Abstract
Interleukin‐15 (IL‐15) is a novel cytokine with actions similar to IL‐2 because of common receptor components. Although IL‐15 is expressed in colonic epithelial cells and may regulate epithelial cell function, its effects on these cells are not fully defined. We explored the regulatory effects of IL‐15 on IL‐8 and monocyte‐chemoattractant protein‐1 (MCP‐1) production in the colonic epithelial cell line Caco‐2 as well as in freshly isolated human colonic epithelial cells. IL‐15 was added to intestinal epithelial cells under various culture conditions. Levels of chemokines were determined by enzyme‐linked immunosorbent assay. To determine the elements of the IL‐2/IL‐15R complex involved we used neutralizing antibodies specific for individual receptor chains. IL‐15 down‐regulates IL‐8 and MCP‐1 production in Caco‐2 cells as well as in freshly isolated human colonic epithelial cells in a dose‐dependent manner. Intestinal epithelial cells became more responsive to IL‐15‐induced suppression when activated with greater IL‐1 doses. Strong chemokine suppression was seen when IL‐15 was given prior to, simultaneous with, or after stimulatory agent. Anti‐IL‐2Rγ antibodies efficiently blocked (82% inhibition) the suppression induced by IL‐15, while anti‐IL‐2Rβ antibodies were less effective. The involvement of β‐chain was further suggested by the finding that a mixture of both monoclonal antibodies (mAb) at a suboptimal concentration (1 µg/ml of each mAb) produced a synergistic inhibitory effect on down‐regulation of epithelial chemokine production. These results show that IL‐15 can suppress IL‐8 and MCP‐1 secretion by intestinal epithelial cells. A microenvironment containing high concentrations of IL‐15 may alter the recruitment of neutrophils to enterocytes at least partly by inhibiting IL‐8 and MCP‐1 production.
Introduction
Interleukin‐15 (IL‐15) belongs to the four‐helix bundle cytokine family and has many functional activities in common with IL‐2: the receptor complexes for IL‐15 and IL‐2 share two subunits, the β and γ chain.1–4 For example, both IL‐2 and IL‐15 promote activation, proliferation and cytokine release of T cells5,6 as well as the proliferation and polyclonal immunoglobulin secretion of activated B‐cells.7 In spite of these similarities to IL‐2, IL‐15 is also quite distinct from IL‐2 in its broad constitutive or inducible gene transcription pattern in multiple cell types and tissues,2,4,8 and in its unusually tightly controlled secretion.9 In addition, a private IL‐15 receptor protein, termed IL‐15Ra, has been described. Although IL‐15Rα did not appear to be involved in signal transduction, its presence is required for high‐affinity binding of IL‐15·10,11
In the intestine, both epithelial cells and macrophages are the major cell types producing IL‐15, which activates signal tranducers and activators of transcription (STAT) 3 and stimulates the proliferation of intestinal epithelial cells.12,13 IL‐15 is the most potent of the known cytokines for intraepithelial lymphocytes, inducing the highest levels of proliferation, interferon‐γ (IFN‐γ) production, and cytotoxicity.14 Elevation of local IL‐15 have been proposed to be a characteristic feature in inflammatory bowel disease (IBD), especially in ulcerative colitis.13,15 In vivo, an increased percentage of IL‐15‐expressing polymorphonuclear cells (PMN) and an elevation of IL‐15‐protein in serum have been shown in IBD patients with moderate and severe disease.15
In a recent report Badolato et al. have demonstrated that IL‐15 may induce IL‐8 and and monocyte chemoattractant protein‐1 (MCP‐1) production in human monocytes, thereby amplifying ongoing inflammatory reactions.16 Because intestinal epithelial cells have also been reported to express binding sites for IL‐1512,14,15 and to produce substantial amounts of IL‐8 and MCP‐1,17–21 we investigated whether IL‐15 can regulate production of these chemokines in colonic epithelial cells. The possible involvement of known IL‐2/IL‐15R components in IL‐15‐mediated effects was analysed by functional blockade with antibodies specific for individual receptor proteins.
Materials and methods
Cell lines and cell culture
The colonic epithelial cell line Caco‐2 (German collection of microorganisms and cell cultures, Department of Human and Animal Cell Cultures, Braunschweig, Germany) was cultured in minimum essential medium (MEM; Gibco, Eggenstein, Germany) as described before.17 The medium contained 1% penicillin/streptomycin and 1 mm l‐glutamine. Cells were cultured with or without 10% AB‐serum. Experiments performed with the colonic epithelial cell line used cells at passage levels of 7–10. The cultures were mycoplasma free and maintained with twice weekly passage. Cells were seeded into 24‐well plates and grown until formation of confluent monolayers (3 days). Monolayers were then incubated for 24 hr in fresh medium containing stimuli as indicated. The supernatants were collected, cleared by centrifugation and passaged through 0·22 µm sterile filters and kept at –70° until evaluation by enzyme‐linked immunosorbent assay (ELISA). Cell number and viability were examined by trypan blue exclusion.
Isolation of human intestinal epithelial cells
Epithelial cells were isolated from histologically normal areas of surgically resected colonic specimens from patients with tumour bowel resections as described by our group.17 Cryosections of the surgical specimens were examined by an independent pathologist to ensure that only histologically normal areas were used for cell isolation. No patient was included in the study who had been on corticosteroids or any other immunosuppressive drug. The study protocol had been approved by the Human Studies Committee of the University of Münster.
In brief, small pieces of resected tissue were incubated in 1 mm/l dithiothreitol (DTT) for 15 min at room temperature and then washed two times in RPMI medium. Subsequently, the tissue was washed for 30 min in 5 mm/l ethylenediamine tetra‐acetic acid (EDTA) four times to remove epithelial cells. Supernatants were then centrifuged for 10 min at 400 g. The resultant pellet was dissolved in Hank's balanced salt solution (HBSS) and centrifuged at 500 g. for 5 min. Epithelial cells were separated from the remaining tissue by passage through a 100‐mm nylon mesh. Epithelial cells were then purified by density gradient centrifugation on a Percoll gradient. From the interface at the top of the Percoll layer intestinal epithelial cells were collected and washed twice with serum‐free RPMI medium. Epithelial cell preparations were free of contaminating B cells, T cells and monocytes/macrophages as assessed by flow cytometry using CD19/CD20, anti‐CD3 and CD33/CD14 markers. As a marker for intestinal epithelial cells we used fluoroscein isothiocyanate (FITC)‐labelled monoclonal antibodies (mAb) against cytokeratin‐18 and cytokeratin‐20 (Progen, Heidelberg, Germany) by immunocytochemistry on cytospin preparations. Purity of epithelial cells was > 85%. Viability of the freshly isolated epithelial cells was assessed by trypan blue exclusion and staining with propidium iodide and was > 95% immediately after isolation, 75–85% after 72 hr, and 73–83% after 96 hr in culture. Epithelial cells were then cultured in RPMI medium with 10% AB‐serum on 24‐well plates, which had been covered with a collagen I (2 mg/ml) layer.
ELISA for IL‐8 and MCP‐1
IL‐8 was determined in culture supernatants using a sandwich ELISA developed by Laboserv (Giessen, Germany). The sensitivity of the IL‐8 enzyme‐linked immunosorbent assay was 50 pg/ml. The lower detection limit of the MCP‐1 ELISA was 16 pg/ml. Culture supernatants were all tested in triplicate. In all tests a standard cytokine preparation (recombinant cytokine at defined concentration) was used as internal control. All assays were tested for cross‐reaction by adding several cytokines and soluble receptors to the different cytokine standards. No significant cross‐reaction could be observed using the following cytokines (except for the recombinant protein which was determined in the assay): human IL‐1β (Promega, Mannheim, Germany), IL‐12, p55 and p75 form of the tumour necrosis factor (TNF) receptor (R & D Systems, Minneapolis, MN), IFN‐γ, IL‐2, IL‐4, IL‐6, IL‐10, IL‐13, IL‐15, granulocyte colony‐stimulating factor (G‐CSF), granulocyte–macrophage colony‐stimulating factor (GM‐CSF) and TNF‐α (Pepro Tech EC Ltd, London, UK).
Neutralization studies
For IL‐2/IL‐15R chain‐blocking experiments, several dilutions of antibody were added to the microwells at the onset of cultures at a final concentration of 10, 5 or 1 µg/ml, in the presence of IL‐15 (100 ng/ml). Intestinal epithelial cells were preincubated for 1 hr at 37° with gentle agitation with either anti‐β (mouse monoclonal immunoglobulin G1) or anti‐γ IL‐2R antibodies (affinity‐purified rabbit polyclonal antibody) in complete medium without antibiotics. IL‐15 (100 ng/ml) was added, and the colonic epithelial cells were incubated for additional 18 hr. Mouse anti‐penicillin monoclonal antibodies and rabbit anti‐chicken immunoglobulin G1, respectively, were used as isotype‐matched controls.
Analysis of data
In the statistical calculations the data were treated as non‐parametric, and results were analysed by the two tailed Wilcoxon's signed rank test. Results are presented as mean ± 95% confidence interval, unless stated otherwise. P‐values < 0·05 were considered significant.
Results
IL‐15 is a potent inhibitor of IL‐8 and MCP‐1 production by colonic epithelial cells
In a recent study our group has shown that activated colonic epithelial cells secrete substantial amounts of MCP‐117 and IL‐8.20 We also showed that in our system the most potent stimulatory agent for MCP‐1 and IL‐8 production in Caco‐2 cells and freshly isolated colonic epithelial cells was IL‐1β.17,20
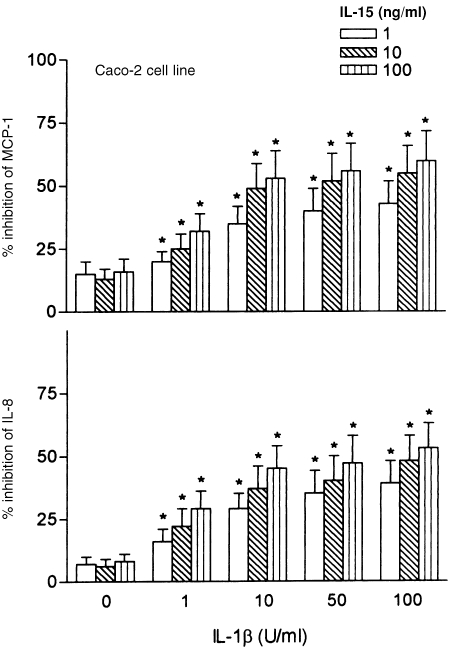
To determine if IL‐15 regulates epithelial chemokine production, we added IL‐15 at doses of 1–100 ng/ml to monolayers of IL‐1β‐activated colonic epithelial cells. At the end of the incubation period of 24 hr, cell‐free supernatants were harvested, and IL‐8 and MCP‐1 protein levels were quantitated by ELISA. As shown in Table 1, IL‐8 and MCP‐1 secretion by both Caco‐2 cells and freshly isolated intestinal epithelial cells rapidly increase after IL‐1β‐exposure, followed by a return to basal levels within 12 hr. IL‐1β‐induced levels of IL‐8 and MCP‐1 from colonic cell lines were significantly down‐regulated by treatment with IL‐15 in a dose‐dependent manner (Fig. 1). At least 1 ng/ml of IL‐15 was required to suppress chemokine production. IL‐15 was found to be increasingly suppressive for IL‐8 and MCP‐1 production when colonic epithelial cells were activated with progressive higher IL‐1 doses (Fig. 1). These data demonstrate that the sensitivity of colonic epithelial cells to IL‐15‐induced suppression can be substantially increased with more potent IL‐1 doses. When intestinal epithelial cells were exposed to IL‐15 without IL‐1β stimulation, a weak but not significant inhibition of IL‐8 and MCP‐1 secretion was observed.
Table 1.
In vitro production of IL‐8 and MCP‐1 by the transformed colonic epithelial cell line Caco‐2 and freshly isolated colonic epithelial cells
| MCP‐1 production (pg/ml) | IL‐8 production (pg/ml) | |||
|---|---|---|---|---|
| Caco‐2 cells | Isolated colonic epithelial cells | Caco‐2 cells | Isolated colonic epithelial cells | |
| No stimulus | <21 | 42 ± 15 | 314 ± 43 | 283 ± 34 |
| IL‐1β (pg/ml) | ||||
| 1 | 170 ± 39 | 153 ± 35 | 1230 ± 215 | 1180 ± 207 |
| 10 | 552 ± 71 | 460 ± 68 | 4100 ± 340 | 4050 ± 329 |
| 50 | 980 ± 142 | 920 ± 95 | 4990 ± 386 | 4630 ± 421 |
| 100 | 1451 ± 168 | 1180 ± 109 | 6860 ± 634 | 7050 ± 711 |
Confluent monolayers of intestinal epithelial cells (1 × 106) were exposed to increasing concentrations of IL‐1β and cultured for 24 hr. Epithelial conditioned media were then harvested, and chemokine levels were determined by ELISA. Each result is the mean ± 95% CI of triplicate cultures in one of three independent experiments that had similar results
Figure 1.
Effects of IL‐15 towards the production of MCP‐1 and IL‐8 in Caco‐2 cells. Confluent monolayers of Caco‐2 cells were cultured in either the absence or the presence of different concentrations of IL‐1β (10–100 ng/ml). IL‐15 at doses of 1–100 ng/ml was added to the cultures of Caco‐2 cells 30 min before stimulation with IL‐1β. IL‐8 and MCP‐1 were measured in 24 hr cell supernatants with ELISA. Results are expressed as percentage of inhibition compared with controls receiving no immunoregulatory cytokine. Each result is the mean ± 95% CI of triplicate cultures in one of three independent experiments that had similar results.
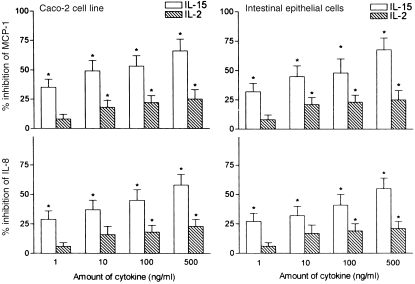
The efficacy of IL‐15 in inhibiting epithelial chemokine production was next compared with that of IL‐2. In our experiments, IL‐2 was about 100–500 times less potent than IL‐15. Optimal suppression of IL‐8 and MCP‐1 was observed when epithelial cells were preincubated with IL‐15 at concentrations of ª 1 ng/ml. These findings reveal an exquisite sensitivity of colonic epithelial cells in their response to IL‐15.
We next addressed whether the degree of epithelial responsiveness to IL‐15 varied between the colonic epithelial cell line and freshly isolated colonic epithelial cells. In our experiments, the IL‐15 dose–response curve we observed in Caco‐2 cells was comparable with that of freshly isolated epithelial cells, suggesting that the latter cells were fully responsive (Fig. 2). We did not observe any differences in cell viability or [3H]leucine incorporation (as an indicator for total protein synthesis) between those intestinal epithelial cells cultured in medium alone and those cultured under the influence of IL‐15. Up to 500 ng/ml of IL‐15 induced no inhibition of total cellular protein synthesis, thus indicating that the observed down‐regulation of IL‐8 and MCP‐1 secretion represents a specific effect (data not shown).
Figure 2.
Effects of IL‐15 and IL‐2 towards the production of MCP‐1 and IL‐8 in Caco‐2 cells and freshly isolated colonic epithelial cells. IL‐15 and IL‐2 at doses of 1–500 ng/ml were added to confluent monolayers of colonic epithelial cell cultures 30 min before stimulation with IL‐1β (10 ng/ml). IL‐8 and MCP‐1 were measured in 24 hr cell supernatants with ELISA. Results are expressed as percentage of inhibition compared with controls receiving no immunoregulatory cytokine. Each result is the mean ± 95% CI of triplicate cultures in one of three independent experiments that had similar results.
Kinetics of suppression of IL‐8 and MCP‐1 production by IL‐15 in intestinal epithelial cells
We were further interested in the effect of the time of addition of IL‐15 on IL‐1β‐activated intestinal epithelial cells. To this end, epithelial cells were incubated for 24 hr in RPMI medium under stimulation with IL‐1β (10 ng/ml) and IL‐15 (100 ng/ml) was added at various times. As shown in Table 2, the IL‐15‐induced suppression of epithelial cell‐derived IL‐8 and MCP‐1 was seen when IL‐15 was given prior to, simultaneous with or 8 hr after addition of IL‐1β. These results show that epithelial cell sensitivity to IL‐15‐induced suppression can not be substantially modulated by the duration of epithelial cell activation. In these experiments, the kinetic responses to IL‐15 by Caco‐2 cells were similar to those of freshly isolated colonic epithelial cells (data not shown).
Table 2.
Addition of IL‐15 to Caco‐2 cells at various times relative to stimulation
| Caco‐2 cells | Intestinal epithelial cells | |||
|---|---|---|---|---|
| Time of IL‐15 addition (h) | MCP‐1 | IL‐8 | MCP‐1 | IL‐8 |
| −6 hr | 49 ± 12 | 42 ± 13 | 44 ± 13 | 38 ± 14 |
| −4 hr | 50 ± 13 | 43 ± 11 | 45 ± 14 | 41 ± 12 |
| −2 hr | 52 ± 11 | 44 ± 12 | 43 ± 14 | 41 ± 12 |
| 0 hr | 55 ± 12 | 46 ± 15 | 46 ± 11 | 42 ± 11 |
| 1 hr | 54 ± 13 | 46 ± 13 | 46 ± 13 | 42 ± 14 |
| 2 hr | 53 ± 11 | 44 ± 13 | 47 ± 13 | 42 ± 15 |
| 4 hr | 51 ± 12 | 44 ± 12 | 44 ± 14 | 40 ± 14 |
| 6 hr | 48 ± 13 | 42 ± 11 | 42 ± 12 | 39 ± 12 |
| 8 hr | 47 ± 11 | 42 ± 14 | 42 ± 11 | 39 ± 13 |
Confluent monolayers of Caco‐2 cells were stimulated with IL‐1β (10 ng/ml). IL‐15 was added at a concentration of 100 ng/ml at different times in relation to stimulation with IL‐1β. IL‐8 and MPC‐1 were measured 24 hr after stimulation in cell supernatants by ELISA. Results are expressed as per cent of inhibiton compared with controls receiving no immunoregulatory cytokine. Each result is the mean ± 95% CI. of duplicate cultures in one of four representative experiments that had similar results.
Neutralization of IL‐15‐induced suppression in chemokine production by epithelial cells
As the β and γ chains of the IL‐2R have been shown to participate in binding and signalling of IL‐15, studies were performed with several dilutions of anti‐IL‐2Rβ and anti‐IL‐2Rγ chain antibodies to inhibit IL‐15 activity. Antibodies against the γ chain of the IL‐2R and, to a lesser extent, anti‐β chain antibodies were effective in partially abrogating IL‐15 activity (Table 3). There was 69 and 82% inhibition of the suppressed chemokine release when the anti‐γ chain antibody was used at 5 and 10 mg/ml, respectively. The involvement of the β chain was further suggested by the finding that a mixture of both mAb at a suboptimal concentration (1 µg/ml of each mAb) produced a synergistic inhibitory effect on down‐regulation of epithelial chemokine production. There was no neutralization of the IL‐15‐induced inhibition of epithelial chemokine production when isotype‐matched control antibodies were used.
Table 3.
Neutralization of IL‐15‐induced down‐regulation of IL‐8 and MCP‐1 in Caco‐2 cells
| Antibody (µg/ml) | MCP‐1 | IL‐8 |
|---|---|---|
| No | 55·95 ± 13·45 | 46·45 ± 11·79 |
| Control Ab | 55·18 ± 14·28 | 46·91 ± 12·48 |
| Anti‐IL‐2Rβ (1) | 51·12 ± 13·40 | 43·25 ± 13·62 |
| Anti‐IL‐2Rβ (5) | 49·20 ± 13·67 | 40·25 ± 12·43 |
| Anti‐IL‐2Rβ (10) | 46·10 ± 11·49 | 38·25 ± 9·31 |
| Anti‐IL‐2Rγ (1) | 29·85 ± 8·21 | 25·25 ± 7·61 |
| Anti‐IL‐2Rγ (5) | 18·17 ± 7·56 | 16·10 ± 6·14 |
| Anti‐IL‐2Rγ (10) | 10·20 ± 3·45 | 8·56 ± 3·29 |
| Anti‐IL‐2Rβ (1) + γ (1) | 25·34 ± 6·22 | 19·25 ± 5·31 |
Antibodies were added at a concentration ranging from 1 to 10 µg/ml at the onset of cultures carried out in the presence of 100 ng/ml human recombinant IL‐15. Results are expressed as per cent of inhibition compared with controls receiving no immunoregulatory cytokine. Data represent means ± SD at least of three separate experiments performed in duplicate.
Discussion
Human colonic epithelial cells were recently shown to represent a cellular target for IL‐15·12,13,22 In this report, we extend the current knowledge on IL‐15/epithelial interactions by showing that recombinant IL‐15 strongly suppress secretion of MCP‐1 and IL‐8 by cytokine‐activated colonic epithelial cells, thereby inhibiting the cascade of events leading to acute inflammation and limiting the accumulation and activation status of the leucocyte infiltrate. Anti‐IL‐2Rγ antibodies efficiently blocked (82% inhibition) the inhibitory effect of IL‐15, while anti‐IL‐2Rβ antibodies were less efficient. Our findings with Caco‐2 cells were verified using freshly isolated human colonic epithelial cells. Production of IL‐8 and MCP‐1 by intestinal epithelial cells in vivo may thus be determined by the profile of IL‐15 released from adjacent cells within the local microenvironment, e.g. monocytes/macrophages.
Our previous work and that of others on patients with IBD have focused attention on IL‐8 and MCP‐1 and have proposed that these potent chemokines are involved in intestinal inflammation.17–21 In our experiments, IL‐15 maintained a substantial inhibition of epithelial IL‐8 and MCP‐1 production over a wide range of time (until 8 hr after stimulation) with substantial inhibitory activity at nanogram concentrations. In contrast, the T helper 2 (Th2) cytokines IL‐10, IL‐13 and IL‐4, which have been shown to negatively regulate inflammatory and cell‐mediated responses in vitro and in vivo, did not significantly affect IL‐8 and MCP‐1 production by activated intestinal epithelial cells when they were added after addition of IL‐1β.17,20,23 These observations indicate that IL‐15 functions as a deactivation factor interfering with both distinct critical early and late steps in the activation cascade of IL‐8 and MCP‐1. We found that supernatants collected from IL‐15‐treated colonic epithelial cells did not have detectable levels of IL‐10, IL‐4, and IL‐13, thus ruling out that other anti‐inflammatory cytokines were responsible for the IL‐15‐induced suppression of epithelial chemokine secretion (data not shown). It is relevant at this point to note that IL‐15 works on IL‐1β‐induced release of IL‐8 and MCP‐1 from colonic epithelial cells, indicating that the inhibitory effect of bioactive IL‐15 on epithelial chemokine secretion would be inflammation dependent. Recent studies by our group could demonstrate that the most potent stimulatory agent for MCP‐1 and IL‐8 production in colonic epithelial cells is IL‐1β,17,20 a physiological cytokine that seems to be very important in intestinal inflammation both in animal models and in human.
In a recent report Badolato et al. showed that IL‐15 may act as a proinflammatory cytokine by stimulating monocytes to produce IL‐8 and MCP‐1.16 They found that production of these chemokines were induced by IL‐15 at concentrations (100 ng/ml) that are reached in pathological conditions such as rheumatoid arthritis.8,16 In our experiments, IL‐15 at concentrations as low as 1 ng/ml significantly inhibit chemokine secretion from intestinal epithelial cells. The comparison between IL‐15‐and IL‐2‐mediated chemokine suppression from colonic epithelial cells revealed that 500‐fold greater concentration of IL‐2 were necessary to reach equivalent biological effects as with IL‐15, although these two cytokines utilize identical receptor molecules (β and γ chain) for cellular binding and signalling. Instead of the IL‐2Rα chain IL‐15 seems to utilize a binding protein that is structurally related to, but different from the IL‐2Rα chain.9,10 IL‐2Rβ and γ chains, but not α chains, have been shown to be present on primary epithelial cells, and the colon carcinoma lines HT‐29 and Caco‐2.22 IL‐15 use the IL‐2Rβ/γc chains expressed on intestinal epithelial cell for signalling, as shown by an increase in tyrosine kinase activity in colonic epithelial cells after stimulation with IL‐15.12 In agreement with previous studies12,22 we detected IL‐15Rα mRNA in both Caco‐2 cells and in freshly isolated colonic epithelial cells (not shown), suggesting that IL‐15Rα may also be involved in our dose–response experiments on colonic epithelial cells. The presence of this receptor component may explain why colonic epithelial cells respond differently to IL‐15 as opposed to IL‐2. However, direct assessment of the role of this receptor is hindered by the current unavailability of anti‐IL‐15Rα mAb.
Little is known about the functional role of IL‐15 in intestinal diseases, and the factors triggering IL‐15 protein release are not clear at present. IL‐15 is synthesized by monocytes and by non‐lymphoid cells and is produced but not secreted by intestinal epithelial cells.12,14 The participation of IL‐15 in the regulation of the chemokine response at the intestinal epithelial cell may be biologically relevant as the co‐ordinated release of a wave of proinflammatory cytokines, such as IL‐1 and TNF‐α, from adjacent cells, particularly from macrophages, may stimulate release of IL‐8 and MCP‐1 by epithelial cells. This, in turn, contributes to chemoattraction and activation of monocytes, T cells and neutrophils. On the other hand, IL‐15 released by monocytes/macrophages may antagonize with IL‐1β and TNF‐α in chemokine production by intestinal epithelial cells, supporting the view that there is a bidirectional interaction between epithelial cells and macrophages in the initiation and maintenance of intestinal inflammation.19,24 In this context, IL‐15 expression has been shown to be induced in macrophages by activators such as lipopolysaccharide and mycobacteria.25,26
In conclusion, we report a novel biological function of human IL‐15 that, at concentrations up to 100 ng/ml, may directly suppress IL‐8 and MCP‐1 production in intestinal epithelial cells. Whether this suppressive response is a result of a functional IL‐2 γ/β complex alone, or in conjunction with the IL‐15Rα chain or other as yet unidentified IL‐15R proteins is uncertain. Although IL‐15 appears to be a significant down‐regulator of IL‐8 and MCP‐1 production in epithelial cells in vitro, its effects are selective, as IL‐15 may act as a proinflammatory cytokine by inducing production of IL‐8 and MCP‐1 in human monocytes16 and neutrophils.27
References
- 1.Burton JD, Bamford RN, Peters C, et al. A. lymphokine, provisionally designated interleukin T and produced by a human adult T‐cell leukemia line, stimulates T‐cell proliferation and the induction of lymphokine‐activated killer cells. Proc Natl Acad Sci USA. 1994;94:4935. doi: 10.1073/pnas.91.11.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giri JG, Anderson DM, Kumaki S, Park LS, Grabstein KH, Cosman D. IL‐15, a novel T cell growth factor that shares activities and receptor components with IL‐2. J Leukocyte Biol. 1995;57:763. doi: 10.1002/jlb.57.5.763. [DOI] [PubMed] [Google Scholar]
- 3.Giri JG, Ahdieh M, Eisenman J, et al. Utilization of the beta and gamma chains of the IL‐2 receptor by the novel cytokine IL‐15. EMBO J. 1994;13:2822. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grabstein KH, Eisenman J, Shanebeck K, et al. Cloning of a T cell growth factor which interacts with the β chain of the interleukin‐2 receptor. Science. 1994;264:965. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 5.Mori A, Suko M, Kaminuma O, et al. IL‐15 promotes cytokine production of human T helper cells. J Immunol. 1996;156:2400. [PubMed] [Google Scholar]
- 6.McInnes I, Al‐mughales J, Field M, et al. The role of interleukin‐15 in T cell migration and activation in rheumatoid arthritis. Nature Med. 1996;2:175. doi: 10.1038/nm0296-175. [DOI] [PubMed] [Google Scholar]
- 7.Armitage RJ, Macduff BM, Eisenman J, Paxton R, Grabstein KH. IL‐15 has stimulatory activity for the induction of B cell proliferation and differentiation. J Immunol. 1995;154:483. [PubMed] [Google Scholar]
- 8.McInnes IB, Leung BP, Sturrock RD, Field M, Liew FY. Interleukin‐15 mediates T. cell‐dependent regulation of tumor necrosis factor‐α production in rheumatoid arthritis. Nat Med. 1997;2:189. doi: 10.1038/nm0297-189. [DOI] [PubMed] [Google Scholar]
- 9.Onu A, Pohl T, Krause H, Bulfone‐paus S. Regulation of IL‐15 secretion via the leader peptide of two IL‐15 isoforms. J Immunol. 1997;158:255. [PubMed] [Google Scholar]
- 10.Giri JG, Kumaki S, Ahdieh M, et al. Identification and cloning of a novel IL‐15 binding protein that is structurally related to the apha chain of the IL‐2 receptor. EMBO J. 1995;14:3654. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson DM, Kumaki S, Ahdieh M, et al. Functional characterization of the human interleukin‐15 receptor α‐chain and close linkage of IL‐15RA and IL‐2RA genes. J Biol Chem. 1995;270:29862. doi: 10.1074/jbc.270.50.29862. [DOI] [PubMed] [Google Scholar]
- 12.Reinecker HC, MacDermott RP, Mirau S, Dignass A, Podolsky DK. Intestinal epithelial cells both express and respond to interleukin‐15. Gastroenterology. 1996;111:1706. doi: 10.1016/s0016-5085(96)70036-7. [DOI] [PubMed] [Google Scholar]
- 13.Sakai T, Kusugami KK, Nishimura H, et al. Interleukin‐15 activity in the rectal mucosa of inflammatory bowel disease. Gastroenterology. 1998;114:1237. doi: 10.1016/s0016-5085(98)70430-5. [DOI] [PubMed] [Google Scholar]
- 14.Ebert EC. Interleukin‐15 is a potent stimulant of intraepithelial lymphocytes. Gastroenterology. 1998;115:1439. doi: 10.1016/s0016-5085(98)70022-8. [DOI] [PubMed] [Google Scholar]
- 15.Kirman I, Nielsen OH. Increased numbers of interleukin‐15‐expressing cells in active ulcerative colitis. Am J Gastroenterol. 1996;91:1789. [PubMed] [Google Scholar]
- 16.Badolato R, Ponzi AN, Millesimo M, Notarangelo LD, Musso T. Interleukin‐15 (IL‐15) induces IL‐8 and monocyte chemotactic protein 1 production in human monocytes. Blood. 1997;90:2804. [PubMed] [Google Scholar]
- 17.Kucharzik T, Lügering N, Pauels HG, Domschke W, Stoll R. IL‐4, IL‐10 and IL. ‐13 down‐regulate monocyte‐chemoattracting protein‐1 (MCP‐1) production in activated intestinal epithelial cells. Clin Exp Immunol. 1998;111:152. doi: 10.1046/j.1365-2249.1998.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daig R, Andus T, Aschenbrenner E, Falk W, Schölmerich J, Gross V. Increased interleukin‐8 expression in the colon mucosa of patients with inflammatory bowel disease. Gut. 1996;38:216. doi: 10.1136/gut.38.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuerer‐maly CC, Eckmann L, Kagnoff MF, Falco T, Maly FE. Colonic epithelial cell lines as a source of interleukin‐8. stimulation of inflammatory cytokines and bacterial lipopolysaccharide. Immunology. 1994;81:85. [PMC free article] [PubMed] [Google Scholar]
- 20.Luegering N, Kucharzik T, Kraft M, Winde G, Stoll R, Domschke W. IL‐13 and IL‐4 are potent inhibitors of IL‐8 regulation by human intestinal epithelial cells. Dig Dis Sci. 1999;44:649. doi: 10.1023/a:1026638330843. [DOI] [PubMed] [Google Scholar]
- 21.Reinecker HC, Loh EY, Ringler DJ, Mehta A, Rombeau JL, MacDermott RP. Monocyte‐chemoattractant protein 1 gene expression in intestinal epithelial cells and inflammatory bowel disease mucosa. Gastroenterology. 1995;108:40. doi: 10.1016/0016-5085(95)90006-3. [DOI] [PubMed] [Google Scholar]
- 22.Stevens AC, Matthews J, Andres P, et al. Interleukin‐15 signals T84 colonic epithelial cells in the absence of the interleukin‐2 receptor β‐chain. Am J Physiol. 1997;272:G1201. doi: 10.1152/ajpgi.1997.272.5.G1201. [DOI] [PubMed] [Google Scholar]
- 23.Luegering N, Kucharzik T, Luegering A, et al. Importance of combined treatment with IL‐10 and IL‐4, but not IL‐13, for inhibition of monocyte release of the Ca2+‐binding protein MRP8/14. Immunology. 1997;91:130. doi: 10.1046/j.1365-2567.1997.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacDermott RP. Alterations of the mucosa immune system in inflammatory bowel disease. J Gastroenterol. 1996;31:907. doi: 10.1007/BF02358624. [DOI] [PubMed] [Google Scholar]
- 25.Doherty TM, Seder RA, Sher A. Induction and regulation of IL‐15 expression in murine macrophages. J Immunol. 1996;156:735. [PubMed] [Google Scholar]
- 26.Nishimura H, Hiromatsu K, Kobayashi N, et al. IL‐15 is a novel growth factor for murine gamma delta T cells induced by Salmonella infection. J Immunol. 1996;156:663. [PubMed] [Google Scholar]
- 27.McDonald P, Russo MP, Ferrini S, Cassatella MA. Interleukin‐15 (IL‐15) induces NF‐KB activation and IL‐8 production in human neutrophils. Blood. 1998;92:4828. [PubMed] [Google Scholar]