Abstract
Human T cells expressing CD161 and an invariant T-cell receptor (TCR) α-chain (Vα24invt T cells) specifically recognize CD1d and appear to have immunoregulatory functions. However, the physiological target cells for this T-cell population, and whether alterations in CD1d expression contribute to the regulation of Vα24invt T-cell responses, remain to be determined. A series of antibodies were generated to assess CD1d expression, structure and regulation on human lymphoid and myeloid cells. CD1d was expressed at high levels by human cortical thymocytes and immunoprecipitation analyses showed it to be a 48 000-MW glycosylated protein. However, after solubilization, the majority of the thymocyte CD1d protein, but not CD1d expressed by transfected cells, lost reactivity with monoclonal antibodies (mAbs) against native CD1d, indicating that it was alternatively processed. Moreover, thymocytes were not recognized by CD1d-reactive Vα24invt T-cell clones. Medullary thymocytes and resting peripheral blood T cells were CD1d–, but low-level CD1d expression was induced on activated T cells. CD1d was expressed by B cells in peripheral blood and lymph node mantle zones, but germinal centres were CD1d–. Resting monocytes were CD1d+ but, in contrast to CD1a, b and c, their surface expression of CD1d was not up-regulated by granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) activation. These results demonstrate constitutive CD1d expression by human professional antigen-presenting cells and that post-translational processing of CD1d may contribute to regulation of the activity of CD1d-specific T cells.
Introduction
The human CD1 locus encodes a family of proteins that are structurally related to major histocompatibility complex (MHC) class I proteins. 1–3 CD1a, b and c are most homologous to each other and are expressed by thymocytes, dendritic cells and activated monocytes, 4–9 while B cells express only CD1c. 10 T cells reactive with CD1a, b, or c have been isolated from peripheral blood, and lipids derived from mycobacteria have been identified as the antigens recognized by some of these clones. 8,11 CD1d is divergent in sequence from CD1a, b and c 3,12 and its tissue distribution is more widespread, including cells outside the lymphoid and myeloid lineages. 13–15 Possible CD1d-presented antigens include hydrophobic peptides,glycosylceramides and glycosylphosphatidylinositol. 16–18
Murine CD1d, even in the absence of an exogenous antigen, is recognized by a population of CD4+ or CD4– CD8– (double negative, DN), CD161+ (NK1) T cells that use an invariant Vα14-Jα281 T-cell receptor (TCR) α-chain. 19–23 A population of human DN or CD4+ T cells that uses a homologous invariant Vα24-JαQ TCR α-chain has been identified (Vα24invt T cells). 24,25 These human T cells also express CD161 (NKR-P1A), which acts as the major costimulatory molecule, and specifically recognize CD1d. 26,27 Functional studies suggest an immunoregulatory role for these Vα24invt T cells, 28–31 but the precise nature of this role and that of CD1d remain to be determined. This report describes the development and use of a new series of CD1d antibodies to assess CD1d expression, structure and regulation on human lymphoid and myeloid cells.
Materials and methods
Cells and culture
C1R cells, a human leucocyte antigen (HLA)-A and -B deficient B-lymphoblastoid cell line, transfected with CD1d cDNA or a CD1d/CD1a chimeric cDNA, were as described previously. 26 Interleukin (IL)-2 used at 100 U/ml was provided by the NIH Biological Response Modifiers Program (Bethesda, MD). Human thymocytes were obtained from paediatric patients undergoing cardiac surgery. CD1d-reactive Vα24invt T-cell clones were derived from the peripheral blood of normal donors, as described previously. 26 Functional studies monitored by proliferation and cytokine production were carried out on resting Vα24invt T-cell clones, as described previously. 26,27 Briefly, Vα24invt T-cell clones (105/well) were incubated with irradiated stimulator cells in 96-well flat-bottom plates and phorbol 12-myristate 13-acetate (PMA) (1 ng/ml) was included, as indicated, to maximize costimulation. 26 Peripheral blood T-cell activation was carried out using phytohaemagglutinin (PHA), as described previously. 26 Monocyte CD1a, b and c expression was induced by culture in granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-4 (100 units/ml of each), 8 plus or minus lipopoly-saccharide (LPS), for 24 hours.
Antibodies
Monoclonal and polyclonal antibodies were generated against CD1d-glutathione-S-transferase (GST) fusion proteins and against a CD1d-immunoglobulin fusion protein. A pool of CD1d-GST fusion proteins containing the α1 domain alone, the α1 and α2 domains, or the α1–α3 domains, were used to immunize rabbits and mice. Rabbit antiserum was affinity purified by passing multiple times through a GST column followed by absorption to a column of agarose beads (Affigel, Bio-Rad, Hercules, CA) covalently conjugated with a mixture of the three CD1d-GST fusion proteins. Monoclonal antibodies (mAbs) against the pooled CD1d-GST fusion proteins were generated by fusing hyperimmune BALB/c spleen cells to murine myeloma (NS-1) cells. One antibody from this screen, termed D5 (immunoglobulin G2b [IgG2b] isotype), reacted in immunoblots against the α1 domain of CD1d (S. Balk, unpublished).
A CD1d-immunoglobulin fusion protein was produced by first generating a BamHI site at the 3′ end of the CD1d α3 domain, using an oligonucleotide (antisense CGGGATCCCCCCAGTAGAGGACGATG) and polymerase chain reaction (PCR) amplification. This BamHI site was then used to fuse the CD1d leader sequence through the α3 domain (ending at the sequence Val–Leu–Tyr–Trp–Gly) to the Fc portion of murine IgG2a, using an immunoglobulin Fc expression vector (kindly provided by Dr Terry Strom, Beth Israel Deaconess Medical Center, Boston, MA). 32 The fusion protein was secreted as a β2-microglobulin (β2m)-associated, disulphide-linked dimer when expressed in hamster (CHO), murine (NSO) or human (C1R) cells (S. Balk, unpublished). CD1d knockout mice (M. Exley et al. submitted) were immunized with the fusion protein and hybridomas were subsequently screened by enzyme-linked immunosorbent assay (ELISA) using the protein. The anti-CD1d mAbs raised against the CD1d-immunoglobulin fusion protein used in this report were 27.1, 42.1, and 51.1. Additional antibodies used in this study included BBM.1, OKT6, 4A76, and M241, which recognize β2m, CD1a, b and c, respectively.
Flow cytometry
Flow cytometry analyses were carried out using ≈ 1 × 106 cells in 50–100 μl of phosphate-buffered saline (PBS) containing 0·05% Na azide and 1% fetal calf serum (FCS). Peripheral blood mononuclear cells (PBMC) were initially blocked by incubation in 10% human serum. For indirect immunofluorescence, the primary antibodies were each used at 10–20 µg/ml for 20–30 min at 4°. The secondary antibodies were fluorescein isothiocyanate (FITC)-conjugated anti-mouse or anti-rabbit F(ab′)2 fragments (DAKO, Carpinteria, CA). Direct Ab conjugates were from Pharmingen or DAKO. The 42.1 anti-CD1d mAb was conjugated to FITC according to the manufacturer's protocol (Molecular Probes, Eugene, OR).
Immunoprecipitation and immunoblotting
Cell-surface proteins were radiolabelled with 125I using lactoperoxidase. 33 Alternatively, cells (1–2 × 107/ml) were surface labelled with 0·5 mg/ml sulphosuccinimidyl-6-(biotinamido) hexanoate (NHS-LC-Biotin; Pierce Chemicals, Rockford, IL) in 25 m m HEPES, pH 8·0, 0·14 m NaCl, for 30 min at 4°. After washing, labelled cells were lysed in immunoprecipitation buffer (0·15 m NaCl, 50 m m Tris, pH 7·8, and 0·5% Nonidet P-40 [NP-40]) containing protease inhibitors. For immunoblotting experiments, lysates from unlabelled cells were prepared similarly.
Immunoprecipitations were performed using antibodies coupled to protein G (IgG1 mAbs) or protein A–Sepharose beads (Pierce). To minimize background from eluted IgG, antibody was covalently coupled to the beads using dimethylpimelimidate. Lysates were cleared by incubation with non-immune serum bound to protein A– and/or protein G–Sepharose beads. They were then incubated with specific antibodies coupled to Sepharose beads, washed, eluted in non-reducing sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) sample buffer and analysed by SDS–PAGE under reducing or non-reducing conditions.
After transfer to nitrocellulose, biotinylated proteins were detected using streptavidin-horseradish peroxidase (HRP) and enhanced chemiluminescence (ECL) (Amersham, Bucks, UK). Non-labelled CD1d was detected by immunoblotting using the affinity-purified rabbit anti-CD1d antibody, followed by anti-rabbit immunoglobulin-HRP conjugates and ECL. β2m was detected by immunoblotting of non-reducing gels with the BBM.1 mAb. N-linked carbohydrates were removed by digestion with N-glycanase (Genzyme, Cambridge, MA), as described previously. 33
Immunohistochemistry
Staining with anti-CD1 mAbs was performed on frozen sections following a 10-min fixation in acetone. Endogenous peroxidase was first blocked by incubation in 1% H2O2 in Tris-buffered saline or in glucose (50 mg/ml) and glucose oxidase (7 U; Sigma Chemical Co., St Louis, MO). Biotinylated horse anti-mouse IgG was used as the secondary antibody followed by Vectostain Elite ABC Reagent (Vector Labs, Burlinghame, CA). The chromogens were 3-amino-9-ethyl-carbazole or 3,3′-diamino-benzidine.
Results
Specificity of anti-CD1d antibodies
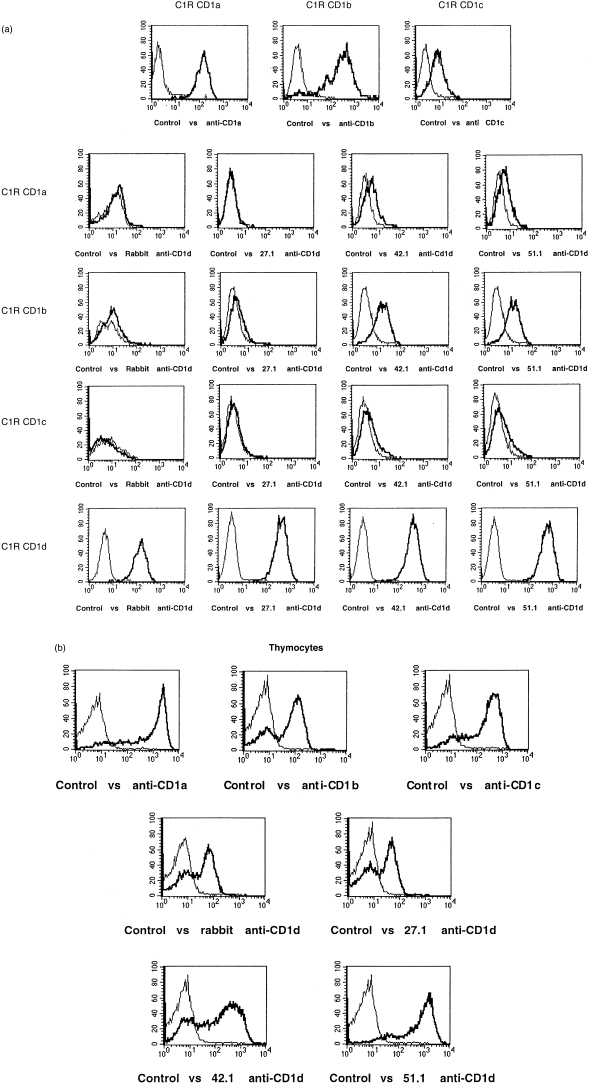
A panel of human C1R B-cell clones transfected with CD1a, b, c, or d were used to determine the specificity of antibodies generated against CD1d-GST and CD1d-immunoglobulin fusion proteins. The affinity-purified rabbit anti-CD1d antibody, generated against CD1d-GST fusion proteins, specifically stained the CD1d transfectant (Fig. 1a, control versus rabbit anti-CD1d). Similarly, the 27.1 mouse anti-human CD1d mAb, raised against a CD1d-IgG fusion protein, specifically recognized CD1d (Fig. 1a, control versus 27.1 anti-CD1d). The 42.1 and 51.1 mouse anti-human CD1d mAbs similarly reacted strongly with CD1d transfectants, although they showed some cross-reactivity with CD1b (Fig. 1a). The 42.1 and 51.1 mAbs may also cross-react very weakly with CD1a and c, as suggested by flow cytometry results (Fig. 1a). The D5 mouse anti-human CD1d mAb, raised against a CD1d-GST fusion protein, did not bind to CD1d-transfected C1R cells or other human CD1d transfectants, as determined by flow cytometry (not shown), but was shown to bind human CD1d on intact, transfected MDCK cells. 34
Figure 1.
Specificity of anti-CD1d antibodies and thymocyte CD1d expression by indirect immunofluorescence. (a) Polyclonal rabbit anti-CD1d and mouse monoclonal 27.1, 42.1 and 51.1 anti-CD1d antibodies were tested by indirect immunofluorescence against C1R cells stably transfected with CD1a, b, c or d, as indicated. The top three panels (C1R CD1a, C1R CD1b and C1R CD1c) are the CD1a, b and c transfectants stained with anti-CD1a (OKT6), anti-CD1b (4A76) and anti-CD1c (M241) monoclonal antibodies (mAbs), respectively. The thick lines represent specific antibodies and the thin lines are control antibodies (normal rabbit or mouse immunoglobulin G [IgG]). (b) Freshly isolated human thymocytes were stained by indirect immunofluorescence with the indicated specific (thick lines) or control (thin lines) antibodies.
CD1d expression by thymocytes
Northern blot analyses showed previously that CD1d transcripts were expressed at very low levels by human thymocytes compared with CD1a. 3 Moreover, CD1d was not detected previously on human thymocytes using a cross-reactive anti-mouse CD1d mAb. 14 Nonetheless, high-level cell-surface expression of CD1d protein, comparable to that on CD1d transfectants, was detected on the majority of thymocytes from multiple donors using each of the anti-CD1d mAbs and the CD1d-specific rabbit polyclonal Ab (Fig. 1b). Thymocyte CD1d expression was also confirmed by immunoprecipitations (see below).
CD1d expression in peripheral blood
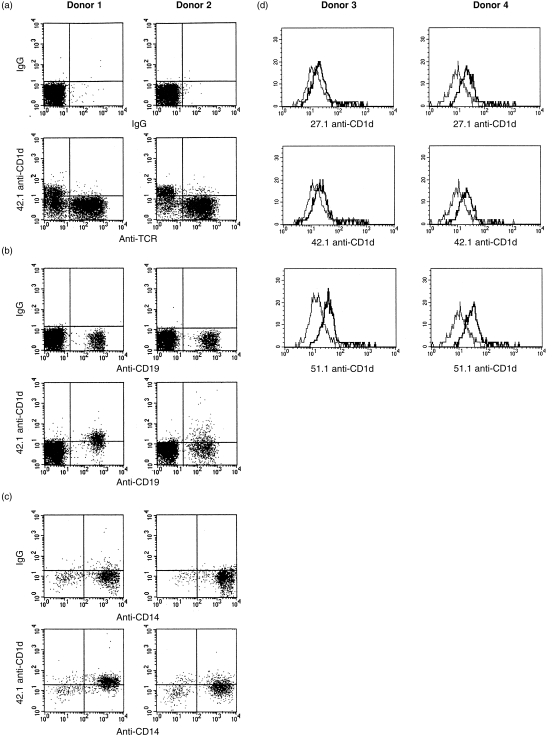
Figure 2 shows an analysis of CD1d expression on peripheral blood cells from two representative normal donors (of ≈ 40 analysed). CD1d expression was undetectable on the vast majority of resting peripheral blood T cells, as determined by using the 27.1, 42.1, 51.1 or rabbit anti-CD1d antibodies, although very small numbers of weakly positive T cells were sometimes observed (Fig. 2a and data not shown). In contrast, CD1d staining of essentially all CD19+ B cells was observed with the 42.1 mAb (Fig. 2B). Analyses of further donors and the use of multiple antibodies confirmed CD1d expression on B cells (Fig. 2d and data not shown). This confirmed the previous finding of CD1d on human B cells using a cross-reactive anti-mouse CD1d mAb. 14 CD1d expression by B cells was not increased after activation in vitro with pokeweed mitogen, anti-immunoglobulin M (anti-IgM), or LPS (results not shown).
Figure 2.
CD1d expression by peripheral blood lymphocytes and monocytes. Peripheral blood mononuclear cells from two representative normal donors were analysed by two-colour immunofluorescence with the indicated directly fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated specific and control antibodies. (a) Anti-T-cell receptor αβ (TCR αβ) versus control or 42.1 anti-CD1d analysis of cells in the resting lymphocyte gate. (b) Anti-CD19 versus control or 42.1 anti-CD1d analysis of cells in the resting lymphocyte gate. (c) Anti-CD14 versus control or 42.1 anti-CD1d analysis of cells in the monocyte gate. (d) Histogram of CD1d expression on B cells from two additional donors, examined as described above, gating of the B-cell (CD19+) population.
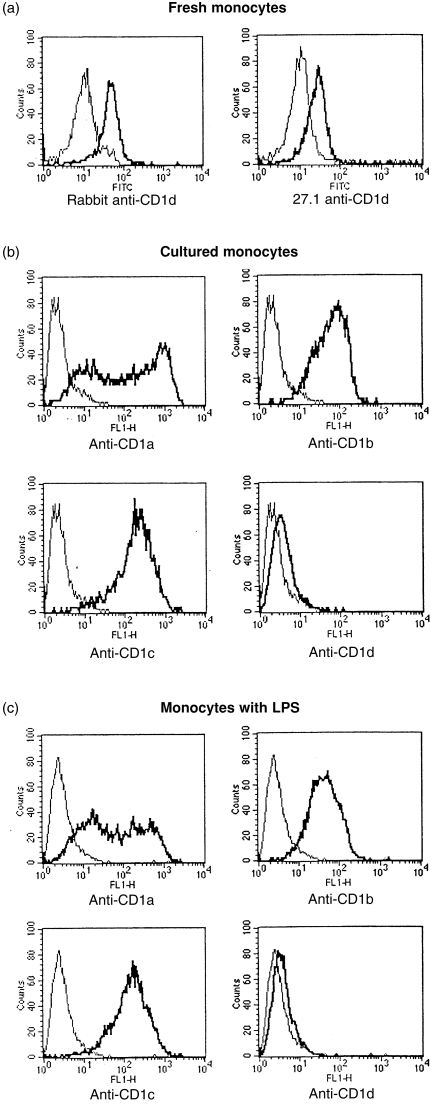
The group 1 CD1 proteins (CD1a, b and c) are not expressed on resting monocytes. However, CD1d expression was clearly detected on freshly isolated CD14+ monocytes (Figs 2c, 3a). It was shown previously that CD1a, b and c expression could be strongly induced on monocytes by stimulation with GM-CSF and IL-4. 8,9 To determine whether CD1d expression was regulated similarly, fresh monocytes were cultured with GM-CSF and IL-4, plus or minus LPS (Fig. 3b, 3c, respectively). In both cases there was induction of CD1a, b and c expression, but surface CD1d expression was not induced under these short-term culture conditions. These results demonstrate that CD1d is expressed constitutively by monocytes and its expression is regulated differently from the group 1 CD1 proteins (CD1a, b and c).
Figure 3.
CD1d expression by activated monocytes. (a) Freshly isolated peripheral blood mononuclear cells in the monocyte gate were analysed by indirect immunofluorescence with rabbit anti-CD1d or 27.1 anti-CD1d antibodies (thick lines) versus control non-immune rabbit or mouse serum (thin lines). (b) Granulocyte–macrophage colony-stimulating factor (GM-CSF)- and interleukin-4 (IL-4)-cultured monocytes were analysed by indirect immunofluorescence with anti-CD1a, b, c or d monoclonal antibodies (mAbs) (thick lines) versus control Abs (thin lines). The CD1d Ab shown was 27.1, but identical results were obtained with the rabbit anti-CD1d antibody. (c) Monocytes cultured in GM-CSF, IL-4 and lipopolysaccharide (LPS) were analysed as described above in (b). FITC, fluorescein isothiocyanate; FL1-H, FITC channel.
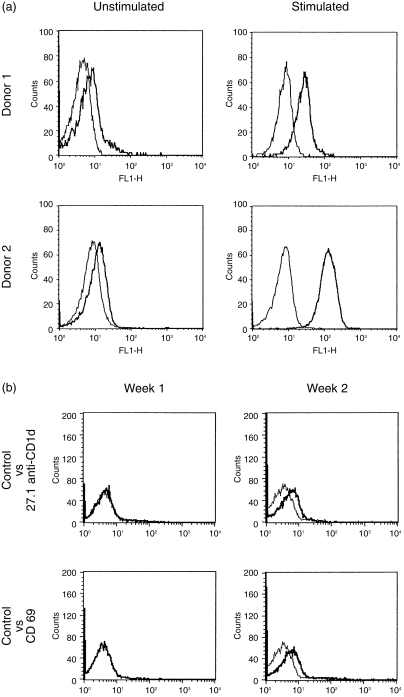
Although peripheral blood T cells were CD1d–, expression of CD1d could be induced by T-cell activation in vitro. CD1d expression was observed within 24 hr of culture (Fig. 4a) and persisted for at least 5 days (results not shown). Significantly, the analysis of peripheral blood from healthy donors revealed some samples with similar low-level CD1d expression by the majority of T cells (Fig 4b, 27.1 anti-CD1d, week 2). CD1d expression by these donors did not appear to reflect polymorphisms or other genetic differences in CD1d regulation, as in all cases previous and/or subsequent samples from the same donors were found to be CD1d– negative (Fig. 4b, 27.1 anti-CD1d, week 1). CD1d expression in each of these samples correlated with modest levels of CD69 expression, indicative of recent activation (Fig. 4b). Therefore, these studies of CD1d expression in peripheral blood from healthy donors indicated that CD1d expression by T cells was induced by activation in vivo.
Figure 4.
CD1d expression by activated T cells. (a) Freshly isolated peripheral blood mononuclear cells (PBMC) from two donors were cultured for 24 hr with or without stimulation and then analysed by indirect immunofluorescence with 42.1 anti-CD1d monoclonal antibody (mAb) (42.1) (thick lines) or isotype-matched control Ab (thin lines). Similar results were obtained with the other anti-CD1d mAbs and the rabbit anti-CD1d Ab (not shown). The very weak staining in the cells cultured without stimulation was not seen in the freshly isolated resting T cells (results not shown). (b) Freshly isolated peripheral blood mononuclear cells isolated 1 week apart from the same donor were analysed by indirect immunofluorescence with the indicated specific mAbs (thick lines) versus control antibodies (thin lines). FL1-H, FITC channel.
Immunohistochemical analysis of CD1d expression in thymus and lymph node
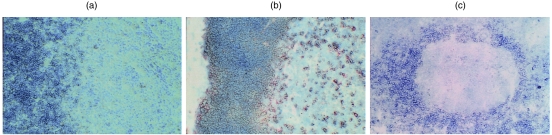
The above flow cytometric analysis showed strong CD1d expression by most thymocytes. Consistent with this, immunohistochemistry showed relatively uniform CD1d expression by cortical thymocytes (Fig. 5a). However, there was a striking down-regulation of CD1d expression by medullary thymocytes, with CD1d+ thymocytes being very rare in the medulla. CD1a expression was also diminished in the transition from cortex to medulla, but many CD1a+ cells could nonetheless be detected in the medulla (Fig. 5b). In lymph node, mantle zone B cells were strongly CD1d+, while CD1d+ cells in germinal centres were extremely rare (Fig. 5c). In the interfollicular T-cell-rich zones, there were scattered, small-to-medium sized mononuclear cells that were strongly CD1d+. Comparable results were obtained with each of the anti-CD1d Abs.
Figure 5.
Immunoperoxidase analysis of CD1 expression in thymus and lymph node. (a) Thymus stained with the 51.1 anti-CD1d monoclonal antibody (mAb). (b) Thymus stained with the OKT6 anti-CD1a mAb. (c) Lymph node stained with 51.1 anti-CD1d mAb. Comparable results were obtained with the 27.1 and 42.1 anti-CD1d mAbs (not shown).
Structure of CD1d expressed by human thymocytes
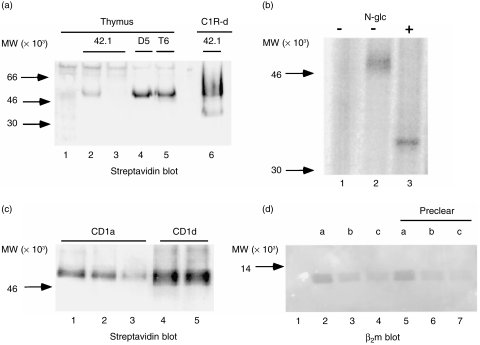
Although flow cytometry with the 27.1, 42.1 and 51.1 mAbs indicated a relatively high level of CD1d expression by thymocytes, sequential immunoprecipitations with the 42.1 mAbs from biotinylated thymocyte lysates yielded relatively small amounts of protein (Fig. 6a, lanes 2 and 3). Comparable low levels of CD1d were immunoprecipitated by the 27.1 and 51.1 mAbs (results not shown). In contrast, substantially more CD1d was precipitated from the same thymocyte lysates by the D5 anti-CD1d mAb (Fig. 6a, lane 4), with the amount of biotin label being comparable to that precipitated by the OKT6 anti-CD1a mAb (lane 5, T6). The proteins precipitated by 42.1 and D5 migrated identically to the major CD1d protein expressed by transfected C1R cells (Fig. 6a, lane 6) (≈ 48 000 MW), and migrated at 37 000 MW after N-glycanase digestion (Fig. 6b), consistent with thymocyte CD1d being fully glycosylated. These results indicated that the epitopes recognized by the 27.1, 42.1, and 51.1 mAbs were lost after biotinylation and detergent solubilization on the majority of the thymocyte cell-surface CD1d.
Figure 6.
CD1d immunoprecipitations from thymocytes and specificity of D5 monoclonal antibody (mAb). (a) Streptavidin blot of immunoprecipitates from biotinylated thymocyte lysates: lane 1, normal mouse serum; lanes 2 and 3, sequential 42.1 anti-CD1d mAb precipitations; lane 4, D5 anti-CD1d mAb; lane 5, OKT6 anti-CD1a precipitation; lane 6, 42.1 precipitation from CD1d-transfected C1R cells. (b) Deglycosylation of CD1d from surface-iodinated thymocyte lysates: lane 1, normal mouse serum immunoprecipitation; lanes 2 and 3, D5 anti-CD1d mAb immunoprecipitates untreated (lane 2) or treated lane 3 with N-glycanase (N-glc). (c) D5 immuno-precipitation from CD1a precleared biotinylated thymocyte lysate: lanes 1–4, lysate immunoprecipitated sequentially with OKT6 (anti-CD1a) beads (lanes 1–3) followed by reimmunoprecipitation with D5 (anti-CD1d) beads (lane 4); lane 5, D5 precipitation from an equal amount of lysate without a CD1a preclear. (d) Immunoprecipitates from unlabelled thymocyte lysates immunoblotted with BBM.1 anti-β2m mAb: lanes 1–4, fresh thymocyte lysate; lanes 5–7, equivalent amounts of D5 anti-CD1d mAb-depleted thymocyte lysate. Immunoprecipitations were as follows: lane 1, normal mouse serum; lanes 2 and 5, anti-CD1a (OKT6) mAb; lanes 3 and 6, anti-CD1b (4A76) mAb; lanes 4 and 7, anti-CD1c (M241) mAb. Samples were analysed on a 15% gel under non-reducing conditions.
The D5 mAb was generated against a CD1d-GST fusion protein and recognizes an epitope in the α1 domain (S. Balk, unpublished). D5 binding to detergent-solubilized CD1d appears to disrupt the association with β2m, as D5 immunoprecipitates CD1d from transfected C1R cells without β2m. 35 Although D5 immunoprecipitates CD1d, and not CD1a, b or c, from the respective transfected C1R cells (reference 35 and data not shown), the specificity of this mAb was further assessed directly on thymocyte lysates. To determine whether the D5 mAb might be cross-reactive with CD1a expressed by thymocytes (which migrates at the same position on SDS–PAGE), thymocyte lysates were initially precleared three times with an anti-CD1a mAb (Fig. 6c, CD1a, lanes 1–3). Immunoprecipitations were then carried out with D5 on the CD1a precleared versus fresh thymocyte lysates. The amount of protein immunoprecipitated by D5 from the precleared lysate (lane 4) versus fresh lysate (lane 5) was comparable, indicating that D5 was not cross-reacting with thymocyte CD1a.
Although cross-reactivity of the D5 mAb with CD1b or CD1c expressed by thymocytes was unlikely, as the apparent molecular weight of these proteins on SDS–PAGE was lower (not shown), this was nonetheless further addressed by determining whether the D5 mAb could immunodeplete CD1a, b, or c from thymocyte lysates. In these experiments, CD1a, b and c were detected by immunoblotting with anti-β2m, as immunoblotting antibodies for each of the group 1 CD1 proteins were not available. D5 was first used to preclear half of an unlabelled thymocyte lysate, followed by CD1a, b and c immunoprecipitation and β2m immunoblotting. Figure 6(d) shows that the amount of CD1a, b and c immunoprecipitated was similar before (lanes 2–4) and after (lanes 5–7) two successive preclears with the D5 mAb, which further confirmed that the D5 mAb was CD1d specific.
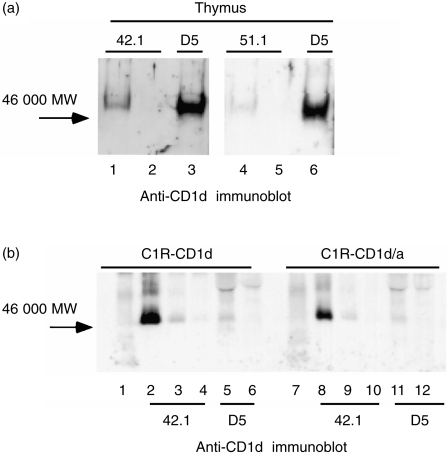
To address the possibility that epitopes on CD1d recognized by the 42.1 and 51.1 mAbs were lost as a result of biotin labelling, and to further confirm the specificity of the mAbs, unlabelled thymocyte lysates were analysed by immunoblotting with the rabbit anti-CD1d Ab. Lysates were cleared by two immunoprecipitations with the 42.1 mAb (Fig. 7a, lanes 1 and 2) or 51.1 mAb (lanes 4 and 5), followed by immunoprecipitation with the D5 mAb (lanes 3 and 6, respectively). Immunoblotting with the rabbit anti-CD1d Ab confirmed that the 42.1 and 51.1 mAbs precipitated only a small fraction of the total cellular CD1d relative to that immunoprecipitated by the D5 mAb. In marked contrast to these results with thymocyte CD1d, most of the CD1d in lysates from CD1d-transfected C1R cells could be cleared by immunoprecipitation with the 42.1 mAb (Fig. 7b, sequential 42.1 preclears in lanes 2–4 versus subsequent D5 immuno-precipitations in lanes 5 and 6). Similar results were obtained with the 27.1 and 51.1 mAbs (results not shown). Taken together, these results confirmed biochemically CD1d expression by human thymocytes and demonstrated a structural difference, detectable as the loss of one or more epitopes after solubilization in non-ionic detergent, between a large fraction of the CD1d protein expressed by thymocytes versus CD1d expressed by transfected C1R cells.
Figure 7.
CD1d immunoprecipitations from thymocytes and transfected cells immunoblotted with rabbit anti-CD1d. (a) Immuno-precipitates from unlabelled thymocytes immunoblotted with affinity-purified rabbit anti-CD1d: lanes 1 and 2, primary and secondary 42.1 monoclonal antibody (mAb) precipitations, respectively; lanes 4 and 5, primary and secondary 51.1 mAb precipitations, respectively; lanes 3 and 6, D5 anti-CD1d precipitations from 42.1 and 51.1 precleared lysates, respectively. (b) Lysates from C1R cells expressing wild-type CD1d (lanes 1–6) or a CD1d/a chimeric protein (lanes 7–12) immunoprecipitated and immunoblotted with affinity-purified rabbit anti-CD1d: lanes 1 and 7, normal mouse serum; lanes 2–4 and 8–10, three sequential immunoprecipitations with 42.1 mAb; lanes 5, 6 and 11, 12, sequential D5 mAb immunoprecipitations of the 42.1-cleared lysates. All samples were analysed on 12% reducing gels.
The cytoplasmic tail of CD1d contains a tyrosine-based endosomal-targeting motif that appears to be necessary for the acquisition of specific lipid antigens. 36 To determine whether the structural difference between thymocyte and C1R CD1d could reflect a post-translational modification that occurs during endosomal processing, a mutant CD1d, without this endosomal targeting motif and containing instead the cytoplasmic tail from CD1a (CD1d/a), was examined. Figure 7(b) shows that the vast majority of the CD1d/a protein could be immunoprecipitated with the 42.1 mAb, indicating that the structural difference between thymocyte and C1R CD1d does not reflect a cytoplasmic tail-dependent processing step.
CD1d-specific Vα24invt T cells do not respond to thymocyte CD1d
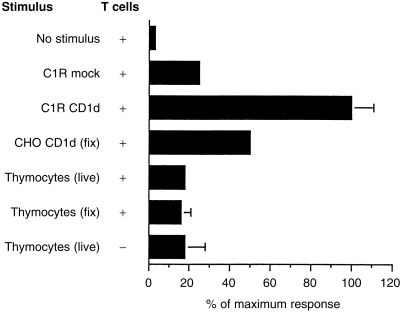
Human Vα24invt T cells can be stimulated by CD1d-transfected cells expressing high levels of CD1d protein, comparable to the levels on thymocytes, without the addition of an exogenous antigen. 26,27 Therefore, the ability of thymocytes to function as stimulator cells for Vα24invt T-cell clones was assessed. In contrast to CD1d-transfected C1R and CHO cells, thymocytes were unable to stimulate Vα24invt T-cell clones (Fig. 8). Similar results were obtained using four distinct Vα24invt T-cell clones (data not shown). Stimulation could not be reconstituted by inclusion of phorbol ester or light glutaraldehyde fixation (Fig. 8), procedures that can circumvent the need for costimulation in the case of some CD1d+ target cells. 26
Figure 8.
Lack of thymocyte CD1d recognition by Vα24invt T cells. A CD1d-reactive Vα24invt clone, DN2.B9, was cultured with the indicated stimulator cells in the presence of phorbol 12-myristate 13-acetate (PMA). Proliferation was determined by 3H-thymidine incorporation. The stimulator cells or DN2.B9 T cells were omitted from the top and bottom experiments, respectively. The response to the C1R CD1d transfectants was set at 100%. fix, light glutaraldehyde fixation; Mock, transfected with empty expression vector.
Discussion
The expression of CD1d in the lymphoid and myeloid lineages was assessed to identify potential physiological CD1d+ target cells for CD1d-reactive T cells and to determine whether CD1d expression was constitutive or regulated. CD1d expression during T-cell development paralleled CD1a, b and c, which are expressed at similar levels on immature cortical thymocytes and down-regulated on mature thymocytes and peripheral blood T cells. 4–6 CD1d expression on peripheral blood B cells paralleled that of CD1c, which is expressed on a large proportion of mature B cells. 10 However, these similarities in the regulation of CD1a, b and c expression did not extend to peripheral blood monocytes, as CD1a, b and c expression by monocytes is activation dependent. 8,9 In contrast, cell-surface CD1d expression by monocytes was constitutive and not up-regulated by in vitro activation. Finally, CD1d expression by mature peripheral blood T cells could be induced in vitro, and appeared to be induced in vivo, by activation.
The lymph node immunohistochemistry was consistent with the peripheral blood analysis, showing CD1d expression primarily in the B-cell-rich mantle zones. Recent reports showed higher level expression of murine CD1d by splenic marginal zone B cells. 37,38 Human lymph nodes do not have well-defined marginal zones,39 but scattered CD1d+ cells observed in the interfollicular T-cell zones could reflect this B-cell population (or dendritic cells). Significantly, CD1d+ cells were not detected in germinal centres, indicating that B-cell CD1d expression is down-regulated at this stage and that CD1d may not play a role in the lymphocyte and dendritic cell interactions that occur in germinal centres.
Immunoprecipitations confirmed that thymocytes ex-pressed relatively high levels of CD1d and showed that it was fully glycosylated. They further showed that thymocyte CD1d, but not CD1d expressed by transfected cells, lost epitopes recognized by conformationally sensitive mAbs after solubilization in non-ionic detergent. The structural differences between CD1d expressed by thymocytes and CD1d transfectants probably reflects post-translational processing at the level of antigen binding or glycosylation. Correspondingly, CD1d expressed by thymocytes also appeared to be functionally distinct from CD1d on transfected cells, as thymocytes expressing high levels of CD1d protein were unable to stimulate CD1d-reactive Vα24invt T cells. These results indicate that while CD1d+ thymocytes may mediate the positive selection of CD1d-reactive T cells, 40 they are not the physiological target cells of mature Vα24invt T cells. Further studies are necessary to determine the relationship between CD1d structure and function on thymocytes. However, the data suggest that the role of alternative CD1d processing by thymocytes may be to promote the positive selection of Vα24invt T cells while preventing negative selection caused by high-affinity binding of CD1d by the Vα24invt TCR.
Significant human thymocyte CD1d expression was not detected previously by flow cytometry or immunohistochemistry using the cross-reactive rat anti-mouse CD1d mAbs, 1H1 and 3C11, 14,15 although these mAbs detected human CD1d on transfected cells, B cells and intestinal epithelial cells. 14,15,33 The 1H1 and 3C11 mAbs similarly failed to immunoprecipitate CD1d from human thymocytes (data not shown), consistent with a distinct structure for thymocyte CD1d. We showed previously, using the 1H1 and 3C11 mAbs, that CD1d was alternatively processed in human intestinal epithelial cells, yielding a non-glycosylated and non-β2m-associated protein, 33 and these results have recently been confirmed with additional antibodies. 41 These observations suggest that post-translational modification may regulate CD1d function in a number of cell types.
The rate of CD1d turnover in thymocytes appeared to be extremely slow, based upon the relatively high level of CD1d protein expression in conjunction with very low message levels. 3 However, immunohistochemistry demonstrated a marked down-regulation of CD1d protein expression in the transition from thymic cortex to the medulla. This suggests a specific mechanism to clear CD1d protein from the cell surface at this developmental stage. This mechanism could involve the tyrosine-based endosomal targeting signal in the cytoplasmic tail of CD1d (as well as CD1b and CD1c), but not CD1a.
In contrast to the results presented here in humans, autoreactive recognition of murine thymocyte CD1d by homologous murine Vα14invt T-cell hybridomas has been observed. 23 This difference could reflect less stringent in vitro requirements for TCR-mediated activation in the murine hybridomas versus the human Vα24invt T-cell clones. Another significant difference between CD1d in humans and mice is that murine CD1d is expressed at low levels by both thymocytes and mature T cells,13, 42 and at slightly higher levels by B cells. 37,38 We suggest that the expression of group 1 and group 2 CD1 proteins by human thymocytes and their absence in mature human T cells may be co-ordinately regulated by CD1 locus-specific enhancer and silencer elements, respectively, and that these elements may have been lost with the deletion of the group 1 (CD1a, b and c) genes from the murine CD1 locus. If this is correct, then the regulated expression of CD1d in the human T-lymphocyte lineage may reflect a physiological role in immunoregulation. Specifically, CD1d expression by activated human T cells may render them as direct in vivo stimulators or targets of CD1d-reactive T cells. A role for Vα24invt T cells in modulating the function of activated T cells would be consistent with the loss of Vα24invt T cells in a number of human autoimmune diseases. 31,43
Acknowledgments
We thank Dr T. Strom (Beth Israel Deaconess Medical Center, Boston) for providing the IgG fusion vector. This work was supported by NIH R01 grants AI42955 to S. P. B.; AI3319 and DK44319 to S. P. B. and R. S. B.; DK51362 to R. S. B., AI45051 to S. B. W, AI40135 and an Arthritis Foundation Investigator Award to S. P.; a Crohns and Colitis Foundation of America grant to D. G.; and an American Hematology Society award to K. T. P.
Glossary
Abbreviations
- GST
glutathione-S-transferase
- β2m
β2-microglobulin
- Vα24invt T cells
Vα24 invariant T cells
References
- 1.Martin LH, Calabi F, Lefebvre FA, Bilsland CA, Milstein C. Structure and expression of the human thymocyte antigens CD1a, CD1b, and CD1c. Proc Natl Acad Sci USA. 1987;84:9189. doi: 10.1073/pnas.84.24.9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aruffo A, Seed B. Expression of cDNA clones encoding the thymocyte antigens CD1a, b, c demonstrates a hierarchy of exclusion in fibroblasts. J Immunol. 1989;143:1723. [PubMed] [Google Scholar]
- 3.Balk SP, Bleicher PA, Terhorst C. Isolation and characterization of a cDNA and gene coding for a fourth CD1 molecule. Proc Natl Acad Sci USA. 1989;86:252. doi: 10.1073/pnas.86.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMichael AJ, Pilch JR, Galfre G, Mason DY, Fabre JW, Milstein C. A human thymocyte antigen defined by a hybrid myeloma monoclonal antibody. Eur J Immunol. 1979;9:205. doi: 10.1002/eji.1830090307. [DOI] [PubMed] [Google Scholar]
- 5.Kahn-perles B, Wietzerbin J, Caillol DH, Lemonnier F. Delineation of three subsets of class I human T antigens (HTA) on Molt-4 cells: serologic and regulatory relationship to HLA class I antigens. J Immunol. 1985;134:1759. [PubMed] [Google Scholar]
- 6.Amiot M, Bernard A, Raynal B, Knapp W, Deschildre C, Boumsell L. Heterogeneity of the first cluster of differentiation: characterization and epitopic mapping of three CD1 molecules on normal human thymus cells. J Immunol. 1986;136:1752. [PubMed] [Google Scholar]
- 7.Fithian E, Kung P, Goldstein G, Rubenfeld M, Fenoglio C, Edelson R. Reactivity of Langerhans' cells with hybridoma antibody. Proc Natl Acad Sci USA. 1981;78:2541. doi: 10.1073/pnas.78.4.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porcelli S, Morita CT, Brenner MB. CD1b restricts the response of human CD4– T lymphocytes to a microbial antigen. Nature. 1992;360:593. doi: 10.1038/360593a0. [DOI] [PubMed] [Google Scholar]
- 9.Kasinrerk W, Baumruker T, Majdic O, Knapp W, Stockinger H. CD1 molecule expression on human monocytes induced by granulocyte–macrophage colony-stimulating factor. J Immunol. 1993;150:579. [PubMed] [Google Scholar]
- 10.Small TN, Knowles RW, Keever C, et al. M241 (CD1) expression on B lymphocytes. J Immunol. 1987;138:2864. [PubMed] [Google Scholar]
- 11.Beckman EM, Porcelli SA, Morita CT, Behar SM, Furlong ST, Brenner MB. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature. 1994;372:691. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 12.Calabi F, Jarvis JM, Martin L, Milstein C. Two classes of CD1 genes. Eur J Immunol. 1989;19:285. doi: 10.1002/eji.1830190211. [DOI] [PubMed] [Google Scholar]
- 13.Bleicher PA, Balk SP, Hagen SJ, Blumberg RS, Flotte TJ, Terhorst C. Expression of murine CD1 on gastrointestinal epithelium. Science. 1990;250:679. doi: 10.1126/science.1700477. [DOI] [PubMed] [Google Scholar]
- 14.Blumberg RS, Terhorst C, Bleicher P, et al. Expression of a nonpolymorphic MHC class I-like molecule, CD1D, by human intestinal epithelial cells. J Immunol. 1991;147:2518. [PubMed] [Google Scholar]
- 15.Canchis PW, Bhan AK, Landau SB, Yang L, Balk SP, Blumberg RS. Tissue distribution of the non-polymorphic major histocompatibility complex class I-like molecule, CD1d. Immunology. 1993;80:561. [PMC free article] [PubMed] [Google Scholar]
- 16.Castano AR, Tangri S, Miller JE, et al. Peptide binding and presentation by mouse CD1. Science. 1995;269:223. doi: 10.1126/science.7542403. [DOI] [PubMed] [Google Scholar]
- 17.Kawano T, Cui J, Koezuka Y, et al. CD1d-restricted and TCR-mediated activation of Va14 NKT cells by glycosylceramides. Science. 1997;278:1626. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 18.Joyce S, Woods AS, Yewdell JW, et al. Natural ligand of mouse CD1d1: cellular glycosylphosphatidylinositol. Science. 1998;279:1541. doi: 10.1126/science.279.5356.1541. [DOI] [PubMed] [Google Scholar]
- 19.Coles MC, Raulet DH. Class I dependence of the development of CD4+ CD8– NK1.1+ thymocytes. J Exp Med. 1994;180:395. doi: 10.1084/jem.180.1.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohteki T, MacDonald HR. Major histocompatibility complex class I related molecules control the development of CD4+ 8– and CD4– subsets of natural killer 1.1+ T cell receptor-alpha/beta+ cells in the liver of mice. J Exp Med. 1994;180:699. doi: 10.1084/jem.180.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4– T cells in mice and humans. J Exp Med. 1994;180:1097. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adachi Y, Koseki H, Zijlstra M, Taniguchi M. Positive selection of invariant V alpha 14+ T cells by non-major histocompatibility complex-encoded class I-like molecules expressed on bone marrow-derived cells. Proc Natl Acad Sci USA. 1995;92:1200. doi: 10.1073/pnas.92.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 24.Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4- alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med. 1993;178:1. doi: 10.1084/jem.178.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dellabona P, Padovan E, Casorati G, Brockhaus M, Lanzavecchia A. An invariant V alpha 24-J alpha Q/V beta 11 T cell receptor is expressed in all individuals by clonally expanded CD4– T cells. J Exp Med. 1994;180:1171. doi: 10.1084/jem.180.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Exley M, Garcia J, Balk SP, Porcelli S. Requirements for CD1d recognition by human invariant Valpha24+ CD4– CD8– T cells. J Exp Med. 1997;186:109. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Exley M, Porcelli S, Furman M, Garcia J, Balk SP. CD161 (NKR-P1A) costimulation of CD1d-dependent activation of human T cells expressing invariant V alpha 24 J alpha Q T cell receptor alpha chains. J Exp Med. 1998;188:867. doi: 10.1084/jem.188.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arase H, Arase N, Nakagawa K, Good RA, Onoe K. NK1.1+ CD4+ CD8– thymocytes with specific lymphokine secretion. Eur J Immunol. 1993;23:307. doi: 10.1002/eji.1830230151. [DOI] [PubMed] [Google Scholar]
- 29.Yoshimoto T, Paul WE. CD4pos, NK1.1pos T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J Exp Med. 1994;179:1285. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshimoto T, Bendelac A, Watson C, Hu-li J, Paul WE. Role of NK1.1+ T cells in a TH2 response and in immunoglobulin E production. Science. 1995;270:1845. doi: 10.1126/science.270.5243.1845. [DOI] [PubMed] [Google Scholar]
- 31.Wilson SB, Kent SC, Patton KT, et al. Extreme Th1 bias of invariant Valpha24JalphaQ T cells in type 1 diabetes. Nature. 1998;391:177. doi: 10.1038/34419. [DOI] [PubMed] [Google Scholar]
- 32.Zheng XX, Steele AW, Nickerson PW, Steurer W, Steiger J, Strom TB. Administration of noncytolytic IL-10/Fc in murine models of lipopolysaccharide-induced septic shock and allogeneic islet transplantation. J Immunol. 1995;154:5590. [PubMed] [Google Scholar]
- 33.Balk SP, Burke S, Polischuk JE, et al. Beta 2-microglobulin-independent MHC class Ib molecule expressed by human intestinal epithelium. Science. 1994;265:259. doi: 10.1126/science.7517575. [DOI] [PubMed] [Google Scholar]
- 34.Rodionov DG, Nordeng TW, Pedersen K, Balk SP, Bakke O. A critical tyrosine residue in the cytoplasmic tail is important for CD1d internalization but not for its basolateral sorting in MDCK cells. J Immunol. 1999;162:1488. [PubMed] [Google Scholar]
- 35.Kim HS, Garcia J, Exley M, Johnson KW, Balk SP, Blumberg RS. Biochemical characterization of CD1d expression in the absence of beta2-microglobulin. J Biol Chem. 1999;274:9289. doi: 10.1074/jbc.274.14.9289. [DOI] [PubMed] [Google Scholar]
- 36.Spada FM, Koezuka Y, Porcelli SA. CD1d-restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J Exp Med. 1998;188:1529. doi: 10.1084/jem.188.8.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amano M, Baumgarth N, Dick MD, et al. CD1 expression defines subsets of follicular and marginal zone B cells in the spleen: beta 2-microglobulin-dependent and independent forms. J Immunol. 1998;161:1710. [PubMed] [Google Scholar]
- 38.Roark JH, Park SH, Jayawardena J, Kavita U, Shannon M, Bendelac A. CD1.1 expression by mouse antigen-presenting cells and marginal zone B cells. J Immunol. 1998;160:3121. [PubMed] [Google Scholar]
- 39.Spencer J, Perry ME, Dunn-walters DK. Human marginal-zone B cells. Immunol Today. 1998;19:421. doi: 10.1016/s0167-5699(98)01308-5. [DOI] [PubMed] [Google Scholar]
- 40.Bendelac A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J Exp Med. 1995;182:2091. doi: 10.1084/jem.182.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Somnay-wadgaonkar K, Nusrat A, Kim HS, et al. Immunolocalization of CD1d in human intestinal epithelial cells and identification of a beta2-microglobulin-associated form. Int Immunol. 1999;11:383. doi: 10.1093/intimm/11.3.383. [DOI] [PubMed] [Google Scholar]
- 42.Brossay L, Jullien D, Cardell S, et al. Mouse CD1 is mainly expressed on hemopoietic-derived cells. J Immunol. 1997;159:1216. [PubMed] [Google Scholar]
- 43.Sumida T, Sakamoto A, Murata H, et al. Selective reduction of T cells bearing invariant V alpha 24J alpha Q antigen receptor in patients with systemic sclerosis. J Exp Med. 1995;182:1163. doi: 10.1084/jem.182.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]