Abstract
By targeted disruption of the MIF gene, we have established a mouse strain deficient in macrophage (Mφ) migration inhibitory factor (MIF). Despite previous reports indicating an essential role of MIF in endotoxaemia, an injection of lipopolysaccharide (LPS) into the MIF-deficient mice (maintained under specific pathogen-free conditions) caused shock. No significant difference was detected between the MIF-deficient mutant and normal mice in susceptibility to LPS for endotoxaemia or tumour necrosis factor-α (TNF-α) formation upon LPS injection. Peritoneal Mφ from the two strains produced TNF-α in response to LPS with similar dose responses. Dexamethasone suppressed the LPS-induced TNF-α response of Mφ, but no difference was detected between the Mφ from the two strains. These results suggest that endogenous MIF has no significant effect on the LPS-induced TNF-α production and no effect on suppression of the response by glucocorticoids. Thus, MIF is not crucial for LPS-induced immune responses leading to shock.
Introduction
Macrophage (Mφ) migration inhibitory factor (MIF) was the first lymphokine to be reported.1,2 This factor was identified by its ability to inhibit the migration of guinea-pig peritoneal Mφ in vitro and shown to play an important role in delayed type hypersensitivity.1–4 The cDNA encoding human MIF was later described as a 700-base transcript that produced a 12 500-MW polypeptide of 115 amino acids.5 Transfection of this cDNA into COS-1 cells resulted in secretion of MIF activity into the culture supernatants.5
The mouse homologue of the human MIF cDNA was isolated and demonstrated 89% identity to the human MIF cDNA, with 90% identity at the amino acid sequence level.6 The 12 500-MW mouse MIF protein inhibits mouse monocyte migration and is thought to play a central role in lipopolysaccharide (LPS)-mediated endotoxaemia.6–9 MIF is a major pituitary cytokine that is released in response to physiological stress induced by LPS.7 Recombinant mouse MIF greatly enhances lethality when co-injected with bacterial LPS, and polyclonal antibodies against the recombinant protein conferred full protection to mice from LPS-induced lethal endotoxaemia.7 MIF is also considered a proinflammatory cytokine that is released by Mφ in response to LPS.10 In addition, Mφ stimulated by the recombinant mouse MIF secrete TNF-α, a potent mediator of endotoxaemia.11,12 The critical role of MIF as an immunoregulator was underscored by the finding that the recombinant MIF was shown to abrogate the glucocorticoid-mediated inhibition of proinflammatory cytokine in LPS-stimulated monocytes.13 These data collectively indicate that MIF plays a central role in the toxic response to endotoxaemia. Interestingly, enzymatic activities of MIF have been demonstrated in two independent in vitro experimental systems. Thus, MIF may have a unique effector function distinct from that of a typical cytokine.14,15
The mouse MIF genomic gene was cloned by three groups and was confirmed as a single functional gene.16–18 In the present experiments, we established a mouse strain deficient in the MIF gene. The MIF null mice appeared to be normal in development. More surprisingly, despite previous evidence for the pathogenic role of MIF in endotoxaemia, administration of LPS to MIF-deficient mice maintained under specific pathogen-free (SPF) conditions resulted in immunological responses that were comparable to those of LPS-treated normal mice.
Materials and methods
Animals
Mice were purchased from Japan SLC (Hamamatsu, Japan). They were maintained in a SPF environment. All procedures were performed in accordance with internal regulations.
LPS and dexamethasone (Dex)
Escherichia coli 0111:B4 LPS was purchased from Difco (Detroit, MI) for in vivo use and from Sigma (St. Louis, MO) for in vitro use. Dex was purchased from Sigma.
Targeted disruption of the MIF gene in mice
The MIF gene was obtained from a λ-phage DNA clone previously isolated from a 129/SVJ mouse genomic library.18 A gene targeting vector was generated using a 6·0-kb XbaI fragment that contains all of the MIF exons subcloned. A 201-bp SacI fragment consisting of the 3′ region of exon 1 and the 5′ region of intron 1 was replaced with a pMC1-neo poly (A) cassette in a forward orientation relative to MIF gene transcription. A DT-A cassette was also introduced at the 3′ flanking region for negative selection.
R1 embryonic stem (ES) cells were cultured, transfected and subjected to positive selection using G418 according to the procedure described previously.19 Resistant colonies were selected, replated individually and subjected to genotype analysis using the polymerase chain reaction (PCR). PCR primers were designed from the thymidine kinase (TK) promoter region of the neo gene (NF1: ATTCGCCAATGACAAGACGCTGG) and from an upstream sequence of the MIF gene (BX2: ACCGGTCGGATGTCTCACTTGTT) that was not included in the targeting construct. Subsequently, BamHI-digested DNA from PCR-positive clones was subjected to Southern analysis using an external probe to confirm that the MIF gene had undergone homologous recombination.
Germline chimeras were generated using the aggregation method, as described by Nagy et al.,20 with slight modifications. Tail DNA samples from agouti pups obtained from mating with C57BL/6 (B6) mice were analysed by the Southern method. Homozygous mutant pups were generated by intercrossing heterozygous mutant mice. Genotype analyses of descendants backcrossed to B6 or BALB/c were performed by PCR using two sets of primers: one for the mutant allele consisting of BX2 and NF1, another for the wild-type allele consisting of BX2 and a sequence of intron1 (WT1: TGTGTCCTCCCTGCAAACCTGT). Approximately 100 ng of genomic DNA obtained from tail cuts was subjected to PCR amplification, as follows, using ExTaq (Takara, Tokyo, Japan): 33 cycles of 95° for 25 seconds, 69° for 5 seconds and 72° for 1 min.
Determination of MIF and tumour necrosis factor-α (TNF-α)
The 12 500-MW MIF protein was detected by Western blotting using the method described previously.16 Soluble fractions of brain were prepared by homogenization with a polytron homogenizer in 50 mm Tris, 1 mm Pefabloc SC (Roche Diagnostics, Mannheim, Germany), 10 mm E-64 (Sigma), and subsequent centrifugation at 13 000 g for 1 hr in a microcentrifuge. Concentration of total protein in the supernatants was calculated as (1·55A280 − 0·76A260) mg/ml.21 The supernatants containing equal amounts of the total protein were incubated with 40 mm dithiothreitol (DTT) and were subjected to Western blot analysis using polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, MA). Serum samples from heart blood and culture supernatants of peritoneal Mφ described below were also subjected to Western blot analysis after treatment with DTT. The membranes were probed with rabbit polyclonal antibodies against mouse MIF.22 To confirm the specificity of the antibody, it was used to affinity purify MIF from the crude extract, and this affinity-purified protein was determined to be MIF by using amino acid sequence analysis. The enhanced chemiluminescence system (ECL) (Amersham Pharmacia Biotech, Uppsala, Sweden) was used for detection. TNF-α was measured using an enzyme-linked immunosorbent assay (ELISA) kit (Endogen, Woburn, MA, USA).
Histological analysis
Mice were killed, autopsied and the tissues were removed for histopathological analysis. The tissues were fixed in 10% neutral-buffered formalin, embedded in paraffin, sectioned and stained with haematoxylin and eosin. Peripheral blood samples were obtained from the retro-orbital plexus using 75-mm heparinized capillary tubes (Funakoshi, Tokyo, Japan), and the blood cells were analysed using a Sysmex automatic microcell counter K-2000 (Toa Medical Electrics, Kobe, Japan).
Flow cytometry analysis
Splenocytes were prepared by dissociation between frosted glass slides. After blocking surface Fc receptors with the 2.4G2 anti-mouse FcR monoclonal antibody (mAb) (prepared from hybridomas obtained from the American Type Culture Collection [ATCC], Rockville, MD), the cells were stained with the following antibodies: fluorescein isothiocyanate (FITC) anti-mouse CD45 (30-F11; Pharmingen, San Diego, CA); biotinylated anti-mouse CD3ε (145–2C11; Pharmingen); streptavidin–phycoerythrin (PE) (Pharmingen); allophycocyanin (APC) anti-mouse CD45R/B220 (RA3-6B2; Pharmingen); PE anti-mouse CD4 (RM4-5; Pharmingen); APC anti-mouse CD8α (53-6.7; Pharmingen); and biotinylated anti-mouse immunoglobulin M (IgM) (331.12; Pharmingen). The stained cells were analysed using a fluorescence-activated cell sorter (FACSVantage; Becton-Dickinson, San Jose, CA) and dead cells were gated out by propidium iodide exclusion.
Preparation of peritoneal Mφ
Peritoneal exudate cells (PEC) were obtained from mice that had been injected intraperitoneally (i.p.) 4 days previously with 1·5 ml of 4% thioglycollate broth (Difco). Cells were harvested by lavage of the peritoneal cavity with 5 ml of ice-chilled phosphate-buffered saline (PBS). The cells were washed twice with PBS, resuspended in RPMI-1640 containing 10% heat-inactivated fetal bovine serum (FBS) (HyClone, Logan, UT) and plated in duplicate at a density of 2·5 × 105 cells/well. After incubation at 37° for 16 hr in a humidified atmosphere with 5% CO2, the cells were washed twice with PBS.
Results
Generation of MIF-deficient mice
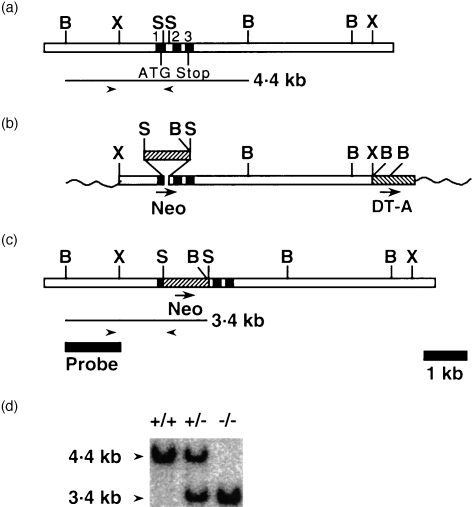
Our targeting vector was designed to replace a 201-bp SacI fragment, spanning exon 1 to intron 1, with a neomycin resistance cassette (Neo) (Fig. 1a, 1b, 1c).
Figure 1.
Targeted disruption of macrophage (Mφ) migration inhibitory factor (MIF) and detection of the disruption in offspring. (a) The endogenous MIF gene. The numbered closed boxes denote exons. BamHI (B), SacI (S) and XbaI (X) sites are indicated. Polymerase chain reaction (PCR) primers used to detect the wild-type allele are shown as right and left arrow heads. (b) The targeting vector. Neo and DT-A indicate neomycin resistance gene and diphtheria toxin A gene, respectively, oriented as indicated by arrows. The wavy lines represent plasmid sequence (not to scale). (c) The targeted MIF gene. A BamHI–XbaI fragment was used as probe for Southern blot analysis. PCR primers used to detect mutant allele are shown as right and left arrow heads. (d) A Southern blot analysis of genomic DNA from offspring of heterozygous crossing digested with BamHI, showing expected fragments for the wild-type (4·4 kb) and targeted (3·4 kb) alleles. Wild-type (+/+), heterozygous (+/–) and homozygous (– /–) offspring are indicated.
Of 189 G418-resistant R1 ES cell clones isolated, 17 were determined to have undergone homologous recombination, as determined by PCR and Southern blot analysis. Two targeted cell lines were used for production of germline chimeras. Both gave rise to chimeric animals that, when mated to B6, subsequently passed the targeted allele to their offspring. Heterozygous mutants were interbred and Southern blot analysis of genomic DNA from their offspring showed the expected DNA fragments for the wild-type (+/+), heterozygous (+/–) and homozygous (–/–) mutant genotypes (Fig. 1c).
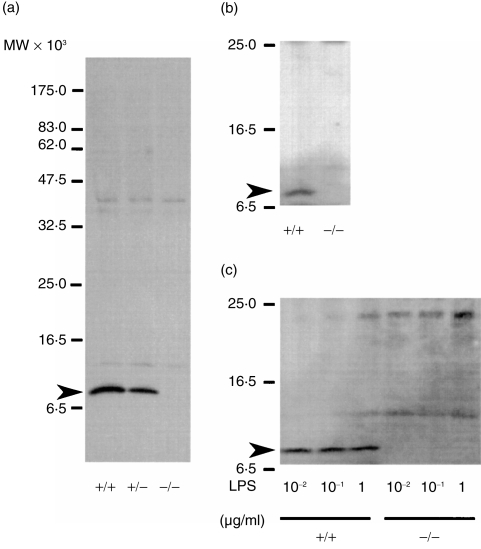
To confirm that this gene targeting had abolished production of MIF protein, Western blot analysis was performed on a soluble fraction from brain using a polyclonal antibody specific for MIF, as MIF protein was previously reported to be detected in brain.23 As illustrated in Fig. 2(a), no 12 5000-MW MIF was detected in MIF –/– mice, whereas in +/– mice, expression level was ≈ 50% as much as that of +/+ mice.
Figure 2.
Detection of macrophage (Mφ) migration inhibitory factor (MIF) protein by Western blot analyses by using a polyclonal antibody to MIF. (a) Soluble fraction of brain. (b) Sera of mice 20 hr after intraperitoneal (i.p.) injection with lipopolysaccharide (LPS) at a dose of 7 mg/kg. (c) Culture supernatants of thioglycollate-elicited peritoneal Mφ stimulated with 0·01–1 μg/ml of LPS for 12 hr. Arrows indicate a band migrating at the expected molecular weight of the MIF protein.
General health and fertility
MIF –/– mutant mice developed normally, appeared healthy and were fertile. Microscopic examination of selected tissues (brain, hypophysis, thymus, spleen, lymph node, femur/sternum [bone and bone marrow], submandibular gland, heart, lung, liver, kidney and testis) from homozygous mutants revealed no significant abnormalities (data not shown). Counts and volumes of white cells, red cells and platelets in peripheral blood of four animals, as measured using an automatic haemocyte counter, were also in the normal ranges. The ratios of CD3+, B220+, CD4+, CD8+ and immunoglobulin M+ (IgM+) cells in CD45+ splenocytes from two animals were also similar to wild-type mice, as determined using flow cytometry.
Genotypic ratios (+/+ : +/–:–/–; raw number) of weanling pups obtained by matings between MIF +/– were as follows: females (68 : 143 : 73), males (98 : 155 : 67).
Survival rate in endotoxic shock
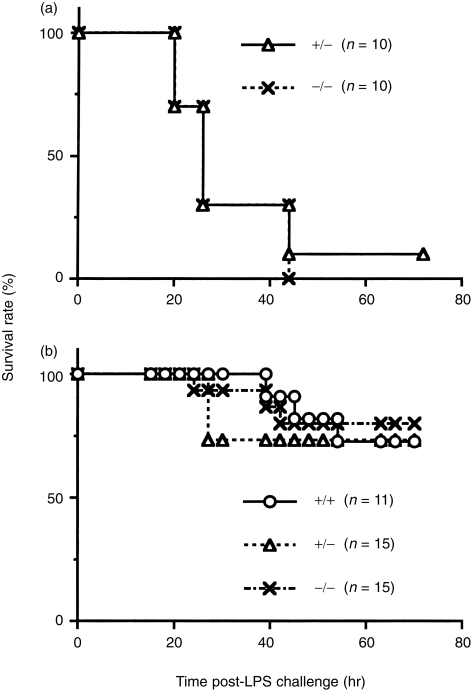
In order to determine the possible role of MIF in endotoxic shock, 10 MIF +/+ mice and 10 –/– mice, produced in the same matings, were injected i.p. with LPS (12 mg/kg). Survival rates for the LPS-injected animals were comparable in the two groups (Fig. 3a). To confirm these findings, groups of MIF +/+, +/– and –/– mice, consisting of 11, 15 and 15 animals, respectively, were injected with LPS at a dose of 7 mg/kg. As shown in Fig. 3(b), no significant differences in the survival rates were found among the three groups. The final survival rates in the MIF +/+, +/– and –/– mice at 80 hr after the LPS injection were 73%, 73% and 80%, respectively. These results indicate that the absence of MIF had no effect on death induced by endotoxic shock.
Figure 3.
Survival rate after lipopolysaccharide (LPS) challenge. Mice were injected with LPS, intraperitoneally (i.p.), at doses of 12 mg/kg (a) or 7 mg/kg (b).
Serum samples were obtained from MIF +/+ and –/– animals 20 hr after an injection of 7 mg/kg LPS and tested for the presence of MIF by Western blotting. As expected, MIF was detected in the serum of MIF +/+ mice but not in the serum of –/– mice (Fig. 2b).
Serum TNF-α levels in LPS-injected mice
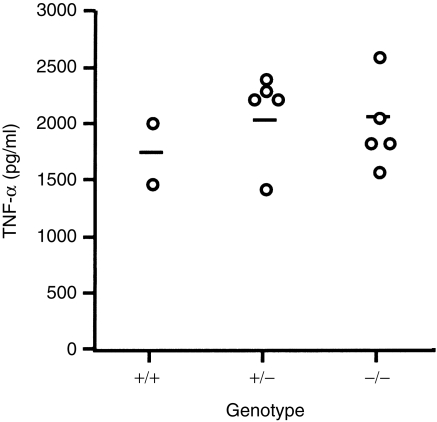
Owing to the critical role of TNF-α in systemic toxicity associated with sepsis,11,12,24 it was determined whether MIF deficient mice could produce TNF-α in response to LPS injection. LPS was injected i.p. into MIF +/+, +/– and –/– mice at a dose of 6 mg/kg. The concentration of TNF-α in their sera was determined by ELISA 1·5 hr after injection, as most serum TNF is produced within the first 1–2 hr after LPS administration.16 The results shown in Fig. 4 indicate relatively large differences among individuals in each group. However, all data were within the range of the levels reported by others25 and differences found among the three groups in the serum concentration of TNF-α was not statistically significant.
Figure 4.
Tumour necrosis factor-α (TNF-α) production after stimulation with lipopolysaccharide (LPS) in vivo. Each circle represents TNF-α level in the serum of an individual mouse 1·5 hr after injection with LPS at a dose of 6 mg/kg. The concentration of TNF-α was measured using enzyme-linked immunosorbent assay (ELISA).
TNF-α production by thioglycollate-elicited peritoneal Mφ stimulated with LPS and its suppression by Dex
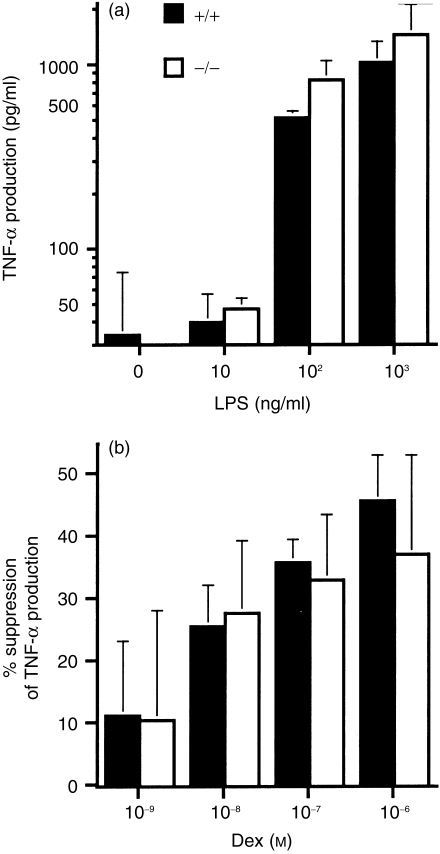
It is well known that stimulation of macrophages with LPS results in the production of TNF-α.26 Calandra et al.10 reported that LPS-stimulated Mφ also produced MIF as well, and suggested that MIF is involved in the production of TNF-α. To confirm their findings, thioglycollate-elicited peritoneal Mφ of MIF +/+ mice and –/– mice were cultured with 10 ng/ml to 1 μg/ml of LPS for 12 hr, and the presence of MIF in the culture supernatants was determined by Western blotting. Mφ from MIF +/+ mice produced a detectable amount of MIF, while those from –/– mice did not (Fig. 2c). The ability of the cells to produce TNF-α was then determined. The Mφ from MIF +/+ mice and –/– mice were cultured for 12 hr in the presence of 10 ng/ml to 1 μg/ml of LPS, and TNF-α in the culture supernatants was determined using ELISA. As shown in Fig. 5(a), no significant difference was detected between the two genotypes in the quantity of TNF-α produced by their Mφ and no significant difference in the sensitivity of the cells to LPS. These results indicate that Mφ-derived MIF does not affect the TNF-α production by Mφ induced with LPS.
Figure 5.
Tumour necrosis factor-α (TNF-α) production by thioglycollate-elicited peritoneal macrophages (Mφ) after stimulation with lipopolysaccharide (LPS) and its suppression by dexamethasone (Dex). (a) TNF-α in culture supernatants of thioglycollate-elicited peritoneal Mφ incubated with 0–1000 ng/ml of LPS for 12 hr. (b) Suppression of TNF-α production by 1 hr of preincubation with Dex in the same system as (a) with 1 μg/ml of LPS. Per cent suppression was calculated using the following formula: % suppression = [(TNF-α production without Dex) − (TNF-α production in culture treated with Dex)]/(TNF-α production without Dex)×100. Data shown are mean ± SD of three animals. The cells from each of the animals were cultured in duplicate. TNF-α was measured by ELISA.
It was previously reported that MIF overrides gluco-corticoid-mediated inhibition of TNF-α production by LPS-stimulated monocytes and suggested that endogenous MIF can act to overcome glucocorticoid inhibition of cytokine production.13 To test this possibility, thioglycollate-elicited peritoneal Mφ from each of three MIF +/+ or –/– mice were precultured in the presence of 10−9−10−6 m Dex, and then stimulated with 1 μg/ml of LPS for 12 hr. Determination of TNF-α in the culture supernatants, shown in Fig. 5(b), indicated that Dex inhibited TNF-α production in a dose-dependent manner, but no significant difference was detected between the MIF +/+ and –/– Mφ in the ability of glucocorticoid to inhibit TNF-α production. In contrast to the previous observations,13 10−12−10−10 m Dex failed to affect TNF-α production by LPS-stimulated Mφ in either cell preparation (data not shown).
Discussion
Mice deficient in the MIF gene are overtly normal, fertile and healthy under SPF conditions. The numbers and proportions of splenic T and B lymphocytes were comparable to those in +/+ mice, indicating that MIF is not involved in the process of lymphocyte development.
Great care was taken to confirm the absence of MIF expression in MIF –/– mice. Previous reports have shown expression of MIF mRNA in the brain, liver and kidney of normal mice.27 Indeed, the 12 500-MW MIF protein was detected in the brain extracts from +/+ mice but not in extracts from MIF-deficient animals (Fig. 2a). As reported by Bernhagen et al.7 and Calandra et al.10 MIF protein was detected in the serum of LPS-injected normal (+/+) mice and in the culture supernatant of LPS-stimulated normal Mφ. In contrast, the protein was not detectable in the serum of LPS-injected MIF-deficient mutant (–/–) or in the culture supernatant of their LPS-stimulated Mφ (Fig. 2b, 2c). Thus, MIF –/– mice are incapable of producing the MIF protein, even after LPS stimulation.
Our experiments showed that the +/+ mice and the –/– mutants were comparable in their susceptibility to LPS for shock and in their serum concentration of TNF-α after LPS injection. Indeed, no significant difference was detected between the +/+ and –/– mice in the ability of their Mφ to produce TNF-α upon LPS stimulation. These findings suggest that endogenous MIF is not involved in the formation of TNF-α, which plays an essential role in LPS-induced shock.11,12
The conclusion obtained from the present experiments using MIF-deficient mice differs from that obtained from previous experiments which utilized anti-MIF antibodies. One explanation for this is that other cytokines may compensate for the defect. Previous reports indicated that not only TNF-α but also interleukin (IL)-1, IL-6, IL-8 and interferon-γ (IFN-γ) participate in the host response to LPS.28–30 These mediators act either alone or in combination to activate Mφ and lymphocytes. Additional inflammatory cell products such as prostaglandins and leukotrienes, as well as reactive oxygen and nitrogen species, also contribute to inflammatory responses.31,32 Despite no significant difference in the TNF-α level in sera of +/+ or –/– mice after LPS treatment, it may be necessary to compare the involvement of the other mediators between MIF-deficient mice and anti-MIF antibody-treated mice.
Another explanation is that the polyclonal antibodies used by Bernhagen et al.7 or the other groups10,33 might have cross-reacted with molecule(s) other than MIF. It is also conceivable that the immune complex itself, consisting of MIF plus antibody, has a protective role in endotoxic shock. Similar contradictory results were obtained in the case of TNF-α for induction of experimental autoimmune encephalomyelitis (EAE). TNF-α-deficient mice clearly indicated that TNF-α is not essential for the development of EAE,34 while a previous report had suggested that TNF-α plays an important role in EAE because severity of EAE was reduced by treatment with anti-TNF-α antibody.35
More recently, Bozza et al. analysed the role of MIF in sepsis using MIF-deficient mice.36 In contrast to our results, they found a significant difference in the survival rate between normal and MIF-deficient mice after LPS administration. In their results, the production of TNF-α after LPS administration was also significantly lower in MIF-deficient mice. They concluded that MIF is critical for LPS-induced sepsis. The reason for this discrepancy from the current data is unclear at this moment. Although the company from which Bozza et al. purchased LPS was different from the one in this work, both LPS preparations were obtained from Escherichia coli serotype O111: B4. The dose of LPS used by Bozza et al. was twice (25 mg/kg) the level used in this work. However, the survival rate of normal B6 mice under our experimental conditions (Fig. 3a) was comparable to that shown in the report of Bozza et al..36 In fact, the LD50 of LPS is known to be rather variable and dependent on the lot number of the product. With this in mind, the experimental systems of the two studies seem to be very similar. It has been suggested that the response to LPS is highly influenced by pre-exposure to LPS,37–42 and it is conceivable that the responses to endotoxin are influenced by the conditions under which the mice are maintained. Our mice were maintained under SPF conditions, while in their report, Bozza et al. did not describe the housing conditions of mice.36 It is possible that their housing conditions made their mice more dependent on MIF in LPS-induced shock reactions. In any event, our results clearly suggested that MIF is not critical in LPS-induced sepsis in mice maintained under some conditions, such as SPF.
Mouse MIF has been shown to be one of the delayed early response genes27 and a glycosylation-inhibiting factor.22 More recently, it was shown to catalyze the tautomerization of d-dopachrome into 5,6-dihydroxyindole-2-carboxolic acid14 and to have protein–thiol oxidoreductase activity.15 More interestingly, Apte et al.43 reported that MIF inhibits natural killer (NK) cells and thereby contributes to immune privilege in the eye, suggesting that MIF is not a simple proinflammatory mediator. While receptor components of MIF have not yet been identified, the binding of MIF on NK cells was demonstrated.44 The involvement of those functions in endotoxaemia remains unclear. However, it is of note that our results indicated that MIF is not an essential regulatory cytokine for the production of TNF-α. In conclusion, our data clearly indicated that the absence of MIF production does not protect against LPS-induced lethal shock. Future studies employing MIF-deficient mice will allow valuable insight into the physiological significance of MIF.
Acknowledgments
We are grateful to Dr K. Ishizaka, Dr Y. Ishii, Dr D. R. Green and Dr W. R. Force for discussion; Dr A. Nagy for providing us with R1 ES cells; and M. Thomas, Y. Takeuchi, N. Kubota and M. Sato for technical assistance.
References
- 1.Bloom BR, Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966;153:80. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- 2.David JR. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell–antigen inter-action. Proc Natl Acad Sci USA. 1966;56:72. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.David JR, David RA. Cellular hypersensitivity and immunity. Inhibition of macrophage migration and the lymphocyte mediators. Prog Allergy. 1972;16:300. [PubMed] [Google Scholar]
- 4.Rockelin RE, Rsen FS, David JR. In vitro lymphocyte response of patients with immunologic deficiency diseases. Correlation of production of macrophage migration inhibitory factor with delayed hypersensitivity. N Engl J Med. 1970;282:1340. doi: 10.1056/NEJM197006112822404. [DOI] [PubMed] [Google Scholar]
- 5.Weiser WY, Temple PA, Witek-giannotti JS, Remold HG, Clark SC, David JR. Molecular cloning of a cDNA encoding a human macrophage migration inhibitory factor. Proc Natl Acad Sci USA. 1989;86:7522. doi: 10.1073/pnas.86.19.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernhagen J, Mitchell RA, Calandra T, Voelter W, Cerami A, Bucala R. Purification, bioactivity, and secondary structure analysis of mouse and human macrophage migration inhibitory factor (MIF) Biochemistry. 1994;33:14144. doi: 10.1021/bi00251a025. [DOI] [PubMed] [Google Scholar]
- 7.Bernhagen J, Calandra T, Mitchell RA, et al. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365:756. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- 8.Brnhagen J, Calandra T, Cerami A, Bucala R. Macrophage migration inhibitory factor is a neuroendocrine mediator of endotoxaemia. Trends Microbiol. 1994;2:198. doi: 10.1016/0966-842x(94)90111-h. [DOI] [PubMed] [Google Scholar]
- 9.Brnhagen J, Calandra T, Bucala R. The emerging role of MIF in septic shock and infection. Biotherapy. 1994;8:123. doi: 10.1007/BF01878495. [DOI] [PubMed] [Google Scholar]
- 10.Calandra T, Bernhagen J, Mitchell RA, Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994;179:1895. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tracey KJ, Fong Y, Hesse DG, et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 12.Mohler KM, Torrance DS, Smith CA, et al. Soluble tumor necrosis factor (TNF) receptors are effective therapeutic agents in lethal endotoxaemia and function simultaneously as both TNF carriers and TNF antagonists. J Immunol. 1993;151:1548. [PubMed] [Google Scholar]
- 13.Calandra T, Bernhagen J, Metz CN, et al. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995;377:68. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- 14.Rosengren E, Bucala R, Aman P, et al. The immunoregulatory mediator macrophage migration inhibitory factor (MIF) catalyzes a tautomerization reaction. Mol Med. 1996;2:143. [PMC free article] [PubMed] [Google Scholar]
- 15.Kleemann R, Kapurniotu A, Frank RW, et al. Disulfide analysis reveals a role for macrophage migration inhibitory factor (MIF) as thiol-protein oxidoreductase. J Mol Biol. 1998;280:85. doi: 10.1006/jmbi.1998.1864. [DOI] [PubMed] [Google Scholar]
- 16.Bozza M, Kolalowski LF, Jr, Jenkins NA, et al. Structural characterization and chromosomal location of the mouse macrophage migration inhibitory factor gene and pseudogenes. Genomics. 1995;27:412. doi: 10.1006/geno.1995.1071. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell R, Bacher M, Bernhagen J, Pushkarskaya T, Seldin MF, Bucala R. Cloning and characterization of the gene for mouse macrophage migration inhibitory factor (MIF) J Immunol. 1995;154:3863. [PubMed] [Google Scholar]
- 18.Honma N, Matsuda Y, Ishii Y, et al. Cloning and mapping of the mouse glycosylation-inhibiting factor gene and four related genes. Mammal Genome. 1996;7:59. doi: 10.1007/s003359900014. [DOI] [PubMed] [Google Scholar]
- 19.Wurst W, Joyner AL. Production of targeted embryonic stem cell clones. In: Joyner AL, editor. Gene Targeting: a Practical Approach. Oxford: IRL Press; 1993. p. 33. [Google Scholar]
- 20.Nagy A, Rossant J, Nagy R, Abramow-newerly W, Roder JC. Derivation of completely cell culture-derived mice from early passage embryonic stem cells. Proc Natl Acad Sci USA. 1993;90:8424. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoscheck CM. Quantitation of protein. In: Deutscher MP, editor. Guide to Protein Purification. New York: Academic Press; 1990. p. 55. [Google Scholar]
- 22.Mikayama T, Nakano T, Gomi H, et al. Molecular cloning and functional expression of a cDNA encoding glycosylation-inhibiting factor. Proc Natl Acad Sci USA. 1993;90:10056. doi: 10.1073/pnas.90.21.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galat A, Rivière S, Bovet F. Purification of macrophage migration inhibitory factor (MIF) from bovine brain cytosol. FEBS Lett. 1993;319:233. doi: 10.1016/0014-5793(93)80553-7. [DOI] [PubMed] [Google Scholar]
- 24.Beutler B, Milsark IW, Cerami AC. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229:869. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- 25.Doherty GM, Lange JR, Langstein HN, Alexander HR, Buresh CM, Norton JA. Evidence for IFN-γ as a mediator of the lethality of endotoxin and tumor necrosis factor-α. J Immunol. 1992;149:1666. [PubMed] [Google Scholar]
- 26.Amiot F, Fitting C, Tracey KJ, Cavaillon JM, Dautry F. Lipopolysaccharide-induced cytokine cascade and lethality in LT alpha/TNF alpha-deficient mice. Mol Med. 1997;3:864. [PMC free article] [PubMed] [Google Scholar]
- 27.Gifford GE, Lohmann-matthes ML. Requirement for the continual presence of lipopolysaccharide for production of tumor necrosis factor by thioglycollate-induced peritoneal murine macrophages. Int J Cancer. 1986;38:135. doi: 10.1002/ijc.2910380121. [DOI] [PubMed] [Google Scholar]
- 28.Lanahan A, Williams JB, Sanders LK, Nathans D. Growth factor-induced delayed early response genes. Mol Cell Biol. 1992;12:3919. doi: 10.1128/mcb.12.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fong Y, Tracey KJ, Moldawer LL, et al. Antibodies to cachectin/tumor necrosis factor reduce interleukin 1 beta and interleukin 6 appearance during lethal bacteremia. J Exp Med. 1989;170:1627. doi: 10.1084/jem.170.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martich GD, Danner RL, Ceska M, Suffredini AF. Detection of interleukin 8 and tumor necrosis factor in normal humans after intravenous endotoxin: the effect of antiinflammatory agents. J Exp Med. 1991;173:1021. doi: 10.1084/jem.173.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Needleman P, Turk J, Jakschik BA, Morrison AR, Lefkowith JB. Arachidonic acid metabolism. Annu Rev Biochem. 1986;55:69. doi: 10.1146/annurev.bi.55.070186.000441. [DOI] [PubMed] [Google Scholar]
- 32.Stuehr DJ, Gross SS, Sakuma I, Levi R, Nathan CF. Activated murine macrophages secrete a metabolite of arginine with the bioactivity of endothelium-derived relaxing factor and the chemical reactivity of nitric oxide. J Exp Med. 1989;169:1011. doi: 10.1084/jem.169.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Juttner S, Bernhagen J, Metz CN, Rollinghoff M, Bucala R, Gessner A. Migration inhibitory factor induces killing of Leishmania major by macrophages: dependence on reactive nitrogen intermediates and endogenous TNF-alpha. J Immunol. 1998;161:2383. [PubMed] [Google Scholar]
- 34.Frei K, Eugster HP, Bopst M, Constantinescu CS, Lavi E, Fontana A. Tumor necrosis factor alpha and lymphotoxin alpha are not required for induction of acute experimental autoimmune encephalomyelitis. J Exp Med. 1997;185:2177. doi: 10.1084/jem.185.12.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruddle NH, Bergman CM, McGrath KM, et al. An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. J Exp Med. 1990;172:1193. doi: 10.1084/jem.172.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bozza M, Satoskar AR, Lin G, et al. Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J Exp Med. 1999;189:341. doi: 10.1084/jem.189.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freudenberg MA, Galanos C. Induction of tolerance to lipopolysaccharide (LPS)-d-galactosamine lethality by pretreatment with LPS is mediated by macrophage. Infect Immun. 1988;56:1352. doi: 10.1128/iai.56.5.1352-1357.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bundschuh DS, Barsig J, Hartung T, et al. Granulocyte–macrophage colony-stimulating factor and IFN-gamma restore the systemic TNF-alpha response to endotoxin in lipopolysaccharide-desensitized mice. J Immunol. 1997;158:2862. [PubMed] [Google Scholar]
- 39.Randow F, Syrbe U, Meisel C, et al. Mechanism of endotoxin desensitization: involvement of interleukin 10 and transforming growth factor beta. J Exp Med. 1995;181:1887. doi: 10.1084/jem.181.5.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans GF, Zuckerman SH. Glucocorticoid-dependent and -independent mechanisms involved in lipopolysaccharide tolerance. Eur J Immunol. 1991;21:1973. doi: 10.1002/eji.1830210902. [DOI] [PubMed] [Google Scholar]
- 41.Weigle WO, Gahring LC, Romball CG, Goodman MG. The effect of lipopolysaccharide desensitization on the regulation of in vivo induction of immunologic tolerance and antibody production and in vitro release of IL-1. J Immunol. 1989;142:1107. [PubMed] [Google Scholar]
- 42.Balkhy HH, Heinzel FP. Endotoxin fails to induce IFN-γ in endotoxin-tolerant mice: deficiencies in both IL-12 heterodimer production and IL-12 responsiveness. J Immunol. 1999;162:3633. [PubMed] [Google Scholar]
- 43.Apte RS, Sinha D, Mayhew E, Wistow GJ, Niederkorn JY. Role of macrophage migration inhibitory factor in inhibiting NK cell activity and preserving immune privilege. J Immunol. 1998;160:5693. [PubMed] [Google Scholar]
- 44.Sugie K, Nakano T, Tomura T, Takakura K, Mikayama T, Ishizaka K. High-affinity binding of bioactive glycosylation-inhibiting factor to antigen-primed T cells and natural killer cells. Proc Natl Acad Sci USA. 1997;94:5278. doi: 10.1073/pnas.94.10.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]