Abstract
Lactoferrin (LF) is a member of the transferrin family of iron-binding glycoproteins to which several anti-inflammatory functions have been ascribed. LF has been shown to down-regulate expression of the pro-inflammatory cytokine tumour necrosis factor-α (TNF-α), although the possibility has been raised that the activity of LF in this regard was indirect and secondary to its ability to bind to and inactivate the bacterial lipopolysaccharide (LPS) used to induce cytokine production. However, the identification of putative membrane receptors for LF raises the possibility that the interaction of LF with its receptor may be one important route through which this protein exerts anti-inflammatory activity. In the present investigations the biological properties of LF have been examined in a model of cutaneous immune function where the allergen-induced migration of epidermal Langerhans cells (LC) from the skin and their subsequent accumulation as dendritic cells (DC) in skin-draining lymph nodes are known to be dependent upon the de novo synthesis of TNF-α, but independent of exogenous LPS. Consistent with the protein having direct anti-inflammatory properties, it was found that the intradermal injection of recombinant murine LF (either iron-saturated or iron-depleted LF) inhibited significantly allergen (oxazolone) -induced LC migration and DC accumulation. That these inhibitory effects were secondary to the inhibition of local TNF-α synthesis was suggested by the findings that first, LF was unable to inhibit LC migration induced by intradermal injection of TNF-α itself, and second, that migration stimulated by local administration of another epidermal cytokine, interleukin 1β, which is also dependent upon TNF-α production, was impaired significantly by prior treatment with LF. Finally, immunohistochemical analyses demonstrated the presence of LF in skin, associated primarily with keratinocytes. Collectively these data support the possession by LF of direct immunomodulatory and/or anti-inflammatory activity, probably associated in this case with inhibition of cytokine production. Furthermore, the results suggest that as a constituent of normal skin, LF may play a role in homeostatic regulation of cutaneous immune function.
Introduction
Lactoferrin (LF) is a member of the transferrin gene family of non-heme iron-binding glycoproteins.1 LF was identified first as an abundant milk protein product of mammary epithelial cells.2 However, the protein is expressed in a variety of glandular epithelial cells and is a major component of exocrine secretions associated with innate host defence, including mucosal, reproductive, salivary and lachrymal secretions.3 In addition, it is produced at high levels in the secondary granules of neutrophil polymorphonuclear cells.4
Several functions have been ascribed to LF, including regulation of iron absorption by intestinal epithelium,5,6 epithelial cell proliferation7,8 and immune defence mechanisms.9 LF can contribute directly to host defence by acting as an antibacterial agent. The antibacterial properties of LF are imparted by two distinct mechanisms, involving discrete domains of the protein. The first is the iron-binding domain of LF, which causes a retardation of bacterial growth due to iron sequestration and deprivation.10 The second domain comprises a cationic region contained within the first 47 amino acids at the amino terminus of human LF.11 This latter domain, when isolated as a peptide, interacts with lipopolysaccharide (LPS) in a manner that causes a disruption of bacterial membranes, leading to a direct bactericidal effect.11
There is, however, growing evidence that, in addition to its bacteriostatic and bactericidal properties, LF may serve as an important regulator of immune responses and inflammatory reactions. Of particular interest are the observations that LF may influence the production of some cytokines, including pro-inflammatory cytokines.9,12,13 Among the cytokines shown to be regulated negatively by LF are tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and granulocyte–macrophage colony-stimulating factor.9,13,14 It has been demonstrated previously that LF administered intravenously to mice prior to an injection of LPS confers protection against endotoxin lethality.15 The proposal is that such protection results from the inhibition by LF of the TNF-α production normally stimulated by LPS.15 Similarly, it has been claimed that LF is able to inhibit directly the production in vitro of TNF-α by mononuclear cells treated with LPS.16 It has been argued, however, that the ability of LF to inhibit the stimulation of TNF-α by LPS may be secondary to its capacity to bind to LPS, thereby compromising the signal for cytokine production, rather than regulating the expression of TNF-α per se.9
With the objective of investigating further the regulation by LF of cytokine-dependent immune processes, we have here examined in mice the effect of homologous recombinant LF on the migration of epidermal Langerhans cells (LC) from the skin and their subsequent accumulation as dendritic cells (DC) in skin-draining lymph nodes. These processes are dependent upon the availability of relevant epidermal cytokines (but independent of a requirement for LPS stimulation). The important signals that initiate the migration of LC from the epidermis in response to topical sensitization with chemical allergen, and possibly other stimuli, are TNF-α, an inducible product of keratinocytes, and IL-1β, a cytokine that in murine epidermis is a constitutive and inducible product of LC.17–20 Both cytokines are up-regulated rapidly in mouse epidermis following exposure to chemical allergen.21
Specific receptors for LF have been identified previously on some epithelial cells, monocytes, macrophages, platelets and lymphocytes,22–24 the assumption being that these receptors mediate the biological activities ascribed to LF, including the regulation of growth factor and cytokine production.25–27 In the context of determining whether LF has the potential to act as an endogenous regulator of cutaneous immune function we have also investigated here the expression of this protein in the skin.
Materials and methods
Mice
Young adult (6- to 8-week-old) male BALB/c strain mice obtained from either the Specific Pathogen-Free Breeding Unit (Alderley Park, Cheshire, UK) or from Harlan Sprague Dawley (Houston, TX) were used throughout these investigations.
Lactoferrin
Recombinant murine LF was produced in Aspergillus awamori using the same expression system as that employed to produce human LF, and which has been described previously.28,29 Briefly, transformants containing the mouse lactoferrin expression vector, p26mLF, were cultured in fungal media for 7 days at 30°. The culture medium was filtered through Miracloth (Calbiochem, La Jolla, CA) followed by ultrafiltration and diafiltration against 0·025 m Tris–HCl/0·05 m NaCl (pH 7·5). The equilibrated medium was passed over a Macro-Prep S Support column (Bio-Rad Laboratories, Hercules, CA) and eluted with 0·5–0·75 m NaCl. Iron-depleted recombinant LF was prepared by dialysis against H2O and 5 mm sodium phosphate, pH 7·5. Iron-resaturated LF was prepared by incubating the protein with FeCl3 in the presence of nitrilotriacetic acid and bicarbonate. Purified recombinant mouse LF was intact, correctly N-terminally processed and similar in size to native mouse breast milk LF. In addition, recombinant mouse LF was shown to be glycosylated and functional in terms of iron binding and release. To investigate the influence of iron on the immunoregulatory properties of LF, one series of experiments was conducted using acid-washed (iron-depleted) LF and acid-washed iron-resaturated LF.28 LF was suspended in phosphate-buffered saline (PBS; pH 7·4) at the concentrations indicated and administered locally 2 hr prior to further treatment. Control mice received bovine serum albumin (BSA) diluted to an equivalent extent in place of LF.
Cytokines
Recombinant murine TNF-α (specific activity 2 × 108 U/mg by L929 cytotoxicity assay; endotoxin level: 0·009 ng/µg) was obtained from Genzyme (West Malling, Kent, UK). Recombinant murine IL-1β (specific activity 1 × 108−2 × 108 U/mg; endotoxin level: < 0·1 ng/µg) was purchased from R & D Systems (Oxon, UK). Cytokines were either supplied as, or reconstituted in, sterile solutions of PBS containing 0·1% BSA as carrier protein. Cytokines were diluted with sterile PBS containing 0·1% BSA. Control mice received an equivalent volume of carrier protein alone.
Administration of LF and cytokines
Samples were administered using 1 ml syringes with 30-gauge stainless steel needles. Mice received 30 µl intradermal injections into both ear pinnae.
Chemicals and exposure
The skin-sensitizing chemical 4-ethoxy-2-phenyloxazol-5-one (oxazolone; Sigma Chemical Co., St Louis, MO) was dissolved in 4 : 1 acetone : olive oil (AOO). Groups of mice received 25 µl of 0·5% oxazolone, or vehicle (AOO) alone, on the dorsum of both ears.
Isolation and enumeration of lymph node dendritic cells
Draining (auricular) lymph nodes were excised at various periods following treatment. Nodes were pooled for each experimental group, and single-cell suspensions of lymph node cells (LNC) were prepared by mechanical disaggregation through 200-mesh stainless steel gauze. LNC were washed with, and suspended in, RPMI-1640 growth medium (Gibco, Paisley, Renfrewshire, UK) supplemented with 25 mm HEPES, 400 µg/ml streptomycin, 400 µg/ml ampicillin and 10% heat-inactivated fetal calf serum (RPMI-FCS). Viable cell counts were performed by exclusion of 0·5% trypan blue and the cell concentration was adjusted to 5 × 106 cells/ml in RPMI-FCS. DC-enriched populations were prepared as described previously.30 Briefly, 2 ml of Metrizamide (Nygaard, Oslo, Norway; 14·5% in RPMI-FCS) was layered gently under 8 ml of cell suspension, and tubes were centrifuged for 15 min (600 g) at room temperature. Cells accumulating at the interface were collected, washed once and resuspended in RPMI-FCS. The frequency of DC in such low buoyant density fractions was assessed routinely by direct morphological examination using phase contrast microscopy.31 Results are expressed as DC/node. DC data were evaluated using analysis of variance allowing for the replicate structure of the experimental design. Differences between individual group means were compared using a two-sided Student's t-test based on the error mean square in the analysis of variance.
Preparation and analysis of epidermal sheets
To prepare epidermal sheets, ears were split with the aid of forceps into dorsal and ventral halves. The dorsal halves were incubated for 2 hr at 37° with 0·02 m ethylenediamine tetraacetic acid (EDTA; Sigma Chemical Co., St Louis, MO) dissolved in PBS. The epidermis was separated from the dermis using forceps and washed in PBS. Epidermal sheets were fixed in acetone for 20 min at − 20°. Following fixation, epidermal sheets were washed in PBS and then incubated at room temperature for 30 min with anti-mouse Ia [clone M5/114 (rat IgG2b); Boehringer Mannheim, Lewes, East Sussex, UK] diluted to 5 µg/ml in PBS containing 0·1% BSA. Sheets were then washed prior to incubation for a further 30 min with fluorescein isothiocyanate (FITC)-conjugated goat F(ab′)2 anti-rat immunoglobulin G (IgG; Bradsure Biologicals Ltd, Loughborough, UK) diluted 1 : 50 in PBS. Finally, sheets were washed in PBS and mounted on microscope slides in Citifluor Ltd, London (UK). Control experiments were performed using rat anti-human leucocyte antigen (HLA) Class I (rat IgG2b; Serotec, Kidlington, UK) in place of the primary antibody.
Samples were examined by fluorescence microscopy and the frequency of stained cells was assessed using an eye piece with a calibrated grid (0·32 mm × 0·213 mm at × 40 magnification). Four epidermal sheets were prepared from each experimental group and for each sample 10 random fields were examined. In no instance was any fluorescence detected following treatment of epidermal sheets with isotype-matched control antibody.
For comparison of LC data, the statistical significance of differences between experimental groups was calculated using Student's t-test.
Immunolocalization of LF in mouse skin
LF protein was localized in mouse skin using the previously well-characterized rabbit anti-mouse LF antiserum.32 Mice were killed and dorsal skin samples were removed and fixed in Bouin's fixative at room temperature overnight, then washed in 70% ethanol. Following fixation, the tissue was dehydrated in graded ethanol solutions, cleared in xylene and embedded in paraffin for sectioning; 5 µm sections were cut and mounted on poly l-lysine slides (Cel Tek Inc., Glenview, IL). Sections were deparaffinized in xylene and rehydrated in graded ethanol solutions. Peroxidase quenching was performed with 3% H2O2 in methanol for 10 min. Sections were blocked with 10% goat serum in PBS for 1 hr, followed by incubation with a 1 : 2000 dilution of polyclonal rabbit anti-mouse lactoferrin serum, or normal rabbit serum, for 3 hr at room temperature. Sections were then washed in PBS three times for 3 min followed by incubation with biotinylated goat anti-rabbit IgG secondary antibody (1 : 2000 dilution, Vector Laboratories) for 1 hr. Following three 3-min washes in PBS, the sections were incubated for 10 min with streptavidin-conjugated peroxidase and rewashed three times for 3 min in PBS. Localization of the primary antibody was visualized with the imidazole-diaminobenzidine (DAB) reaction for 3 min, producing a brown-coloured stain. Sections were then counterstained in haematoxylin, dehydrated through graded ethanol solutions, cleared in xylene and coverslipped for brightfield microscopy. Images were acquired digitally using a Zeiss Axioskop microscope (Carl Zeiss Inc., Thornwood, NY) coupled with a Hamamatsu C5810 CCD camera (Hamamatsu Corporation, Bridgewater, NJ) and were processed for contrast adjustment, gamma correction, and cropping using adobe photoshop 4·0 (Adobe Systems Inc., San Jose, CA). Images were printed using a Codonics dye-sublimation printer.
Results
LF inhibits allergen-induced accumulation of DC in draining lymph nodes
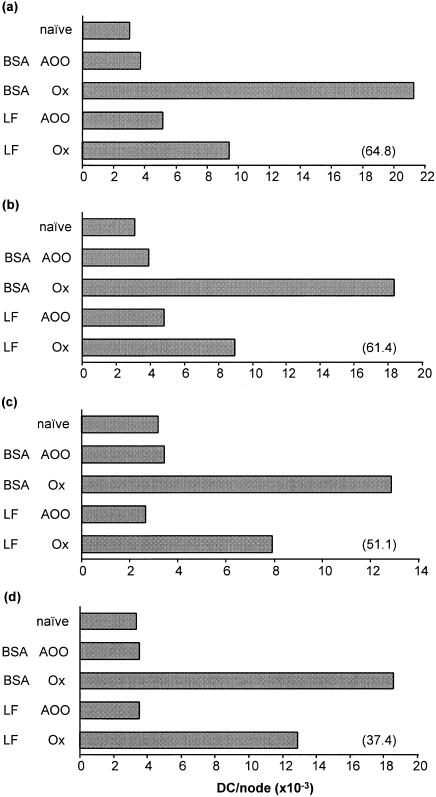
Topical exposure of mice to skin sensitizing chemical allergens, such as oxazolone, causes the migration of DC to draining lymph nodes. Groups of mice were injected with various concentrations of LF (or with a control protein, BSA) 2 hr prior to sensitization at the same site with oxazolone. Control groups were treated topically with an equal volume of vehicle (AOO) alone. The frequency of DC in draining lymph nodes was measured 18 hr following topical exposure. The experiments illustrated in Fig. 1 demonstrate that, at all doses examined (3·0, 1·5, 0·75 and 0·3 µg/ear), LF resulted in a substantial inhibition of oxazolone-induced DC accumulation compared with control mice that had received identical amounts of BSA. The latter control protein had no effect on the integrity of DC migration compared with that in mice that had received an intradermal injection of PBS alone (data not shown). The results shown in Fig. 1 serve to demonstrate also that LF (and BSA) was without influence on the frequency of DC within resting lymph nodes draining the site of exposure to vehicle. The most substantial inhibition (64·8%) of DC accumulation was recorded following treatment of mice with 3·0 µg/ear LF, although even with the lowest dose tested (0·3 µg/ear) LF still resulted in an inhibition of 37·4% in the experiment illustrated in Fig. 1.
Figure 1.
Influence of LF on oxazolone-induced DC accumulation: a dose–response analysis. Groups of mice (n = 10) received 30 µl intradermal injections into both ear pinnae of LF or an equivalent amount of control protein (BSA); (a) 3·0 µg/ear, (b) 1·5 µg/ear, (c) 0·75 µg/ear, (d) 0·3 µg/ear. Two hours later mice were exposed on the dorsum of both ears to 25 µl of 0·5% oxazolone (Ox) or to vehicle (AOO) alone. Control mice were untreated (naive). Draining auricular lymph nodes were removed 18 hr later and the frequency of DC/node was measured. A representative experiment for each concentration tested is shown. Values in parentheses indicate the percentage inhibition caused by LF of oxazolone-induced DC accumulation.
In order to examine the species selectivity of the induced effects of exogenous LF, additional experiments were conducted to determine the ability of heterologous (human) LF to influence allergen-induced DC accumulation in mice. In two independent experiments, only intradermal administration of murine LF (3·0 µg/ear) prior to application of oxazolone resulted in substantial inhibition of lymph node DC accumulation (53·2% and 51·2%). Under the same conditions of exposure, equivalent amounts of the human recombinant protein only minimally prevented this response (7·9% and 11·9%).
Both iron-free LF and iron-saturated LF inhibit the accumulation of DC induced by oxazolone
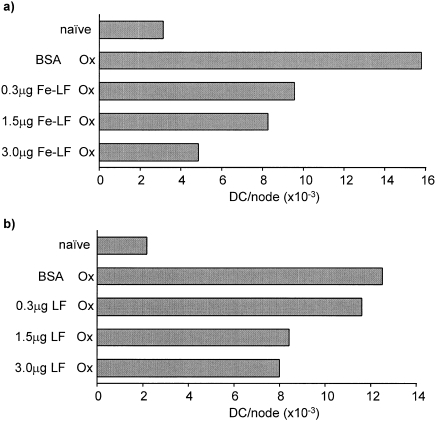
Mice were injected intradermally with various amounts of either acid-washed (iron-depleted) LF or acid-washed, iron-resaturated LF (Fe-LF). Two hours later mice were exposed topically to oxazolone. Control mice were injected with 3·0 µg of BSA in place of LF preparations, or were untreated (naive). Representative experiments are illustrated in Fig. 2 in which the frequency of DC in draining lymph nodes was measured 18 hr following exposure to oxazolone. The results reveal that both iron-free and iron-saturated LF were able, when injected locally, to cause a dose-related inhibition of DC accumulation in draining nodes, although in the experiments illustrated the iron-saturated LF preparation appeared to be somewhat more active. In control experiments, acid-washed BSA, when compared with untreated BSA, was without effect on the integrity of oxazolone-induced DC accumulation (data not shown).
Figure 2.
Influence of iron on the immunomodulatory activity of LF. Groups of mice (n = 10) received 30 µl intradermal injections into both ear pinnae of either (a) acid-washed iron-resaturated LF (Fe-LF), or (b) acid-washed (iron-depleted) LF. Two hours later mice were exposed on the dorsum of both ears to 25 µl of 0·5% oxazolone (Ox). Control mice were untreated (naive) or were injected with 3·0 µg of BSA in place of LF. Draining auricular lymph nodes were removed 18 hr later and the number of DC/node was counted. Representative experiments for each form of LF tested are shown.
Intradermal LF inhibits LC migration induced by IL-1β, but is without effect on migration stimulated by TNF-α
It has been demonstrated previously that allergen induced migration of LC from the epidermis requires the participation of both TNF-α and IL-1β and that this response is blocked by antibodies directed against either TNF-α or IL-1β.18,20 However, intradermal injection of either cytokine alone is sufficient to cause the migration of LC, albeit with different tempos.19,20 The proposal is that the endogenous constitutive levels of IL-1β produced by LC are sufficient to act with exogenously administered TNF-α to induce LC mobilization, while IL-1β when administered alone by intradermal injection is adequate to induce synthesis of TNF-α by keratinocytes.33 Thus, LC migration resulting from IL-1β exposure requires the de novo production of TNF-α by keratinocytes.
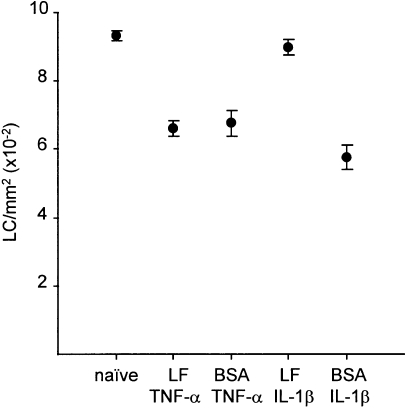
We hypothesized that LF may inhibit allergen-induced LC migration by interacting directly with keratinocytes to inhibit production of TNF-α. Furthermore, if this were the case, we would predict that LF should inhibit LC migration induced by IL-1β, but not by TNF-α. We therefore examined the influence of LF on cytokine-induced LC migration from the epidermis. Groups of mice received intradermal injections of LF (0·75 µg/ear) 2 hr prior to receipt at the same site of either TNF-α or IL-1β. Control mice were injected with an equivalent amount of BSA in place of LF, or received no treatment (naive). A representative experiment is shown in Fig. 3. Intradermal administration of TNF-α resulted in a significant reduction in the frequency of epidermal LC measured 30 min following exposure, which was unaffected by either LF or BSA. In similar fashion, IL-1β caused significant LC migration from the skin, in this case measured at 17 hr. However, in contrast to the activity of TNF-α, the reduction in epidermal LC frequency stimulated by IL-1β exposure was almost completely inhibited in mice that had been pretreated at the same site with LF (Fig. 3.).
Figure 3.
Effect of LF on LC migration induced by TNF-α or IL-1β. Groups of mice (n = 3) received 30 µl intradermal injections into both ear pinnae of LF (0·75 µg/ear), or an equivalent amount of control protein (BSA), 2 hr prior to receipt of a second intradermal injection into the same site of cytokine (TNF-α or IL-1β; 50 ng/ear). Control mice were untreated. Ears were removed 30 min following administration of TNF-α or 17 hr after treatment with IL-1β and epidermal sheets were prepared. The frequency of major hsitocompatibility complex class II+ LC was measured by indirect immunofluorescence. Results are expressed as the mean number of cells/mm2 (± SE) derived from examination of 10 fields per sample for each of four samples. Treatment of mice with either TNF-α or IL-1β, in the presence of control protein (BSA), caused a significant decrease in the frequency of LC compared with untreated controls (P < 0·005). Pretreatment of mice with LF resulted in a significantly higher frequency of LC in mice exposed subsequently to IL-1β (P < 0·005). In contrast, LF failed to affect significantly LC frequency in TNF-α-treated mice.
Intradermal LF also causes differential inhibition of TNF-α- and IL-1β-induced DC accumulation in draining lymph nodes
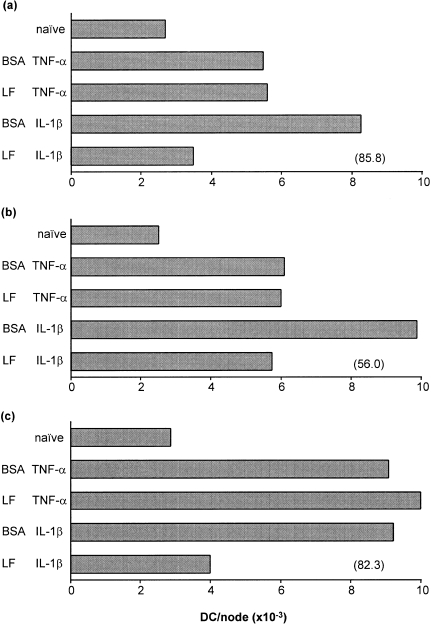
Comparisons were made of the ability of LF to inhibit the accumulation of DC in draining lymph nodes caused by either TNF-α or IL-1β. Experiments were designed as detailed above and the frequency of DC was measured in draining lymph nodes 4 hr following exposure to TNF-α, or 17 hr following exposure to IL-1β. Three independent experiments are illustrated in Fig. 4. In all experiments, treatment with either TNF-α or IL-1β was associated with a substantial increase in the frequency of lymph node DC. In each case exposure of mice to LF prior to treatment at the same site with IL-1β caused a substantial inhibition of DC migration. In contrast, LF had no influence on the integrity of DC accumulation caused by TNF-α.
Figure 4.
Influence of LF on the accumulation of DC in draining lymph nodes provoked by TNF-α or IL-1β. Groups of mice (n = 10) received 30 µl intradermal injections into both ear pinnae of LF (0·75 µg/ear), or an equivalent amount of control protein (BSA), 2 hr prior to receipt of a second intradermal injection into the same site of cytokine (TNF-α or IL-1β; 50 ng/ear). Control mice were untreated. Draining auricular lymph nodes were removed 4 hr following exposure to TNF-α, or 17 hr after IL-1β treatment and the frequency of DC/node was measured. Three independent experiments (a–c) are shown. Values in parentheses represent the percentage inhibition caused by LF of IL-1β-induced DC accumulation. IL-1β-induced DC accumulation was reduced significantly in the presence of LF (P < 0·01) compared with BSA pretreatment. No statistically significant differences were observed between the TNF-α-treated groups.
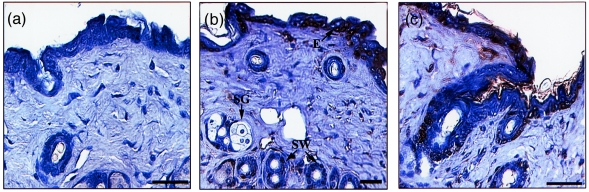
LF is expressed in normal adult mouse skin
To determine whether LF may be an endogenous component of mouse skin, the distribution of LF protein in adult mouse skin was investigated using immunocytochemical localization. Transverse sections of skin were analysed using a polyclonal antiserum specific for murine LF.32 Positively stained epithelial cells were restricted to the epidermis, sweat glands and follicles (Fig. 5b,c). Within the epidermis staining for LF did not appear to be restricted to the mitotic basal layer, but was observed in scattered keratinocytes throughout the epithelium. Some positively stained cells were also observed in the dermis. Staining was specific for the LF antiserum and was not observed using normal rabbit serum as a control (Fig. 5a).
Figure 5.
Immunolocalization of LF in mouse skin. (a–c) Transverse sections of adult skin, 5 µm thick, incubated with normal rabbit control serum (a) or with rabbit anti-mouse LF serum (b,c). Scale bars are 30 µm; E, epidermis; SW, sweat gland; SG, sebaceous gland.
Discussion
Epidermal LC, members of the wider family of DC, are considered to play pivotal roles in the induction and regulation of cutaneous immune responses.34 Topical exposure of mice to skin-sensitizing chemicals results in the mobilization of a proportion of local LC and their directed movement, via afferent lymphatics, to draining lymph nodes where they accumulate as immunostimulatory DC.30,31,35,36 Many of the DC that arrive in the paracortical regions of lymph nodes bear high levels of the inducing antigen and are able to activate responsive T lymphocytes.30,31,35 If the integrity of LC migration, and the delivery of antigen-bearing cells to lymph nodes, are compromised then cutaneous immune function is impaired. Thus, inhibition of allergen-induced LC migration caused by systemic exposure of mice to neutralizing anti-TNF-α antibody has been shown to result in a significant reduction in contact sensitization.18 The importance of LC migration for the maintenance of immunological competence at skin surfaces is indicated also by investigations of the effects of ultraviolet B (UVB) irradiation on responses to antigen encountered in the skin. It has been suggested that the local immune suppression associated with exposure to UVB is at least in part attributable to the UVB-induced movement of responsive LC away from the epidermis.37
The ability of exogenous LF to compromise both LC migration and DC accumulation in draining lymph nodes as recorded here is consequently of some interest. The data suggest that the inhibition of LC migration associated with exposure to LF may be secondary to regulation of local TNF-α production. Thus, it was observed that whereas responses to allergen and IL-1β were compromised by LF, LC migration stimulated by TNF-α was unaffected. The mobilization of LC following exposure to allergen or injection of IL-1β is in both cases dependent upon the de novo production of TNF-α,19,20 whereas migration induced by TNF-α is not. These results are reminiscent of the effects on LC migration induced by the systemic administration to mice of dexamethasone,38 a drug that is known to inhibit the transcription of a variety of cytokines, including IL-1β and TNF-α.39,40 It was shown that whereas treatment of mice with dexamethasone inhibited allergen (oxazolone)- and IL-1β-induced LC migration, responses to TNF-α were unaffected.38 The interpretation was that migration in response to allergen and IL-1β was suppressed owing to the transcriptional inhibition of TNF-α, and furthermore, that while de novo IL-1β production is also undoubtedly inhibited by dexamethasone, there are sufficient amounts of this cytokine available constitutively to act together with exogenous TNF-α to permit LC mobilization to proceed as normal.38
It is tempting to speculate that the same selective inhibition of LC migration resulting from local exposure to LF is due to a similar impairment of local cytokine production, with the effect on TNF-α production being the critical event. Nevertheless, it is not possible currently to conclude that the influence of LF on LC migration is attributable to changes in TNF-α production in the local microenvironment. Investigations of the effect of LF on cutaneous cytokine production are currently in progress. It is, however, possible to exclude non-specific effects of LF on the process of migration per se. The fact that LC migration and DC accumulation induced by TNF-α are unaffected by treatment with LF provides strong evidence that the protein is not impairing the tissue architecture necessary for the movement of cells from the skin or compromising directly the integrity or viability of LC themselves. Furthermore, the fact that human LF, a recombinant protein produced in an identical manner to murine LF, failed to inhibit markedly DC accumulation induced by oxazolone suggests that a contaminant of the production process is not responsible for the observed effects; these data also suggesting that the activity of LF in this respect is species selective. Finally, the observation that both iron-depleted and iron-saturated LF elicit an inhibitory response suggests that iron may not be required for this response.
In advance of confirmation of the mechanisms through which the effects on LC mobilization are mediated, the results presented here provide compelling evidence that LF is able to influence directly immunological processes that are known to be initiated and controlled by cytokines. As such, these data support the view that LF can modulate immune and inflammatory responses and host defences in ways that are independent of the bacteriostatic and bactericidal properties of the protein and which are not secondary to the affinity of LF for LPS.
The ability of exogenous LF to modify cutaneous immune function raises the intriguing question whether the endogenous protein has the potential to perform a similar function. While there is no direct evidence to indicate that this is the case, evidence described herein suggests that such may in theory be possible. Thus, immunolocalization studies revealed the presence in skin of LF itself. In principle therefore, LF has the potential to serve as a local regulator of immune function acting to influence and control local cytokine production.
Acknowledgments
We are grateful to Dr Christina Teng, National Institutes for Environmental Health Services (C.T.T.), National Institute for Health, Research Triangle Park, NC, for kindly providing us with mouse anti-lactoferrin antibody.
Glossary
Abbreviations
- BSA
bovine serum albumin
- DC
dendritic cells
- IL-1β
interleukin-1β
- LC
Langerhans cells
- LF
lactoferrin
- LPS
lipopolysaccharide
- Ox
oxazolone
- PBS
phosphate-buffered saline
- TNF-α
tumour necrosis factor α
References
- 1.Metz-boutigue MH, Jolles J, Mazurier J, et al. Human lactotransferrin: amino acid sequence and structural comparisons with other transferrins. Eur J Biochem. 1984;145:659. doi: 10.1111/j.1432-1033.1984.tb08607.x. [DOI] [PubMed] [Google Scholar]
- 2.Masson PL, Heremans JF. Lactoferrin in milk from different species. Comp Biochem Physiol. 1971;39:119. doi: 10.1016/0305-0491(71)90258-6. [DOI] [PubMed] [Google Scholar]
- 3.Masson PL, Heremans JF, Dive C. An iron binding protein common to many external secretions. Clin Chem Acta. 1966;14:735. [Google Scholar]
- 4.Masson PL, Heremans JF, Schonne E. Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J Exp Med. 1969;130:643. doi: 10.1084/jem.130.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fransson G-B, Lonnerdal B. Iron in human milk. J Pediatr. 1980;96:380. doi: 10.1016/s0022-3476(80)80676-7. [DOI] [PubMed] [Google Scholar]
- 6.Davidson LA, Lonnerdal B. Fe-saturation and proteolysis of human lactoferrin: effect on brush-border receptor-mediated uptake of Fe and Mn. Am J Physiol. 1989;257:6930. doi: 10.1152/ajpgi.1989.257.6.G930. [DOI] [PubMed] [Google Scholar]
- 7.Nichols BL, McKee K, Putman M, Henry JF, Nichols VN. Human lactoferrin supplementation of infant formulas increases thymidine incorporation into the DNA of rat crypt cells. J Pediatr Gastroent Nutr. 1989;8:102. doi: 10.1097/00005176-198901000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Nichols BL, McKee KS, Huebers HA. Iron is not required in the lactoferrin stimulation of thymidine incorporation into the DNA of rat crypt enterocytes. Pediatr Res. 1990;27:525. doi: 10.1203/00006450-199005000-00022. [DOI] [PubMed] [Google Scholar]
- 9.Brock J. Lactoferrin: a multifunctional immunoregulatory protein? Immunol Today. 1995;16:417. doi: 10.1016/0167-5699(95)80016-6. [DOI] [PubMed] [Google Scholar]
- 10.Bullen JJ, Rogers HJ, Griffiths E. Role of iron in bacterial infection. Curr Top Microbiol Immunol. 1978;80:1. doi: 10.1007/978-3-642-66956-9_1. [DOI] [PubMed] [Google Scholar]
- 11.Bellamy W, Takase M, Yamauchi K, Wakabayaski H, Kawase K, Tomita M. Identification of the bactericidal domain of lactoferrin. Biochem Biophys Acta. 1992;1121:130. doi: 10.1016/0167-4838(92)90346-f. [DOI] [PubMed] [Google Scholar]
- 12.Lonnerdal B, Iyer S. Lactoferrin: molecular structure and biological function. Annu Rev Nutr. 1995;15:93. doi: 10.1146/annurev.nu.15.070195.000521. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez L, Calvo M, Brock JH. Biological role of lactoferrin. Arch Dis Childhood. 1992;67:657. doi: 10.1136/adc.67.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penco S, Pastorino S, Bianchi-scarra G, Garre C. Lactoferrin down-modulates the activity of the granulocyte macrophage colony-stimulating factor promoter in interleukin-1β-stimulated cells. J Biol Chem. 1995;270:12263. doi: 10.1074/jbc.270.20.12263. [DOI] [PubMed] [Google Scholar]
- 15.Machnicki M, Zimecki M, Zagulski T. Lactoferrin regulates the release of tumour necrosis factor α and interleukin 6 in vivo. Int J Exp Path. 1993;74:433. [PMC free article] [PubMed] [Google Scholar]
- 16.Crouch SPM, Slater KJ, Fletcher J. Regulation of cytokine release from mononuclear cells by the iron-binding protein lactoferrin. Blood. 1992;80:235. [PubMed] [Google Scholar]
- 17.Cumberbatch M, Kimber I. Dermal tumour necrosis factor-α induces dendritic cell migration to draining lymph nodes, and possibly provides one stimulus for Langerhans cell migration. Immunology. 1992;75:257. [PMC free article] [PubMed] [Google Scholar]
- 18.Cumberbatch M, Kimber I. Tumour necrosis factor-α is required for accumulation of dendritic cells in draining lymph nodes and for optimal contact sensitization. Immunology. 1995;84:31. [PMC free article] [PubMed] [Google Scholar]
- 19.Cumberbatch M, Dearman RJ, Kimber I. Interleukin 1β and the stimulation of Langerhans cell migration: comparisons with tumour necrosis factor alpha. Arch Dermatol Res. 1997;289:277. doi: 10.1007/s004030050193. [DOI] [PubMed] [Google Scholar]
- 20.Cumberbatch M, Dearman RJ, Kimber I. Langerhans cells require signals from both tumour necrosis factor-α and interleukin-1β for migration. Immunology. 1997;92:388. doi: 10.1046/j.1365-2567.1997.00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enk AH, Katz SI. Early molecular events in the induction phase of contact sensitivity. Proc Natl Acad Sci USA. 1992;89:1398. doi: 10.1073/pnas.89.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birgens HS, Hansen WE, Karle H, Kristensen LO. Receptor binding of lactoferrin by human monocytes. Br J Haematol. 1983;54:383. doi: 10.1111/j.1365-2141.1983.tb02113.x. [DOI] [PubMed] [Google Scholar]
- 23.Leveugle B, Mazurier J, Legrand D, Mazurier C, Montreuil J, Spik G. Lactotransferrin binding to its platelet receptor inhibits platelet aggregation. Eur J Biochem. 1993;213:1205. doi: 10.1111/j.1432-1033.1993.tb17871.x. [DOI] [PubMed] [Google Scholar]
- 24.Mazurier J, Legrand D, Hu W-L, Montreuil J, Spik G. Expression of human lactoferrin receptors in phytohemagglutinin-stimulated human peripheral blood lymphocytes. Isolation of the receptors by antiligand-affinity chromatography. Eur J Biochem. 1989;179:481. doi: 10.1111/j.1432-1033.1989.tb14578.x. [DOI] [PubMed] [Google Scholar]
- 25.Zucali JR, Broxmeyer HE, Ulatowski JA. Specificity of lactoferrin as an inhibitor of granulocyte-macrophage colony-stimulating activity production from fetal mouse liver cells. Blood. 1979;54:951. [PubMed] [Google Scholar]
- 26.Zucali JR, Broxmeyer HE, Levy D, Morse C. Lactoferrin decreases monocyte-induced fibroblast production of myeloid colony-stimulating activity by suppressing monocyte release of interleukin-1. Blood. 1989;74:1531. [PubMed] [Google Scholar]
- 27.Hangoc G, Falkenburg JHF, Broxmeyer HE. Influence of T-lymphocytes and lactoferrin on the survival-promoting effects of IL-1 and IL-6 on human bone marrow granulocyte-macrophage and erythroid progenitor cells. Exp Haematol. 1991;19:697. [PubMed] [Google Scholar]
- 28.Ward PP, Piddington CS, Cunningham GA, Zhou X, Wyatt RD, Conneely OM. A system for production of commercial quantities of human lactoferrin: a broad spectrum natural antibiotic. Bio/Technology. 1995;13:498. doi: 10.1038/nbt0595-498. [DOI] [PubMed] [Google Scholar]
- 29.Ward PP, Chu H, Zhou X, Conneely OM. Expression and characterization of recombinant murine lactoferrin. Gene. 1997;204:171. doi: 10.1016/s0378-1119(97)00539-8. [DOI] [PubMed] [Google Scholar]
- 30.Macatonia SE, Edwards AJ, Knight SC. Dendritic cells and the initiation of contact sensitivity to fluorescein isothiocyanate. Immunology. 1986;59:509. [PMC free article] [PubMed] [Google Scholar]
- 31.Cumberbatch M, Kimber I. Phenotypic characteristics of antigen-bearing cells in the draining lymph nodes of contact sensitized mice. Immunology. 1990;71:404. [PMC free article] [PubMed] [Google Scholar]
- 32.Teng CT, Pentecost BT, Chen YH, Newbold RR, Eddy EH, McLachlan JA. Lactotransferrin gene expression in the mouse uterus and mammary gland. Endocrinology. 1989;124:992. doi: 10.1210/endo-124-2-992. [DOI] [PubMed] [Google Scholar]
- 33.Enk AH, Angeloni VL, Udey MC, Katz SI. An essential role for Langerhans cell-derived IL-1β in the initiation of primary immune responses in the skin. J Immunol. 1993;150:3698. [PubMed] [Google Scholar]
- 34.Kimber I, Dearman RJ, Cumberbatch M, Huby RJD. Langerhans cells and chemical allergy. Curr Opin Immunol. 1998;10:614. doi: 10.1016/s0952-7915(98)80078-2. [DOI] [PubMed] [Google Scholar]
- 35.Kinnaird A, Peters SW, Foster JR, Kimber I. Dendritic cell accumulation in draining lymph nodes during the induction phase of contact allergy in mice. Int Arch Allergy Appl Immunol. 1989;89:202. doi: 10.1159/000234947. [DOI] [PubMed] [Google Scholar]
- 36.Kripke ML, Munn CG, Jeevan A, Tang J-M, Bucana C. Evidence that cutaneous antigen-presenting cells migrate to regional lymph nodes during contact sensitization. J Immunol. 1990;145:2833. [PubMed] [Google Scholar]
- 37.Moodycliffe AM, Kimber I, Norval M. Role of tumour necrosis factor-α in ultraviolet B light-induced dendritic cell migration and suppression of contact hypersensitivity. Immunology. 1994;81:79. [PMC free article] [PubMed] [Google Scholar]
- 38.Cumberbatch M, Dearman RJ, Kimber I. Inhibition by dexamethasone of Langerhans cell migration: influence of epidermal cytokine signals. Immunopharmacology. 1999;41:235. doi: 10.1016/s0162-3109(99)00037-5. [DOI] [PubMed] [Google Scholar]
- 39.Lee SW, Tsou A-P, Chan H, et al. Glucocorticoids selectively inhibit the transcription of the interleukin 1β gene and decrease the stability of interleukin 1β mRNA. Proc Natl Acad Sci USA. 1988;85:1204. doi: 10.1073/pnas.85.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Remick DG, Strieter RM, Lynch JP, III, Nguyen D, Eskandari M, Kunkel SL. In vivo dynamics of murine tumour necrosis factor-α gene expression. Kinetics of dexamethasone-induced suppression. Lab Invest. 1989;60:766. [PubMed] [Google Scholar]