Abstract
To investigate the role of the CD40–CD154 interaction in rheumatoid arthritis (RA), we analysed the expression of CD154 on CD3+ and CD4+ T cells in synovial fluid (SF) from patients with RA and in peripheral blood (PB) from patients and normal controls. As interleukin (IL)-15 is a potent activator of synovial T cells we wanted to study whether IL-15 also regulated the expression of CD154 on these T cells. Freshly isolated synovial T cells did not express significant levels of CD154, as evaluated using flow cytometry, whereas the expression of CD86 and human leucocyte antigen (HLA)-DR was significantly elevated on SF T cells when compared with PB T cells from patients or controls. Synovial T cells could up-regulate their CD154 expression following activation with phorbol 12-myristate 13-acetate (PMA)+ionomycin or anti-CD3+ anti-CD28 monoclonal antibodies (mAbs), but the maximal level of expression remained lower than in control T cells. IL-15 significantly increased the expression of CD154 on SF and PB T cells from patients, whereas IL-2 had minimal effects. Furthermore, IL-15 induced extensive proliferation in SF T cells. Our results show that SF T cells up-regulate the expression of CD154 in the presence of IL-15, a cytokine present in the synovium of patients with RA. These results further emphasize the role of IL-15 in the pathogenesis of RA.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by accumulation of T cells, plasma cells, macrophages (Mφ) and dendritic cells (DC) in the synovium, leading to the destruction of cartilage and bone. It has been suggested that T cells are crucially involved in the onset and perpetuation of this inflammation.1 The majority of synovial T cells from patients with RA are CD4+ and they express CD45RO, a marker for activated and memory T cells.2, 3 They also express elevated levels of activation markers such as human leucocyte antigen (HLA)-DR, CD69 and SLAM (CDw150).2, 4 However, synovial T cells express low levels of CD25 and fail to proliferate properly and to produce interleukin (IL)-2 in response to mitogens and recall antigens.1, 2 Activation pathways through T-cell receptor (TCR)/CD3 seem to be defective in these cells.5 Also, the costimulatory pathway through CD28 has been suggested to be hyporesponsive.6
Induction and maintenance of T-cell activation is regulated by a number of soluble and surface-bound molecules. CD154 is the ligand for CD40 (CD40 ligand) and it is primarily expressed on activated CD4+ T cells.7 Studies in CD154 and CD40 knockout mice have revealed the essential role of this ligand–receptor pair on proliferation of B cells, formation of germinal centres and memory B cells, and isotype switching.7 Similar observations were made earlier in patients with hyperimmunoglobulin M (hyper-IgM) syndrome, deficient for CD154 expression. The CD154–CD40 interaction also plays an important role in the activation and differentiation of antigen-presenting cells (APC). Ligation of CD40 on Mφ and monocytes leads to the expression of cytokines, such as IL-1α, IL-6, tumour necrosis factor-α (TNF-α) and IL-10, by these cells.7 Immature DC mature in response to CD40 stimulation and start to produce high levels of IL-12, which is crucial for the induction of an inflammatory, T helper 1 (Th1) type response.8 High expression of CD154 on CD4+ T cells is observed only transiently after stimulation with mitogens and antigens in vitro. In contact with B cells, CD154 is rapidly internalized, decreasing further stimulation via CD40 ligation.9 On the other hand, primed CD4+ T cells contain preformed CD154 that can rapidly be exposed on the cell surface.10 Recently, CD4+ T cells from synovium of patients with RA were shown to express low but enhanced levels of functional CD154 when compared with peripheral blood (PB) T cells from patients and normal controls.11 Moreover, another recent study shows that low levels of CD154 are expressed on CD4+ and CD8+ cells in the lymphoid follicles of synovial tissue (ST).12
IL-15 is a member of the four α-helix bundle cytokine family and shares functional similarities with IL-2, including the induction of proliferation of activated CD4+ and CD8+ T cells and natural killer (NK) cells.13 Furthermore, IL-15 induces proliferation and immunoglobulin synthesis of B cells activated by CD40 ligand, and it also activates mast cells. The receptor for IL-15 in T and NK cells utilizes the IL-2 receptor β-chain as well as the common γ-chain, but has its own α-chain. In contrast to IL-2, IL-15 is produced by various cell types, such as Mφ, DC, fibroblasts and endothelial cells, but is not produced by T cells.13, 14 Recently, IL-15 was reported to be expressed at high concentrations in synovial fluid (SF) and ST from patients with RA.15, 16 IL-15 was found to contribute to the chemoattractant activity of SF and to play a role in the accumulation of CD4+ T cells in the synovium.14, 16 Furthermore, it has been proposed that the increased expression of CD69 on synovial T cells is mediated by IL-15, 17 further suggesting that IL-15 is an important regulator of the inflammation in RA.
To evaluate the capacity of synovial T cells to activate CD40+ APC in the synovium, 7, 18, 19 the expression of CD154 on synovial T cells, its kinetics in response to polyclonal activation and its regulation by IL-15 were investigated. SF T cells expressed diminished levels of CD154 in response to stimulation with phorbol 12-myristate 13-acetate (PMA) + ionomycin and anti-CD3 + anti-CD28 monoclonal antibodies (mAbs) when compared with normal PB T cells, possibly owing to the activation status and impaired activation of SF T cells through the TCR/CD3. Interestingly, exogenous IL-15 increased the expression of CD154, both in SF and PB T cells from patients, whereas exogenous IL-2 had only a slight effect. In conclusion, the results of this work suggest that SF T cells up-regulate the expression of CD154 in the presence of IL-15. These data favour the conclusion that IL-15 probably contributes to the chronic inflammatory process in patients with RA.
MATERIALS AND METHODS
Patients
Twenty-six patients with RA were enrolled in this study. PB and SF samples were taken concurrently from the patients. SF samples from the inflamed knee joints were collected by needle aspiration into heparinized tubes. PB samples from healthy volunteers were used as controls. Tonsillar tissue was obtained at tonsillectomy from otherwise symptomless patients suffering from recurrent tonsillitis.
RA was determined according to the criteria of the American College of Rheumatology (formerly the American Rheumatism Association).20 Median age of the patients was 57 years (range 28–89 years) and median duration of disease was 11 years (range 0·6–31 years). Seventeen of the patients were treated with disease-modifying antirheumatic drugs, 17 with corticosteroids and 17 were receiving non-steroidal anti-inflammatory drugs. This study was approved by the ethical committee of Turku University Central Hospital.
Reagents
Fluorescein isothiocyanate (FITC)-conjugated anti-human CD3, CD4 and non-specific mouse immunoglobulin G (IgG); phycoerythrin (PE)-conjugated anti-human CD3, CD4, CD80, HLA-DR and non-specific mouse IgG; and peridinin chlorophyll protein (PerCP)-conjugated anti-human CD4 and non-specific mouse IgG were all purchased from Becton-Dickinson (San Jose, CA). FITC-conjugated anti-human CD40; PE-conjugated anti-human CD86, CD154 and CD45RO; and non-conjugated anti-human CD28 were obtained from PharMingen (San Diego, CA). Non-conjugated anti-human CD3 was from Becton-Dickinson. Ionomycin and PMA were purchased from Sigma (St. Louis, MO). Purified recombinant human (rh) IL-15 was obtained from R & D Systems (Minneapolis, MN), IL-2 was kindly provided by Dr G. Zurawski (DNAX Research Institute, Palo Alto, CA) and IL-10 was obtained from Shering-Plough Research Institute (Kenilworth, NJ).
Cell preparations
Some SF samples were treated with hyaluronidase (10 µg/ml) for 15 min at 37°. Tonsillar tissue was first cut into pieces in medium on Petri dishes on ice, then minced through a stainless steel mesh. Synovial fluid mononuclear cells (SFMC), peripheral blood mononuclear cells (PBMC) and tonsillar mononuclear cells were isolated by using Ficoll–Paque (Pharmacia, Uppsala, Sweden) density-gradient centrifugation, washed twice with Hanks’ buffered solution (HBS) and resuspended in Iscove’s modified Dulbecco’s medium (IMDM) (Gibco BRL, Grand Island, NY) supplemented with 10% heat-inactivated fetal calf serum (FCS; HyClone Laboratories, Logan, UT), 0·1 mm 2-mercaptoethanol (Sigma), 10 mm HEPES (Gibco BRL) and 100 µg/ml of gentamycin (Biological Industries, Kibbutz Beit Haemek, Israel). In five experiments studying the kinetics of the surface molecule expression on SF T cells, the non-adherent cells were isolated by incubating the cells on tissue culture Petri dishes (Falcon 1001; Becton-Dickinson Labware, Lincoln Park, NJ) for 1 hr at 37°. The results were the same when either the total or non-adherent cells were used.
Activation assays
SFMC and PBMC (at a concentration of 1·0 × 106/ml) were cultured in IMDM + 10% FCS with PMA (1 ng/ml) + ionomycin (0·5 µg/ml), or with anti-human CD3 (1 µg/ml) + anti-human CD28 (10 µg/ml) mAbs, in 24-well plates (Costar, Cambridge, MA). The cells were harvested after 6, 18, 48 and 72 hr of culture at 37° in a humidified atmosphere containing 5% CO2. To study the up-regulation of surface antigens on tonsillar T cells, tonsillar mononuclear cells were cultured for 6 hr with PMA (1 ng/ml) + ionomycin (0·5 µg/ml). When studying the effects of cytokines on surface marker expression, 1·0 × 106/ml SFMC and PBMC were cultured in IMDM + 10% FCS with IL-15 (50 ng/ml), IL-2 (100 U/ml), IL-10 (100 U/ml) or medium only, and harvested after 72 hr. To study cell proliferation in response to cytokines, 0·1 × 106 cells were cultured in triplicate in 200 µl of IMDM + 10% FCS in flat-bottom 96-well microtitre plates (Costar) for 4 days. IL-2 and IL-15 were added at the start of culture. [3H]Thymidine (1·0 µCi/well; Du Pont, Boston, MA) was added for the final 18 hr of culture, and the plates were harvested and analysed by scintillation counting (Wallac, Turku, Finland).
Immunofluorescence analysis
SFMC and PBMC were stained before and after four different time-periods of culture. The freshly isolated SFMC and PBMC or harvested cells were washed twice with phosphate-buffered saline (PBS) supplemented with 2% FCS and incubated with saturating concentrations of FITC- or PE-conjugated mAbs for 30 min at 4°, which was followed by two further washes. The expression of surface molecules was studied on gated CD4+ or CD3+ cells. CD3 gate was used after PMA + ionomycin stimulation because the expression of CD4 was strongly down-regulated. Propidium iodide (Sigma)-staining was used to exclude the dead cells. Washed tonsillar mononuclear cells were stained with FITC-, PE- or PerCP-conjugated mAbs after 6 hr of culture. The expression of surface molecules on gated CD3+ CD45RO+ and CD3+ CD45RO– populations was examined. Labelled cells were analysed using a fluorescence-activated cell sorter (FACScan flow cytometer) and CellQuest software (both from Becton-Dickinson). Cells with light scatter characteristics of lymphocytes were gated.
Statistical analysis
Statistical analysis was performed using the Wilcoxon signed-rank test for paired samples and the Mann–Whitney U-test for unpaired data.
RESULTS
The expression of CD154 on freshly isolated SF T cells
The expression of CD154 was studied on CD3+ and CD4+ SF and PB lymphocytes derived from patients with RA or from healthy volunteers. The results presented in Table 1 indicate that neither SF CD3+ cells nor PB CD3+ cells from patients or controls expressed detectable levels of CD154. Similarly, no significant expression of CD154 was detected on SF and PB CD4+ T cells from patients. The phenotype of SF T cells was further investigated by analysing the expression of CD40, CD80, CD86 and HLA-DR on these cells. These markers are expressed on activated human T cells.7, 21 As shown in Table 1, CD3+ and CD4+ SF T cells expressed significantly higher levels of HLA-DR than control PB cells. The expression of CD40 on T cells was minimal in all samples, irrespective of the source of the cells. Interestingly, the frequency of CD86+ cells was significantly increased in SF T cells, whereas the frequency of CD80+ cells was only moderately higher in SF T cells when compared with PB cells from patients and normal controls.
Table 1.
The expression of surface molecules on CD3+ or CD4+ cells from synovial fluid (SF) and peripheral blood (PB) of patients with rheumatoid arthritis (RA) and in PB from normal controls
| %CD3+ | %CD4+ | |||||
|---|---|---|---|---|---|---|
| RA SF | RA PB | Control PB | RA SF | RA PB | Control PB | |
| CD154 | 0.7±0.4 | 0.2±0.1 | 0.6±0.5 | 1.6±1.5 | 1.7±0.5 | 3.9±1.4 |
| CD40 | 0.3±0.1 | 0.6±0.2 | 0.8±0.2 | 0.9±0.7† | 0.8±0.5† | 0.3±0.1† |
| CD80 | 5.7±2.4 | 0.5±0.2 | 0.5±0.2 | 5.9±2.6 | 1.4±0.4 | 3.2±2.0 |
| CD86 | 16.4±3.0*** | 2.2±0.6 | 1.3±0.2 | 17.0±3.0** | 3.5±0.7 | 1.3±0.4 |
| HLA-DR | 87.9±3.9*** | 24.7±4.8 | 16.6±3.3 | 78.1±13.3** | 18.9±6.4 | 15.4±2.4 |
*Results are expressed as frequencies (mean %±SEM) of positive cells. Nine samples were studied in CD3+ cells and seven in CD4+ cells.
n = 5.
P<0.01
P<0.05, when compared with RA PB and control PB cells.
The kinetics of CD154 expression on activated SF T cells
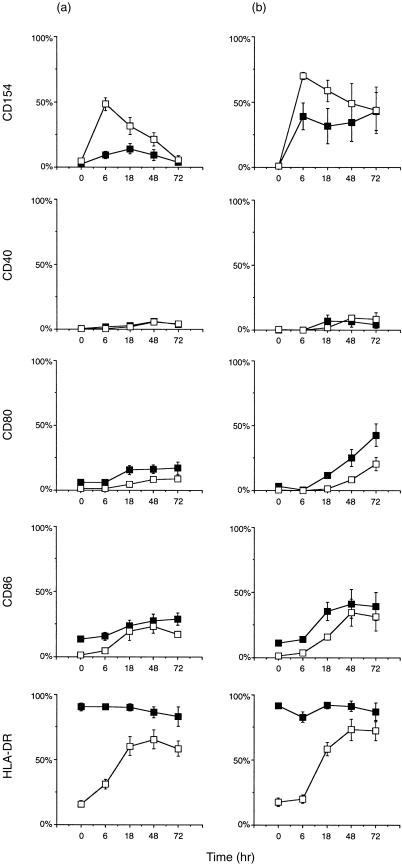
We then studied the kinetics of CD154 expression in response to in vitro polyclonal T-cell activation. The combination of PMA + ionomycin has been shown to induce maximal expression of CD154 on normal PB T cells after 6 hr.22 SFMC and control PBMC were cultured with PMA + ionomycin or with anti-CD3 + anti-CD28 mAbs for 6, 18, 48 or 72 hr and the expression of CD154, CD40, CD80, CD86 and HLA-DR was studied on gated CD3+ or CD4+ cells. As shown in Fig. 1, synovial T cells were able to up-regulate CD154 expression in response to stimulation with PMA + ionomycin and anti-CD3 + anti-CD28 mAbs. The expression of CD154 was lower on SF T cells than on control PB T cells at 6 hr of stimulation with anti-CD3 + anti-CD28 mAbs (P = 0·002). The difference in the expression levels of CD154 on SF and PB T cells stimulated with PMA + ionomycin for 6 hr did not reach statistical significance. At other time-points the expression of CD154 was generally lower in samples from patients when stimulated with anti-CD3 + anti-CD28 mAbs, but no clear differences were observed upon stimulation with PMA + ionomycin. The expression of CD154 peaked at 6 hr on both the SF and control PB T cells in response to PMA + ionomycin. In response to anti-CD3 + anti-CD28 mAbs the peak expression was generally observed later, at 18 hr on SF T cells and at 6 hr on control PB T cells. Furthermore, the expression of CD154 was poorly induced on SF T cells in response to stimulation with anti-CD3 + anti-CD28 mAbs. The expression of CD40, CD80, CD86 and HLA-DR could be up-regulated or induced on both SF and normal T cells by PMA + ionomycin and anti-CD3 + anti-CD28 mAbs. The expression of HLA-DR peaked at 48 hr in control PB T cells but did not reach the level of expression of SF T cells. The expression of CD40 could be weakly induced on both SF T cells and control T cells. Furthermore, the expression of CD80 and CD86 was up-regulated more rapidly and to a significantly higher level on SF T cells.
Figure 1.
(a) CD4+ cells cultured with anti-CD3 + anti-CD28 monoclonal antibodies (mAbs) for 0, 6, 18, 48 or 72 hr. Six samples were studied (except for five at 18 hr). (b) CD3+ cells cultured with phorbol 12-myristate 13-acetate (PMA) + ionomycin. Five samples were studied (except for the expression of CD40, which was studied in four samples). (▪), synovial fluid mononuclear cells (SFMC) from patients with rheumatoid arthritis (RA); (□), peripheral blood mononuclear cells (PBMC) from normal controls. Results are expressed as frequencies (mean percentage ± SEM) of positive cells. HLA, human leucocyte antigen.
The expression of CD154 on activated memory and naive tonsillar T cells
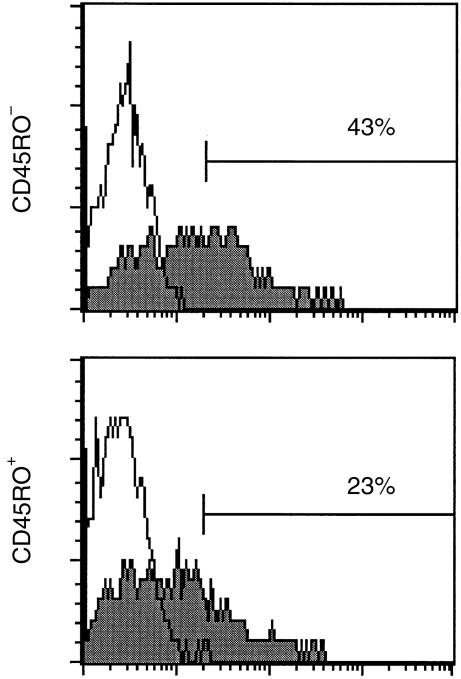
To study whether diminished induction of CD154 in SF T cells was generally related to the memory or activated phenotype of these cells, tonsillar mononuclear cells were cultured with PMA + ionomycin for 6 hr and the expression of CD154 on gated CD3+ CD45 RO+ and CD3+ CD45 RO– T cells was compared. Tonsillar mononuclear cells contain a higher percentage of CD45RO+ cells than PBMC. Also, the majority of SF T cells express CD45RO.3 As illustrated in Fig. 2, the expression of CD154 was clearly lower on CD45RO+ cells (29 ± 7%; mean ± SEM, n = 4) than on CD45RO– cells (45 ± 8%) in all the samples studied, suggesting that the reduced up-regulation of CD154 on SF T cells may be associated with the activation state of these cells. Moreover, these results suggest that tonsillar T cells are a more appropriate control than resting PB T cells for studies on SF T cells.
Figure 2.
Representative histograms illustrate the expression of CD154 on CD3+ CD45RO– and CD3+ CD45RO+ tonsillar mononuclear cells activated with phorbol 12-myristate 13-acetate (PMA) + ionomycin for 6 hr. Filled histograms show the staining with anti-CD154 monoclonal antibody (mAb) and open histograms the staining with non-specific control antibody. Four independent experiments were carried out.
The effect of IL-15 on CD154 expression on SF T cells
Although synovial T cells are deficient in responding to mitogens, they seem to proliferate well in the presence of exogenous IL-15.16, 17 Therefore, we next addressed the question of whether IL-15 is able to modulate the expression of CD154 on synovial T cells. As shown in Table 2, IL-15 significantly up-regulated the expression of CD154 on SF, as well as on PB, T cells from patients with RA. This up-regulation was clearly observed in all the samples studied. Moreover, IL-15 increased the expression of CD154 in two out of four normal control PB samples. IL-2 increased the expression of CD154 on these cells only slightly. IL-15 also significantly increased the expression of CD86 and HLA-DR on SF and control PB T cells, whereas IL-2 had a significant augmenting effect only on the CD86 expression of SF T cells. Results were similar with a 10-fold higher concentration of IL-2 (data not shown). IL-15 had a stronger proliferative effect than IL-2 on both synovial fluid (13 600 ± 5600 counts per minute [c.p.m.] versus 2700 ± 1700 c.p.m., mean ± SEM of three individual experiments) and control cells (4400 ± 2600 c.p.m. versus 1100 ± 800 c.p.m.), which is in line with a previous study.17
Table 2.
Synovial fluid mononuclear cells (SFMC) and peripheral blood mononuclear cells (PBMC) from normal controls were cultured with interleukin (IL)-2, IL-15 or in medium alone for 72 hr. The expression of surface molecules on CD4+ lymphocytes is shown*
| Medium | IL-15 | IL-2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RA SF | RA PB | Control PB | RA SF | RA PB | Control PB | RA SF | RA PB | Control PB | ||
| CD154 | % | 1.4±0.5 | 2.4±0.5 | 4.3±1.7 | 13.7±5.5*** | 6.2±1.0** | 6.7±1.6 | 3.5±2.2 | 2.8±0.6 | 1.5±0.7 |
| MFIR | 1.3±0.1 | 1.5±0.1 | 1.5±0.1 | 2.4±0.8*** | 1.5±0.1 | 1.6±0.2 | 1.3±0.1 | 1.6±0.1 | 1.3±0.1 | |
| CD86 | % | 19.4±6.7 | 4.0±0.9 | 3.1±0.7 | 43.0±9.4*** | 5.6±1.8 | 7.8±0.8** | 30.5±7.2*** | 5.6±1.3 | 3.7±0.9 |
| MFIR | 2.0±0.5 | 1.3±0.1 | 1.2±0.0 | 5.5±2.0*** | 1.3±0.1 | 1.4±0.0** | 3.4±1.0*** | 1.3±0.1 | 1.1±0.1 | |
| HLA-DR | % | 86.6±2.9† | 28.3±9.0 | 27.2±5.7† | 95.6±1.1†** | 36.1±4.0 | 46.4±3.7†** | 86.5±3.7† | 32.7±7.8 | 30.8±5.8† |
| MFIR | 27.2±8.4† | 7.9±4.6 | 2.6±0.4† | 155.4±18.4†** | 3.5±0.7 | 7.2±1.2†** | 45.2±6.5† | 4.2±1.1 | 3.4±0.7† | |
Results are presented as frequencies of positive cells and as mean fluorescence intensity ratios (MFIR) (MFI of the anti-cell surface monoclonal antibody: MFI of the negative control) (mean±SEM) from six SFMC samples from patients with PBMC samples from normal controls, and five PBMC samples from patients.
n = 5.
P<0.03
P<0.05, as compared with cells cultured in medium only.
Previous studies have shown that a substantial amount of IL-15 is produced in rheumatoid synovium. Considering this, there seems to be a discrepancy between the lack of constitutive CD154 expression and the up-regulation of CD154 by IL-15 on SF T cells. We therefore wanted to study whether IL-10 would block the effects of IL-15 on SF T cells because IL-10 has several anti-inflammatory functions on both APC and T cells in synovial inflammation. In two separate experiments, IL-10 was not observed to modulate the expression of CD154 on SF T cells when added to the culture together with IL-15 (Fig. 3). Nor was the up-regulation of CD86 or HLA-DR inhibited by IL-10 (data not shown).
Figure 3.
Representative histograms illustrate the expression of CD154 on CD4+ synovial fluid mononuclear cells (SFMC), from patients with rheumatoid arthritis (RA), cultured in the presence of interleukin (IL)-15, IL-15 + IL-10, IL-2, IL-2 + IL-10, or in medium alone, for 72 hr. Filled histograms show the staining with anti-CD154 monoclonal antibody (mAb) and open histograms the staining with non-specific control antibody. Two independent experiments were carried out.
DISCUSSION
IL-15, but not IL-2, clearly up-regulated CD154 expression on SF T cells. Recent studies indicating that IL-15 is produced in rheumatoid synovium and that IL-15 has a potent growth-promoting activity on synovial T cells16, 17 suggest that IL-15 plays an important role in synovial inflammation. The effect of IL-15 on CD154 expression on SF T cells further supports that IL-15 is an important regulator in synovial inflammation. Memory or preactivated phenotypes of SF T cells may explain their increased responsiveness to IL-15.23 It is probable that SF T cells express increased levels of receptors for IL-15 when compared with resting PB T cells. The IL-15 receptor α-chain is generally increased on activated or memory T cells.13 By up-regulating CD154 expression, IL-15 can regulate APC function in the synovium. Synovial T cells in RA are biased towards the Th1 phenotype.24–27 Furthermore, IL-12 has been shown to be produced in the synovium from patients with RA, 28 indicating that APC in the rheumatoid synovium are actively skewing the Th1 response. Collagen-induced arthritis in mice can be prevented by blocking the CD40–CD154 interaction29 and prevention of IL-12 production by this mechanism may be involved in attenuation of the disease. We propose the interesting possibility that IL-15 may be involved in skewing the Th1 response by increasing CD154 expression, and thus IL-12 production, through increased CD40 triggering on DC, but this remains to be shown. Furthermore, the exact mechanism by which IL-15 influences CD154 expression, whether it is by inducing de novo protein synthesis or translocation of preformed cytosolic molecules, remains unconfirmed. There seems to be a contradiction between lack of constitutive CD154 expression and IL-15 production in the synovium. However, it is possible that CD154 expression is down-regulated as a consequence of active contact with CD40+ cells in the synovium.9 Another possibility is that CD154 is expressed on ST T cells but not on SF T cells.
Freshly isolated SF T cells did not express significant levels of CD154, whereas the expression of HLA-DR and CD86 was up-regulated on these cells, when compared with PB T cells from patients and normal controls. The up-regulation of CD154 was diminished on SF T cells in response to polyclonal activation in vitro, reflecting the general unresponsiveness of synovial T cells to mitogenic stimulation and defects in signal transduction. There is a defect proximally in the signal transduction pathway via the TCR/CD3 complex; the phosphorylation of p38 is defective and tyrosine phosphorylation of the CD3 ζ-chain is decreased in SF T cells from patients with RA.30 The differences between up-regulation of CD154 on normal and synovial T cells was best observed when cells were stimulated with anti-CD3 + anti-CD28 mAbs, which simulates physiological T-cell activation more than PMA + ionomycin stimulation. When PMA + ionomycin was used the difference was not as obvious, suggesting that PMA + ionomycin can partially bypass a block in the signal transduction pathway. Furthermore, we also showed that activated tonsillar CD3+ CD45RO+ cells expressed lower levels of CD154 than CD3+ CD45RO– cells, suggesting that decreased expression of CD154 is a result of the memory phenotype of SF T cells. It has been reported previously that the up-regulation of CD154 is impaired in anergic T cells, which are defective in their capacity to stimulate B cells.31 Synovial T cells from patients with RA have characteristics of anergic T cells:32 they do not proliferate properly and seem to produce little IL-2 and other cytokines after activation.1 Diminished induction of CD154 also reflects the anergic status of SF T cells. Anergy of CD4+ T cells can be induced by stimulation via the TCR/CD3 complex in the absence of a proper costimulus. However, APC in the synovium have been shown to express enhanced levels of costimulatory molecules, as well as HLA-DR, and to be potent stimulators of T cells.19, 33 One possible mechanism mediating the unresponsiveness of SF T cells is the high levels of production of IL-10 in synovium.34, 35 IL-10 not only inhibits the cytokine production and expression of costimulatory molecules by APC but can also suppress the proliferation and cytokine production of T cells.36 Furthermore, IL-10 induces directly an anergic state in human CD4+ T cells.37 In chronic stimulation, IL-10 can induce the differentiation of CD4+ T-cell clones, characterized by production of high levels of IL-10 and low levels of IL-2, and poor proliferation.37
The enhanced expression of HLA-DR on synovial T cells is well established, whereas the expression of CD40, CD80 and CD86 has been examined in less detail. There are a few reports on the increased expression of CD80 on synovial T cells from RA.18, 38 It has been shown that synovial T cells can activate allogeneic T cells and that addition of anti-CD80 mAbs decreases their alloresponse.38 It is possible that the increased expression of these molecules primarily reflects the activation state of T cells, and the molecules may not in fact interact with their ligands. There is evidence that CD86 is expressed in a non-glycosylated, non-functional form on T cells.39 However, a recent report shows that the expression of CD86 is restricted to human CD45RO+ memory T cells, and that this CD86 costimulates the responses of naive T cells.40 Present results suggest that CD86 plays a more important role than CD80 in synovial inflammation. Our previous results on synovial Mφ also favour this suggestion.19
Acknowledgments
We thank Marianne Laine for expert technical assistance. Dr Riitta Saario and Dr Timo Möttönen are acknowledged for providing samples from patients. We also thank Dr Janne Komi for critical evaluation of the manuscript. These studies were supported by the Turku Graduate School of Biomedical Sciences, the Academy of Finland and special funds for the Turku University Central Hospital.
REFERENCES
- 1.Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 2.Cush JJ, Lipsky PE. Phenotypic analysis of synovial tissue and peripheral blood lymphocytes isolated from patients with rheumatoid arthritis. Arthritis Rheum. 1988;31:1230. doi: 10.1002/art.1780311003. [DOI] [PubMed] [Google Scholar]
- 3.Thomas R, McIlraith M, Davis LS, Lipsky PE. Rheumatoid synovium is enriched in CD45RBdim mature memory T cells that are potent helpers for B cell differentiation. Arthritis Rheum. 1992;35:1455. doi: 10.1002/art.1780351209. [DOI] [PubMed] [Google Scholar]
- 4.Isomäki P, Aversa G, Cocks BG, et al. Increased expression of signaling lymphocytic activation molecule in patients with rheumatoid arthritis and its role in the regulation of cytokine production in rheumatoid synovium. J Immunol. 1997;159:2986. [PubMed] [Google Scholar]
- 5.Maurice MM, van der Voort EAM, Leow A, Levarht N, Breedveld FC, Verweij CL. CD28 co-stimulation is intact and contributes to prolonged ex vivo survival of hyporesponsive synovial fluid T cells in rheumatoid arthritis. Eur J Immunol. 1998;28:1554. doi: 10.1002/(SICI)1521-4141(199805)28:05<1554::AID-IMMU1554>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 6.Hernández-García C, Fernández-Gutiérrez B, Morado IC, Banares A, Jover JA. The CD69 activation pathway in rheumatoid arthritis synovial fluid T cells. Arthritis Rheum. 1996;39:1277. doi: 10.1002/art.1780390803. [DOI] [PubMed] [Google Scholar]
- 7.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 8.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T–T help via APC activation. J Exp Med. 1996;184:747. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yellin MJ, Sippel K, Inghirami G, et al. CD40 molecules induce down-modulation and endocytosis of T cell surface T cell–B cell activating molecule/CD40-L. Potential role in regulating helper effector function. J Immunol. 1994;152:598. [PubMed] [Google Scholar]
- 10.Casamayor-Palleja M, Khan M, MacLennan ICM. A subset of CD4+ memory T cells contains preformed CD40 ligand that is rapidly but transiently expressed on their surface after activation through the T cell receptor complex. J Exp Med. 1995;181:1293. doi: 10.1084/jem.181.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacDonald KPA, Nishioka Y, Lipsky PE, Thomas R. Functional CD40 ligand is expressed by T cells in rheumatoid arthritis. J Clin Invest. 1997;100:2404. doi: 10.1172/JCI119781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner UG, Kurtin PJ, Wahner A, et al. The role of CD8+ CD40L+ T cells in the formation of germinal centers in rheumatoid synovitis. J Immunol. 1998;161:6390. [PubMed] [Google Scholar]
- 13.Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol. 1999;17:19. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 14.Oppenheimer-Marks N, Brezinschek RI, Mohamadzadeh M, Vita R, Lipsky PE. Interleukin-15 is produced by endothelial cells and increases the transendothelial migration of T cells in vitro and in the SCID mouse–human rheumatoid arthritis model in vitro. J Clin Invest. 1998;101:1261. doi: 10.1172/JCI1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thurkow EW, van der Heijden IM, Breedveld FC, et al. Increased expression of IL-15 in the synovium of patients with rheumatoid arhritis compared with patients with yersinia-induced arhritis and osteoarthritis. J Pathol. 1997;181:444. doi: 10.1002/(SICI)1096-9896(199704)181:4<444::AID-PATH778>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 16.McInnes IB, Al-Mughales J, Field M, et al. The role of interleukin-15 in T cell migration and activation in rheumatoid arthritis. Nat Med. 1996;2:175. doi: 10.1038/nm0296-175. [DOI] [PubMed] [Google Scholar]
- 17.McInnes IB, Leung BP, Sturrock RD, Field M, Liew FY. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-α production in rheumatoid arthritis. Nat Med. 1997;3:189. doi: 10.1038/nm0297-189. [DOI] [PubMed] [Google Scholar]
- 18.Ranheim EA, Kipps TJ. Elevated expression of CD80 (B7/BB1) and other accessory molecules on synovial fluid mononuclear cell subsets in rheumatoid arthritis. Arthritis Rheum. 1994;37:1637. doi: 10.1002/art.1780371113. [DOI] [PubMed] [Google Scholar]
- 19.Möttönen M, Isomäki P, Saario R, Toivanen P, Punnonen J, Lassila O. Interleukin-10 inhibits the capacity of synovial macrophages to function as antigen-presenting cells. Br J Rheumatol. 1998;37:1207. [PubMed] [Google Scholar]
- 20.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1998;31:315. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 21.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 22.Fuleihan R, Ramesh N, Horner A, et al. Cyclosporin A inhibits CD40 ligand expression in T lymphocytes. J Clin Invest. 1994;93:1315. doi: 10.1172/JCI117089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 24.Dolhain RJEM, Van der Heiden AN, Ter Haar NT, Breedveld FC, Miltenburg AMM. Shift toward T lymphocytes with a T helper 1 cytokine-secretion profile in the joints of patients with rheumatoid arthritis. Arthritis Rheum. 1996;39:1961. doi: 10.1002/art.1780391204. [DOI] [PubMed] [Google Scholar]
- 25.Morita Y, Yamamura M, Kawashima M, et al. Flow cytometric single-cell analysis of cytokine production by CD4+ T cells in synovial tissue and peripheral blood from patients with rheumatoid arthritis. Arthritis Rheum. 1998;41:1669. doi: 10.1002/1529-0131(199809)41:9<1669::AID-ART19>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 26.Isomäki P, Luukkainen R, Lassila O, Toivanen P, Punnonen J. Synovial fluid T cells from patients with rheumatoid arthritis are refractory to the T helper type 2 differentiation-inducing effects of interleukin-4. Immunology. 1999;96:358. doi: 10.1046/j.1365-2567.1999.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skapenko A, Wendler J, Lipsky PE, Kalden JR, Schulze-Koops H. Altered memory T cell differentiation in patients with early rheumatoid arthritis. J Immunol. 1999;163:491. [PubMed] [Google Scholar]
- 28.Morita Y, Yamamura M, Nishida K, et al. Expression of interleukin-12 in synovial tissue from patients with rheumatoid arthritis. Arthritis Rheum. 1998;41:306. doi: 10.1002/1529-0131(199802)41:2<306::AID-ART15>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 29.Durie FH, Fava RA, Foy TM, Aruffo A, Ledbetter JA, Noelle RJ. Prevention of collagen-induced arthritis with an antibody gp39, the ligand for CD40. Science. 1993;261:1328. doi: 10.1126/science.7689748. [DOI] [PubMed] [Google Scholar]
- 30.Maurice MM, Lankester AC, Bezemer AC, et al. Defective TCR-mediated signaling in synovial T cells in rheumatoid arthritis. J Immunol. 1997;159:2973. [PubMed] [Google Scholar]
- 31.Bowen F, Haluskey J, Quill H. Altered CD40 ligand induction in tolerant T lymphocytes. Eur J Immunol. 1995;25:2830. doi: 10.1002/eji.1830251018. [DOI] [PubMed] [Google Scholar]
- 32.Salojin KV, Zhang J, Madrenas J, Delovitch TL. T-cell anergy and altered T-cell receptor signalling: effects on autoimmune disease. Immunol Today. 1998;19:468. doi: 10.1016/s0167-5699(98)01326-7. [DOI] [PubMed] [Google Scholar]
- 33.Thomas R. Antigen-presenting cells in rheumatoid arthritis. Springer Semin Immunopathol. 1998;20:53. doi: 10.1007/BF00831999. [DOI] [PubMed] [Google Scholar]
- 34.Isomäki P, Luukkainen R, Saario R, Toivanen P, Punnonen J. Interleukin-10 functions as an antiinflammatory cytokine in rheumatoid arthritis. Arthritis Rheum. 1996;39:386. doi: 10.1002/art.1780390306. [DOI] [PubMed] [Google Scholar]
- 35.Katsikis PD, Chu C-Q, Brennan FM, Maini RN, Feldmann M. Immunoregulatory role of interleukin-10 in rheumatoid arthritis. J Exp Med. 1994;179:1517. doi: 10.1084/jem.179.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Waal Malefyt R, Yssel H, De Vries JE. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J Immunol. 1993;150:4754. [PubMed] [Google Scholar]
- 37.Groux H, O'garra A, Bigler M, Rouleau M, Antonenko S, De Vries JE, Roncarolo M-G. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 38.Verwilghen J, Lovis R, De Boer M, et al. Expression of functional B7 and CTLA-4 on rheumatoid synovial T cells. J Immunol. 1994;153:1378. [PubMed] [Google Scholar]
- 39.Höllsberg P, Scholz C, Anderson DE, et al. Expression of hypoglycosylated form of CD86 (B7-2) on human T cells with altered binding properties to CD28 and CTLA-4. J Immunol. 1997;159:4799. [PubMed] [Google Scholar]
- 40.Jeannin P, Herbault N, Delneste Y, et al. Human effector memory T cells express CD86: a functional role in naive T cell priming. J Immunol. 1999;162:2044. [PubMed] [Google Scholar]