Abstract
Studies over the past three decades have clearly established a central role for complement in the promotion of a humoral immune response. The primary function of complement, in this regard, is to opsonize antigen or immune complexes for uptake by complement receptor type 2 (CR2, CD21) expressed on B cells, follicular dendritic cells (FDC) and some T cells. A variety of mechanisms appear to be involved in complement-mediated promotion of the humoral response. These include: enhancement of antigen (Ag) uptake and processing by both Ag-specific and non-specific B cells for presentation to specific T cells; the activation of a CD21/CD19 complex-mediated signalling pathway in B cells, which provides a stimulus synergistic to that induced by antigen interaction with the B-cell receptor (BCR); and promotion of the interaction between B cells and FDC, where C3d-bearing immune complexes participate in intercellular bridging. Finally, current studies suggest that CR2 may also play a role in the determination of B-cell tolerance towards self-antigens and thereby hold the key to the previously observed correlation between deficiencies of the early complement components and autoimmune disease.
In vivo evidence for the role of complement in the acquired immune response
While it is only in recent years that the role of complement in the induction and regulation of acquired immunity has come to be fully appreciated, the first indication that this might be the case dates back to the mid-1970s to the pioneering studies of Mark Pepys, who showed that depleting mice of complement by injection of cobra venom factor (CVF) markedly impaired their humoral responses to primary antigen1,2 (see Table 1 for a historical overview). Evidence for involvement of the classical pathway (CP) of complement activation was derived from the observation that C2 or C4 deficiency3–5 results in similar impairment in immune responsiveness to that seen with C3-depleted mice, or with C3-deficient guinea-pigs 6 and dogs.7 Support for this view was also provided by the finding that the enhancement of a humoral response achieved by administering immunoglobulin M (IgM) class antibodies of appropriate specificity, concurrently with the immunizing antigen, failed to occur when a mutant IgM monoclonal antibody (mAb) with impaired complement-activating ability was employed.8 Somewhat paradoxically, CP component deficiencies in humans4 (reviewed in reference 9) and guinea-pigs10 were also found to be predisposing for autoimmune conditions, suggesting that the complement system may additionally be involved in the induction and/or maintenance of tolerance at the humoral level.
Table 1.
Milestones in the study of the role of complement in the immune response
| In vivo studies | In vitro studies | |
|---|---|---|
| 1970–1980 | C3 depletion abrogates the HIR1,2 | |
| 1980–1990 | Classical pathway deficiencies result in impaired HIR3–7 | Identification of CR2 on B cells108 and FDC67 |
| In vitro demonstration of synergy in BCRand CR2 signalling41–44 | ||
| 1990–2000 | Blockade of CR2 receptor function abrogates the primary HIR16–19 | Identification of the CR2/CD19 signalling complex47,48 |
| Coupling of C3d fragments to antigen ehnahces the HIR20 | Opsonized immune complexes of Ag and natural Ab are shown to induce the HIR32,36 | |
| Knockout mice define the roles of CR2 on B cellsand FDC in the HIR25,26,77 | Characterization of BCR, CR2/CD19 andFcγRIIb signalling pathways51–66 | |
| Cr2 and CD19 shown to regulate B-cell self-tolerance106,107 |
Ab, antibody; Ag, antigen; BCR, B-cell receptor; FDC, follicular dendritic cells; HIR, humoral immune response.
Initial in vivo studies of the mechanism(s) underlying the contribution of complement to acquired immunity, focused attention on its role in ensuring the retention of antigen by the follicular dendritic cells (FDC) in germinal centres, 11–15 thereby providing a constant source of antigenic stimulus to activated, antigen-specific B cells. Concomitant with subsequent in vitro studies, revealing that the C3-fragment- (iC3b and C3dg) binding complement receptor type 2 (CR2/CD21) can act synergistically with the B-cell antigen receptor (BCR) in B-cell activation (see below), a range of in vivo investigations provided clear evidence that CR2 was also involved in the induction of a primary humoral response (Table 1). These included:
Studies showing that blockade of murine CR2 with a mAb which interfered with ligand binding, abrogated the primary immune response to both T-dependent16 and T-independent antigens 17 without impairing T helper (Th) cell induction.18
The demonstration that whereas neutralization of CR2 function by competing soluble CR2 diminished the humoral response, 19 ligation of C3d fragments to the immunizing antigen markedly enhanced the response. Thus, immunization of mice with the engineered chimeric protein, hen egg lysozyme-(HEL-)C3d3, resulted in a 100-fold and 10 000-fold enhancement in response, respectively, compared to immunization with HEL in Freund's complete adjuvant or with HEL alone.20
Studies with CR2-knockout mice.21,22 These, in common with their C3- and C4-knockout counterparts, 23 displayed marked inhibition in the production of Abs arising from class switching (i.e. immunoglobulin G [IgG]2a, IgG2b and IgG3), as well as the generation of fewer, and smaller, germinal centres.
(With regard to the aforementioned observations, it should be noted that complement receptor type 1 (CR1/CD35) and CR2 in mice are encoded from the same gene, Cr2, where CR1 is expressed as a longer isoform of CR2.24 Thus, the effects of a lack of one or other of these receptors cannot readily be distinguished.)
While these studies demonstrated unequivocally that CR2 is implicated in the induction of a humoral response, they did not shed any light on the relative contributions to this process made by CR2 expressed on B cells and FDC, respectively. This was achieved using two reconstitution mouse models. In the first model, RAG-2 mice were reconstituted with B cells from CR2-knockout mice to provide CR2-negative B cells against a CR2-positive FDC background 24 In the second model, Cr2–/– mice were implanted with Cr2+/+ bone marrow to create the reverse situation 26 (Table 2). In the former case of B-cell CR2 deficiency, the phenotype resembled that of the total CR2 knockouts (namely, an impaired initial response and failure of class-switch). By contrast, mice with selective CR2 deficiency on their FDC displayed a normal initial response, although the long-term IgG antibody response was depressed and there was a lack of memory induction.
Table 2.
The role of complement receptor type 2 (CR2) on B cells and follicular dendritic cells (FDC) in the development of a humoral immune response
| Immune response | |||||
|---|---|---|---|---|---|
| Chimera construction memory | Cell phenotype | Primary Abresponse | Class switchresponse | Long-termresponse | IgG response |
| Cr2–/– es cellsin | CR2– B cells+ | ↓ | ↓ | ↓ | ↓ |
| RAG-2–/– blastocytes | CR2+ FDC | ||||
| Cr2+/+ BM cellsin | CR2+ B cells+ | Normal | Normal | ↓ | ↓ |
| irradiated Cr2–/– mice | CR2– FDC | ||||
BM, bone marrow, es cells, embryonic stem cells; FDC, follicular dendritic cells; RAG-2, recombination-activating gene-2; ↓, decreased.
It should be noted that an observation common to many of the aforementioned studies was that the contribution of complement to induction of a humoral response was most apparent upon immunization with low doses of antigen and was diminished or even abolished by administering the antigen in higher doses. However, it can be argued that immunization with low doses of antigen may well reflect the type of challenge that arises from many naturally occurring infections, and thus be closely representative of the physiological state.
Complement opsonization of antigens
The observations that C2, C4, C3 and CR2 deficiencies result in impairment of the humoral immune response whereas C5 deficiency does not,8 all point to C3 fragment engagement of the CR2 receptor as being the pivotal interaction in the induction process. However, equally decisive is the physical association of the C3 fragment with the immunizing antigen.20 Under normal physiological conditions, this association may arise by covalent deposition of C3 fragments on antigens capable of activating the alternative pathway (AP), classical pathway (CP) or lectin pathway (LP) directly. Alternatively, the formation of immune complexes (IC) with circulating antibodies, which are then capable of activating complement via the CP, would result in C3 fragment attachment to the antigen and/or the adjacent antibodies. The latter mechanism may be operative, even in the primary immune response, owing to the existence of natural antibodies, which are polyreactive immunoglobulins of IgM, IgG or immunoglobulin A (IgA) isotypes. These Abs recognize a great variety of self- and foreign antigens with low affinity, and are present in the serum of all individuals without previous deliberate immunization (reviewed in references 27–29). Natural antibodies express heavy chain variable region (VH) genes in virtually non-mutated configuration 30 and are often directed against public epitopes and antigens that are well conserved during evolution. They are thought to play an important role in the first line of defence during the period necessary to mount a specific antibody response, and may be involved in immune regulation by facilitating formation of complement-activating IC with antigen and subsequent binding to antigen-presenting cells (APC) via complement receptors (see below).
Mechanisms whereby complement enhances the immune response
The attachment of C3 to an antigen has been demonstrated to affect various phases of the acquired immune response. With respect to the function of B cells, it appears to be involved in:
The promotion of antigen uptake, processing and presentation by B cells to antigen-specific T cells;
Direct activation of B cells; and
Facilitation of B-cell interactions with FDC.
Promotion of antigen uptake, processing and presentation by B cells
In 1988, Arvieux et al. demonstrated that coupling of C3b or C4b to an antigen, tetanus toxin (TT), enhanced the proliferative and cytotoxic responses of antigen-specific Th cell clones.31 The role of complement, in this connection, was to target the antigen to CR2 and CR1 on Epstein–Barr virus (EBV)-transformed B cells employed for antigen presentation to the T cells. More recently, it has been observed that the binding, processing and presentation of antigens, in the form of IC, are substantially enhanced by incorporation of C3 fragments into the complexes.32,33 Furthermore, CR1 and, particularly, CR2 on the B cells were shown to be of key importance for the binding of the opsonized IC.32–34 Notably, studies with both C3b-TT 35 and opsonized IC32,33,36 have indicated that the interaction of C3 fragments with CR2 (and CR1) allows non-specific B cells to participate in antigen presentation to specific T cells – albeit less efficiently than antigen-specific B cells32– thereby greatly enhancing the efficiency of antigen presentation. Subsequently it was shown that only the antigen-specific B cells were reciprocally stimulated for antibody synthesis upon culture of peripheral blood mononuclear cells (PBMC) with IC, indicating that regulatory mechanisms exist to prevent polyclonal B-cell activation.36
Recent studies indicate that attachment of C3 to antigens not only enhances the antigen uptake by B cells but also modulates downstream events, such as endosomal targeting of antigen, as well as the processing and binding of peptides to major histocompatibility complex (MHC) class II molecules. Thus, covalent attachment of C3b to TT results in enhanced and prolonged stimulation of specific T cells by both non-specific and TT-specific B-cell clones, presumably owing to delayed proteolysis of C3b–TT by an endosomal enzyme, cathepsin D.37 It has been suggested that the delayed endosomal proteolysis results in an improved peptide loading on MHC class II molecules and an increased stability of these molecules in the lysosomes.38 Consistent with this observation is the finding that attachment of C3b to the heavy chain of murine IgG results in a 100-fold reduction in the amount of IgG required for human B-cell lines to stimulate heavy chain-specific T-cell clones, without enhancing antigen presentation to light chain-specific T-cell clones.39
Apart from facilitating antigen presentation, IC may play an additional enhancing role by inducing the expression of costimulatory molecules. Thus, the costimulatory molecule CD80,32 which binds to CD28 on T cells, has been demonstrated to be up-regulated by the ligation of IC-associated IgG to B-cell FcγRII (CD32), with CR2 playing a synergistic role. Likewise, IC ligation to either CR2 or FcγRII can activate another costimulatory molecule, lymphocyte function-associated antigen-1 (LFA-1; CD11a/CD18), that binds to intercellular adhesion molecule-1 (ICAM-1, CD54) on T cells.36 (Note: this enhancing role of FcγRII in immune regulation is in contrast to the down-regulatory signalling through this receptor seen in relation to B-cell activation – see below.) It has also been reported that the expression of CD86, which also binds CD28, could be induced by cross-linking CR2,40 although, according to another study, T-cell factors are required for the up-regulation of CD86 on B cells by IC.33
As mentioned above, natural antibodies may be involved in the formation of complement-activating IC, which could play a decisive role in mediating immune responses to primary antigens. Indeed, it has been reported that incubation of keyhole limpet haemocyanin (KLH) with normal human serum results in the formation of complement-opsonized IC with the aforementioned effects on B-cell antigen binding and processing.32 Furthermore, we have shown recently that natural autoantibodies and complement, under normal conditions may, likewise, mediate the binding of human thyroglobulin to peripheral B cells and subsequently elicit Th cell proliferation (C.H. Nielsen, R.G.Q. Leslie, M.D. Kazatchkine, S. Kaveri, E.M. Fischer, unpublished).
Direct B-cell stimulation
The first indication that complement may be directly involved in B-cell activation was derived from in vitro studies showing that cross-linking of CR2 with the BCR enhanced calcium mobilization and the T cell-independent proliferative response of B cells induced by BCR aggregation,41,42 and that polyvalent ligands for CR2 were capable of priming B cells for enhanced response to stimulation via the BCR,43,44 whereas monovalent ligands proved to be inhibitory.44 The crucial observation, regarding the mechanism for this enhancement, was that CR2, which possesses only a short 34 amino acid (aa) or 35 aa cytoplasmic tail, in the case of human and murine CR2, respectively,45,46 associates non-covalently with the CD19/TAPA(CD81)/Leu-13 signalling complex.47,48 CD19, when co-ligated directly with the BCR, was found to lower markedly the threshold for stimulation via the BCR.49,50
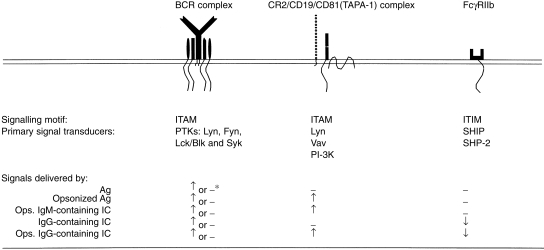
Synergy between the BCR and CD19 appears to be achieved through both mutual stimulation and the parallel activation of signalling pathways. Thus, aggregation of the BCR activates constitutively associated protein tyrosine kinases (PTK) of the Src-family (i.e. Lyn, Fyn, Blk and/or Lck) (see Fig. 1), which then phosphorylate immunoreceptor tyrosine-based activation motifs (ITAMs) in the cytoplasmic tails of the immunoglobulin-α (CD79a) and immunoglobulin-β (CD79b) components of the BCR complex (reviewed in reference 51). This enables recruitment of the cytosolic PTK, Syk, which plays a key role in the activation of a wide range of downstream pathways. These include phosphatidylinositol cleavage and mobilization of intracellular free calcium ions as well as activation of protein kinase C and the mitogen-activated protein kinases (MAP kinases), which are responsible for the regulation of nuclear transcription.51 Central to the activation process is the recruitment to the BCR complex of two adaptor molecules, BLNK/SLP-65 and Shc, which are responsible for the binding of essential downstream signalling elements such as phospholipase C-γ (PLC-γ), the guanidine nucleotide exchange factor, Vav, and phosphatidylinositol-3 kinase (PI-3K). While CD19 is also constitutively associated with Lyn52 and, possibly, the nucleotide exchange factor, Vav,53 phosphorylation of tyrosyl residues in the ITAM of its cytoplasmic tail by BCR-associated PTK54 results in enhanced binding of Vav53,55 and recruitment of the Src-family PTK, Fyn, and PI-3K56 (Fig. 1). Thus, CD19 functions as an adaptor protein, participating co-operatively with BLNK/SLP-65 and Shc in BCR signalling.
Figure 1.
Signalling processes induced by ligation of B-cell receptor (BCR), complement receptor type 2 (CR2) and FcγRII with opsonized antigen and immune complexes. Ag, antigen; IC, immune complexes; IgG, immunoglobulin G; IgM, immunoglobulin M; ITAM, immunoreceptor tyrosine-based activation motif; ITIM, immunoreceptor tyrosine-based inhibition motif; Ops. opsonized; PI-3K, phosphatidylinositol-3 kinase; PTK, protein tyrosine kinase; SHIP, inositol phosphate 5-phosphatase; SHP-2, protein tyrosine phosphatase, SHP-2; Vav, guanidine nucleotide exchange factor, Vav. ↑, activation; ↓, inhibition; –, no effect; *, for Ag-specific or non-specific B cells, respectively.
The role of CR2 is apparently to amplify association of the CD19/CD81/Leu-13 with the activated BCR, via cross-linking with complement-opsonized Ag, where the CD19 molecule itself provides the synergistic signalling, whilst CD81 is involved in promoting homotypic cellular interactions essential for the activation process.57 The role of Leu-13 remains, as yet, undefined. It has also been reported that the CR2 cytoplasmic domain, when phosphorylated, is capable of inducing signal transduction independently of CD19, apparently through the binding of a p53 anti-oncoprotein, a p68 calcium-binding protein and the nuclear p120 ribonucleoprotein as well as by activation of PI-3K.58,59
In the case of B-cell stimulation by IC containing IgG antibodies, a down-regulatory signalling pathway is also drawn into the picture. The B-cell Fcγ receptors (CD32, FcγRIIb1 and FcγRIIb2), in contrast to their myeloid cell counterpart, FcγRIIa, bear immunoreceptor tyrosine-based inhibitory motifs60 (ITIMs) in their cytoplasmic domains. These motifs, upon phosphorylation by the BCR-associated PTK, recruit the SH2 domain-containing inositol polyphosphate 5-phosphatase, SHIP61 and, in the case of humans, the protein tyrosine phosphatase, SHP-2,62(Fig. 1). When cross-linked to the BCR by IgG-containing IC, FcγRIIb acts to down-regulate antigen-mediated stimulation through a variety of mechanisms, including inhibition of calcium influx,61 dephosphorylation of the PTK, Syk,63 and inhibition of the MAP kinase cascade.64,65
B-cell activation by complement-opsonized IC can thus be described as being under the control of a signalling triad, consisting of the BCR and CR2–CD19 complex, as stimulatory components, and FcγRIIb as a negative regulator of the activation process (Fig. 1). In the case of antigen-specific B cells, the binding of IgG-containing opsonized IC will result in engagement of the full triad, whereas non-specific B cells will be engaged only via CR2 and FcγRIIb. IgM-containing complexes, on the other hand, will, when opsonized, engage either CR2 alone (in the case of non-specific B cells) or both the BCR and CR2. The, as yet, limited in vitro data available on the dynamics of signalling via the triad suggests that whereas engagement of FcγRIIb markedly inhibits signalling via either BCR or CR2 alone, triple engagement results in a similar degree of activation to that attained by single stimulation via the BCR.66 However, it is probable that the nature of the B-cell response will be determined not only by the types of receptor engaged but also upon the degree to which the various receptors are represented in the final signalling complex.
Promotion of B-cell interactions with FDC
Follicular dendritic cells of the spleen and lymph nodes express three receptors for C3 fragments (CR1, CR2 and CR3, CD11b/CD1867) as well as a receptor for the Fc portion of IgG, FcγRIIb.68 These receptors enable the FDC to take up and retain on their surfaces, in a process primarily involving CR1 and CR2,15,68,69 opsonized IC for presentation to activated, antigen-specific B cells. The consequences of the interaction appear to be twofold. First, rescue of the antigen-activated B cells from apoptosis;70,71 and second, the promotion of somatic hypermutation72,73 and class switch,26,74,75 concomitant with the development of a memory B-cell population. With regard to rescue from apoptosis, at least, it would appear that CR2 plays a central role in the process.76,77 This occurs via a pathway independent of that involving CD40,78 and the required activation of CR2 on B cells takes place either by its association with C3 fragments deposited on the FDC or through association with FcγRII (CD23) borne on the FDC.79
Recently, it has been demonstrated that antigen presentation may be enhanced by direct C3 fragment deposition on murine B lymphoblasts or macrophages.80 It had previously been established that B cells81 and FDC82,83 (which constitutively express CR2), as well as non-CR2-expressing murine and human macrophages,80,84,85 are the targets for the covalent deposition of C3 fragments. In the case of human B cells, it has been shown that this deposition is mediated by CR286,87 which, by virtue of its capacity to bind the hydrolysed form of C3 (iC3), assembles an AP convertase at its ligand-binding site.88 Nascent C3b fragments, generated by the convertase, then attach themselves to secondary acceptor sites on the B-cell surface.89 Although the full significance of this deposition for the development of an immune response remains to be clarified, there is some evidence to suggest that it may play a subsidiary role by promoting intercellular interactions involving the binding of C3 fragments deposited on one cell to CR2 expressed on another.80 Thus, covalent C3 deposition on B cells may enhance their interaction with CR2 on FDC (and vice versa) or on the subset of T cells, which expresses this receptor.90
Finally it should be noted that, in addition to its role in regulating B-cell responses, complement also plays a role in modulating the cytokine profile of helper T cells. Thus, it has been reported that interferon-γ (IFN-γ) synthesis is reduced in C1q-deficient mice91 while interleukn-4 (IL-4) production remains normal. This deficiency has a dual effect. It blocks the switch to IgG2a and IgG3 production, which is under IFN-γ control,92,93 and it ablates the induction of localized C3 synthesis in lymph nodes activated by antigen challenge, thereby diminishing the potency of the amplification mechanisms described above. In other words, it would appear that complement exerts a comprehensive and integrated influence on all aspects of the acquired immune response.
The role of complement in maintenance of immune tolerance
Although the prevalence of homozygous complement deficiency is low, association of complement deficiency in the early components of the classical pathway with systemic lupus erythematosus (SLE) is important. This relationship is paradoxical, as complement deficiency is associated with an impaired antibody response to foreign antigens, whereas SLE is characterized by high levels of antibodies against intracellular and cell-surface antigens. The susceptibility to SLE and the severity of the disease are related to the position of the missing complement component in the classical activation pathway (reviewed in reference 94). Thus, individuals with complete C1q deficiency have the highest prevalence of SLE and the most severe disease manifestations. By contrast, just a minority of the patients with C3 deficiency develop clinical features associated with autoimmune disease, and mild, lupus-like illness has been reported in only three families.
Animal deficiencies in C2, C4 and C3 do not result in spontaneous disease, although C2- and C4-deficient guinea-pigs develop elevated levels of IgM and rheumatoid factors, which are not seen in C3-deficient dogs (reviewed in reference 95). In contrast, dogs with C3 deficiency develop a glomerulonephritis that is very similar to that reported in humans.96 The genes for C3, C4, CR1/2, Factor B and C1q have been successfully targeted in mice. No phenotype associated with spontaneous autoimmune disease has been observed, with the exception of C1q-deficient mice. A significant percentage of C1qÁ − / − animals develop high titres of antinuclear antibodies and glomerulonephritis, and exhibit high numbers of apoptotic bodies in the glomeruli. Recent findings indicate that C1q is involved in the clearance and processing of self-antigens contained within surface blebs generated by apoptotic cells.97,98 Thus, SLE associated with C1q deficiency may be related to an impairment of the clearance of self-antigens, which then chronically stimulate an autoimmune response.99
The development of SLE in humans is associated with abnormalities of B-cell functions, and an altered expression pattern of both CR1 and CR2 on B lymphocytes has been reported.100 A similar decrease in C3 receptor expression was found in MRL/lpr mice – considered as a spontaneous model of human SLE – and was shown to precede the development of clinical and autoimmune manifestations.101 A number of lines of evidence (discussed above) indicate that the CD19/CD21 complex regulates transduction thresholds governing humoral immunity to T-dependent antigens. In addition, CD19 plays a key role in the development of the CD5+ B-1 subset of B cells in mice. B-1 cells constitute a small population of the B lineage, which is the major producer of serum IgM but generates only a restricted antibody repertoire dominated by a specific set of V genes, many of which encode autoreactive antibodies.102 B-1 cells produce natural antibodies (IgM and IgG3) of low affinity that have a broad specificity for bacterial polysaccharides, lipids and proteins, as well as autoantigens such as single-strand DNA (ssDNA) and IgG. A dominant feature of CD19-deficient mice is a dramatic decrease of CD5+ B-1 cells and of anti-DNA autoantibodies, while mice that overexpress CD19 have significantly increased numbers of CD5+ B cells and high titres of anti-DNA antibodies of IgG isotype.103,104 Deficient Cr2–/– mice were also found to have a reduction in the CD5+ population of peritoneal B-1 cells, although their serum levels of IgM were within the range of normal mice.21 Interestingly, Cr2–/– mice lack Ab with specificity for certain antigens commonly found in wild-type mice, such as those towards lipopolysaccharide (LPS), Escherichia coli and hypoxia antigen, demonstrating an altered antibody repertoire.105 Thus, the binding of C3 fragment-coated self-antigens to CD19/CD21, together with low-affinity BCRs of B-1 cells, may promote clonal expansion of this subset and be involved in the selection of the repertoire of natural antibodies.
Although it remains unclear whether pathogenic IgG autoantibodies are derived from switched B-1 cells or from conventional B-2 cells, the inappropriate or prolonged generation of C3 fragments during inflammatory or infectious episodes may alter signalling thresholds of B cells, leading to breakdown of tolerance and positive selection of autoreactive B-cell clones. Indeed, recent studies have established a role for the CD19/CD21 complex in the control of peripheral tolerance. The role of CD19 in tolerance regulation was examined by crossing mice that overexpress a human CD19 transgene with transgenic mice expressing a model autoantigen (sHEL) and high-affinity HEL-specific antigen-receptor (IgHEL).106 Whereas B cells of double-transgenic mice sHEL/IgHEL were functionally anergic and did not produce autoantibodies, overexpression of CD19 resulted in the breakdown of peripheral tolerance and the production of anti-HEL antibodies. Thus, by augmenting antigen receptor signalling, CD19 overexpression shifts the balance to autoimmunity, suggesting that inappropriate CD19 expression or function contributes to autoimmunity by disrupting tolerance.
Involvement of complement in the negative selection of self-reactive B cells has also been reported.107 B cells from double-transgenic mice (HEL/IgHEL) crossbred with Cr2 mice accumulated in secondary lymphoid organs and responded to stimulation with autoantigen by calcium mobilization, suggesting a defect in anergy development of the B cells. However, no autoantibodies against HEL were detected in the serum of the animals. Similar results were obtained in double-transgenic mice crossed with C4–/– mice but not with C3–/– mice, suggesting a predominant role of C4 in the negative selection of self-reactive B cells. The authors propose that uptake of C4b-coated antigens by CD35(CR1-)-positive cells within the bone marrow and secondary lymphoid organs would provide a mechanism for concentration of self-antigens and ensure contact with immature self-reactive B cells. This new role for complement in negative selection of self-reactive B cells would provide an explanation for the apparently paradoxical association between complement deficiency and autoimmunity.
Conclusions
In this review, we have described in outline the considerable body of evidence that links the complement system not only to the development of humoral immunity but also to the induction of self-tolerance in developing B cells. While the former serves to underline the high degree of interdependency that exists between the innate and acquired elements of the immune system, the latter provides a rationale for the, at first sight, paradoxical association between deficiencies of the early complement components and autoimmune disease. While the past decade has witnessed major advances in our understanding of the role(s) of complement in the induction of acquired immunity and B-cell tolerance, there remain, nevertheless, unanswered questions of both a conceptual and practical nature. For the first, the contention that complement provides the organism with a means of distinguishing between harmless antigens and dangerous (microbial) antigens, on the basis of their capacity to activate complement directly, requires re-examination in light of the findings that many primary antigens can form complement-activating IC with natural antibodies. In this case, it is the natural Ab repertoire that is decisive, and this includes not only specificity for carbohydrate antigens of microbial origin but also for a wide range of autoantigens.
The role of complement in tolerance induction illustrates once again the familiar paradigm concerning the initiation of cellular activation or death at different stages of development of the cell. While the mechanism for induction presumably involves tagging circulating autoantigens with C4b, it remains to be clarified how this labelling takes place: is it through a spontaneous activation process or is it consequent upon complex formation with natural autoantibodies?
The new insights that have been acquired regarding the role of complement in the development and regulation of humoral immune responses are likely to result in the development of novel strategies for immunization against pathogenic micro-organisms, on the one hand, and open the way to new forms of treatment for immune complex-associated autoimmune diseases, on the other.
References
- 1.Pepys MB. Role of complement in induction of antibody production in vivo. Effect of cobra venom factor and other C3-reactive agents on thymus-dependent and thymus-independent antibody responses. J Exp Med. 1974;140:126. doi: 10.1084/jem.140.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pepys MB. Role of complement in the induction of immunological responses. Transplant Rev. 1976;32:93. doi: 10.1111/j.1600-065x.1976.tb00230.x. [DOI] [PubMed] [Google Scholar]
- 3.Böttger EC, Hoffmann T, Hadding U, Bitter-suermann D. Influence of genetically inherited complement deficiencies on the humoral response in guinea pigs. J Immunol. 1985;135:4100. [PubMed] [Google Scholar]
- 4.Jackson CG, Ochs HD, Wedgwood RJ. Immune response of a patient with deficiency of the fourth component of complement and systemic lupus erythematosus. N Engl J Med. 1979;300:1124. doi: 10.1056/NEJM197905173002002. [DOI] [PubMed] [Google Scholar]
- 5.Ochs HD, Wedgwood RJ, Frank MM, Heller SR, Hosea SW. The role of complement in the induction of antibody responses. Clin Exp Immunol. 1983;53:208. [PMC free article] [PubMed] [Google Scholar]
- 6.Böttger EC, Metzger S, Bitter-suermann D, Stevenson G, Kleindienst S, Burger R. Impaired humoral response in complement C3-deficient guinea pigs: absence of a secondary antibody response. Eur J Immunol. 1986;16:1231. doi: 10.1002/eji.1830161008. [DOI] [PubMed] [Google Scholar]
- 7.O'neil KM, Ochs HD, Heller SR, Cork LC, Morris JM, Winkelstein JA. Role of C3 in humoral immunity. Defective antibody production in C3-deficient dogs. J Immunol. 1988;140:1939. [PubMed] [Google Scholar]
- 8.Heyman B, Pilström L, Shulman MJ. Complement activation is required for the IgM-mediated enhancement of the antibody response. J Exp Med. 1988;167:1999. doi: 10.1084/jem.167.6.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan KE. Complement deficiency and autoimmunity. Curr Opin Pediatr. 1998;10:600. doi: 10.1097/00008480-199810060-00011. [DOI] [PubMed] [Google Scholar]
- 10.Böttger EC, Hoffmann T, Hadding U, Bitter-suermann D. Guinea pigs with inherited deficiencies of complement components C2 or C4 have characteristics of immune complex disease. J Clin Invest. 1986;78:689. doi: 10.1172/JCI112628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papamichail M, Gutierrez C, Embling P, Johnson P, Holborow EJ, Pepys MB. Complement dependence of localisation of IgG in germinal centres. Scand J Immunol. 1975;4:343. doi: 10.1111/j.1365-3083.1975.tb02635.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen LL, Frank AM, Adams JC, Steinman RM. Distribution of horseradish peroxidase (HRP)-anti-HRP immune complexes in mouse spleen with special reference to follicular dendritic cells. J Cell Biol. 1978;79:184. doi: 10.1083/jcb.79.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enriquez-rincon F, Klaus GG. Follicular trapping of hapten–erythrocyte–antibody complexes in mouse spleen. Immunology. 1984;52:107. [PMC free article] [PubMed] [Google Scholar]
- 14.Van Den Eertwegh AJ, Laman JD, Schellekens MM, Boersma WJ, Claassen E. Complement-mediated follicular localisation of T-independent type-2 antigens: the role of the marginal zone macrophages revisited. Eur J Immunol. 1992;22:719. doi: 10.1002/eji.1830220315. [DOI] [PubMed] [Google Scholar]
- 15.Van Den Berg TK, Dopp EA, Daha MR, Kraal G, Dijkstra CD. Selective inhibition of immune complex trapping by follicular dendritic cells with monoclonal antibodies against rat C3. Eur J Immunol. 1992;22:957. doi: 10.1002/eji.1830220412. [DOI] [PubMed] [Google Scholar]
- 16.Heyman B, Wiersma EJ, Kinoshita T. In vivo inhibition of the antibody response by a complement receptor-specific monoclonal antibody. J Exp Med. 1990;172:665. doi: 10.1084/jem.172.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thyphronitis G, Kinoshita T, Inoue K, et al. Modulation of mouse complement receptors 1 and 2 suppresses antibody responses in vivo. J Immunol. 1991;147:224. [PubMed] [Google Scholar]
- 18.Gustavsson S, Kinoshita T, Heyman B. Antibodies to murine complement receptor 1 and 2 can inhibit the antibody response in vivo without inhibiting T helper cell induction. J Immunol. 1995;154:6524. [PubMed] [Google Scholar]
- 19.Hebell T, Ahearn JM, Fearon DT. Suppression of the immune response by a soluble complement receptor of B lymphocytes. Science. 1991;254:102. doi: 10.1126/science.1718035. [DOI] [PubMed] [Google Scholar]
- 20.Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271:348. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 21.Ahearn JM, Fischer MB, Croix D, et al. Disruption of the Cr2 locus results in a reduction in B-1a cells and in an impaired B cell response to T-dependent antigen. Immunity. 1996;4:251. doi: 10.1016/s1074-7613(00)80433-1. [DOI] [PubMed] [Google Scholar]
- 22.Molina H, Holers VM, Li B, et al. Markedly impaired humoral response in mice deficient in complement receptors 1 and 2. Proc Natl Acad Sci. 1996;93:3357. doi: 10.1073/pnas.93.8.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer MB, Ma M, Goerg S, et al. Regulation of the B cell response to T-dependent antigens by classical pathway complement. J Immunol. 1996;157:549. [PubMed] [Google Scholar]
- 24.Molina H, Kinoshita T, Inoue K, Carel JC, Holers VM. A molecular and immunochemical characterisation of mouse CR2. Evidence for a single gene model of mouse complement receptors 1 and 2. J Immunol. 1990;145:2974. [PubMed] [Google Scholar]
- 25.Croix DA, Ahearn JM, Rosengard AM, et al. Antibody response to a T-dependent antigen requires B cell expression of complement receptors. J Exp Med. 1996;183:1857. doi: 10.1084/jem.183.4.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang Y, Xu C, Fu YX, Holers VM, Molina H. Expression of complement receptors 1 and 2 on follicular dendritic cells is necessary for the generation of a strong antigen-specific IgG response. J Immunol. 1998;160:5273. [PubMed] [Google Scholar]
- 27.Kaveri SV, Lacroix-desmazes S, Mouthon L, Kazatchkine MD. Human natural autoantibodies: lessons from physiology and prospects for therapy. Immunologist. 1998;6:227. [Google Scholar]
- 28.Casali P, Notkins AL. CD5+ B lymphocytes, polyreactive antibodies and the human B-cell repertoire. Immunol Today. 1989;10:364. doi: 10.1016/0167-5699(89)90268-5. [DOI] [PubMed] [Google Scholar]
- 29.Dighiero G. Natural antibodies, tolerance, and autoimmunity. Ann N Y Acad Sci. 1997;815:182. doi: 10.1111/j.1749-6632.1997.tb52059.x. [DOI] [PubMed] [Google Scholar]
- 30.Sanz I, Casali P, Thomas JW, Notkins AL, Capra JO. Nucleotide sequences of eight human natural antibody VH regions reveal apparent restricted use of VH families. J Immunol. 1989;142:4054. [PubMed] [Google Scholar]
- 31.Arvieux J, Yssel H, Colomb MG. Antigen-bound C3b and C4b enhance antigen-presenting cell function in activation of human T-cell clones. Immunology. 1988;65:229. [PMC free article] [PubMed] [Google Scholar]
- 32.Thornton BP, Vetvicka V, Ross GD. Natural antibody and complement-mediated antigen processing and presentation by B lymphocytes. J Immunol. 1994;152:1727. [PubMed] [Google Scholar]
- 33.Nielsen CH, Matthiesen S, Lyng I, Leslie RGQ. The role of complement receptor 1 (CR1, CD35) in determining complex distribution between whole blood cells: kinetic analysis of the buffering capacity of erythrocytes. Immunology. 1997;90:129. doi: 10.1046/j.1365-2567.1997.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boacle SA, Morris MA, Holers VM, Karp DR. Complement opsonization is required for presentation of immune complexes by resting peripheral blood B cells. J Immunol. 1998;161:6537. [PubMed] [Google Scholar]
- 35.Villiers M-B, Villiers CL, Jacquier-sarlin MR, Gabert FM, Journet AM, Colomb MG. Covalent binding of C3b to tetanus toxin: influence on uptake/internalisation of antigen by antigen-specific and non-specific B cells. Immunology. 1996;89:348. doi: 10.1046/j.1365-2567.1996.d01-747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thornton BP, Vetvicka V, Ross GD. Function of C3 in a humoral response: iC3b/C3dg bound to an immune complex generated with natural antibody and a primary antigen promotes antigen uptake and the expression of co-stimulatory molecules by all B cells, but only stimulates immunoglobulin synthesis by antigen-specific B cells. Clin Exp Immunol. 1996;104:531. doi: 10.1046/j.1365-2249.1996.57761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacquier-sarlin MR, Gabert FM, Villiers MB, Colomb MG. Modulation of antigen processing and presentation by covalently linked complement C3b fragment. Immunology. 1995;84:164. [PMC free article] [PubMed] [Google Scholar]
- 38.Serra VA, Cretin F, Pépin E, Gabert FM, Marche PN. Complement C3b fragment covalently linked to tetanus toxin increases lysosomal sodium dodecyl sulphate-stable HLA-DR dimer production. Eur J Immunol. 1997;27:2673. doi: 10.1002/eji.1830271029. [DOI] [PubMed] [Google Scholar]
- 39.Santoro L, Drouet C, Reboul A, Mach JP, Colomb MG. Covalent binding of C3b to monoclonal antibodies selectively up-regulates heavy chain epitope recognition by T cells. Eur J Immunol. 1994;24:1620. doi: 10.1002/eji.1830240725. [DOI] [PubMed] [Google Scholar]
- 40.Kozono Y, Abe R, Kozono H, Kelly RG, Azuma T, Holers VM. Cross-linking CD21/CD35 or CD19 increases both B7 and B7 expression on murine splenic cells. J Immunol. 1998;160:1565. [PubMed] [Google Scholar]
- 41.Carter RH, Spycher MO, Ng YC, Hoffman R, Fearon DT. Synergistic interaction between complement receptor type 2 and membrane IgM on B lymphocytes. J Immunol. 1988;141:457. [PubMed] [Google Scholar]
- 42.Fingeroth JD, Heath ME, Ambrosino DM. Proliferation of resting B cells is modulated by CR2 and CR1. Immunol Lett. 1989;21:291. doi: 10.1016/0165-2478(89)90022-9. [DOI] [PubMed] [Google Scholar]
- 43.Carter RH, Fearon DT. Polymeric C3dg primes human B lymphocytes for proliferation induced by anti-IgM. J Immunol. 1989;143:1755. [PubMed] [Google Scholar]
- 44.Tsokos GC, Lambris JD, Finkelman FD, Anastassiou ED, June CH. Monovalent ligands of complement receptor 2 inhibit whereas polyvalent ligands enhance anti-Ig-induced human B cell intracytoplasmic free calcium concentration. J Immunol. 1990;144:1640. [PubMed] [Google Scholar]
- 45.Moore MD, Cooper NR, Tack BF, Nemerow GR. Molecular cloning of the cDNA encoding the Epstein–Barr virus/C3d receptor (complement receptor type 2) of human B lymphocytes. Proc Nat Acad Sci. 1987;84:9194. doi: 10.1073/pnas.84.24.9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fingeroth JD, Benedict MA, Levy DN, Strominger JL. Identification of murine complement receptor type 2. Proc Natl Acad Sci USA. 1989;86:242. doi: 10.1073/pnas.86.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsumoto AK, Kopicky-burd J, Carter RH, Tuveson DA, Tedder TF, Fearon DT. Intersection of the complement and immune systems: a signal transduction complex of the B lymphocyte-containing complement receptor type 2 and CD19. J Exp Med. 1991;173:55. doi: 10.1084/jem.173.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bradbury LE, Kansas GS, Levy S, Evans RL, Tedder TF. The CD19/CD21 signal transducing complex of human B lymphocytes includes the target of antiproliferative antibody-1 and Leu-13 molecules. J Immunol. 1992;149:2841. [PubMed] [Google Scholar]
- 49.Carter RH, Fearon DT. CD19: lowering the threshold for antigen stimulation of B lymphocytes. Science. 1992;256:105. [PubMed] [Google Scholar]
- 50.Mongini PK, Vilensky MA, Highet PF, Inman JK. The affinity threshold for human B cell activation via the antigen receptor complex is reduced upon co-ligation of the antigen receptor with CD21 (CR2) J Immunol. 1997;159:3782. [PubMed] [Google Scholar]
- 51.Campbell KS. Signal transduction from the B cell antigen-receptor. Curr Opin Immunol. 1999;11:256. doi: 10.1016/s0952-7915(99)80042-9. [DOI] [PubMed] [Google Scholar]
- 52.Van Noesel CJ, Lankester AC, Van Schijndel GMW, Van Lier RAW. The CR2/CD19 complex on human B cells contains the Src-family kinase Lyn. Int Immunol. 1993;5:699. doi: 10.1093/intimm/5.7.699. [DOI] [PubMed] [Google Scholar]
- 53.Fujimoto M, Poe JC, Jansen PJ, Sato S, Tedder TF. CD19 amplifies B lymphocyte signal transduction by regulating Src-family protein tyrosine kinase activation. J Immunol. 1999;162:7088. [PubMed] [Google Scholar]
- 54.Carter RH, Doody GM, Bolen JB, Fearon DT. Membrane IgM-induced tyrosine phosphorylation of CD19 requires a CD19 domain that mediates association with components of the B cell antigen receptor complex. J Immunol. 1997;158:3062. [PubMed] [Google Scholar]
- 55.Li X, Sandoval D, Freeberg L, Carter RH. Role of CD19 tyrosine 391 in synergistic activation of B lymphocytes by coligation of CD19 and membrane Ig. J Immunol. 1997;158:5649. [PubMed] [Google Scholar]
- 56.Tuveson DA, Carter RH, Soltoff SP, Fearon DT. CD19 of B cells as a surrogate kinase insert region to bind phosphatidylinositol 3-kinase. Science. 1993;260:986. doi: 10.1126/science.7684160. [DOI] [PubMed] [Google Scholar]
- 57.Matsumoto AK, Martin DR, Carter RH, Klickstein LB, Ahearn JM, Fearon DT. Functional dissection of the CD21/CD19/TAPA-1/Leu-13 complex of B lymphocytes. J Exp Med. 1993;178:1407. doi: 10.1084/jem.178.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barel M, Balbo M, Gauffre A, Frade R. Binding sites of the Epstein–Barr virus and C3d receptor (CR2, CD21) for its three intracellular ligands, the p53 anti-oncoprotein, the p68 calcium-binding protein and the nuclear p120 ribonucleoprotein. Mol Immunol. 1995;32:389. doi: 10.1016/0161-5890(95)00005-y. [DOI] [PubMed] [Google Scholar]
- 59.Bouillie S, Barel M, Frade R. Signalling through the EBV/C3d receptor (CR2, CD21) in human B lymphocytes: activation of phosphatidylinositol 3-kinase via a CD19-independent pathway. J Immunol. 1999;162:136. [PubMed] [Google Scholar]
- 60.Muta T, Kurosaki T, Misulovin Z, Sanchez M, Nussenzweig MC, Ravetch JV. A 13-amino-acid motif in the cytoplasmic domain of FcγRIIB modulates B-cell receptor signalling. Nature. 1994;368:70. doi: 10.1038/368070a0. [DOI] [PubMed] [Google Scholar]
- 61.Ono M, Bolland S, Tempst P, Ravetch JV. Role of inositol phosphatase SHIP in negative regulation of the immune system by the receptor Fc(γ)RIIB. Nature. 1996;383:263. doi: 10.1038/383263a0. [DOI] [PubMed] [Google Scholar]
- 62.Sármay G, Koncz G, Pecht I, Gergely J. Fcγ receptor type IIb induced recruitment of inositol and protein phosphatases to the signal transductory complex of human B-cells. Immunol Lett. 1997;57:159. doi: 10.1016/s0165-2478(97)00055-2. [DOI] [PubMed] [Google Scholar]
- 63.Dustin LB, Plas DR, Wong J, et al. Expression of dominant-negative Src-homology domain 2-containing protein tyrosine kinase activity and B cell activation. J Immunol. 1999;162:2717. [PubMed] [Google Scholar]
- 64.Tridandapani S, Phee H, Shivakumar L, Kelley TW, Coggeshall KM. Role of SHIP in FcγRIIb-mediated inhibition of Ras activation in B cells. Mol Immunol. 1998;35:1135. doi: 10.1016/s0161-5890(98)00097-2. [DOI] [PubMed] [Google Scholar]
- 65.Koncz G, Pecht I, Gergely J, Sármay G. Fcγ receptor-mediated inhibition of human B cell activation: the role of SHP-2 phosphatase. Eur J Immunol. 1999;29:1980. doi: 10.1002/(SICI)1521-4141(199906)29:06<1980::AID-IMMU1980>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 66.Koncz G, Gergely J, Sármay G. FcγRIIb inhibits both B cell receptor- and CD19-induced Ca2+ mobilisation in FcγR-transfected human cells. Int Immunol. 1998;10:141. doi: 10.1093/intimm/10.2.141. [DOI] [PubMed] [Google Scholar]
- 67.Reynes M, Aubert JP, Cohen JH, et al. Human follicular dendritic cells express CR1, CR2 and CR3 complement receptor antigens. J Immunol. 1985;135:2687. [PubMed] [Google Scholar]
- 68.Yoshida K, Van Den Berg TK, Dijkstra CD. Two functionally different dendritic cells in secondary lymphoid follicles of mouse spleen, as revealed by CR1/2 and FcγRII-mediated immune complex trapping. Immunology. 1993;80:34. [PMC free article] [PubMed] [Google Scholar]
- 69.Vora KA, Ravetch JV, Manser T. Amplified follicular immune complex deposition in mice lacking the Fc receptor γ-chain does not alter maturation of the B cell response. J Immunol. 1997;159:2116. [PubMed] [Google Scholar]
- 70.Liu YJ, Joshua DE, Williams GT, Smith CA, Gordon J, MacLennan IC. Mechanism of antigen-driven selection in germinal centres. Nature. 1989;342:929. doi: 10.1038/342929a0. [DOI] [PubMed] [Google Scholar]
- 71.Lindhout E, Mevissen ML, Kwekkeboom J, Tager JM, De Groot C. Direct evidence that human follicular dendritic cells (FDC) rescue germinal centre B cells from death by apoptosis. Clin Exp Immunol. 1993;91:330. doi: 10.1111/j.1365-2249.1993.tb05904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nie X, Basu S, Cerny J. Immunization with immune complex alters the repertoire of antigen-reactive B cells in the germinal centres. Eur J Immunol. 1997;27:3517. doi: 10.1002/eji.1830271253. [DOI] [PubMed] [Google Scholar]
- 73.Apel M, Berek C. Somatic mutations in antibodies expressed by germinal centre B cells early after primary immunization. Int Immunol. 1990;2:813. doi: 10.1093/intimm/2.9.813. [DOI] [PubMed] [Google Scholar]
- 74.WUJ, Qin D, Burton GF, Szakal AK, Tew JG. Follicular dendritic cell-derived antigen and accessory activity in initiation of memory IgG responses in vitro. J Immunol. 1996;157:3404. [PubMed] [Google Scholar]
- 75.Fu YX, Molina H, Matsumoto M, Huang G, Min J, Chaplin DD. Lymphotoxin-α (LTα) supports development of splenic follicular structure that is required for IgG responses. J Exp Med. 1997;185:2111. doi: 10.1084/jem.185.12.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kozono Y, Duke RC, Schleicher MS, Holers VM. Co-ligation of mouse complement receptors 1 and 2 with surface IgM rescues splenic B cells and WEHI-231 cells from anti-surface IgM-induced apoptosis. Eur J Immunol. 1995;25:1013. doi: 10.1002/eji.1830250423. [DOI] [PubMed] [Google Scholar]
- 77.Fischer MB, Goerg S, Shen L, et al. Dependence of germinal centre B cells on expression of CD21/CD35 for survival. Science. 1998;280:582. doi: 10.1126/science.280.5363.582. [DOI] [PubMed] [Google Scholar]
- 78.Koopman G, Keehnen RM, Lindhout E, Zhou DF, De Groot C, Pals ST. Germinal centre B cells rescued from apoptosis by CD40 ligation or attachment to follicular dendritic cells, but not by engagement of surface immunoglobulin or adhesion receptors, become resistant to CD95-induced apoptosis. Eur J Immunol. 1997;27:1. doi: 10.1002/eji.1830270102. [DOI] [PubMed] [Google Scholar]
- 79.Maeda K, Burton GF, Padgett DA, et al. Murine follicular dendritic cells and low affinity receptors for IgE (FcεRII) J Immunol. 1992;148:2340. [PubMed] [Google Scholar]
- 80.Kerekes K, Prechl J, Bajtay Z, Mihály J, Erdei A. A further link between innate and adaptive immunity: C3 deposition on antigen-presenting cells enhances the proliferation of antigen-specific T cells. Int Immunol. 1998;10:1923. doi: 10.1093/intimm/10.12.1923. [DOI] [PubMed] [Google Scholar]
- 81.Marquart HV, Svehag S-E, Leslie RGQ. CR2 is the primary acceptor site for C3 during alternative pathway activation of complement on human peripheral B lymphocytes. J Immunol. 1994;153:307. [PubMed] [Google Scholar]
- 82.Yamakawa M, Imai Y. Complement activation in the follicular light zones of human lymphoid tissues. Immunology. 1992;76:378. [PMC free article] [PubMed] [Google Scholar]
- 83.Qin D, Wu J, Carroll MC, Burton GF, Szakal AK, Tew JG. Evidence for an important interaction between a complement-derived CD21 ligand on follicular dendritic cells and CD21 on B cells in the initiation of IgG responses. J Immunol. 1998;161:4549. [PubMed] [Google Scholar]
- 84.Erdei A, Bajtay Z, Fábry Z, Gergely J. Appearance of acceptor-bound C3b on HLA-DR positive macrophages and on stimulated U937 cells; inhibition of Fcγ-receptors by the covalently fixed C3 fragments. Mol Immunol. 1988;25:295. doi: 10.1016/0161-5890(88)90021-1. [DOI] [PubMed] [Google Scholar]
- 85.Maison CM, Villiers CL, Colomb MG. Secretion, cleavage and binding of complement component C3 by the human monocytic cell line, U937. Biochemistry. 1989;261:407. doi: 10.1042/bj2610407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mold C, Nemerow GR, Bradt BM, Cooper NR. CR2 is a complement activator and the covalent binding site for C3 during alternative pathway activation by Raji cells. J Immunol. 1988;140:1923. [PubMed] [Google Scholar]
- 87.Olesen EH, Johnson AA, Damgaard G, Leslie RGQ. The requirement of localised, CR2-mediated, alternative pathway (AP) activation of complement for covalent deposition of C3 fragments on normal B cells. Immunology. 1998;93:177. doi: 10.1046/j.1365-2567.1998.00429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schwendinger MG, Spruth M, Schoch J, Dierich MP, Prodinger WM. A novel mechanism of alternative pathway complement activation accounts for the deposition of C3-fragments on CR2-expressing homologous cells. J Immunol. 1997;158:5455. [PubMed] [Google Scholar]
- 89.Leslie RGQ. The influence of complement receptor type 1 (CD35) and decay-accelerating factor (CD55) on complement receptor type 2-(CD21-) mediated alternative pathway activation by B cells. Immunology. 1999;97:371. doi: 10.1046/j.1365-2567.1999.00846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fischer E, Delibrias C, Kazatchkine MD. Expression of CR2 (the C3dg/EBV receptor, CD21) on normal human peripheral blood T lymphocytes. J Immunol. 1991;146:865. [PubMed] [Google Scholar]
- 91.Cutler AJ, Botto M, Van Essen D, et al. T cell-dependent immune response in C1q-deficient mice: defective interferon γ production by antigen-specific T cells. J Exp Med. 1998;187:1789. doi: 10.1084/jem.187.11.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Snapper CM, Paul WE. Interferon-γ and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 93.Snapper CM, McIntyre TM, Mandler R, et al. Induction of IgG3 secretion by interferon γ: a model for T cell-independent class switching in response to T cell-independent type 2 antigens. J Exp Med. 1992;175:1367. doi: 10.1084/jem.175.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Walport MJ, Davies KA, Morley BJ, Botto M. Complement deficiency and autoimmunity. Ann N Y Acad Sci. 1997;815:267. doi: 10.1111/j.1749-6632.1997.tb52069.x. [DOI] [PubMed] [Google Scholar]
- 95.Frank MM. Animal models for complement deficiencies. J Clin Immunol. 1995;15:113S. doi: 10.1007/BF01540901. [DOI] [PubMed] [Google Scholar]
- 96.Winkelstein JA, Cork LC, Griffin DE, Griffin JW, Adams RJ, Price DL. Genetically determined deficiency of the third component of complement in the dog. Science. 1981;212:1169. doi: 10.1126/science.7233211. [DOI] [PubMed] [Google Scholar]
- 97.Korb LC, Ahearn JM. C1q binds directly and specifically to surface blebs of apoptotic human keratinocytes: complement deficiency and systemic lupus erythematosus revisited. J Immunol. 1997;158:4525. [PubMed] [Google Scholar]
- 98.Rosen A, Casciola-rosen L, Ahearn J. Novel packages of viral self-antigens are generated during apoptosis. J Exp Med. 1995;181:1157. doi: 10.1084/jem.181.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Walport MJ, Davies KA, Botto M. C1q and systemic lupus erythematosus. Immunobiology. 1998;199:265. doi: 10.1016/S0171-2985(98)80032-6. [DOI] [PubMed] [Google Scholar]
- 100.Marquart HV, Svendsen A, Rasmussen JM, et al. Complement receptor expression and activation of the complement cascade on B lymphocytes from patients with systemic lupus erythematosus (SLE) Clin Exp Immunol. 1995;101:60. doi: 10.1111/j.1365-2249.1995.tb02277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Takahashi K, Kozono Y, Waldschmidt T, et al. Mouse complement receptors type 1 (CR1; CD35) and type 2 (CR2; CD21): expression on normal B subpopulations and decreased levels during the development of autoimmunity in MRL/lpr mice. J Immunol. 1997;159:1557. [PubMed] [Google Scholar]
- 102.Murakami M, Honjo T. B-1 cells and autoimmunity. Ann N Y Acad Sci. 1995;764:402. [PubMed] [Google Scholar]
- 103.Engel P, Zhou LJ, Ord DC, Sato S, Koller B, Tedder TF. Abnormal B lymphocyte development, activation, and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity. 1995;3:39. doi: 10.1016/1074-7613(95)90157-4. [DOI] [PubMed] [Google Scholar]
- 104.Sato S, Ono N, Steeber DA, Pisetsky DS, Tedder TF. CD19 regulates B lymphocyte signalling thresholds critical for the development of B-1 lineage cells and autoimmunity. J Immunol. 1996;157:4371. [PubMed] [Google Scholar]
- 105.Carroll MC, Prodeus AP. Linkages of innate and adaptive immunity. Curr Opin Immunol. 1998;10:36. doi: 10.1016/s0952-7915(98)80028-9. [DOI] [PubMed] [Google Scholar]
- 106.Inaoki M, Sato B, Weintraub BC, Goodnow CC, Tedder TF. CD19-regulated signalling thresholds control peripheral tolerance and autoantibody production in B lymphocytes. J Exp Med. 1997;186:1923. doi: 10.1084/jem.186.11.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Prodeus AP, Georg S, Shen LM, et al. A critical role for complement in maintenance of self-tolerance. Immunity. 1998;9:721. doi: 10.1016/s1074-7613(00)80669-x. [DOI] [PubMed] [Google Scholar]
- 108.Weis JJ, Tedder TF, Fearon DT. Identification of a 145 000 Mr protein as the C3d receptor (CR2) of human B lymphocytes. Proc Natl Acad Sci USA. 1984;81:881. doi: 10.1073/pnas.81.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]