized groups. A comparison of the lymphocyte counts among the three immune/challenged groups revealed no statistically significant differences in the CD4+ or B220+ counts, and only a marginally significant difference in the CD8+ counts.
Figure 3.
Numbers of vaginal lymphocytes in immunized and non‐immunized mice before and 20 hr after vaginal challenge with wild‐type herpes simplex virus type‐2 (HSV‐2). The numbers of CD4+, CD8+ and B220+ lymphocytes in the vaginal mucosa were relatively small in immunized and non‐immunized mice without vaginal challenge and also in non‐immunized mice after vaginal challenge. The numbers of CD4+ cells were not significantly different among these five groups (P = 0·23), nor were the numbers of CD8+ cells (P = 0·24), while there was a marginally significant difference in the numbers of B220+ cells (P = 0·026, five‐group analysis of variance [anova]). At 20 hr after vaginal challenge, the numbers of all three lymphocyte types in the vagina were markedly increased in the three immunized groups but not in the non‐immunized group. The numbers of CD4+ cells in the immune/challenged groups were not significantly different (P = 0·15), nor were the numbers of B220+ cells (P = 0·42), while there was a marginally significant difference in the numbers of CD8+ cells (P = 0·041, three‐group anova).
Epithelial infection
Infection of the vaginal epithelium after HSV‐2 challenge was observed in all non‐immune mice and in most immunized mice (Table 2). The greatest resistance to challenge infection was observed in the vaginal‐DP group, as seen by the lower numbers of mice in which epithelial infection was detected, the decreased percentage of epithelium that was infected and in the reduced titres of shed virus protein in the vaginal lumen at both 20 hr and 48 hr after challenge. The least resistance to challenge infection was found in the nasally immunized mice. Epithelial infection was intermediate in the vaginal‐E‐scar group, but in comparison to the nasal group the differences were not statistically significant. All immunized mice resisted the neurological illness that developed in non‐immunized mice after challenge with the wild‐type virus.
Table 2.
Immunity to vaginal challenge 11 weeks after immunization with attenuated herpes simplex virus type‐2 (HSV‐2)
| Infection of vaginal epithelium at 20hr | Log3 GMT and GMT of shed virus protein in vagina | ||||
|---|---|---|---|---|---|
| Site of immunization | No. of mice* | % (mean±SEM)† | 20 hr‡ | 48 hr§ | Illness score |
| None | 9/9 | 3.9±1.1 | 6.7±0.3 | 7.8±0.1 | 3.0¶ |
| (1,600) | (5,300) | ||||
| Vaginal‐DP | 4/10 | 0.11±0.06 | 2.0±0.3 | 2.3±0.4 | 0.0** |
| (9.0) | (13) | ||||
| Vaginal‐E‐scar | 7/10 | 0.36±0.14 | 3.8±0.3 | 4.3±0.5 | 0.0 |
| (65) | (110) | ||||
| Nasal | 8/10 | 0.70±0.25 | 4.4±0.4 | 5.5±0.4 | 0.0 |
| (130) | (420) | ||||
Infection was identified by immunostaining of HSV‐2 proteins in equivalent total lengths of vaginal epithelium in four independent sections from each mouse.
The percentage of vaginal epithelium that was HSV‐2 infected was significantly different in the three immunized groups (P = 0.0003, Kruskal–Wallis non‐parametric analysis of variance ANOVA).
The log3 GMT titre in the vaginal‐DP group was significantly lower than in the other immunized groups (P < 0.001; three‐group ANOVA), whereas the log3 GMT in the vaginal‐scar and nasal groups were not significantly different (P = 0.27; two‐tailed t‐test).
The log3 GMT in the vaginal‐DP group was significantly lower than in the other immunized groups (P < 0.001; three‐group ANOVA), whereas the log3 GMT in the vaginal‐scar and nasal gruops were not significantly different (P = 0.080; two‐tailed t‐test).
All mice died or became so ill that euthanasia was desirable by 9 days after challenge.
Mice never showed any signs of illness (7).7
Discussion
The IgA response in the female genital tract after intranasal immunization with non‐replicating vaccines has been extensively studied (see References 4 and 6). Less attention has been given to immunization using live attenuated vaccines, comparisons of nasal and vaginal immunizations, cell‐mediated immunity in the genital tract, and the effectiveness of nasal immunization in preventing infections with clinically relevant pathogens of the genital tract. In the present study we compared nasal immunization of mice, using live attenuated HSV‐2, to two forms of vaginal immunization with the same virus. We found that antiviral IgG titres in serum and antiviral IgA titres in vaginal secretions were similar in the three groups, but the vaginal immunizations produced higher IgG titres in vaginal secretions, probably higher memory T‐cell responses to challenge, and better protection against challenge infection.
Our results indicate that nasal immunization failed to seed the vagina with IgA plasma cells and did not induce a higher antiviral IgA titre in vaginal secretions than immunization in the vagina. Other studies have reported that nasal immunization led to local production of specific IgA antibody in the female genital tract.13,14 It is possible that nasal immunization may cause specific IgA production by plasma cells in the uterus, rather than the vagina, and that the IgA in uterine secretions may then drain into the vaginal lumen. It is also possible that previous ELISA measurements of the specific activity of IgA antibody (titre per concentration of total immunoglobulin) were higher in secretions than in serum and thus indicated local production of specific antibody because a non‐S‐IgA standard was used for both concentration measurements. The non‐S‐IgA standard would be accurate for the serum measurements but would underestimate S‐IgA in secretions by two‐ to threefold,15 resulting in apparently higher specific activity in secretions than in serum. And while the S‐IgA in secretions at several mucosal sites is mainly caused by local production, because serum IgA has been shown to contribute little to S‐IgA at those sites, this has not been shown for the vagina and seems an unsafe assumption in progestin‐treated mice with a mucified vaginal epithelium. Nasal immunization also failed to seed the vagina with IgG plasma cells. In contrast, vaginal immunization, especially in progestin‐treated mice, markedly increased the number of IgG plasma cells in the vagina. The local IgG plasma cells were associated with high antiviral IgG titres in vaginal secretions and with higher secretion/serum titre ratios than in nasally immunized mice. Similar results were obtained in a previous study that compared vaginal immunization with parenteral immunizations in the footpads, pelvis, or peritoneal cavity.9 In that study it was shown, by comparison of specific antibody activities in secretions and serum, that the elevated secretion/serum IgG titre ratio in vaginally immunized mice was caused by local production of virus‐specific IgG in the vagina that persisted for at least 10 months. Local production of IgG after vaginal immunization in women has also been reported.16 Given the failure of parenteral and nasal immunizations, to seed IgG plasma cells into the vagina, the absence of antiviral IgG production in the vagina after parenteral immunizations and the relatively low secretion/serum IgG titre ratio after nasal immunization in the present study, it seems unlikely that nasal immunization induced significant antiviral IgG production in the vagina. However, VanCott et al.14 reported that nasal immunization in mice, using glycoprotein 160 (gp160) of human immunodeficiency virus‐1 (HIV‐1), caused local production of specific IgG in the genital tract. Further studies are needed to clarify the basis of these differing results.
The occurrence of IFN‐γ secretion in situ in the vagina 20 hr after immunized mice were challenged in the vagina with HSV‐2 demonstrates the presence of memory T cells in the vagina because this response reached a maximum within 8 hr and did not occur in non‐immunized mice.17 As cell‐mediated immunity is an important component of protection against genital tract pathogens such as HSV‐27,18 and Chlamydia trachomatis,19 it is important to know whether the number of memory T cells in the female genital tract is dependent on the site of immunization. Previous studies have suggested that memory T cells preferentially recirculate through the tissues where they were originally activated.20 In the present study, the mean titres of IgG antiviral antibody in serum and the mean IFN‐γ responses to vaginal challenge were the same in the two vaginally immunized groups. In nasally immunized mice, the antiviral IgG titre in serum was one‐half of that in the vaginally immunized groups, while the IFN‐γ response to vaginal challenge was less than one‐third of that in the vaginal groups. This suggests a slight preference of memory T cells to home to the vagina after vaginal immunization, but further studies are needed to quantify this effect and to evaluate its importance in immune protection.
Vaginal immunization using scarification in oestradiol‐treated mice provided less protection against challenge infection than did vaginal immunization in progestin‐treated mice. Serum antiviral IgG titres were essentially the same in the two groups, as were the IFN‐γ responses in situ after vaginal challenge with HSV‐2. The main difference between the two vaginally immunized groups was in the numbers of IgG and IgA plasma cells in the vagina and the titres of antiviral antibodies in vaginal secretions. Why vaginal immunization with scarification in oestradiol‐treated mice should recruit fewer IgG plasma cell precursors to the vagina is unknown, but hormonal effects on the number and localization of Langerhans' cells in the vaginal epithelium at the time of immunization21 might be involved.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (HD 17337). The authors thank Dr Mark McDermott for supplying attenuated and wild‐type HSV‐2 and Vero cell lysates, and Sheila Scillufo and Maureen Doran for their excellent technical assistance.
Glossary
Abbreviations
- DP
Depo‐Provera®
- ELISA
enzyme‐linked immunosorbent assay
- GMT
geometric mean titre
- HSV‐2
herpes simplex virus‐type 2
- IFN‐γ
interferon‐γ
- IgA
immunoglobulin A
- IgG
immunoglobulin G
- PBS
phosphate‐buffered saline
- PFU
plaque‐forming units
- S‐IgA
secretory immunoglobulin A
References
- 1.Parr MB, Parr EL. Female genital tract immunity in animal models. In: Ogra PL, Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, editors. Mucosal Immunology. 2. San Diego, CA: Academic Press, Inc; 1999. p. 1395. [Google Scholar]
- 2.Parr EL, Parr MB. Immunoglobulin G is the main protective antibody in mouse vaginal secretions after vaginal immunization with attenuated herpes simplex virus type 2. J Virol. 1997;71:8109. doi: 10.1128/jvi.71.11.8109-8115.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parr MB, Parr EL. Mucosal immunity in the female and male reproductive tracts. In: Ogra PL, Mestecky J, Lamm ME, Strober W, McGhee JR, Bienenstock J, editors. Handbook of Mucosal Immunology. 1. San Diego, CA: Academic Press, Inc; 1994. p. 677. [Google Scholar]
- 4.Parr MB, Parr EL. Protective immunity against HSV‐2 in the mouse vagina. J Reprod Immunol. 1997;36:77. doi: 10.1016/s0165-0378(97)00055-7. [DOI] [PubMed] [Google Scholar]
- 5.Murphy BR. Mucosal immunity to viruses. In: Ogra PL, Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, editors. Mucosal Immunology. 2. San Diego, CA: Academic Press, Inc; 1999. p. 695. [Google Scholar]
- 6.Hook EW, Pate MS, Hedges SR, Russell MW, Mestecky J. Mucosal immunology of sexually transmitted diseases. In: Ogra PL, Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, editors. Mucosal Immunology. 2. San Diego, CA: Academic Press, Inc; 1999. p. 1463. [Google Scholar]
- 7.Parr MB, Parr EL. Mucosal immunity to herpes simplex virus type 2 infection in the mouse vagina is impaired by in vivo depletion of T lymphocytes. J Virol. 1998;72:2677. doi: 10.1128/jvi.72.4.2677-2685.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parr MB, Kepple L, McDermott MR, Drew MD, Bozzola JJ, Parr EL. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab Invest. 1994;70:369. [PubMed] [Google Scholar]
- 9.Parr EL, Parr MB. Immunoglobulin G, plasma cells, and lymphocytes in the murine vagina after vaginal or parenteral immunization with attenuated herpes simplex virus type 2. J Virol. 1998;72:5137. doi: 10.1128/jvi.72.6.5137-5145.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parr EL, Bozzola JJ, Parr MB. Immunity to vaginal infection by herpes simplex virus type 2 in adult mice: characterization of the antibody in vaginal mucus. J Reprod Immunol. 1998;38:15. doi: 10.1016/s0165-0378(97)00081-8. [DOI] [PubMed] [Google Scholar]
- 11.Franco MA, Greenberg HB. Role of B cells and cytotoxic T lymphocytes in clearance of and immunity to rotavirus infection in mice. J Virol. 1995;69:7800. doi: 10.1128/jvi.69.12.7800-7806.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishizaka ST, Piacente P, Silva J, Mishkin EM. IgG subtype is correlated with efficiency of passive protection and effector function of anti‐herpes simplex virus glycoprotein D monoclonal antibodies. J Infect Dis. 1995;172:1108. doi: 10.1093/infdis/172.4.1108. [DOI] [PubMed] [Google Scholar]
- 13.Gallichan WS, Rosenthal KL. Specific secretory immune responses in the female genital tract following intranasal immunization with a recombinant adenovirus expressing glycoprotein B of herpes simplex virus. Vaccine. 1995;13:1589. doi: 10.1016/0264-410x(95)00100-f. [DOI] [PubMed] [Google Scholar]
- 14.Vancott TC, Kaminski RW, Mascola JR, et al. HIV‐1 neutralizing antibodies in the genital and respiratory tracts of mice intranasally immunized with oligomeric gp160. J Immunol. 1998;160:2000. [PubMed] [Google Scholar]
- 15.Tomasi TB, Bienenstock J. Secretory immunoglobulins. Adv Immunol. 1968;9:1. doi: 10.1016/s0065-2776(08)60441-1. [DOI] [PubMed] [Google Scholar]
- 16.Kozlowski PA, Cu‐uvin S, Neutra MR, Flanigan TP. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect Immun. 1997;65:1387. doi: 10.1128/iai.65.4.1387-1394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parr MB, Parr EL. The role of gamma interferon in immune resistance to vaginal infection by herpes simplex virus type 2 in mice. Virology. 1999;258:282. doi: 10.1006/viro.1999.9739. [DOI] [PubMed] [Google Scholar]
- 18.Milligan GN, Bernstein DI, Bourne N. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J Immunol. 1998;160:6093. [PubMed] [Google Scholar]
- 19.Rank RG, Bavoil PM. Prospects for a vaccine against chlamydia genital disease. 2. Immunity and vaccine development. Bull Inst Pasteur. 1996;94:55. [Google Scholar]
- 20.Mackay CR. The concept of memory T Cells. In: Snow EC, editor. Handbook of B and T Lymphocytes. New York: Academic Press; 1994. [Google Scholar]
- 21.Young WG, Newcomb GM, Hosking AR. The effect of atrophy, hyperplasia and keratinization accompanying the estrous cycle on Langerhans' cells in mouse vaginal epithelium. Am J Anat. 1985;174:173. doi: 10.1002/aja.1001740207. [DOI] [PubMed] [Google Scholar]