Abstract
We previously reported that CA074, a specific inhibitor of cathepsin B, modulates specific immune responses from the T helper 2 (Th2) type to Th1 type in BALB/c mice infected with Leishmania major. In the present study, we found that a similar type of immune deviation was also induced in mice immunized with ovalbumin (OVA). However, treatment of mice with pepstatin A, a specific cathepsin D inhibitor, suppressed the OVA-specific proliferation of lymphocytes and blocked the development of both Th1 and Th2 cellular responses. These inhibitors did not appear to have any direct influence in vitro on functions of naive lymphocytes. OVA antigen (47 000 MW) was digested mainly into 40 000 MW protein in vitro by lysosomal proteases from naive BALB/c mice, and its digestion was markedly inhibited by the addition of CA074, but not by addition of pepstatin A, during incubation. However, pepstatin A strongly suppressed the degradation of the major histocompatibility complex class II-associated invariant chain (Ii) molecule in vivo and in vitro. Thus, cathepsin B appears to process antigens directed to preferential activation of Th2 cells, while cathepsin D may be responsible for the degradation of Ii, the processing of which is essential in initiating the antigen-specific activation of Th1 and Th2 CD4+ T cells. These lysosomal proteases may have different functions in regulating immune responses.
Introduction
Exogenous antigens are processed by lysosomal proteases within antigen-presenting cells (APC) to create antigenic peptides and then presented by major histocompatibility complex (MHC) class II molecules prior to the activation of CD4+ T cells. 1,2 Enzymes such as aspartate proteases (e.g. cathepsin D and E) and cysteine proteases (e.g. cathepsin B, L and S) are proposed to be involved in this process. Although cathepsins B and D are located mainly in macrophages and have been reported to be involved in the processing of various antigens and also in the degradation of the invariant chain (Ii) of MHC class II molecules, 3–13 the majority of this evidence was obtained by in vitro, and not in vivo, studies. Recently, it was reported that cathepsin S, but not cathepsins B and D, is necessary for maturation of MHC class II molecules and for antigen presentation. 14 Another aspartate protease, cathepsin E, was also considered to be involved in antigen processing, although the exact subcellular localization of cathepsin E in APC is not known. 1,13 Furthermore, it is still remains unclear whether each lysosomal enzyme plays a different role in creating antigenic epitopes, and whether these proteases influence the development of functional subsets of CD4+ T cells.
It is known that activated CD4+ T cells differentiate into one of two functional subsets: T helper 1 (Th1) and T helper 2 (Th2). Th1 cells produce interleukin (IL)-2 and interferon-γ (IFN-γ), which are involved in the cell-mediated inflammatory reaction. Th2 cells secrete IL-4, IL-5 and IL-10, which are responsible for mediating B-cell immune responses. 15,16 When immunized intraperitoneally (i.p.) with ovalbumin (OVA) adsorbed in alum, BALB/c mice are preferentially induced to exhibit Th2-type immune responses, i.e. high levels of IL-4 and IL-5 and an increased production of antigen-specific immunoglobulin G1 (IgG1) and immunoglobulin E (IgE), while immunization of mice with OVA in Freund's complete adjuvant (FCA) develops Th1-type immune responses. 17,18
In the present study, lysosomal cathepsins are suggested to be proteases in antigen processing. The functional differences between these proteases in antigen processing and in inducing immune responses was studied by using their specific inhibitors, e.g. pepstatin A for cathepsin D and CA074 for cathepsin B, in BALB/c mice immunized with OVA in alum. It was observed that the cathepsin D inhibitor suppressed the development of both Th1 and Th2 responses, while the cathepsin B inhibitor directed the OVA-induced Th2-type immune response into a Th1-type response. CA074 modulated in vitro the processing of OVA antigen with lysosomal proteases from naive BALB/c mice. On the other hand, pepstatin A suppressed the degradation of the MHC class II-associated Ii molecule. Thus, various lysosomal proteases may be involved in class II-restricted immune regulation through different pathways.
Materials and methods
Animals
BALB/c Cr Slc (BALB/c) mice were purchased from Japan Shizuoka Laboratory Animal Center (Hamamatsu, Japan). Female mice aged 8–10 weeks were used in all experiments.
Cathepsin inhibitors
Pepstatin A (Peptide Institute, Osaka, Japan), a specific inhibitor of aspartyl proteases such as cathepsin D and pepsin, has previously been shown to cause prolonged inhibition of cathepsin D in mice, particularly in the spleen, liver and kidney. 19 Pepstatin A was dissolved in dimethylsulphoxide (DMSO) and was further diluted in phosphate-buffered saline (PBS), at least 25 times, to avoid the toxic effect of a high concentration of DMSO. A DMSO control was included in the pepstatin A experiments. CA074 [N-(L-3 trans-propylarbamoyloxirane-2-carbonyl)- l-isoleucyl- l-proline], synthesized in our laboratory, could be rapidly dissolved in PBS or RPMI-1640, and showed selective inhibition of cathepsin B in vitro and in vivo.
Immunization and delayed-type hypersensitivity response
BALB/c mice were immunized i.p. with 1 μg of OVA (Sigma, St. Louis, MO) adsorbed to 4 mg of alum (LSL, Tokyo, Japan). CA074 or pepstatin A (each at 0·25 mg/mouse) was administered 2 hr before and after immunization, and every 12 hr for 10 days thereafter. For the delayed-type hypersensitivity (DTH) assay, mice were challenged with 50 μl of OVA (10 μg) in alum injected into the left hind footpad 10 days after immunization. An identical volume of alum was injected into the right hind footpad as a control. The DTH response was evaluated by comparing the size of the swollen and non-swollen footpads 24 hr after challenge.
Enumeration of cytokine-producing cells by enzyme-linked immunospot (ELISPOT) assay
Multiscreen-IP plates were coated overnight with anti-mouse IFN-γ monoclonal antibody (mAb) (R4-6A2), anti-mouse IL-4 mAb (11B11) or anti-mouse IL-5 mAb (Genzyme, Cambridge, MA), respectively, each at 5 μg/ml. After blocking for 90 min, splenocytes were plated at 106 cells per well with OVA (500 μg/ml) or phytohaemagglutinin (PHA) (5 μg/ml) and cultured for 36 hr. Subsequently, the cells were removed by washing, biotinylated detection antibody was added, and the plates were incubated overnight. The plate-bound secondary antibody was then visualized by adding streptavidin-alkaline phosphatase and alkaline phosphatase conjugate substrate (Bio-Rad, Hercules, CA). The number of spots was assessed with the aid of a dissection microscope. The number of spot-forming cells reflects the frequency of cells per million that produce cytokine after the antigen challenge. 20
Detection of antigen-specific immunoglobulins in serum
Volumes of 1 mg/ml OVA were covalently coated onto Covalink 96-well microplates (InterMed Nunc, Kamstrup, Denmark) for 2 hr using N-hydroxysulphosuccinimide (Pierce, Rockford, IL) and I-ethyl-3-(3-dimethylaminoprophyl) carbodiimide (Sigma). After blocking overnight with 0·1% bovine serum albumin (BSA; Gibco BRL, Grand Island, NY) in PBS, serum samples serially diluted with blocking buffer were incubated for 6 hr in microplates. They were then examined with alkaline phosphatase-conjugated anti-mouse IgE (Southern Biotechnology, Birmingham, AL) or IgG2a (Cappel, Durham, NC) followed by p-nitrophenyl phosphate (Sigma). The reaction was stopped with 3N NaOH and the absorbance at 415 nm was read using a microplate reader (Bio-Rad).
Antigen-specific proliferation
Splenocytes (1 × 106 cells/well) from three experimental groups were cultured with OVA (500 μg/ml), concanavalin A (Con A) (5 μg/ml) or plate-coated anti-mouse CD3 mAb (145-2C11) in 0·2 ml of RPMI-1640 containing 10% fetal bovine serum (FBS), 100 U/ml of penicillin, 100 μg/ml of streptomycin and 5 × 10−5m 2-mercaptoethanol (2-ME) in 96-well flat-bottom microplates at 37°, in an atmosphere of 5% CO2 for 72 hr. [3H]Thymidine ([3H]TdR) (1 μCi) was added to each well8 hr before harvesting. The incorporation of [3H]TdR wasmeasured in a liquid scintillation counter (Aloka, Tokyo).
Flow cytometry
One million splenocytes were stained with a combination of anti-CD4-phycoerythrin (PE)-labelled mAb and anti-CD8-fluorescein isothiocyanate (FITC)-labelled mAb(Pharmingen, San Diego, CA) or anti-Thy-1·2 (30-H12)-PElabelled mAb and anti-B220 (RA3-6B2)-FITC-labelled mAb, or with FITC-labelled In-1 mAb specific for the mouse invariant chain, 8 and were analysed by using a fluorescence-activated cell sorter (FACScan; Becton-Dickinson, Mountain View, CA).
Mitochondria/lysosome (ML) fraction
Lysosomes were isolated to assess proteolysis after gentle homogenization of samples of minced spleen with a Teflon pestle in 0·25 m cold sucrose. The suspension was centrifuged at 3500 g for 10 min at 4°, and the supernatant was centrifuged at 25 000 g for 20 min at 4°. The resulting pellet was resuspended in 50 mm acetate buffer (pH 5·0), and the suspension fluid was freeze/thawed three times to disrupt lysosomal membranes. The fluid was then centrifuged and the supernatant used as the ML fraction.
Protein-digestion assay
OVA was digested at pH 5·0 (the pH of endocytic vesicles) at 37° for 3 hr with lysosomal enzymes, prepared from the ML fraction of splenocytes from naive mice, in the presence or absence of CA074 or pepstatin A. After digestion, samples were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE). The digested products were directly stained with Coomassie Brilliant Blue R-250(Bio-Rad).
Immunoprecipitation
A20 cell suspensions were washed and solubilized on ice for 30 min in 1 ml of lysis buffer (1% Nonidet P-40 [NP-40]/PBS in the presence of protease inhibitors). After ultracentrifugation to remove nuclei and cell debris, the supernatants were precleared twice by incubation with 5 μl of normal mouse serum and 100 μl of protein A–agarose (Pierce) for 2 hr. Samples were immunoprecipitated overnight with In-1 mAb and protein A–agarose. An irrelevant antibody (anti-rat IgG) was used as a negative control. Agarose pellets were washed five times in TNE buffer (1% NP-40, 50 mm Tris HCl, 150 mm NaCl, 5 mm EDTA, 2 mm pepstatin A and 2 mm leupeptin, pH 7·8), resuspended in sample buffer containing 10% (v/v) 2-ME, and separated in 15% SDS–PAGE gels. Gels were stained using silver-staining reagents (Daiichi Pure Chemicals, Tokyo, Japan).
Results
Cathepsin inhibitors modulate cytokine production
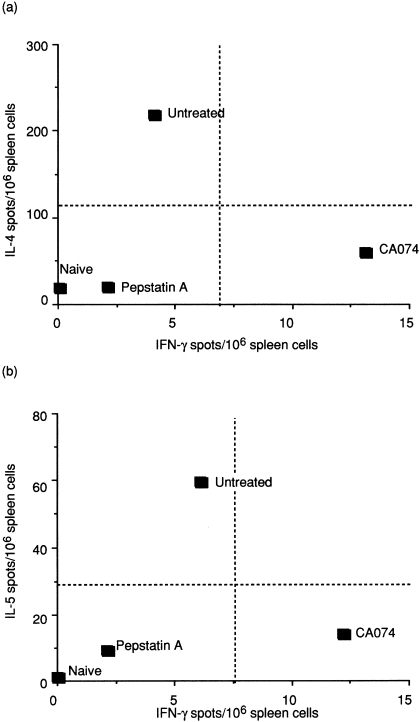
To clarify whether the Th phenotype was different in pepstatin A- and CA074-treated mice during immunization, drained splenocytes were restimulated in vitro, with or without OVA, 10 days after immunization. The antigen-specific cytokine profile was assessed using ELISPOT in order to calculate the number of cytokine-secreting cells. As shown in Fig. 1, splenocytes from untreated animals comprised a high number of IL-4- and IL-5-secreting cells, but a low number of IFN-γ-secreting cells, indicating that the OVA-specific Th cells were Th2 dominant. In contrast, mice treated with CA074 showed a different response, of Th1 type, generating very low levels of IL-4 and IL-5, and a high level of IFN-γ. Unlike the mice treated with CA074, the pepstatin A-treated mice produced only minimal levels of Th2-type cytokines IL-4 and IL-5 and Th1-type levels of IFN-γ (Fig. 1), similar to naive mice. Thus, treatment with CA074 exclusively suppresses development of the Th2-type response but activates the Th1-type response, while treatment with pepstatin A suppresses both Th1 and Th2 responses.
Figure 1.
T helper (Th) phenotype in mice treated with cathepsin inhibitors. Mice were immunized intraperitoneally (i.p.) with ovalbumin (OVA) adsorbed with alum. CA074 or pepstatin A (0·25 mg of each/mouse) was injected i.p. 2 hr before and after immunization and every 12 hr thereafter for 10 days. Frequencies of splenic interleukin (IL)-4, IL-5 and interferon-γ (IFN-γ)-secreting cells from naive, untreated, CA074- or pepstatin A-treated mice were determined by using an enzyme-linked immunospot (ELISPOT) assay. Spots were counted, and the results are expressed per 106 cells. Standard deviations (SD) were < 10% of total spots counted for each cytokine. Results are representative of three separate experiments.
The effect of treatment with cathepsin inhibitors on specific antibody production
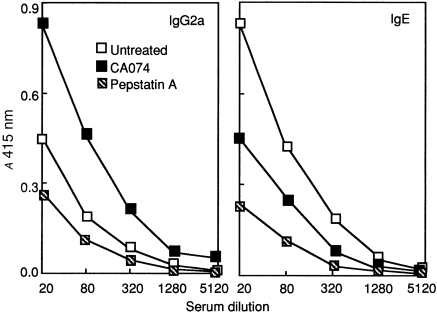
Cytokines regulate immunoglobulin isotype production by B cells; the Th1-type cytokines preferentially direct class switching to IgG2a production, whilst Th2-type cytokines, such as IL-4 and IL-5, govern switching to IgE production. 21 To confirm whether immunoglobulin class switching to Th2-mediated antibodies was impaired in the CA074-treated mice, serum samples were obtained from BALB/c mice to assess the production of OVA-specific IgG2a and IgE by enzyme-linked immunosorbent assay (ELISA). Immunization with OVA formulated in alum primed splenocytes to produce IL-4 and IL-5 and elicited high titres of IgE antibody and low titres of IgG2a (Fig. 2), suggesting the development of a Th2-mediated immune response in untreated mice. Animals treated with CA074 showed a reduced level of OVA-specific IgE, whereas the production of IgG2a was significantly increased. On the other hand, pepstatin A-treated mice showed suppression of all classes of OVA-specific antibody production compared with untreated or CA074-treated mice (Fig. 2). These results imply that CA074 preferentially induces Th1 effector cells, which facilitate switching to IgG2a by enhancing the production of IFN-γ or by inhibiting the secretion of IL-4 and IL-5, while both Th1 and Th2 effector cells were suppressed in pepstatin A-treated mice.
Figure 2.
Levels of ovalbumin (OVA)-specific antibodies in mice treated with cathepsin inhibitors. Mice were treated as indicated in the Materials and methods, and serum titres of antigen-specific immunoglobulin G2a (IgG2a) and immunoglobulin E (IgE) from untreated, CA074- or pepstatin A-treated animals (n = 6) were assessed by using enzyme-linked immunosorbent assay (ELISA) 10 days after immunization. Results are representative of five separate experiments.
The effect of treatment with cathepsin inhibitors on OVA-specific DTH
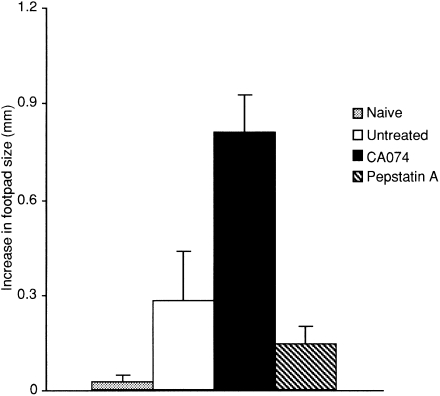
We further confirmed the modulatory effects of these inhibitors on in vivo immune responses by examining the development of the OVA-specific DTH response, which is mediated by Th1 cells. 22 Groups of experimental mice in which DTH was elicited with OVA in alum were injected into the left hind footpad 10 days after immunization. Mice treated with CA074 showed remarkably augmented OVA-specific DTH responses compared with those of untreated mice, which demonstrated a Th2-based immune response. In striking contrast, mice treated with pepstatin A showed a lower suppression of the DTH response than untreated mice (Fig. 3). Thus, these results further demonstrated that CA074 treatment exclusively results in a Th1-type response. However, pepstatin A suppresses both Th1- and Th2-type responses.
Figure 3.
The delayed-type hypersensitivity (DTH) response in mice treated with cathepsin inhibitors. Mice (n = 3–5) were challenged subcutaneously (s.c.) with 10 μg of ovalbumin (OVA) in alum in the left hind footpad 10 days after immunization with OVA. The size of the footpad swelling in naive, untreated, CA074- or pepstatin A-treated mice was measured by comparing the swollen footpad with the non-swollen footpad 24 hr after challenge. The results of the DTH reaction are representative of three individual experiments.
Treatment with cathepsin D inhibitor suppresses antigen-specific activation of CD4+ T lymphocytes
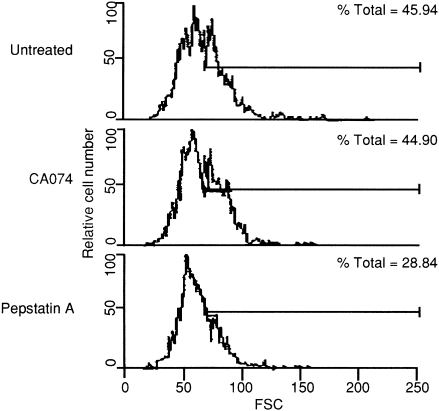
It is important to clarify the levels of immunological modulation that result from the treatment of cathepsin B or D inhibitors. To analyse the type of T lymphocyte that was influenced by treatment of these inhibitors, the number of T-cell subsets in mice treated with each inhibitor was analysed by flow cytometry 10 days after immunization. Total cellularity in spleen did not differ significantly among these three groups, but the number of Thy-1·2+ blastoid cells decreased as a result of treatment with pepstatin A. That is, the proportion of Thy1·2+ blastoid cells was 30% less in pepstatin A-treated mice than in untreated or CA074-treated mice (Fig. 4). As large cells are generally assumed to be activated blastoid cells, T cells in pepstatin A-treated mice would not be considered as activated. As shown in Table 1, the proportion of activated CD4+ T cells in pepstatin A-treated mice was reduced to 67·8% compared with that of untreated mice, while the proportions in CA074-treated mice were similar to those of control mice. Further treatment with either CA074 or pepstatin A did not directly affect the number and antibody secretion of B220+ cells (data not shown).
Figure 4.
Flow cytometry analysis of activated splenocytes in mice treated with cathepsin inhibitors. Cells from untreated (upper panel), CA074- (middle panel), or pepstatin A- (lower panel) treated mice were evaluated by fluorescence-activated cell sorter (FACScan) using the double-staining method. These panels were shown as the percentage of Thy-1·2+ blastoid cells. FSC, forward scatter.
Table 1.
The cell proportion of splenocytes from BALB/c mice immunized with ovalbumin (OVA) adsorbed in alum
| CD4+ | CD8+ | CD4–/CD8– | |||||
|---|---|---|---|---|---|---|---|
| Total cell number (× 107) | Small | Large | Small | Large | Small | Large | |
| Untreated | 3·3 | 59·2 | 45·2 | 61·9 | 40·7 | 37·4 | 63·0 |
| CA074 | 5·2 | 58·5 | 46·7 (103·1) | 58·7 | 45·0 (110·4) | 38·3 | 63·4 (100·6) |
| Pepstatin A | 4·8 | 72·8 | 30·8 (67·8) | 66·6 | 36·7 (89·9) | 41·3 | 59·1 (93·7) |
The splenocytes were collected 10 days after immunization and stained with a combination of fluorescein isthiocyanate (FITC) anti-CD8+ and phycoerythrin (PE) anti CD4+. The number in parenthesis represents the control percentage.
The effect of treatment with cathepsin inhibitors on specific and non-specific proliferation
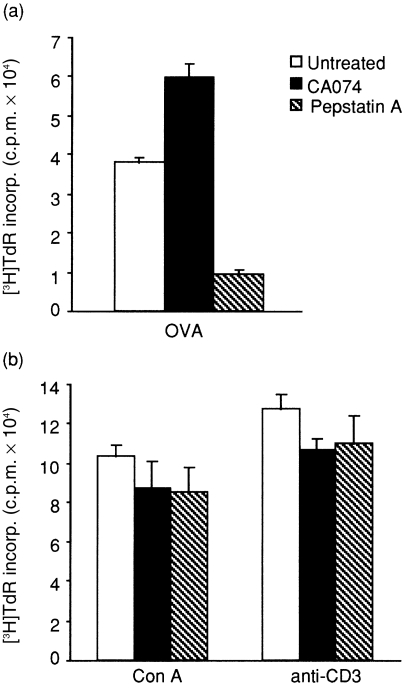
Next, the proliferative responses of splenocytes to specific or non-specific stimuli were examined using immunized mice treated with each inhibitor. Splenocytes from CA074-treated mice proliferated similarly to those of untreated mice, in response to specific stimulation with OVA, whereas this proliferation in immunized mice was significantly suppressed by treatment with pepstatin A (Fig. 5a). No difference was seen among the three groups in proliferative response to non-specific stimuli such as Con A or anti-CD3 mAb (Fig. 5b). Moreover, neither cathepsin inhibitor showed any direct influence on the functions of naive T cells, such as proliferation, cytokine production and MHC class I-restricted cytotoxic T lymphocytes (CTL) (data not shown). Hence, these results suggest that CA074 treatment does not suppress proliferative responses to either antigen-specific stimulation with OVA or to non-specific stimulation, while pepstatin A suppresses the antigen-specific proliferation, but does not suppress non-specific proliferation.
Figure 5.
Ovalbumin (OVA)-specific or non-specific proliferative response of splenocytes in mice treated with cathepsin inhibitors. Mice were immunized and treated as indicated in the Materials and methods, and cells were isolated and cultured for 72 hr with OVA (a), concanavalin A (Con A) (b) or anti-CD3 (b). Proliferation was measured by [3H]thymidine incorporation. The data are means of triplicate wells ± SD and are representative of five separate experiments. c.p.m., counts per minute; incorp., incorporation.
Taken together, these results indicate that the treatment of mice with cathepsin D inhibitor blocked the activation of CD4+ T cells and influenced the differentiation of both Th1 and Th2 cells, probably affecting the priming process of naive T cells.
Cathepsin B inhibitor modulates the digestion pattern of OVA with lysosomal proteases
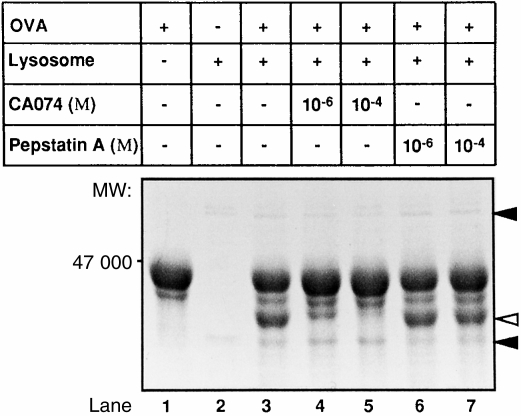
The specificity of these cathepsin inhibitors was confirmed. Each inhibitor showed an inhibitory activity of > 50%, and was highly specific in vitro and in vivo (data not shown). Next, we examined, by protein digestion assay, whether the OVA-digesting activities of lysosomal proteases of naive mice are modulated when CA074 or pepstatin A is added during incubation with OVA and the proteases, as described in the Materials and methods. As shown in Fig. 6, a 47 000-MW OVA antigen decreased in size as a result of digestion with lysosomal enzymes, whereas the 40 000-MW protein increased in size compared with undigested OVA. CA074 blocked the digestion of OVA antigen with lysosomal proteases and the 40 000-MW protein band almost disappeared. These results suggest that lysosomal cathepsin B, but not cathepsin D, contributes mainly to the digestion of OVA into the 40 000-MW protein. On the other hand, pepstatin A did not influence the digestion of OVA, suggesting that immune suppression resulting from treatment with pepstatin A is not caused by digestion of OVA in the lysosome. This difference may have been a result of the difference in the function of CD4+ T cells between CA074- and pepstatin A-treated mice.
Figure 6.
In vitro digestion of ovalbumin (OVA) by lysosomal proteases. Five micrograms of OVA was digested with 10 μg of mitochondria/lysosome (ML) fraction in the absence (lane 3) or in the presence of CA074 (lanes 4 and 5) or pepstatin A (lanes 6 and 7) (each at 1 μg/ml) for 3 hr at 37°. The OVA digests were then stained with Coomassie Brilliant Blue following separation by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE). The untreated OVA control is shown in lane 1, and control lysosomes in lane 2. Filled arrowheads indicate the lysosomal band, and the open arrowhead indicates the band of digested OVA.
Role of proteases on the degradation of MHC class II-associated Ii chain
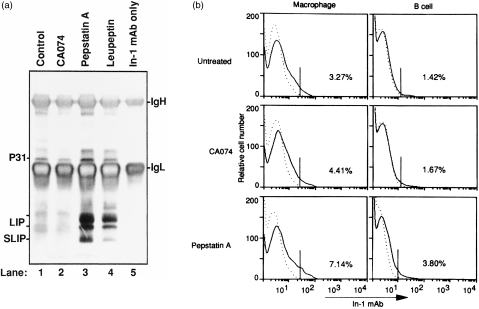
Next, the role of cathepsins B and D was studied on the maturation of MHC class II molecules for T-cell priming. To determine the effect of these inhibitors on the degradation of Ii molecules, a murine B-cell line (A20) was incubated for 4 hr with or without CA074, pepstatin A, or leupeptin. The breakdown of Ii was determined by immunoprecipitation with the In-1 mAb that reacts with degrading fragments containing the N-terminal portion of Ii. 23 Ii-p31 has been described as the major isoform in B cells 24 (Fig. 7a). Leupeptin, an inhibitor of cysteine proteases, interferes with the maturation of MHC class II by blocking the complete destruction of Ii, 10,25 and contributes to the increasing overall intermediates of Ii degradation, such as the 20–24 000 MW leupeptin-induced peptide (LIP) and the 12 000 MW small LIP (SLIP) fragments (Fig. 7a, lane 4). CA074, a specific cathepsin B inhibitor, did not induce the accumulation of LIP and SLIP, indicating that cathepsin B does not contribute to the degradation of Ii. In contrast, treatment with pepstatin A, an aspartate protease inhibitor, resulted in marked alteration of Ii processing, indicating the accumulation of both LIP and SLIP Ii fragments in this experimental group. Moreover, no fragments of Ii were found in an irrelevant antibody control (data not shown).
Figure 7.
Degradation of invariant chain (Ii) molecules in cathepsin inhibitor-treated A20 cells (a) and BALB/c mice (b). (a) Cells (108) were incubated for 4 hr in the absence (lane 1) or in the presence of CA074 (lane 2), pepstatin A (lane 3), or leupeptin (lane 4) (each at 100 μg/ml). The cells were then lysed and immunoprecipitated with In-1 (anti-Ii) monoclonal antibody (mAb), as described in the Materials and methods. Immunoprecipitates were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and stained using silver-staining reagents. In-1 mAb only is shown in lane 5. IgH is the H chain of immunoglobulin, and IgL is the L chain of immunoglobulin. (b) BALB/c mice were treated with CA074 or pepstatin A for 10 days. Splenocytes were collected and blocked with normal mouse serum for 30 min at 37° and then stained with fluorescein isothiocyanate (FITC)-conjugated anti-mouse Ii mAb (solid lines) Dotted lines indicate the unstained control.
To further confirm that in vivo degradation of Ii was affected by cathepsin inhibitors, BALB/c mice were treated with pepstatin A and CA074 for 10 days. The expression of Ii on APC was examined by flow cytometry analysis. As shown in Fig. 7(b), the expression of Ii on macrophages and B cells was significantly increased by treatment with pepstatin A compared with that of CA074-treated and -untreated mice. Thus, pepstatin A appears to suppress the activation of CD4+ T cells by blocking the maturation of MHC class II molecules. That is, cathepsin D may mediate the cleavage and release of MHC class II-bound Ii.
Discussion
The development of CD4+ helper T cells from naive CD4+ T cells has been one of the most important issues to be addressed in the immunological field. There are many reports pertaining the regulatory mechanisms of Th1 and Th2 responses, e.g. the route of antigen entry, structure of antigen, type of adjuvant used and antigen dosages employed. 26–29 We previously proposed that lysosomal proteases play a crucial role in the differentiation of functional CD4+ T cells. A candidate protease is cathepsin B, as CA074, a specific inhibitor of lysosomal cathepsin B, deviates the immune response from Th2 to Th1 type in BALB/c mice infected with Leishmania major and in genetically susceptible mice that have become resistant to this infection. 28 At present, however, detailed molecular mechanisms on how the inhibitor of cathepsin B induces switching of polarity from Th2 to Th1 has not been clarified.
In the present study, we showed that pepstatin A, an inhibitor of cathepsin D, also influences the development of specific immunities in mice immunized with OVA adsorbed with alum, but differently from CA074. That is, CA074 preferentially induced Th1 responses, while pepstatin A suppressed the development of both Th1 and Th2 responses. Thus, OVA antigen processed by lysosomal cathepsin B in APC may preferentially promote development of the Th2 response, while cathepsin D contributes to the degradation of Ii molecules and then initiates the activation of either Th1- or Th2-type CD4+ T cells.
Early-stage antigen-processing compartments of APCs are endosomes where various lysosomal enzymes, such as cathepsin B and D, are located. We previously reported that soluble Leishmania antigen (SLA) was digested in vitro by lysosomal proteases and its digestion was markedly modified by the addition of CA074 or pepstatin A during the incubation, but in a different manner. CA074 specifically interfered with the digestion of 16, 28 and 31 000-MW peptides of SLA by lysosomal enzymes, while pepstatin A mainly blocked that of the 28 000-MW peptide of SLA. Inactivation of lysosomal cathepsin B or D with their corresponding inhibitors resulted in the modulation of CD4+ T-cell differentiation, and the helper T cells failed to instruct B cells to completely produce diverse specific antibodies, as seen in L. major-infected and untreated BALB/c mice. Thus, these lysosomal proteases appear to have different functions in regulating immune responses by creating specific antigenic fragments. L. major antigens processed by cathepsin D may be essential for the induction of Th0, a prototype of Th1 and Th2, or for both types of CD4+ T cells. 29 In the processing of OVA antigen, however, cathepsin D was not involved (Fig. 6). As discussed above, pepstatin A-treated mice generally showed suppression of both Th1 and Th2 responses to specific stimulation with OVA, although T cells from these mice proliferated and generated both Th1- and Th2-type cytokines in response to non-specific stimulation with Con A. Thus, it is possible that cathepsin D plays a role in antigen presentation and degradation of MHC class II-associated Ii molecules, whilst the specific inhibitor, pepstatin A, completely suppresses the differentiation of class II-restricted CD4+ T cells in OVA-immunized mice.
MHC class II molecules bind antigenic peptides generated in APC and display them at the cell surface to present the peptide to CD4+ T cells. Newly synthesized class II α/β heterodimers associate with Ii in the endoplasmic reticulum, and this complex is transported to endocytic compartments where Ii is partially degraded into small fragments by acid proteases, whilst maintaining the binding groove of the class II molecules. 30,31 Recent experimental evidence has illustrated that cysteine proteases play an important role in the degradation of MHC class II-bound Ii. Inhibition of cysteine proteases with leupeptin causes accumulation of degraded Ii intermediates in A20 cells expressing class II molecules of I-Ad haplotypes. 10,24,25 In the present study, we also found that specific inhibition of cathepsin D with pepstatin A in vivo and in vitro led to the accumulation of Ii fragments, suggesting that cathepsin D is able to degrade Ii from MHC class II–Ii complexes for antigen presentation and activation of CD4+ T cells. However, this observation differs from the work of Riese et al. using cathepsin D knockout mice. 14 In their report, they described that cathepsin S, but not cathepsins B or D, is necessary for the digestion of Ii from MHC class II molecules. One possible reason for this discrepancy is that pepstatin A may be an inhibitor for aspartate proteases, including pepsin and cathepsins D and E, and not just cathepsin D. Conceivably, cathepsin E may be also a factor in the degradation of Ii, although the exact subcellular localization of cathepsin E in B cells is unclear. 13 These results indicate that aspartate proteases also contribute to degradation of the Ii chain in addition to cysteine proteases, such as cathepsin S. Thus, subjecting mice to pepstatin A may have impaired OVA-specific proliferation and activation of CD4+ T cells by suppressing maturation of the MHC class II molecule and antigen presentation.
The present study shows that administration of CA074 to OVA-immunized mice suppresses Th2 responses, but augments Th1 responses, whereas treatment with pepstatin A suppresses both Th1 and Th2 responses. In other words, cathepsin B appears to induce Th2 exclusively, whereas cathepsin D may be essential for inducing activation of CD4+ T cells via degradation of the Ii chain. Thus, some lysosomal proteases may be, in part, responsible for immune regulation by creating antigenic peptides/motifs or digesting the Ii chain. This viewpoint may provide a new direction on approaches for T-cell differentiation.
Acknowledgments
We thank Dr Toshiaki Mizuochi and Dr Michiyuki Kasai for their assistance with the invariant chain degradation experiments; Dr Hiroshi Kido and Dr Eiki Kominami for helpful suggestions; and Ms Kimie Ohgame for preparation of the manuscript. This work was supported in part by Grants-in-aid from the Ministry of Education, Science and Culture, Japan (grant nos: 10166216, 10167214, 10172222, 10470067, 10044297, 10770140 and 09877055).
Glossary
Abbreviations
- ELISPOT
enzyme-linked immunospot
- Ii
invariant chain
- LIP
leupeptin-induced peptide
- ML
mitochondria and lysosomes
- SLA
soluble Leishmania antigen
- SLIP
small leupeptin-induced peptide
References
- 1.Watts C. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu Rev Immunol. 1997;15:821. doi: 10.1146/annurev.immunol.15.1.821. [DOI] [PubMed] [Google Scholar]
- 2.Germain RN. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 1994;76:287. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 3.Puri J, Factorovich Y. Selective inhibition of antigen presentation to cloned T cells by protease inhibitors. J Immunol. 1988;141:3313. [PubMed] [Google Scholar]
- 4.Takahashi H, Cease KB, Berzofsky JA. Identification of proteases that process distinct epitopes on the same protein. J Immunol. 1989;142:2221. [PubMed] [Google Scholar]
- 5.Rodriguez GM, Diment S. Destructive proteolysis by cysteine proteases in antigen presentation of ovalbumin. Eur J Immunol. 1995;25:1823. doi: 10.1002/eji.1830250705. [DOI] [PubMed] [Google Scholar]
- 6.Diment S. Different roles for thiol and aspartyl proteases in antigen presentation of ovalbumin. J Immunol. 1990;145:417. [PubMed] [Google Scholar]
- 7.Rodriguez GM, Diment S. Role of cathepsin D in antigen presentation of ovalbumin. J Immunol. 1992;149:2894. [PubMed] [Google Scholar]
- 8.Mizuochi T, Yee S, Kasai M, Kakiuchi T, Muno D, Kominami E. Both cathepsin B and cathepsin D are necessary for processing of ovalbumin as well as for degradation of class II MHC invariant chain. Immunol Lett. 1994;43:189. doi: 10.1016/0165-2478(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 9.Van Noort JM, Jacob MJM. Cathepsin D, but not cathepsin B, releases T cell stimulatory fragments from lysozyme that are functional in the context of multiple murine class II MHC molecules. Eur J Immunol. 1994;24:2175. doi: 10.1002/eji.1830240936. [DOI] [PubMed] [Google Scholar]
- 10.Maric MA, Taylor DM, Blum JS. Endosomal aspartic proteinases are required for invariant-chain processing. Proc Natl Acad Sci USA. 1994;91:2171. doi: 10.1073/pnas.91.6.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Noort JM, Van der Drift ACM. The selectivity of cathepsin D suggests an involvement of the enzyme in the generation of T-cell epitopes. J Biol Chem. 1989;264:14159. [PubMed] [Google Scholar]
- 12.Van Noort JM, Boon J, Van der Drift ACM, Wagenaar JPA, Boots AMH, Boog CJP. Antigen processing by endosomal proteases determines which sites of sperm-whale myoglobin are eventually recognized by T cells. Eur J Immunol. 1991;21:1989. doi: 10.1002/eji.1830210904. [DOI] [PubMed] [Google Scholar]
- 13.Hewitt EW, Treumann A, Morrice N, Tatnell PJ, Kay J, watts C. Natural processing sites for human cathepsin E and cathepsin D in tetanus toxin. J Immunol. 1997;159:4693. [PubMed] [Google Scholar]
- 14.Riese RJ, Wolf PR, Bromme D, Natkin LR, Villadangos JA, Ploegh HL, Chapman HA. Essential role for cathepsin S in MHC class II-associated invariant chain processing and peptide loading. Immunity. 1996;4:357. doi: 10.1016/s1074-7613(00)80249-6. [DOI] [PubMed] [Google Scholar]
- 15.Mosmann TR, Cherwinski H, Bond MW, Giendlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348. [PubMed] [Google Scholar]
- 16.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 17.Beck L, Spiegelberg HL. The polyclonal and antigen-specific IgE and IgG subclass response of mice injected with ovalbumin in alum or complete Freund's adjuvant. Cell Immunol. 1989;123:1. doi: 10.1016/0008-8749(89)90263-3. [DOI] [PubMed] [Google Scholar]
- 18.Yang X, Hayglass KT. Allergen-dependent induction of interleukin-4 synthesis in vivo. Immunology. 1993;78:74. [PMC free article] [PubMed] [Google Scholar]
- 19.Greenbaum LM, James HR. Host cathepsin D response to tumor in the normal and pepstatin-treated mouse. Cancer Res. 1983;43:2584. [PubMed] [Google Scholar]
- 20.Czerkinsky C, Andersson G, Ekre H, Nilsson L, Klareskog L, Ouchterlony O. Reverse ELISPOT assay for clonal analysis of cytokine production. I. Enumeration of gamma-interferon-secreting cells. J Immunol Methods. 1988;110:29. doi: 10.1016/0022-1759(88)90079-8. [DOI] [PubMed] [Google Scholar]
- 21.Kopf M, Gros GL, Bachmann M, Lamers MC, Bluethmann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 22.Fong T, Annie T, Mpsmann TR. The role of IFN-γ in delayed-type hypersensitivity mediated by Th1 clones. J Immunol. 1989;143:2887. [PubMed] [Google Scholar]
- 23.Koch N, Koch S, Hammerling GJ. Ia invariant chain detected on lymphocyte surface by monoclonal antibody. Nature. 1982;299:644. doi: 10.1038/299644a0. [DOI] [PubMed] [Google Scholar]
- 24.Kampgen E, Koch N, Koch F, et al. Class II major histocompatibility complex molecules of murine dendritic cells: synthesis, sialylation of invariant chain, and antigen processing capacity are down-regulated upon culture. Proc Natl Acad Sci USA. 1991;88:3014. doi: 10.1073/pnas.88.8.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amigorena S, Webster P, Drake J, Newcomb J, Cresswell J, Mellman J. Invariant chain cleavage and peptide loading in major histocompatibility complex class II vesicles. J Exp Med. 1995;181:1729. doi: 10.1084/jem.181.5.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Constant SL, Bottomly K. Induction of Th1 and Th2, CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 27.Hosken NA, Shibuya K, Heath AW, Murphy KM, O’Garra AO. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-transgenic model. J Exp Med. 1995;182:1579. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maekawa Y, Himeno K, Ishikawa H, et al. Switch of CD4+ T-cell differentiation from Th2 to Th1 by treatment with cathepsin B-inhibitor in experimental leishmaniasis. J Immunol. 1998;161:2120. [PubMed] [Google Scholar]
- 29.Zhang T, Maekawa Y, Ishikawa H, et al. Different roles of lysosomal cathepsins B and D in antigen-processing and T helper cell activation in experimental leishmaniasis. Int Immunol. 2000 in press. [Google Scholar]
- 30.Perrin-cocon LA, Marche PN, Villiers CL. Purification of intracellular compartments involved in antigen processing: a new method based on magnetic sorting. Biochem J. 1999;338:123. [PMC free article] [PubMed] [Google Scholar]
- 31.Weber DA, Evavold BD, Jensen PE. Enhanced dissociation of HLA-DR-bound peptides in the presence of HLA-DM. Science. 1996;274:618. doi: 10.1126/science.274.5287.618. [DOI] [PubMed] [Google Scholar]