Abstract
Most tumours do not stimulate effective antitumour immune responses in vivo. In order to enhance the immunogenicity of human tumour cells, we fused a variety of tumour cell lines with an Epstein–Barr virus transformed B‐lymphoblastoid cell line (EBV B‐LCL) in vitro, to produce stable hybrid cells. Hybrid cell lines showed a marked increase in their ability to stimulate primary allogeneic T‐cell responses in vitro, as compared with the parent tumour cells. The hybrid cells induced proliferation of naive (CD45RA+) as well as memory (CD45RO+) T lymphocytes, and both CD4+ and CD8+ subpopulations of T cells were directly stimulated. The stimulatory hybrids expressed human leucocyte antigen (HLA) class I and II, and a wide range of surface accessory molecules, including the T‐cell co‐stimulatory ligand molecules CD40, CD80 (B7.1) and CD86 (B7.2), the expression of which was required for optimal stimulation of T‐cell responses. Fusion of the EBVB‐LCL with a melanoma cell line (518.A2) yielded hybrid cells that expressed the melanoma‐associated antigens MAGE‐1 and MAGE‐3, and presented these antigens to antigen‐specific, HLA class I‐restricted cytotoxic T‐lymphocyte clones with greater efficiency than the parent melanoma cell line. These findings suggest that the generation of human antigen‐presenting cell/tumour cell hybrids offers promise as an approach to cancer immunotherapy.
Introduction
The concept of exploiting the potential of the immune system in the treatment of cancer has existed for many years. Advances in our understanding of the processes by which the immune system recognizes and eliminates cancer cells have led to a renewed optimism for the development of effective immunotherapies for cancer. The importance of tumour‐specific T cells, especially major histocompatibility complex (MHC) class I‐restricted cytotoxic T lymphocytes (CTL), in the rejection of tumour cells has been shown in both animal and human studies.1–4 A range of human tumour‐associated antigens has been identified, which contain HLA class I‐restricted epitopes recognized by tumour‐specific CTL.5 Where known, these antigens represent attractive targets for use in cancer vaccination, and clinical trials have been undertaken using a number of these antigens (or peptides derived from them) as immunogens.6–8 In most human tumour cell types, however, the relevant tumour‐associated antigens are unknown, and alternative strategies must be developed.
It is generally accepted that the majority, if not all, tumour cells express proteins that the immune system is capable of recognizing as antigenic, but fail to present these antigens to the immune system in a manner which generates effective antitumour immune responses. One explanation for this is that the cells from which the tumours arise are poorly immunogenic because of their inability to deliver the second signals that are required, in addition to the T‐cell receptor/antigen–MHC complex interaction, to activate naive, antigen‐specific T cells. These second signals include the co‐stimulatory receptor/ligand interactions CD28/B79 and CD40/CD40 ligand,10 and are further enhanced by additional accessory receptor/ligand interactions, e.g. lymphocyte function‐associated antigen‐1 (LFA‐1)/intracellular adhesion molecule‐1 (ICAM‐1) and CD2/LFA‐3,11,12 and by the presence of certain cytokines, e.g. interleukin (IL)‐2, IL‐7 and IL‐12.13,14 In the absence of appropriate second signals, the naive T cell may be rendered anergic to subsequent exposure to the antigen.15
Several approaches have been adopted to enhance the immunogenicity of tumour cells with a view to generating genetically modified tumour cells for use as candidate cancer vaccines, including transfection of allogeneic MHC molecules,16,17 cytokines,18 ligands for T‐cell co‐stimulatory molecules such as the B7 family,19 and transfection of a combination of these molecules, e.g. MHC class II and B7.1.20 In essence, the aim of these approaches is to make the tumour cell resemble, or bypass the need for, ‘professional’ antigen‐presenting cells (APC) in the induction of the primary, tumour‐specific T‐cell response. Professional APC (B cells, monocyte/macrophages and dendritic cells) are potent stimulators of antigen‐specific T‐cell responses, through their ability to process and present antigens in the context of both MHC class I and class II molecules, and by providing the appropriate second signals to the naive T cell to stimulate cell activation and clonal expansion.21
We have reasoned that, by fusing tumour cells with professional APC, we should generate hybrid cells with the antigen expression of the parent tumour cell and the antigen processing and immunostimulatory capacity of the parent APC. We report here the generation of such human APC/tumour cell hybrids, using an Epstein–Barr virus‐transformed B‐lymphoblastoid cell line (EBV B‐LCL) as the APC partner, and show that the resulting APC/tumour cell hybrids stimulate strong allogeneic T‐cell responses, and express and present relevant tumour‐associated antigens to cloned, antigen‐specific CTL in vitro. These data indicate in preclinical studies the potential of hybrid cells as immunogens in cancer immunotherapy, and provide a basis for their use in clinical trials.
Materials and methods
Cell lines
The EBV B‐LCL parent cell line used in the generation of each APC/tumour cell hybrid was HMy2.22 This cell line is resistant to ouabain, but sensitive to hypoxanthine, aminopterin and thymidine (HAT), allowing for chemical selection of hybrid cells in vitro. The EBV‐negative parent tumour cell lines used were the promyelocytic leukaemia‐derived cell line, HL60,23 the T‐cell leukaemia‐derived cell line, CEM,24 the erythro‐leukaemia‐derived cell line, K562,25 and the melanoma‐derived cell line, 518.A2. All cell lines were human leucocyte antigen (HLA) genotyped, with respect to their HLA‐A, ‐B and ‐DR alleles, by the polymerase chain reaction (PCR) using sequence‐specific primers, as described previously.26 The HLA types were as follows. HMy2: HLA‐A2, A3, B35, B62/76, DR4, DR12; HL60: HLA‐A1, B57, DR7; CEM: HLA‐A1, B8, B60, DR3, DR7; K562: HLA‐A24, B18, B60, DR3, DR4; and 518.A2: HLA‐A1, A2, B8, DR3, DR15. EBV B‐LCL and tumour cell lines were maintained in growth medium (GM) consisting of RPMI‐1640 supplemented with 10% fetal bovine serum (FBS), 2 mm l‐glutamine, penicillin (100 U/ml) and streptomycin (100 µg/ml), with the exception of the 518.A2 melanoma cell line, which was grown in Dulbecco's modified Eagle's minimal essential medium (DMEM), supplemented as described above. Hybrid cell lines were cultured in selection medium (SM) consisting of GM supplemented with 2% HAT and 1–2·5 mm ouabain. All cell lines were cultured at 37°, in 5% CO2 and saturating humidity.
Cell fusions
Cell fusions between HMy2 and tumour cell lines were carried out using established cell fusion procedures in the presence of polyethylene glycol (PEG; MW 1500).27,28 Briefly, 107 cells from each parental cell line were washed in serum‐free RPMI‐1640 medium, mixed in a 1 : 1 ratio, pelleted by centrifugation and fused by the addition of PEG (50% w/v) for 1 min. The PEG‐treated cells were washed and resuspended in GM. After 24 hr, the cells were transferred into SM, permitting growth only of EBV B‐LCL/tumour cell hybrids. After chemical selection, the cell hybrids were cloned by limiting dilution, to establish stable hybrid cell lines.
Cell phenotype analysis
Parent and hybrid cell lines were analysed for the expression of cell surface markers by monoclonal antibody (mAb) staining of viable cells using immunofluorescence (IF) techniques, followed by flow cytometric analysis using a fluorescence‐activated cell sorter (FACScan; Becton‐Dickinson Ltd, Cowley, UK). Cell surface molecules analysed were HLA class I (mAb W6/3229), HLA class II (mAb L24330), HLA‐A1 (mAb 142.2), HLA‐A2 (mAb BB7.231) and HLA‐A3 (GAP.A332); cell lineage‐specific markers CD3, CD4 and CD8 (T cell), CD13, CD15 and CD33 (myelocyte/monocyte), CD19 and CD20 (B cell) and Glycophorin A (erythrocyte); the adhesion molecules CD11a (LFA‐1), CD50 (ICAM‐3), CD54 (ICAM‐1) and CD58 (LFA‐3); and the co‐stimulatory ligand molecules CD40, CD80 (B7.1) and CD86 (B7.2). mAbs W6/32, L243, BB7.2 and GAP.A3 were used as tissue culture supernatants. mAb 142.2 was used as ascites fluid diluted 1 : 2000 in phosphate‐buffered saline (PBS). mAbs CD4, CD8, CD15, CD33 and Glycophorin A were fluorescein isothiocyanate (FITC) conjugated, whilst CD3, CD13, CD45RA and CD45RO were R‐phycoerythrin (RPE) conjugated, and used in direct IF assays. All other antibodies were used in indirect IF assays and detected using FITC‐conjugated F(ab′)2 fragment of rabbit anti‐mouse immunoglobulin G (IgG) as the secondary antibody.
EBV genome and antigen expression
Expression of the EBV latent gene transcripts Epstein–Barr nuclear antigen‐1 (EBNA‐1) and latent membrane protein‐1 (LMP‐1), and antigens EBNA‐1, ‐2, ‐3a and LMP‐1, were determined by Southern and Western blotting, respectively, as described previously.27
Responder T cells, and separation of T‐cell populations from peripheral blood mononuclear cells
Responder T cells for use in in vitro assays were obtained from peripheral blood mononuclear cells (PBMC) that were isolated from heparinized blood of healthy, allogeneic donors and separated by density gradient centrifugation over Lymphoprep™ (Nycomed Diagnostics, Oslo, Norway). The HLA types and EBV‐serological status (presence of immunoglobulin M [IgM] antibodies against the EBV capsid protein and/or IgG antibodies against Epstein–Barr nuclear antigens) of the donors used in the study were as follows: Responder 1: HLA‐A3, B7, B35, DR4, DR10, EBV seronegative; Responder 2: HLA‐A1, A2, B37, B62, DR4, DR10, EBV seronegative; Responder 3: HLA‐A3, A23/24, B62, DR7, EBV seronegative; Responder 4: HLA‐A1, A23/24, B35, B63, DR11, DR13, EBNA IgG seropositive.
In certain experiments, purified, ‘untouched’ CD3+ T cells were obtained from PBMC by negative selection using a pan T cell magnetic microbead isolation kit (Miltenyi Biotec Ltd, Bisley, UK), according to the manufacturer's instructions. CD3+ T‐cell populations were further separated, by negative selection, into CD4+, CD8+, CD45RA+ and CD45RO+ subpopulations, using CD8‐, CD4‐, CD45RO‐ and CD45RA‐specific magnetic microbeads, respectively. The purity of separated T‐cell populations was determined by mAb staining using direct IF techniques and flow cytometric analysis, as described above, and was routinely greater than 90%.
Mixed lymphocyte/tumour cell cultures
Stimulation of lymphocyte proliferation was estimated in mixed lymphocyte/tumour cell microcultures (MLTC). All co‐cultures were set up in triplicate in 96‐well U‐bottomed microtitre plates. Typically, 105 allogeneic PBMC/well were co‐cultured with stimulator cells (104–105 stimulator cells/well, pretreated with mitomycin C, 50 µg/ml, 30 min), for 6 days at 37°, 5% CO2 in GM. Microcultures were pulsed with 1 µCi/well of [3H]thymidine and harvested after incubation (8 hr, 37°, 5% CO2) with a Skatron cell harvester onto glass‐fibre filters. Incorporated radioactivity was measured (as counts per minute [c.p.m.]) in a liquid scintillation counter.
Blocking of co‐stimulatory molecule function
MLTC assays were carried out as described above, except that stimulator cells were preincubated with anti‐CD40 mAb (mAb89, Coulter Electronics Ltd, Luton, UK), cytotoxic T lymphocyte antigen‐4 (CTLA‐4) immunoglobulin fusion protein (Ancell, Nottingham, UK), a combination of both, or with mouse monoclonal IgG1 as an isotype antibody control, at saturating concentrations (0·5 µg/ml final concentration) for 30 min prior to the addition of responder PBMC. The presence of the blocking antibody was maintained throughout the period of culture.
Induction of CTL activity
The ability of parent and hybrid cell lines to induce a cell‐specific CTL response was determined by 51Cr‐release assays, essentially as previously described.33 Briefly, 107 negatively separated CD3+ T cells were cultured in GM in vitro (37°, 5% CO2) with 2 × 106 mitomycin C‐treated stimulator cells, and restimulated after 7 days (in the presence of IL‐2, 20 U/ml) with the same stimulator cell line. CTL activity was determined on day 14. Target cells were radiolabelled by incubation with 0·5 mCi of [51Cr]sodium chromate, followed by thorough washing to remove excess radioactivity. Test and control wells were set up in duplicate in 96‐well U‐bottomed microtitre plates using 5 × 103 target cells/well and effector : target (E : T) cell ratios of 20 : 1, 10 : 1 and 3 : 1. Following 4 hr of incubation, 50 µl of supernatant from each test and control well was removed and analysed for 51Cr activity in a universal gamma counter. CTL‐induced target cell lysis was determined by the following calculation:
 |
Expression of melanoma‐associated antigens
The expression of the melanoma‐associated antigens MAGE‐1, ‐2 and ‐3, tyrosinase and Melan A in parent and melanoma hybrid cells was determined by reverse transcription–PCR (RT–PCR), essentially as described by Mulcahy et al.34 Briefly, total cellular RNA was extracted, 2 µg of RNA was reverse transcribed using Superscript‐RT (Gibco‐BRL, Paisley, UK) and then subjected to PCR using primer sequences and thermal cycling conditions as previously described.34 Appropriately sized, antigen‐specific PCR products were visualized using ethidium bromide staining and UV transillumination following agarose‐gel electrophoresis. PCR products from test samples were compared with PCR products from a positive‐control RNA (2 µg of total RNA from cell line MZ2, reverse transcribed as above and subjected to PCR at 1 : 1, 1 : 10 and 1 : 100 dilutions), to enable semiquantitative interpretation of antigen expression.
Antigen presentation to tumour‐associated antigen‐specific CTL clones
Processing and presentation of known tumour antigens to antigen‐specific, MHC class I‐restricted CTL clones was tested using an EBV B‐LCL/melanoma hybrid cell line, HMy2 × 518.A2, in 51Cr‐release assays. CTL clones 82/30 and 297/22, raised against the melanoma tumour antigens MAGE‐1 and MAGE‐3, respectively, were generated as previously described.35–37 CTL assays were performed at E : T cell ratios of 10 : 1, 3 : 1, 1 : 1 and 0·1 : 1. Specific cell lysis was determined as described above.
Results
This study addressed the feasibility of generating stable human APC/tumour hybrid cells by fusion of EBV B‐LCL with a range of tumour cell types, and describes their characterization in vitro.
Generation and morphology of EBV B‐LCL/tumour cell hybrids
Heterologous human cell fusions were generated between EBV B‐LCL as the APC and a variety of both lymphoid and non‐lymphoid tumour cell lines, to produce stable cell hybrids. The hybrid cell lines grew continuously in culture in the presence of double chemical selection. The hybrid cell lines were HLA genotyped with respect to their HLA‐A, ‐B and ‐DR alleles. All hybrid cells contained HLA‐A, ‐B and ‐DR alleles from both parent cells, with the exception of HMy2 × K562 clone A4, which contained only the HMy2 parent HLA alleles (data not shown). Periodic analysis of expression of HLA class I and class II molecules, and markers specific to each parental cell line, showed phenotypic stability of the hybrid cells over prolonged periods (data not presented).
EBV B‐LCL/tumour cell hybrids stimulate primary allogeneic T‐cell responses in vitro
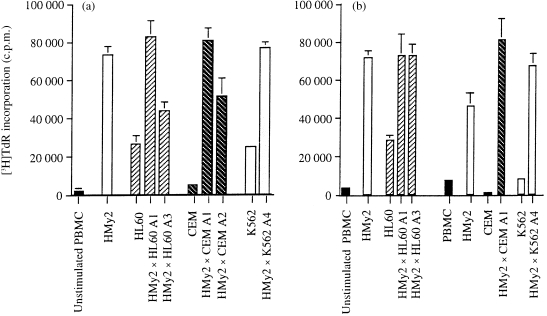
To investigate the immunostimulatory capacity of the hybrid cell lines, we assessed their ability to stimulate primary allogeneic T‐cell proliferation in vitro, using PBMC from normal, allogeneic donors as responder cells. Data representative of a number of experiments are shown throughout. In all cases, the parent EBV B‐LCL stimulated strong T‐cell proliferative responses (Fig. 1). In contrast, the parent tumour cell lines showed little or no stimulation of T‐cell proliferation. APC/tumour cell hybrid clones showed a marked increase in their ability to induce T‐cell proliferation as compared with the parent tumour cell lines, with responses of individual hybrid clones being equivalent to those of the parent EBV B‐LCL (Fig. 1a, 1b). To rule out an effect of EBV antigen expression and presentation by the hybrid cells in the induction of T‐cell responses, these experiments were carried out using responder PBMC from both EBV‐seronegative and EBV‐seropositive donors. Comparable induction of lymphocyte proliferation was seen (Fig. 1a, 1b, respectively), demonstrating that the observed responses were not caused by presentation of EBV antigens by the hybrid cells. In all, these experiments were carried out using PBMC from four EBV‐seronegative donors, and no qualitative differences in responses between EBV‐seronegative and EBV‐seropositive donors were observed.
Figure 1.
Proliferative responses of peripheral blood mononuclear cells (PBMCs) to stimulation with parent and hybrid cells. (a) Proliferative responses of PBMCs from Responder 1, a normal allogeneic, Epstein–Barr virus (EBV)‐seronegative donor to stimulation with 3·3×104 mitomycin C‐treated parent EBV transformed B‐lymphoblastoid cell line (B‐LCL) (HMy2) cells, parent tumour cells (HL60, CEM, K562), or EBV B‐LCL/tumour cell hybrid clones (HMy2×HL60, HMy2×CEM, HMy2×K562) in mixed lymphocyte/tumour cell microcultures (MLTC) in vitro. Cell proliferation is expressed in terms of [3H]thymidine incorporation (mean ± SD, n = 3). (b) Proliferative responses of PBMCs from Responder 4, a normal, allogeneic, EBV‐seropositive donor, to stimulation, as described above.
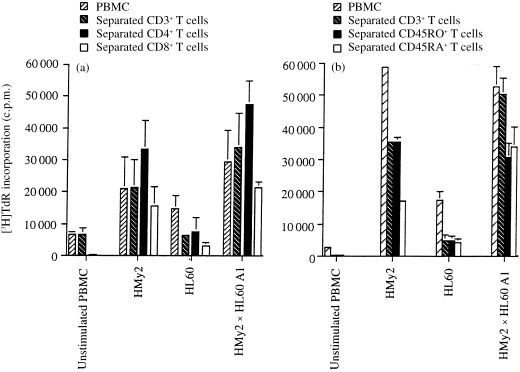
To confirm that the responding cells in the cultures were T lymphocytes, we repeated the experiments using highly purified populations of negatively selected CD3+, CD4+ and CD8+ T cells, as well as unseparated PBMC, as responder cells. Representative results are shown in Fig. 2(a), using purified responder T cells from an EBV‐seronegative donor. The responses of separated (CD3+) T cells to stimulation with the hybrid cell line were essentially equivalent to those of unseparated PBMC throughout, confirming that T lymphocytes represented the dominant responder cell type. Further separation of T cells into CD4+ and CD8+ subpopulations showed that both CD4+ and CD8+ T cells were directly stimulated by the hybrid cells, although purified CD4+ T cells consistently showed greater proliferative responses to stimulation than purified CD8+ T cells (Fig. 2a). These experiments also indicated that the hybrid cells acted directly as APCs in stimulating the responding T cells, by removing the potential for uptake, processing and presentation of antigens by APC of responder origin in the cultures. As in previous experiments, the parental tumour cells showed considerably less stimulation of T‐cell responses in vitro, as compared with the parent EBV B‐LCL or the APC/tumour cell hybrids. To determine the ability of the hybrid cells to induce naive T‐cell activation and proliferation, T cells were separated into CD45RA+ (naive) and CD45RO+ (memory) subpopulations. The responses of both naive and memory T cells to HMy2, HL60 or the hybrid cell line (HMy2 × HL60 clone A1) showed that both naive and memory T cells were directly stimulated to a similar extent by the hybrid cells (Fig. 2b). Similar results were obtained using HMy2 × CEM and HMy2 × K562 hybrids as stimulator cells (data not shown).
Figure 2.
Proliferative responses of separated T‐cell populations to stimulation with parent and hybrid cells. (a) Proliferative responses of peripheral blood mononuclear cells (PBMCs), separated CD3+ T cells, separated CD4+ T cells, and separated CD8+ T cells from Responder 2, a normal, allogeneic, Epstein–Barr virus (EBV)‐seronegative donor, to stimulation with 3·3×104 mitomycin C‐treated parent EBV B‐lymphoblastoid cell line (B‐LCL) (HMy2) cells, parent tumour cells (HL60), or HMy2×HL60 A1 hybrid cells, in mixed lymphocyte/tumour cell microcultures (MLTC) in vitro. (b) Proliferative responses of PBMCs, separated CD3+ T cells, separated CD45RO+ T cells and separated CD45RA+ T cells from Responder 3, a normal, allogeneic, EBV‐seronegative donor, to stimulation with 3·3×104 mitomycin C‐treated parent EBV B‐LCL (HMy2) cells, parent tumour cells (HL60), or HMy2×HL60 A1 hybrid cells, in MLTC in vitro.
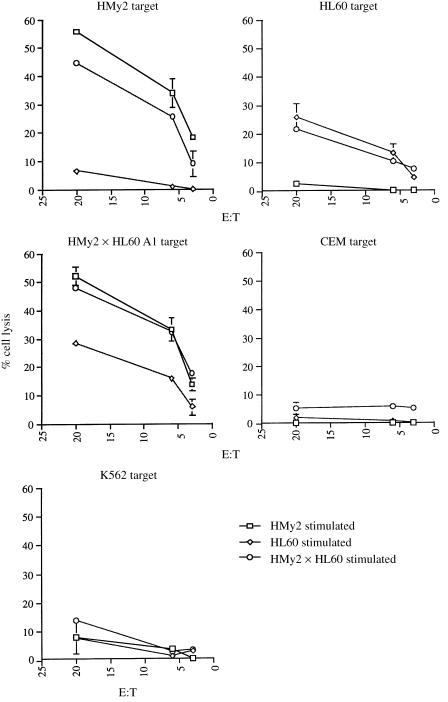
Next, we investigated the ability of the APC/tumour cell hybrids to induce CTL activity in vitro, following co‐culture of negatively selected T cells from an EBV‐seronegative donor with each parent cell line, with APC/tumour cell hybrids or in the absence of stimulator cells. Representative data presented in Fig. 3 demonstrate the ability of HMy2 × HL60 clone A1 hybrid cells to induce CTL activity against both HMy2 and HL60 parent cells and itself, whilst parent HMy2 and HL60 cells induced CTL activity only against HMy2 and HL60 targets, respectively. In all cases, little or no CTL activity was induced against the unrelated CEM cell line, showing the specificity of CTL activity for the stimulating cell type. The natural killer (NK)‐cell sensitive K562 cell line was used as a target cell to show NK cell‐mediated cell lysis.
Figure 3.
Induction of cytotoxic T‐lymphocyte (CTL) activity. Separated T cells from Responder 1, a normal, Epstein–Barr virus (EBV)‐seronegative donor, were cultured in vitro with mitomycin C‐treated HMy2, HL60, or HMy2×HL60 A1 hybrid cells, restimulated after 7 days (in the presence of interleukin‐2, 20 U/ml), and CTL activity was determined against target cells HMy2, HL60, HMy2×HL60 A1, and the unrelated tumour cell line, CEM, on day 14 by 51Cr‐release assays. Each parent cell line stimulated activity against itself, but not against the other parent cell or against CEM, whereas HMy2×HL60 A1 hybrid cells stimulated CTL activity against both parent cell lines and itself, but not against CEM targets. Natural killer (NK)‐cell activity is shown using the NK‐sensitive K562 cell line as target cells.
Surface phenotype and EBV status of immunostimulatory cell hybrids
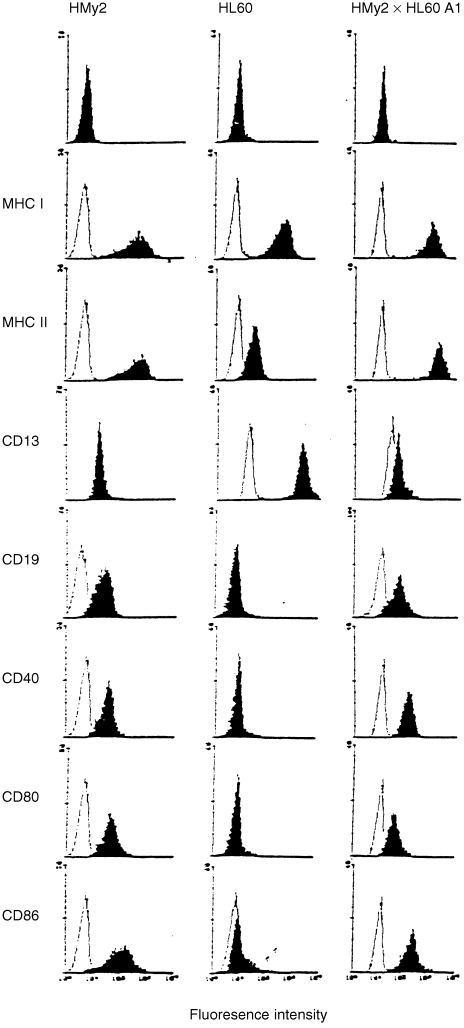
To be effective as cell‐based cancer vaccines, the hybrid cells would probably need to present antigens to both CD4+ and CD8+ T cells, requiring expression of both MHC class I and class II molecules, and to express a range of accessory and co‐stimulatory ligand molecules characteristic of professional APC. Immunostimulatory hybrid cell lines constitutively expressed MHC class I and II antigens, the T‐cell co‐stimulatory ligand molecules CD40, CD80 and CD86 (Fig. 4), and the accessory/adhesion molecules CD11a, CD50, CD54 and CD58 (data not shown). Hybrid cells also expressed lineage‐specific markers from both parent cells, e.g. HMy2 × HL60 clone A1 hybrid cells expressed both the B‐cell marker, CD19, and the myelocyte/monocyte cell marker, CD13 (Fig. 4). The stimulatory hybrid clones were EBV positive by Southern blotting, and expressed the EBV nuclear antigens EBNA‐1 and EBNA‐2, and the EBV latent membrane protein, LMP‐1, characteristic of the LCL‐like pattern of EBV latent protein expression27 (data not shown).
Figure 4.
Phenotypic characterization of parent and hybrid cells. Cell surface expression of major histocompatibility complex (MHC) class I and II antigens, CD13 (myeloid/monocyte marker), CD19 (B‐cell marker), CD40, CD80 and CD86 (co‐stimulatory ligand molecules) was examined on HMy2, HL60 and HMy2×HL60 A1 cell lines. Solid areas represent staining with specific monoclonal antibodies (mAbs). Open areas represent staining with secondary mAb only.
Role of T‐cell co‐stimulatory ligand molecules CD40, CD80 and CD86
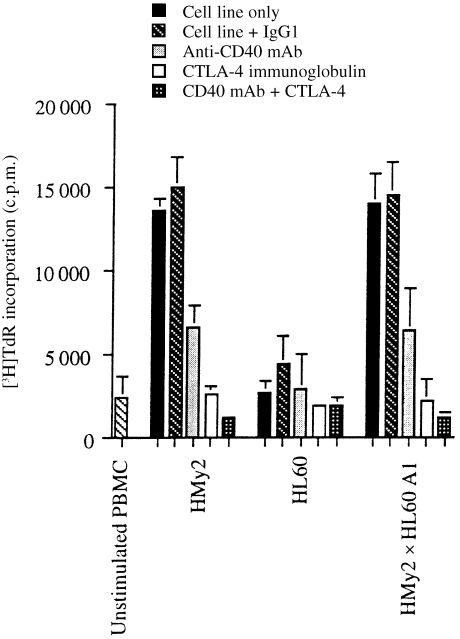
Expression of the co‐stimulatory ligand molecules, CD40, CD80 and CD86, by the stimulatory APC/tumour cell hybrids, but not the parent tumour cell lines, suggested that these molecules might play an important role in the ability of the hybrid cells to stimulate primary T‐cell responses. The dependence of T‐cell proliferative responses in MLTC on the interaction between these molecules and their respective co‐receptors on the responding T cells is shown in Fig. 5. Blocking of the interaction of B7 (CD80 and CD86) on the APC and CD28/CTLA‐4 on the responding T cells by the addition of CTLA‐4 immunoglobulin fusion protein in MLTC assays, reduced PBMC proliferative responses almost to background levels (Fig. 5). Similarly, the addition of anti‐CD40 mAb reduced the proliferative response to cells expressing the CD40 molecule, although to a lesser extent than the blocking of B7 molecules. Together with the immunophenotyping data, these experiments indicate the importance of the expression of T‐cell co‐stimulatory ligand molecules on the APC/tumour cell hybrids in their enhanced ability, compared with the parent tumour cell lines, to stimulate primary T‐cell responses.
Figure 5.
CD40 and B7.1 B7.2 (CD80 and CD86) are required for optimal stimulation of primary allogeneic responses in vitro. Monoclonal antibody (mAb) blocking of allogeneic T‐cell (Responder 3) proliferative responses to stimulation with 104 mitomycin C‐treated parent Epstein–Barr virus transformed B‐lymphoblastoid cell line (EBV B‐LCL) (HMy2) cells, parent tumour cells (HL60), or HMy2×HL60 A1 hybrid cells, in mixed lymphocyte/tumour cell microculture (MLTC) in vitro, showing inhibition of proliferation in the presence of anti‐CD40 mAb, CTLA‐4 immunoglobulin and CD40 mAb+CTLA‐4 immunoglobulin, compared to the cell line alone, but not with a non‐specific mAb, IgG1.
Expression, processing and presentation of melanoma antigens by an EBV B‐LCL/melanoma cell hybrid
Finally, for APC/tumour cell hybrids to be effective as cancer vaccines, they must retain expression of relevant tumour‐associated antigens and present these antigens efficiently to antigen‐specific CTL. In order to investigate the ability of APC/tumour cell hybrids to express, process and present relevant tumour‐associated antigens to CTL, we generated hybrid cells by fusion of HMy2 with a melanoma cell line, 518.A2, which expresses the melanoma antigens MAGE‐1 and MAGE‐3, as determined by RT–PCR (Table 1), as well as the HLA molecules HLA‐A1 and HLA‐A2 (data not shown) through which these antigens are presented to CTL. The resulting EBV B‐LCL/tumour cell hybrids (HMy2 × 518.A2) expressed HLA‐A1 and ‐A2 at levels equivalent to or greater than the parent cell line 518.A2, as determined by mAb staining with mAbs 142.2 and BB7.2, respectively (data not shown). In addition, the hybrid cells retained expression of the melanoma‐associated antigens MAGE‐1, ‐2 and ‐3, as determined by antigen‐specific RT–PCR (Table 1) and, in the case of hybrid clone 2, expressed these at greater levels than the parent cells. Expression of MAGE‐3 by the parent EBV B‐LCL was unexpected, but the specificity of the PCR product was confirmed by Southern blotting. Neither the parent tumour cell line nor the hybrid cells expressed the tyrosinase or Melan A antigens.
Table 1.
Melanoma‐associated antigen (MAGE) expression in parent and melanoma hybrid cells as determined by semiquantitative reverse transcription–polymerase chain reaction (RT–PCR)
| MAGE‐1 | MAGE‐2 | MAGE‐3 | Tyrosinase | MelanA | |
|---|---|---|---|---|---|
| HMy2 | W | – | + +* | – | – |
| 518.A2 | W | W | + + | – | – |
| HMy2 × 518.A2 bulk | ++ | W | + | – | – |
| HMy2 × 518.A2 clone 2 | +++ | + | +++ | – | – |
MAGE‐3 specific PCR product confirmed by Southern blotting using a MAGE‐specific probe.
W, detectable PCR product, but less intense than 1 : 100 of control sample.
+, intensity of PCR product < 1 : 10; > 1 : 100 of control.
+ +, intensity of PCR product < 1 : 1; > 1 : 10 of control.
+ + +, intensity of PCR product > 1 : 1 of control.
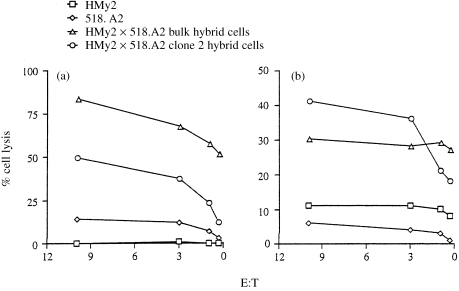
MAGE‐1‐ and ‐3‐specific CTL clones lysed the parent melanoma cell line, 518.A2, with relatively low efficiency (Fig. 6a, 6b). The bulk APC/tumour cell hybrids, and a subclone derived from it (HMy2 × 518.A2 clone 2), were lysed by the MAGE‐1‐ and MAGE‐3‐specific CTL clones with greater efficiency than the parent melanoma cell line (Fig. 6). The expression of MAGE‐3 by the parent EBV B‐LCL, as determined by RT–PCR, is supported by the low‐level recognition of this cell line by the MAGE‐3‐specific CTL clone (Fig. 6b). The reasons why the hybrid cells were lysed with greater efficiency than the parent tumour cell line are not clear, but probably relate to levels of antigen expression by the hybrid cells compared with the parent melanoma cell line (see Table 1). Surface expression of HLA‐A2 on the hybrid cells was ≈ threefold higher than on the parent tumour cells, but expression of HLA‐A1 was essentially equivalent. There were no qualitative differences in levels of expression of the adhesion molecules LFA‐1, LFA‐3, ICAM‐1 and ICAM‐3 between the parent melanoma and hybrid cell lines (data not presented). Both parent and hybrid cells expressed transporter associated with antigen processing (TAP) and LMP‐2 and ‐7 gene products, although at relatively low levels compared with the parent EBV B‐LCL, as determined by Western blot.38 Levels of TAP and LMP gene products in both parent melanoma and hybrid cells were significantly up‐regulated by culture of the cells in interferon‐γ (IFN‐γ) (data not shown). Overall, the data indicate that the hybrid cells retained expression of relevant tumour‐associated antigens, and processed and presented these efficiently in association with MHC class I molecules to antigen‐specific CTL.
Figure 6.
Epstein–Barr virus transformed B‐lymphoblastoid cell line (EBV B‐LCL)/melanoma hybrids retain expression of melanoma antigens, and process and present them to antigen‐specific, human leucocyte antigen (HLA) class I‐restricted cytotoxic T‐lymphocyte (CTL) clones with greater efficiency than the parent melanoma cell line. (a) Presentation of the melanoma‐associated antigen MAGE‐1 by EBV B‐LCL (HMy2) cells, melanoma cells (518.A2), HMy2×518.A2 bulk cells and HMy2×518.A2 clone 2 hybrid cells to MAGE‐1, HLA‐A1‐restricted CTL clone 82/30. (b) Presentation of the melanoma antigen MAGE‐3 by HMy2, 518.A2, HMy2×518.A2 bulk and HMy2×518.A2 clone 2 hybrid cells to MAGE‐3, HLA‐A2‐restricted CTL clone 297/22.
Discussion
Our data demonstrate that human APC/tumour cell hybrids, formed by fusion of EBV B‐LCL and a range of tumour cell lines, have a markedly enhanced immunostimulatory capacity as compared with the parent tumour cells, and are capable of stimulating naive (CD45RA+) and memory (CD45RO+), as well as CD4+ and CD8+, T‐cell populations in vitro. Optimal T‐cell stimulation was dependent on the expression of the T‐cell co‐stimulatory ligand molecules CD40, CD80 and CD86 by the hybrid cells. Hybrid cells retained expression of relevant tumour‐associated antigens, processed and presented these efficiently to antigen‐specific, MHC class I‐restricted CTL clones, and stimulated CTL induction in vitro.
These findings support the idea that APC/tumour cell hybrids represent a promising approach to cancer immunotherapy.39 Using a similar approach, Gong and co‐workers40 showed the induction of strong antitumour activity in mice immunized with a murine dendritic cell/carcinoma cell hybrid, which not only protected the animals against a primary tumour cell challenge, but also mediated rejection of pre‐established metastatic disease.40 Similar findings were observed by Guo and colleagues, who fused rat hepatocarcinoma cells with activated B cells to produce hybrid cells which, when injected into syngeneic rats, protected against subsequent tumour cell challenge and mediated rejection of pre‐established hepatomas.41
Not all of the hybrid clones derived from each fusion described in this paper had the enhanced immunostimulatory phenotype. However, the non‐stimulatory hybrid clones could be distinguished from their stimulatory counterparts in that they were phenotypically similar to the tumour cell parent, did not express the T‐cell co‐stimulatory ligand molecules CD40, CD80 and CD86, and expressed only the EBNA‐1 EBV latent protein (characteristic of the type of EBV latency associated with Burkitt lymphoma cells) rather than the full range of EBV latent proteins, typical of B‐LCL27 (A. L. Cywinski, D. J. Dunnian, V. C. Tucker et al., manuscript in preparation). This point reinforces the significance of the T‐cell co‐stimulatory molecules in the ability of the hybrid cells to stimulate primary T‐cell responses (as illustrated in Fig. 5), and emphasizes the importance of cloning and in vitro characterization of the hybrid cells following fusion, in order to identify those hybrid cells that may be of potential use as cancer vaccines.
Our choice of EBV B‐LCL as APC for these experiments was a result largely of their continuous growth and ease of culture. We have subsequently made several attempts to generate hybrids using dendritic cells as APC and the tumour cell lines K562 and CEM. The tumour cell lines were rendered sensitive to HAT by incubation in increasing concentrations of 8‐azaguanine, to allow non‐fused parental tumour cells to be killed in culture after hybrid generation. Dendritic cells were generated from adherent PBMC by culture in IL‐4 and granulocyte–macrophage colony‐stimulating factor (GM‐CSF), as described previously.42 Using 106 cells of each parent cell line and the method described, we generated small numbers of adherent cells with dendritic cell morphology, which remained viable in culture in the presence of HAT selection for over 8 weeks in culture, but which failed to proliferate. We were unable, in any of these experiments, to generate sufficient numbers of these cells for further analysis. From this, we conclude that human dendritic cells, whilst representing the most potent form of APC, are unlikely to be suitable for the generation of hybrid cells for use as therapeutic cancer vaccines.
This paper presents a detailed description and in vitro characterization of human APC/tumour cell hybrids as a strategy for developing therapeutic cell‐based cancer vaccines. We have shown: the generation in vitro of stable human APC/tumour cell hybrids, using EBV B‐LCL as the parent APC; that the hybrid cells are capable of stimulating primary T‐cell responses in vitro to levels similar to those induced by the APC on its own; that both naive and memory T‐cell populations are activated; and that the hybrid cells retain expression of relevant tumour‐associated antigens, and process and present these to MHC class‐I restricted, antigen‐specific CTL clones. Proof of the efficacy of APC/tumour cell hybrids as cancer vaccines will require in vivo studies showing induction of tumour‐specific immune responses and tumour regression in vivo. The data presented in this study, however, provide a justification for the clinical evaluation of APC/tumour cell hybrids as candidate cancer vaccines. Taken together with the results of animal experiments,40,41 our data support the idea that the generation of APC/tumour cell hybrids represents a promising strategy for the immunotherapy of human cancer, but also highlights the need for characterization of the hybrids in vitro prior to their use in in vivo studies.
Acknowledgments
We thank Dr T. Horsburgh for HLA‐DR typing and Dr A. Kelly for antibodies to TAP and LMP. This work was supported by grants from the Association of International Cancer Research, the Medical Research Council, the Peel Medical Trust, the Association Contre le Cancer, Brussels, Belgium, and from CGER‐Assurances and VIVA, Brussels, Belgium.
References
- 1.Greenberg PD. Adoptive T‐cell therapy of tumors – mechanisms operative in the recognition and elimination of tumor cells. Adv Immunol. 1991;49:281. doi: 10.1016/s0065-2776(08)60778-6. [DOI] [PubMed] [Google Scholar]
- 2.Kast WM, Offringa R, Peters PJ, et al. Eradication of adenovirus E‐1 induced tumors by EIA‐specific cytotoxic T lymphocytes. Cell. 1989;59:603. doi: 10.1016/0092-8674(89)90006-8. [DOI] [PubMed] [Google Scholar]
- 3.Papadopoulos EB, Landanyi M, Emanuel D, et al. Infusions of donor leukocytes to treat Epstein–Barr virus‐associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med. 1994;330:1185. doi: 10.1056/NEJM199404283301703. [DOI] [PubMed] [Google Scholar]
- 4.Rooney CM, Smith CA, Ng CYC, et al. Use of gene‐modified virus‐specific T‐lymphocytes to control Epstein–Barr‐virus‐related lymphoproliferation. Lancet. 1995;45:9. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 5.Browning M, Dunnion D. HLA and cancer: implications for cancer immunotherapy and vaccination. Eur J Immunogenet. 1997;24:293. doi: 10.1111/j.1365-2370.1997.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 6.Borysiewicz LK, Flander A, Nimako M, et al. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet. 1996;347:1523. doi: 10.1016/s0140-6736(96)90674-1. [DOI] [PubMed] [Google Scholar]
- 7.Marchand M, Wegmants P, Rankin E, et al. Tumor regression responses in melanoma patients treated with a peptide encoded by gene MAGE‐3. Int J Cancer. 1995;63:883. doi: 10.1002/ijc.2910630622. [DOI] [PubMed] [Google Scholar]
- 8.Mukherji B, Chakraborty NG, Yamasaki S, et al. Induction of antigen‐specific cytolytic T cells in situ in human melanoma by immunization with synthetic peptide‐pulsed autologous antigen presenting cells. Proc Natl Acad Sci USA. 1995;92:8078. doi: 10.1073/pnas.92.17.8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 10.Grewal IS, Flavell RA. The role of CD40 ligand in costimulation and T cell activation. Immunol Rev. 1996;153:85. doi: 10.1111/j.1600-065x.1996.tb00921.x. [DOI] [PubMed] [Google Scholar]
- 11.Bachmann MF, Mckall‐faienza K, Schmits R, et al. Distinct roles for LFA‐1 and CD28 during activation of naive T cells: adhesion versus costimulation. Immunity. 1997;7:549. doi: 10.1016/s1074-7613(00)80376-3. [DOI] [PubMed] [Google Scholar]
- 12.Wingren AG, Parra E, Vargo M, et al. T cell activation pathways: B7, LFA‐3 and ICAM‐1 shape unique T cell profiles. Crit Rev Immunol. 1995;15:235. doi: 10.1615/critrevimmunol.v15.i3-4.30. [DOI] [PubMed] [Google Scholar]
- 13.Gately MK, Desai BB, Wolitzky AG, et al. Regulation of human lymphocyte proliferation by a heterodimeric cytokine, IL‐12 (cytotoxic lymphocyte maturation factor) J Immunol. 1991;147:874. [PubMed] [Google Scholar]
- 14.Londei M, Verhoef A, Hawrylowicz C, Groves J, Deberardinis P, Feldmann M. Interleukin‐7 is a growth factor for mature human T‐cells. Eur J Immunol. 1990;20:425. doi: 10.1002/eji.1830200228. [DOI] [PubMed] [Google Scholar]
- 15.Becker JC, Brabletz T, Czerny C, Termeer C, Brocker EB. Tumor escape mechanisms from immunosurveillance – induction of unresponsiveness in a specific MHC‐restricted CD4+ human T‐cell clone by the autologous MHC class‐II+ melanoma. Int Immunol. 1993;5:1501. doi: 10.1093/intimm/5.12.1501. [DOI] [PubMed] [Google Scholar]
- 16.Nabel GJ, Nabel EJ, Yang ZY, et al. Direct gene‐transfer with DNA liposome complexes in melanoma expression, biological activity, and lack of toxicity in humans. Proc Natl Acad Sci USA. 1993;90:11307. doi: 10.1073/pnas.90.23.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wahl WL, Strome SE, Nabel GJ, et al. Generation of therapeutic T‐lymphocytes after in vivo tumor transfection with an allogeneic class‐I major histocompatibility complex gene. J Immunol. 1995;17:1. doi: 10.1097/00002371-199501000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Colombo MP, Forni G. Cytokine gene‐transfer in tumor‐inhibition and tumor‐therapy – where are we now? Immunol Today. 1994;15:48. doi: 10.1016/0167-5699(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Linsley PS, Hellström KE. Costimulation of T‐cells for tumor‐immunity. Immunol Today. 1993;14:483. doi: 10.1016/0167-5699(93)90262-J. [DOI] [PubMed] [Google Scholar]
- 20.Basker S, Glimcher L, Nabavi N, Jones RT, Ostrand‐rosenberg S. Major histocompatibility complex class II+B7.1+ tumor cells are potent vaccines for stimulating tumor rejection in tumor‐bearing mice. J Exp Med. 1995;181:619. doi: 10.1084/jem.181.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 22.Edwards PAW, Smith CM, Munro‐neville A, O'hare MJ. A human‐human hybridoma system based on a fast‐growing mutant of the ARH‐77 plasma cell leukemia‐derived cell line. Eur J Immunol. 1982;12:641. doi: 10.1002/eji.1830120804. [DOI] [PubMed] [Google Scholar]
- 23.Collins SJ, Gallo RC, Gallager RE. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977;270:347. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- 24.Foley GE, Lazarus H, Farber S, Uzman BG, Boone BA, Mccarthy RE. Continuous culture of human lymphoblasts from peripheral blood of a child with acute leukaemia. Cancer. 1965;18:522. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 25.Lozzio CB, Lozzio BB. Human chronic myelogenous leukemic cell line with positive Philadelphia chromosome. Blood. 1975;45:321. [PubMed] [Google Scholar]
- 26.Bunce M, O'Neill C, Barnardo MCNM, et al. Phototyping: comprehensive DNA typing for HLA‐A, B, C, DRB1, DRB3, DRB4, DRB5 and DQB1 by PCR with 144 primer mixes utilising sequence‐specific primers (PCR–SSP) Tissue Antigens. 1995;46:355. doi: 10.1111/j.1399-0039.1995.tb03127.x. [DOI] [PubMed] [Google Scholar]
- 27.Kerr BM, Lear AL, Rowe M, et al. Three transcriptionally distinct forms of Epstein–Barr virus latency in somatic cell hybrids: cell phenotype dependence of virus promoter usage. Virology. 1992;187:189. doi: 10.1016/0042-6822(92)90307-b. [DOI] [PubMed] [Google Scholar]
- 28.Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 29.Barnstable CJ, Bodmer WF, Brown G, et al. Production of monoclonal antibodies to Group A erythrocytes, HLA and other cell surface antigens – new tools for genetic analysis. Cell. 1978;14:9. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- 30.Lampson LA, Levy R. Two populations of Ia‐like molecules on a human B cell line. J Immunol. 1980;125:293. [PubMed] [Google Scholar]
- 31.Brodsky FM, Parham P, Barnstable CJ, Crompton MJ, Bodmer WF. Monoclonal antibodies for analysis of the HLA system. Immunol Rev. 1979;47:3. doi: 10.1111/j.1600-065x.1979.tb00288.x. [DOI] [PubMed] [Google Scholar]
- 32.Berger AE, Davis JE, Cresswell P. Monoclonal antibody to HLA‐A3. Hybridoma. 1982;1:87. doi: 10.1089/hyb.1.1982.1.87. [DOI] [PubMed] [Google Scholar]
- 33.Browning MJ, Huang AS, Reiss CS. Cytolytic T lymphocytes from the BALB/c‐H‐2dm2 mouse recognize the vesicular stomatitis virus glycoprotein and are restricted by class II MHC antigens. J Immunol. 1990;145:985. [PubMed] [Google Scholar]
- 34.Mulcahy KA, Rimoldi D, Brasseur F, et al. Infrequent expression of the MAGE gene family in uveal melanomas. Int J Cancer. 1996;66:738. doi: 10.1002/(SICI)1097-0215(19960611)66:6<738::AID-IJC5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 35.Coulie PG, Brichard V, Van Pel A, et al. A new gene coding for the differentiation antigen recognized by autologous cytolytic T lymphocytes on HLA‐A2 melanomas. J Exp Med. 1994;180:35. doi: 10.1084/jem.180.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Den Eynde B, Hinaut P, Hérin M, et al. Presence on a human melanoma of multiple antigens recognized by autologous CTL. Int J Cancer. 1989;44:634. doi: 10.1002/ijc.2910440413. [DOI] [PubMed] [Google Scholar]
- 37.Van Der Bruggen P, Bastin J, Gajewski T, et al. A peptide encoded by human gene MAGE‐3 and presented by HLA‐A2 induces cytolytic T lymphocytes that recognise tumor cells expressing MAGE‐3. Eur J Immunol. 1994;24:3038. doi: 10.1002/eji.1830241218. [DOI] [PubMed] [Google Scholar]
- 38.De La Salle H, Hanau D, Fricker D, et al. Homozygous human TAP peptide transporter mutation in HLA class I deficiency. Science. 1994;265:237. doi: 10.1126/science.7517574. [DOI] [PubMed] [Google Scholar]
- 39.Hart I, Colaco C. Fusion induces tumor rejection. Nature. 1997;388:626. doi: 10.1038/41662. [DOI] [PubMed] [Google Scholar]
- 40.Gong J, Chen D, Kashiwaba M, Kufe D. Induction of anti‐tumor activity by immunization with fusions of dendritic and carcinoma cells. Nature Med. 1997;3:558. doi: 10.1038/nm0597-558. [DOI] [PubMed] [Google Scholar]
- 41.Guo Y, Wu M, Chen H, et al. Effective tumor vaccine generated by fusion of hepatoma cells with activated B cells. Science. 1994;263:518. doi: 10.1126/science.7507262. [DOI] [PubMed] [Google Scholar]
- 42.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by GM‐CSF plus IL‐4 and downregulated by TNFα. J Exp Med. 1994;179:1109. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]