Abstract
Mature B lymphocytes respond to antigen receptor ligation by phenotypic changes, including upregulation of major histocompatibility complex class II molecules and expression of B7.2, which are required for initiating and sustaining a productive interaction with T helper cells. We have previously demonstrated that neonatal B cells fail to show a similar up-regulation of class II and B7.2 expression following B-cell receptor (BCR) ligation, although these responses could be induced by other stimuli. Here we demonstrate that immature B cells from adult bone marrow exhibit even more profound defects in these responses, as they fail to up-regulate class II in response to either BCR ligation or interleukin-4. Moreover, bone marrow-derived, immature B cells could not be induced to express B7.2 either by receptor cross-linking or by lipopolysaccharide. These differences in the inducible expression of class II and B7.2 appear to be intrinsic to the B cells, as they were retained in purified populations of B-lineage cells and could not be induced in mature B cells by coculture with bone marrow cells. Furthermore, short-term culture of bone marrow permitted B-cell maturation, which was accompanied by acquisition of responsiveness to the same stimuli as mature, splenic B cells. The inability of immature B cells to show these responses provides a molecular explanation for their reported deficiency in interacting with T cells. Failure of immature B cells to inducibly express B7.2 may also be important for the establishment of self tolerance in the B-cell compartment.
INTRODUCTION
Class II major histocompatibility complex (MHC) molecules (MHC-II) play a critical role in immune responses. They are responsible both for the positive selection of T cells in the thymus, which shapes the T-cell repertoire, and for the presentation of antigenic peptides to CD4+ T cells. Expression of MHC class II molecules is developmentally regulated and mainly restricted to specialized cells such as thymic epithelial cells and professional antigen-presenting cells (APC) including macrophages, dendritic cells and B lymphocytes. Several studies show that the nature of the cell acting as APC is important in determining the type of response elicited from T cells1, 2 and that B cells are essential at least for responses to some antigens.3–5 Amongst cells with APC capability the level of constitutive expression of MHC-II is tightly controlled during development and may be up-regulated upon cellular activation. In the B-cell compartment, class II expression can be induced in pre-B cells by interleukin-4 (IL-4).6 Mature B cells constitutively express class II molecules at modest levels and show a dramatic hyperexpression on activation, 7, 8 and terminal differentiation to the plasma cell phenotype is accompanied by loss of class II expression.9
The level of MHC-II expression has been shown to influence T-cell activation directly10 and, taken together with evidence that processed peptides are targeted to newly synthesized class II molecules, 11 this suggests that the ability of B cells to up-regulate MHC-II expression following activation is likely to be critical in their ability to function as APC.
There is now substantial evidence that, in addition to ligation of T-cell receptors (TCR) by peptide-loaded MHC-II, successful T-cell activation requires the delivery of other co-stimulatory signals. In particular, signalling via CD28 (when this molecule is ligated by B7.1 or B7.2 molecules on the APC) appears to be critical.12 Co-stimulation through B7.1 and B7.2 have been shown to influence differentially the outcome of T-cell activation, biasing responses to either those of the T helper type 1 (Th1) or Th2 type.13 B cells do not constitutively express B7.1 or B7.2, but both are induced on activation. Some stimuli, such as lipopolysaccharide (LPS), induce expression of both molecules, whilst others preferentially induce one or the other. Ligation of B-cell antigen receptors (BCR) has been shown to induce expression of B7.2 but not B7.1.14 These findings suggest that the ability of B cells to act as effective APC for T-cell activation is likely to be dependent on their ability to respond to antigen binding both by up-regulating MHC-II expression and by showing induced expression of B7.2.
We have recently shown that immature B cells from neonatal spleen exhibit a specific inability to up-regulate MHC class II expression following ligation of their BCR, 15 although induction of class II hyperexpression by other stimuli, including ligation of IL-4 receptors and CD40, was as effective as on mature, adult B cells. Furthermore, we have also demonstrated that neonatal B cells fail to show induced expression of B7.2 after BCR cross-linking.16 These findings correlate strongly with observations by Morris and co-workers17 that the ability of B cells to act as effective APC is developmentally regulated. In this report, B cells from neonatal spleen were shown to be unable to present an antigen which required processing to an antigen-specific T-cell line. This correlation suggests a causal relationship between the ability of B cells to modulate MHC-II and B7.2 expression and their APC activity. Here we report experiments which provide further support for this relationship.
In addition to showing that neonatal B cells were ineffective APC, Morris and colleagues also found that B cells from freshly explanted adult bone marrow failed to present antigen to T cells. Interestingly, marrow-derived B cells were shown to acquire APC capacity equivalent to that of mature, splenic B cells after short-term culture. We have now investigated inducible class II hyperexpression and B7.2 expression by immature B cells from adult bone marrow. Our data indicate that freshly explanted bone marrow B cells, like neonatal B cells, fail to show both surface immunoglobulin (sIg) -induced MHC-II hyperexpression and B7.2 expression. Indeed they fail to up-regulate class II in response to stimuli which were effective on neonatal B cells. However, after culture for several days, bone marrow B cells became responsive to the same stimuli as mature adult B cells. These findings show that the regulation of molecules critically involved in T-cell activation is developmentally controlled in the B-cell lineage and they provide a possible molecular basis for the acquisition of APC activity by B cells.
MATERIALS AND METHODS
Mice
BALB/c mice were bred in our animal facility and were used for experiments at 10–12 weeks of age unless otherwise stated.
Antibodies and reagents
The following antibodies were used in this study. Rat anti-mouse κ-chain (mAb187.1); rat anti-mouse I-Ad (NIM-R4); biotin and fluorescein-coupled rat anti-mouse B220 (CD45; RA3-6B2, Pharmingen, San Diego, CA); rat anti-mouse B7.2 (CD86; 2D10, a kind gift of Dr D. Faherty, Hoffmann–La Roche, NJ); rat anti-mouse immunoglobulin D (IgD; 1.19); F(ab′)2, goat anti-mouse µ-chain (Chemicon, Harrow, UK); FGK65, rat anti-mouse CD40 (a kind gift from Dr J. Andersson, Basel Institute for Immunology, Basel, Switzerland). Non-commercial antibodies were partially purified by ammonium sulphate precipitation and/or chromatography on protein G–Sepharose (Calbiochem, Nottingham, UK) and, where appropriate, coupled with biotin or fluorescein by standard methods. Biotinylated antibodies were revealed using PE-streptavidin (Serotec, Oxford, UK). Recombinant murine IL-4 was obtained from Genzyme (Cambridge, MA). LPS (Escherichia coli 055B5) was obtained from Difco, Detroit, MI. Ionomycin and (–)-indolactam V were obtained from Sigma (Poole, UK).
Cell preparation and culture
Adult B cells were purified from spleen cell suspensions as described previously.15 Bone marrow cells were prepared by flushing out the contents of the femurs removed from adult mice with RPMI-1640 using a syringe equipped with a 23-gauge needle. Clumps were disrupted by gentle tituration through a 23-gauge needle. The cell suspension was washed in RPMI-1640 and erythrocytes were removed by lysis in 0·83% w/v ammonium chloride for 2–5 min. After two further washes, cells were resuspended in RPMI-1640 containing 10% v/v selected fetal calf serum (FCS), 1 mm sodium pyruvate, 2 mm l-glutamine, 50 U/ml penicillin, 50 mg/ml streptomycin and 5×10−5 m 2-mercaptoethanol. For short-term culture, cells were incubated at 2×106/ml in supplemented RPMI-1640 for 24–48 hr in 24-well plates with the stimuli indicated. Cultured marrow cells were derived by culturing the cells at 2×106−4×106/ml for 72 hr in supplemented RPMI-1640, non-adherent and loosely adherent cells were recovered by gentle pipetting and were washed twice before being used for experiments.
Magnetic activated cell sorting (MACS) separation
For MACS separation, bone marrow cells prepared as above, were suspended at 108/ml in phosphate-buffered saline (PBS) containing 1% w/v bovine serum albumin (BSA), 1 mm EGTA and 0·005% w/v sodium azide (MACS buffer). Then, 10 µl of anti-B220 coupled magnetic microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) were added to each 90 µl of cells and incubated at 4° for 15 min. After one wash in 5–10 ml MACS buffer the cells were resuspended at 1×107−5×107/ml in MACS buffer, applied to columns and B220 positive cells were eluted according to the manufacturer’s instructions. Cells purified in this way were always > 95% B220+.
Flow cytometry
Cells (106) were washed in PBS containing 0·2% w/v BSA and 0·2% w/v sodium azide (PBS-BA) and stained with fluorescein isothiocyanate (FITC) -coupled reagents (10–50 µg/ml) for 30 min on ice. The cells were then washed, incubated with biotin-coupled reagent 10–50 µg/ml for a further 30 min on ice, washed again and incubated with phycoerythrin (PE)-coupled streptavidin (0·1 mg/ml) for 20 min and washed twice more prior to analysis.
Flow cytometry was performed using a fluorescence-activated cell sorter (FACScan) cytometer (Becton Dickinson, Oxford, UK). Dead cells were excluded on the basis of their forward- and side-scatter characteristics. For spleen cells an electronic gate was set to include lymphoid cells, the analysis of bone marrow cells was based on those which fell within an extended lymphoid gate, set to include > 90% of sIg-positive cells. Results shown are based on 5000–20 000 events.
RESULTS
IL-4 does not induce elevation of MHC class II expression on immature B cells from adult bone marrow
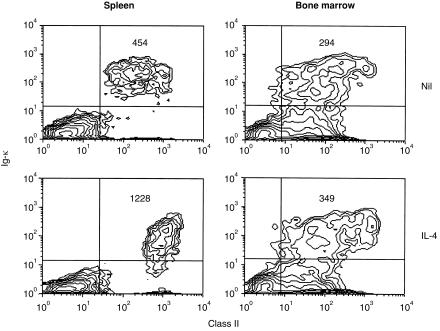
It has been widely shown that IL-4 is a potent inducer of class II hyperexpression on B cells; even immature B cells from neonatal spleen, which fail to up-regulate class II in response to BCR ligation, remain responsive to this stimulus.15 We examined the response of bone marrow B cells to IL-4 by culturing freshly explanted bone marrow cells in the presence of the cytokine and comparing MHC class II expression with that induced in mature, splenic B cells by two-colour immunofluorescence. Levels of class II expression on unstimulated bone marrow (κ+) B cells were noticeably lower than those on splenic B cells (Fig. 1) as has been observed by others, a small fraction (6%) of cells (presumably mature B cells – see Fig. 2a) expressed higher class II levels. As expected, mature, splenic B cells showed a marked (three- to five-fold) increase in class II expression in the presence of IL-4. In contrast, overall expression of class II on κ+ bone marrow B cells was unaffected by this stimulus; although some cells expressing high levels of κ (again presumably mature B cells) did show a modest increase in class II. These cells represented less than 20% of total κ+ cells.
Figure 1.
Bone marrow B cells fail to up-regulate MHC class II in response to IL-4. BALB/c spleen and bone marrow cells (2×106/ml) cultured in medium (Nil) or with 50 U/ml of recombinant murine IL-4 for 24 hr, were stained to reveal expression of Igκ and MHC class II and analysed by flow cytometry. The numbers in the upper right quadrant of each plot indicate the mean fluorescence intensity (MFI) of class II staining on the cells in that quadrant. Cells were gated on their forward- and side-scatter characteristics to collect data from lymphoid cells, for bone marrow an extended lymphoid gate was employed. The results shown are based on analysis of 10 000 gated events and are representative of five similar experiments.
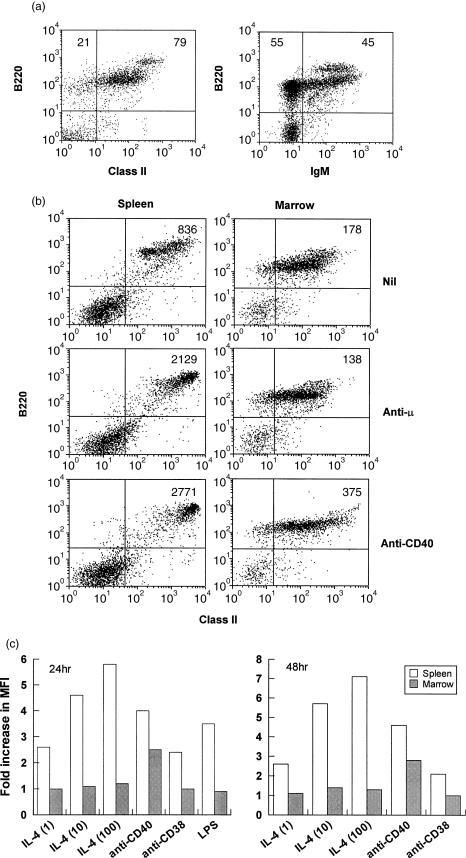
Figure 2.
Bone marrow B cells fail to up-regulate MHC class II in response to BCR ligation but are responsive to CD40 ligation. (a) Bone marrow cells were stained to reveal expression of MHC class II and IgM on B220+ cells. Figures in the upper quadrants show the percentage of B220+ cells bearing MHC class II (left panel) and IgM (right panel). (b) Spleen and bone marrow cells were cultured at 2×106/ml in medium or with 10 µg/ml F(ab)2 anti-µ or 5 µg/ml anti-CD40 as shown. After 24 hr the cells were stained for B220 and MHC class II expression and analysed by flow cytometry. Gating was as for Fig. 1. The results shown represent 5000 gated events, and the figures in the upper right quadrant indicate the MFI of class II staining of the various populations. (c) Spleen and bone marrow cells were cultured as above with the indicated stimuli for 24 or 48 hr before being analysed for MHC class II expression. IL-4 was used at 1, 10 and 100 U/ml, anti-CD40 at 5 µg/ml, anti-CD38 at 20 µg/ml and LPS at 10 µg/ml. The results shown indicate the fold increase in the mean fluorescence of MHC class II on B220+ cells compared to that of control, unstimulated cells. The control values were: spleen (24 hr) 355; bone marrow (24 hr) 150; spleen (48 hr) 376; bone marrow (48 hr) 205. The data shown were calculated from analysis of 10 000 gated events in each case.
Induction of class II up-regulation by other stimuli
Since bone marrow B cells were refractory to IL-4-induced class II hyperexpression, their response to other stimuli which modulate class II expression was investigated. To analyse the response to ligation of sIg it was necessary to examine class II expression on B220+ cells, because ligation of sIg with anti-µ antibodies caused a marked down-regulation of sIg expression, precluding its use as a marker for B cells. Preliminary experiments (Fig. 2a) revealed that approximately 80% of bone marrow cells falling within an extended lymphoid gate were B220+, 80% of these were class II+ and 45–50% were IgM+. A small fraction (∼10%) of the cells expressed B220 and class II at levels comparable to those seen on splenic B cells (B220hi), essentially all of these were µ+ δ+ (data not shown). When stimulated with anti-µ, immature B cells from adult bone marrow failed to up-regulate class II, but ligation of CD40 with anti-CD40 mAb did provoke a two- to three-fold increase in class II expression on B220+ cells (Fig. 2b). In contrast, both stimuli induced marked class II hyperexpression in mature, splenic B cells. Bone marrow B cells (κ+) also failed to show increased class II expression when cultured with anti-CD38 mAb or LPS, even after 48 hr, although both stimuli induced class II hyperexpression on splenic B cells (Fig. 2c). Bone marrow B cells remained refractory to IL-4-induced class II hyperexpression after 48 hr in culture and at levels of the cytokine 100 times greater than those which induced a clear response in mature, splenic B cells.
Differences in class II up-regulation are an inherent property of immature, bone marrow B cells
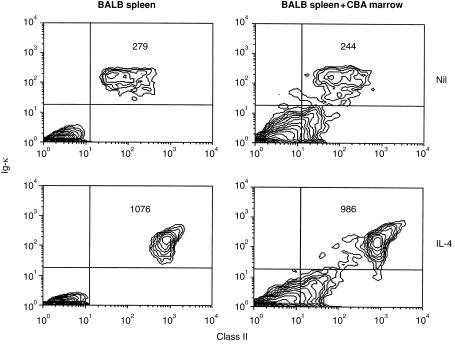
The inability of bone marrow B cells to hyperexpress MHC class II in response to several stimuli which induced up-regulation in mature B cells could result from inhibitory effects on the B cells exerted by other marrow cells present in the culture. To investigate this possibility, mature, splenic B cells were co-cultured with bone marrow cells and the effect of this on their ability to up-regulate class II was assessed. In order to examine the response of only the splenic B cells, advantage was taken of the fact that the reagent used to detect MHC class II (NIM-R4) reacts with I-Ad but not I-Ak (E. Andrews and S. Marshall-Clarke, unpublished results). BALB/c (I-Ad) splenic B cells were therefore co-cultured with a two-fold excess of CBA (I-Ak) bone marrow cells and class II hyperexpression was induced by addition of IL-4. The data (Fig. 3) indicate that the presence of an excess of bone marrow cells did not affect the ability of the mature BALB/c B cells to hyperexpress class II in response to IL-4. It therefore seems unlikely that the lack of this response in immature, bone marrow B cells is due to the influence of non-B-lineage cells; rather it appears to be an inherent property of the bone marrow B cells themselves.
Figure 3.
Co-culture with bone marrow does not influence class II up-regulation by splenic B cells. BALB/c spleen cells (1×106) were cultured with CBA bone marrow cells (2×106) in 2 ml cultures, with or without murine IL-4 (50 U/ml) for 24 hr before being stained and analysed for Igκ and class II expression by flow cytometry. Staining with NIM-R4 reveals MHC class II on cells of H2d but not H2k haplotype. Figures in the upper right quadrant indicate the MFI of class II fluorescence of the cells within that quadrant. Results shown are based on 10 000 gated events and are representative of two similar experiments.
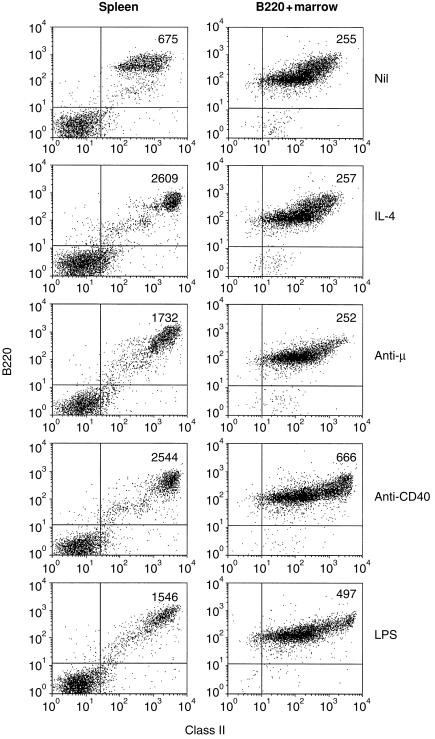
This conclusion was strengthened when class II modulation was assessed on purified B220+ cells (Fig. 4). Even in the complete absence of non-B-lineage cells, bone marrow B cells still failed to show class II hyperexpression after treatment with IL-4 or anti-µ. Ligation of anti-CD40 did, however, induce a two- to three-fold increase in class II fluorescence and, interestingly, a modest increase was also seen after stimulation with LPS, although this stimulus was ineffective on unseparated bone marrow B cells.
Figure 4.
Purified B220+ bone marrow cells remain refractory to MHC-II up-regulation. Spleen cells or MACS-purified B220+ bone marrow cells were cultured (2×106/ml) with recombinant murine IL-4 (50 U/ml), goat F(ab)2 anti-µ (10 µg/ml), rat anti-mouse CD40 (10 µg/ml) or LPS (10 µg/ml) for 24 hr before being stained and analysed for B220 and class II expression by flow cytometry. Figures in the upper right quadrant indicate the MFI of class II fluorescence of the cells within that quadrant. Results shown are based on analysis of 10 000 gated events.
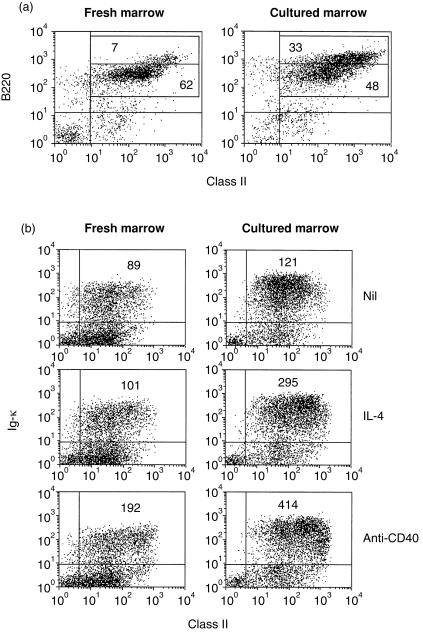
Immature B cells from adult marrow acquire the ability to up-regulate class II after short-term culture
Short-term culture of bone marrow cells has been shown to allow the maturation of B cells from their precursors.18 Interestingly, in the experiments of Morris and co-workers, freshly explanted bone marrow cells were unable to effectively process and present antigen to a T-cell clone, but acquired the capability to do so after 72 hr in culture.17 The ability of freshly isolated and cultured bone marrow B cells to up-regulate MHC class II expression in response to various stimuli was therefore compared (Table 1 and Figs 5 and 6). After 3 days in culture bone marrow cell suspensions contained more κ+ B cells and these expressed higher levels of both sIg and MHC class II (Table 1) there was also a shift towards a higher proportion of B220hi cells (Fig. 5a) and of mature sIgD+ cells (data not shown). The cultured cells also showed functional maturation since cultured B cells were clearly induced to hyperexpress class II in response to both IL-4 (Fig. 5b) and anti-µ (Fig. 6). Their response to CD40 ligation was also improved (Fig. 5b). These data correlate well with those on the effectiveness of bone marrow cells as APC, indicating that the ability to hyperexpress class II MHC molecules may be a necessary prerequisite for a productive B-cell:T-cell interaction.
Table 1.
Surface expression of Igκ, MHC class II and B220 on freshly isolated and cultured bone marrow cells
| Cells | %Igκ | MFI* (Igκ) | MFI (class II) † | %B220 |
|---|---|---|---|---|
| Fresh marrow | 26 | 113 | 193 | 71 |
| Cultured marrow | 54 | 222 | 293 | 83 |
Cells were isolated, cultured and stained as described in the Materials and Methods and fluorescence was analysed on cells falling within an extended lymphoid gate.
Mean fluorescence index
mean fluorescence of class II staining on Igκ cells.
Figure 5.
Acquisition of IL-4-induced class II hyperexpression by cultured bone marrow B cells. Freshly explanted bone marrow cells or cells harvested after culture at 2×106/ml for 3 days, were either (a) stained for analysis of MHC class II and B220 expression, or (b) incubated for 20 hr with IL-4 (100 U/ml) or anti-CD40 (5 µg/ml) prior to staining for expression of Igκ and MHC class II. Cell staining and fluorescence analysis was carried out as described in Fig. 1. Figures in the upper right quadrant indicate the MFI of class II fluorescence on Igκ+ cells. Data shown are based on the analysis of 5000 gated events and are representative of five similar experiments. Expression of class II on control (unstimulated) cells was compared to that on stimulated cells by Student’s t-test. Fresh marrow: control versus IL-4, 0·1 < P > 0·05; control versus anti-CD40, P < 0·0005. Cultured marrow: control versus IL-4, P < 0·0005; control versus anti-CD40, P < 0·0005.
Figure 6.
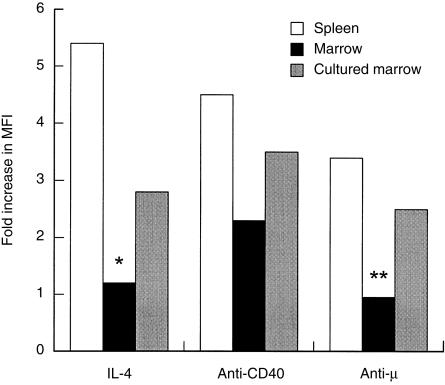
Responsiveness of cultured bone marrow B cells to BCR ligation. Spleen cells or freshly isolated or cultured (3 days at 2×106/ml) bone marrow cells as indicated, were incubated for 20 hr with IL-4 (100 U/ml), F(ab)2 anti-µ (10 µg/ml) or anti-CD40 mAb (10 µg/ml) before being analysed for expression of MHC class II by flow cytometry. Analysis was carried out as in Fig. 2. The data shown indicate the fold increase in the mean fluorescence of MHC class II on B220+ cells compared to that of control, unstimulated cells. The control values (mean ± SEM) were: spleen, 289 ± 6·4; bone marrow, 135 ± 5·2; cultured bone marrow, 235 ± 4·3. The data shown were calculated from analysis of 5000 gated events in each case. Class II fluorescence on control and stimulated cells was compared by Student’s t-test. Except for responses of freshly isolated cells to IL-4 (*0·5 < P > 0·25) and anti-µ (**0·15 < P > 0·1), all other responses were significantly different from controls (P < 0·0005).
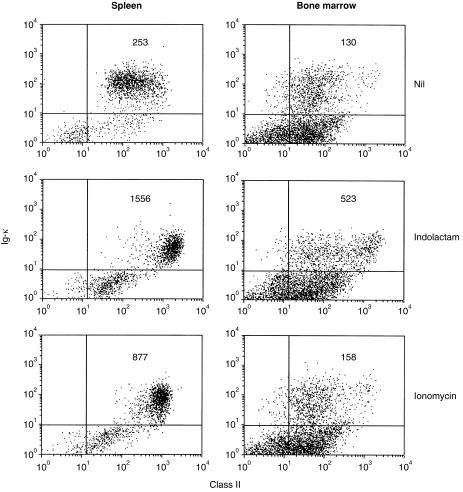
Class II up-regulation can be induced in bone marrow B cells by activation of protein kinase C, but not by elevation of intracellular Ca2+
Taken together with our previous data on immature B cells from neonatal spleen, these findings suggest the existence of several distinct pathways by which class II hyperexpression can be signalled. They also provide evidence that the availability of these pathways is differentially regulated during B-cell development and differentiation. To explore this further we investigated the effects on class II expression of protein kinase C (PKC) activation using (–)-indolactam V and elevation of intracellular Ca2+ by treatment with ionomycin. Both agonists induced class II hyperexpression in mature, adult B cells (Fig. 7) and in immature B cells from neonatal spleen (data not shown). However, although freshly isolated bone marrow B cells were induced to up-regulate class II after PKC activation with (–)-indolactam V they were refractory to the effects of ionomycin (Fig. 7). It thus appears that the immature B cells in murine bone marrow possess the relevant PKC isoform which participates in this response and that this can be activated. In contrast, they apparently lack those pathways which connect an elevation of intracellular Ca2+ concentrations to increased transcription of class II genes.
Figure 7.
Induction of MHC-II up-regulation in bone marrow B cells by indolactam and ionomycin. Spleen or bone marrow cells were cultured at 2×106/ml for 20 hr with (–)-indolactam V (30 ng/ml) or ionomycin (0·1 µm). Cell staining and fluorescence analysis were carried out as described in Fig. 5.
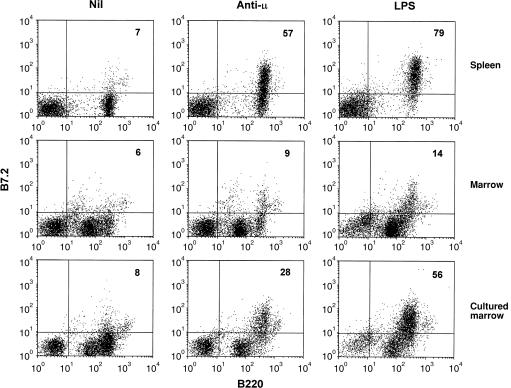
Bone marrow B cells show a selective failure in B7.2 induction following sIg ligation
In mature B cells antigen receptor cross-linking induces the expression of B7.2 (CD86). The expression of this molecule is essential for B cells to act as effective APC since it provides co-stimulatory signals by ligating CD28 on the T cells whose TCR has been engaged by peptide-loaded MHC class molecules. We therefore considered it possible that the failure of bone marrow B cells to act as APC could result from an inability to show B7.2 induction. To test this, the induction of B7.2 by immunoglobulin ligation with anti-µ and simulation with LPS was compared on mature B cells from adult spleen and B cells from freshly isolated and cultured marrow. As can be seen from Fig. 8, splenic B cells were induced to express B7.2 by both stimuli. Amongst bone marrow-derived cells the B220hi cells were induced to express B7.2 after culture with LPS but only a fraction (30%) of these responded to sIg cross-linking. Cells which were B220lo did not respond to either stimulus. Cultured bone marrow contained a higher proportion of B cells and more of these expressed B220 at a level comparable with that seen on mature, splenic B cells. Like splenic B cells, these cells responded to both BCR ligation and LPS treatment by strongly up-regulating B7.2. The residual B220lo cells (which were > 30% Ig+) did not show this response. Thus it appears that immature B cells in the bone marrow are unable to up-regulate B7.2 expression and that culturing marrow for 72 hr allows B cells to mature both in terms of their surface expression of B220 (and IgD, data not shown) and in their ability to show induced expression of this important co-stimulatory molecule.
Figure 8.
Immature bone marrow B cells fail to express B7.2 on stimulation with LPS or by BCR ligation. Spleen or freshly isolated or cultured (3 days) bone marrow cells were incubated for 20 hr with F(ab)2 anti-µ (10 µg/ml) or LPS (10 µg/ml) before being analysed for expression of B7.2 on B220+ cells by flow cytometry. The numbers in the upper right quadrant indicate the percentage of B220+ cells expressing B7.2. Data are based on analysis of 10 000–20 000 gated events and are representative of three similar experiments.
DISCUSSION
The response of B lymphocytes to antigen receptor ligation depends on their differentiation status. Immature B cells, whether from fetal liver, neonatal spleen, or adult bone marrow, have been shown to be highly susceptible to tolerance induction, especially in the absence of T cells.19, 20 Recent experiments have suggested that this sensitivity results from an increased susceptibility to clonal deletion since mature adult B cells require a higher affinity interaction with self antigen to be deleted in a transgenic model, 21 are refractory to the induction of apoptosis by soluble anti-immunoglobulin antibodies22 and are only induced to undergo apoptosis when sIg is hypercrosslinked.23 These differences are presumed to reflect developmental regulation of BCR-coupled signalling pathways but, although some differences have been reported, 24–26 the critical elements underpinning this differential responsiveness remain to be resolved.
We have examined the ability of immature B cells to up-regulate MHC class II expression in response to BCR ligation and other stimuli and reported that immature B cells from neonatal spleen were shown to exhibit a selective defect in this response which affected anti-immunoglobulin induced class II hyperexpression, but not that induced by other stimuli including IL-4 and anti-CD40.15 Furthermore, BCR ligation of immature B cells from neonatal spleen failed to induce surface expression of the co-stimulatory molecule, B7.2, although other stimuli could induce its expression.16 Here we have extended these findings by examining the induction of class II hyperexpression and inducible B7.2 expression in immature B cells from adult bone marrow. B cells freshly explanted from adult marrow did not show up-regulation of MHC class II in response to stimulation with either anti-immunoglobulin antibodies or IL-4. In this regard they differed from both mature, adult B cells which respond to both stimuli, and to immature, neonatal B cells which respond to IL-4 but not to immunoglobulin cross-linking.15 Culture of bone marrow B cells with an agonistic anti-CD38 monoclonal antibody27 also failed to induce class II up-regulation, although mature adult B cells responded to this stimulus. This result provides a further example of the apparently tight coupling of responses induced by CD38 ligation to the signalling capacity of the BCR.28, 29 However, short-term culture of bone marrow, prior to stimulation, allows B-cell maturation and the concomitant development of the ability to hyperexpress MHC class II. Taken together these findings demonstrate the existence of multiple pathways for signalling the induced hyperexpression of MHC class II and show that these are differentially regulated in different locations and at different stages of B-cell maturation. It has recently been elegantly demonstrated that the gene encoding the MHC transactivator, CIITA, which ultimately regulates MHC class II expression, is under the control of several different promoters which are differentially utilized by cells of different lineages.30 It seems very probable that the complex control of constitutive and inducible MHC class II expression during B-cell differentiation and ontogeny is achieved by the differential use of these promoters, and we are currently investigating this possibility.
The differences in inducible MHC class II expression exhibited by bone marrow and splenic B cells could be an inherent property of the cells or, alternatively might result from the influence of some external factor, present in the bone marrow environment but not in the spleen. In two recent reports, type 1 interferons have been shown to affect the development of B lymphocytes when administered in vivo31 and influence their ability to proliferate in response to immunoglobulin ligation.32 It was thus possible that cytokines or other stromal cell-derived factors could be exerting an inhibitory effect on the ability of bone marrow B cells to show class II hyperexpression. Our data suggest that this is unlikely because mature splenic B cells co-cultured with bone marrow cells remained responsive to both IL-4 and anti-immunoglobulin, and B220-purified bone marrow B cells were as unresponsive as unseparated cells. There were, however, some differences in the induction of MHC class II hyperexpression in response to LPS. This stimulus failed to provoke class II hyperexpression in B cells in intact marrow (Fig. 2), but did stimulate up-regulation in B220-purified cells (Fig. 4), suggesting that extrinsic, stromal factors may influence the ability of developing B cells to respond to LPS. The fact that bone marrow B cells could hyperexpress MHC class II following ligation of CD40 also argues against this possibility and shows that the response elements required for class II up-regulation are intact in immature B cells. It thus appears that the inability of immature B cells in adult marrow to show up-regulation of MHC class II expression in response to both BCR ligation and IL-4 is an inherent property of the B cells.
Our data also show that the ability to show inducible expression of B7.2 is tightly linked to the maturation status of B cells. In freshly explanted marrow a minority of B cells showing high-level expression of B220 which are of the µ+ δ+ phenotype are induced to express B7.2 by LPS and these cells show some response to BCR ligation. After short-term culture a higher proportion of cells are B220hi and these show similar reactivity to both LPS and anti-µ to mature, adult, splenic B cells. Immature B cells, characterized by low expression of B220 were not induced to express B7.2 by any stimulus tested, nor did they become capable of this response after short-term culture.
Two pieces of evidence demonstrate that bone marrow B cells are unable to interact effectively with helper T cells. First, in a precocious series of experiments, Playfair and Purves showed that bone marrow B cells were much less effective than splenic B cells in their ability to collaborate with T cells in the response to sheep red blood cells.33 Second, in an in vitro system, Morris et al.17 showed that freshly explanted bone marrow B cells were unable to present an antigen which required processing to an antigen specific T-cell line, although they were able to present a peptide fragment of the antigen. Recent experiments have provided direct evidence that the initiation of signalling through the TCR requires multivalent engagement of peptide–MHC ligands, 34 highlighting the importance of the level of MHC class II expression on the APC in mediating T-cell activation. This strongly suggests that the failure of bone marrow B cells to up-regulate class II in response to BCR ligation demonstrated here could account for their defective APC function. Interestingly, in the report by Morris et al. bone marrow cells which had been cultured for 72 hr in the presence of LPS became as potent at presenting antigen as mature splenic B cells. Our finding that, after short-term culture, bone marrow B cells become able to up-regulate MHC class II expression and show inducible expression of the important co-stimulatory molecule, B7.2, provides a basis for this acquisition of APC activity.
An alternative explanation of the lack of APC activity of bone marrow B cells could be that immature B cells are inefficient in internalizing and processing antigen. Against this, we have found that immature B cells from both adult bone marrow and neonatal spleen internalize labelled anti-IgM antibody as rapidly as do mature, splenic B cells and that once taken up the receptor-associated surrogate antigen is also effectively degraded (S. Marshall-Clarke, unpublished observations).
It thus seems probable that the reported ineffectiveness of bone marrow B cells as APC, both in vitro and in vivo, results from their inability to express a high density of peptide-loaded MHC, compounded by the fact that they are unable to provide co-stimulatory signals to T cells via the interaction between B7.2 and CD28.35 Furthermore, regulation of the ability to show inducible expression of B7.2 may be critical for maintaining self tolerance. In a series of recent experiments, 36, 37 Rathmell and others have shown that the forced expression of B7.2 in anergic B cells from HEL/anti-HEL transgenic animals not only protected the cells from Fas-mediated apoptosis but also, in the presence of specific T cells, allowed the B cells to proliferate and differentiate to the stage of antibody production, effectively overriding self tolerance and producing a state of autoimmunity. Our observation that immature B cells fail to show B7.2 induction would thus be expected to render them prone to Fas-mediated deletion by T cells circulating to the marrow. Experiments to test this suggestion are currently underway.
During the preparation of this manuscript, Benschop et al.38 have reported similar findings to those described here. In their experiments immature B cells from immunoglobulin transgenic animals failed to respond to BCR ligation by up-regulation of MHC class II, B7.2, or CD69. Rather, they were induced to undergo receptor editing, a response which was not triggered in mature, splenic B cells. Treatment of immature B cells with ionomycin also failed to induce MHC class II hyperexpression, just as we found. Our data complement and extend these observations by showing that immature B cells from normal animals display similar characteristics.
REFERENCES
- 1.Chang TL, Shea CM, Urioste S, Thompson RC, Boom WH, Abbas AK. Heterogeneity of helper/inducer T lymphocytes. III. Responses of IL-2- and IL-4-producing (Th1 and Th2) clones to antigens presented by different accessory cells. J Immunol. 1990;145:2803. [PubMed] [Google Scholar]
- 2.Gajewsky TF, Pinnas M, Wong T, Fitch FW. Murine Th1 and Th2 clones proliferate optimally in response to distinct antigen-presenting cell populations. J Immunol. 1991;146:1750. [PubMed] [Google Scholar]
- 3.Stockinger B, Zal T, Zal A, Gray D. B cells solicit their own help from T cells. J Exp Med. 1996;183:891. doi: 10.1084/jem.183.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Constant S, Schweitzer N, West J, Ranney P, Bottomly K. B lymphocytes can be competent antigen-presenting cells for priming CD4+ T cells to protein antigens in vivo. J Immunol. 1995;155:3734. [PubMed] [Google Scholar]
- 5.Serreze DV, Fleming SA, Chapman HD, Richard SD, Leiter EH, Tisch RM. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Immunol. 1998;161:3912. [PubMed] [Google Scholar]
- 6.Polla BS, Ohara J, Paul WE, et al. Differerential induction of class II gene expression in murine pre-B-cell lines by B-cell stimulatory factor-1 and by antibodies to B-cell surface antigens. J Mol Cell Immunol. 1988;3:363. [PubMed] [Google Scholar]
- 7.Lala PK, Johnson GR, Battye FL, Nossal GJ. Maturation of B lymphocytes. I. Concurrent appearance of increasing Ig, Ia, and mitogen responsiveness. J Immunol. 1979;122:334. [PubMed] [Google Scholar]
- 8.Mond JJ, Seghal E, Kung J, Finkelman FD. Increased expression of I-region-associated antigen (Ia) on B cells after cross-linking of surface immunoglobulin. J Immunol. 1981;127:881. [PubMed] [Google Scholar]
- 9.Halper J, Fu SM, Wang CY, Winchester R, Kunkel HG. Patterns of expression of human ‘Ia-like’ antigens during the terminal stages of B cell development. J Immunol. 1978;120:1480. [PubMed] [Google Scholar]
- 10.Janeway CA, Bottomly K, Babich J, et al. Quantitative variation in Ia antigen expression plays a central role in immune regulation. Immunol Today. 1984;5:99. doi: 10.1016/0167-5699(84)90043-4. [DOI] [PubMed] [Google Scholar]
- 11.Davidson H, Reid P, Lanzavecchia A, Watts C. Processed antigen binds to newly synthesized MHC class II molecules in antigen-specific B lymphocytes. Cell. 1991;67:105. doi: 10.1016/0092-8674(91)90575-j. [DOI] [PubMed] [Google Scholar]
- 12.Linsley PS, Brady W, Grosmaire L, Aruffo A, Damle NK, Ledbetter JA. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991;173:721. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lombardi G, Arnold K, Uren J, et al. Antigen presentation by interferon-gamma-treated thyroid follicular cells inhibits interleukin-2 (IL-2) and supports IL-4 production by B7-dependent human T cells. Eur J Immunol. 1997;27:62. doi: 10.1002/eji.1830270110. [DOI] [PubMed] [Google Scholar]
- 14.Lenschow DJ, Sperling AI, Cooke MP, et al. Differential up-regulation of the B7.1 and B7.2 costimulatory molecules after Ig receptor engagement by antigen. J Immunol. 1994;153:1990. [PubMed] [Google Scholar]
- 15.Tasker L, Marshall-Clarke S. Immature B cells from neonatal mice show a selective inability to up-regulate MHC class II expression in response to antigen receptor ligation. Int Immunol. 1997;9:475. doi: 10.1093/intimm/9.4.475. [DOI] [PubMed] [Google Scholar]
- 16.Marshall-Clarke S, Reen D, Tasker L, Hassan J. Neonatal immunity: How well is it grown up? Immunol Today. 2000;21:35. doi: 10.1016/s0167-5699(99)01548-0. [DOI] [PubMed] [Google Scholar]
- 17.Morris JF, Hoyer JT, Pierce SK. Antigen presentation for T cell interleukin-2 secretion is a late acquisition of neonatal B cells. Eur J Immunol. 1992;22:2923. doi: 10.1002/eji.1830221125. [DOI] [PubMed] [Google Scholar]
- 18.Raff M, Owen J, Cooper M, Lawton ARD, Megson M, Gathings W. Differences in susceptibility of mature and immature mouse B lymphocytes to anti-immunoglobulin-induced immunoglobulin suppression in vitro. Possible implications for B-cell tolerance to self. J Exp Med. 1975;142:1052. doi: 10.1084/jem.142.5.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nossal GJ, Pike BL. Evidence for the clonal abortion theory of B-lymphocyte tolerance. J Exp Med. 1975;141:904. [PMC free article] [PubMed] [Google Scholar]
- 20.Metcalf E, Klinman N. In vitro tolerance induction of bone marrow cells: a marker for B cell maturation. J Immunol. 1977;118:2111. [PubMed] [Google Scholar]
- 21.Lang J, Jackson M, Teyton L, Brunmark A, Kane K, Nemazee D. B cells are exquisitely sensitive to central tolerance and receptor editing induced by ultralow affinity, membrane-bound antigen. J Exp Med. 1996;184:1685. doi: 10.1084/jem.184.5.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norvell A, Mandik L, Monroe J. Engagement of the antigen-receptor on immature murine B lymphocytes results in death by apoptosis. J Immunol. 1995;154:4404. [PubMed] [Google Scholar]
- 23.Parry SL, Hasbold J, Holman M, Klaus GG. Hypercross-linking surface IgM or IgD receptors on mature B cells induces apoptosis that is reversed by costimulation with IL-4 and anti-CD40. J Immunol. 1994;152:2821. [PubMed] [Google Scholar]
- 24.Yellen AJ, Glenn W, Sukhatme VP, Cao X, Monroe JG. Signaling through surface IgM in tolerance-susceptible immature murine B lymphocytes. Developmentally regulated differences in transmembrane signaling in splenic B cells from adult and neonatal mice. J Immunol. 1991;146:1446. [PubMed] [Google Scholar]
- 25.Wechsler RJ, Monroe JG. Immature B lymphocytes are deficient in expression of the src-family kinases p59 fyn and p55 fgr-1. J Immunol. 1995;154:1919. [PubMed] [Google Scholar]
- 26.Carman JA, Wechsler reya RJ, Monroe JG. Immature stage B cells enter but do not progress beyond the early G1 phase of the cell cycle in response to antigen receptor signaling. J Immunol. 1996;156:4562. [PubMed] [Google Scholar]
- 27.Harada N, Santos-Argumedo L, Chang R, et al. Expression cloning of a cDNA encoding a novel murine B cell activation marker. Homology to human CD38. J Immunol. 1993;151:3111. [PubMed] [Google Scholar]
- 28.Lund FE, Yu N, Kim KM, Reth M, Howard MC. Signaling through CD38 augments B cell antigen receptor (BCR) responses and is dependent on BCR expression. J Immunol. 1996;157:1455. [PubMed] [Google Scholar]
- 29.Lund FE, Solvason NW, Cooke MP, et al. Signaling through murine CD38 is impaired in antigen receptor-unresponsive B cells. Eur J Immunol. 1995;25:1338. doi: 10.1002/eji.1830250531. [DOI] [PubMed] [Google Scholar]
- 30.Muhlethaler Mottet A, Otten LA, Steimle V, Mac HB. Expression of MHC class II molecules in different cellular and functional compartments is controlled by differential usage of multiple promoters of the transactivator CIITA. EMBO J. 1997;16:2851. doi: 10.1093/emboj/16.10.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin Q, Dong C, Cooper MD. Impairment of T and B cell development by treatment with a type I interferon. J Exp Med. 1998;187:79. doi: 10.1084/jem.187.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demengeot J, Vasconcellos R, Modigliani Y, Grandien A, Coutinho A. B lymphocyte sensitivity to IgM receptor ligation is independent of maturation stage and locally determined by macrophage-derived IFN-beta. Int Immunol. 1997;9:1677. doi: 10.1093/intimm/9.11.1677. [DOI] [PubMed] [Google Scholar]
- 33.Playfair JH, Purves EC. Separate thymus dependent and thymus independent antibody forming cell precursors. Nat New Biol. 1971;231:149. doi: 10.1038/newbio231149a0. [DOI] [PubMed] [Google Scholar]
- 34.Boniface JJ, Rabinowitz JD, Wulfing C, et al. Initiation of signal transduction through the T cell receptor requires the peptide multivalent attachment of MHC ligands. Immunity. 1998;9:459. doi: 10.1016/s1074-7613(00)80629-9. [DOI] [PubMed] [Google Scholar]
- 35.Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T cell clones. Nature. 1992;356:607. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 36.Rathmell JC, Cooke MP, Ho WY, et al. CD95 (fas) -dependent elimination of self-reactive B cells upon interaction with CD4+ T cells. Nature. 1995;376:181. doi: 10.1038/376181a0. [DOI] [PubMed] [Google Scholar]
- 37.Rathmell JC, Fournier S, Weintraub BC, Allison JP, Goodnow CC. Repression of B7.2 on self-reactive B cells is essential to prevent proliferation and allow Fas-mediated deletion by CD4+ T cells. J Exp Med. 1998;188:651. doi: 10.1084/jem.188.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benschop R, Melamed D, Nemazee D, Cambier J. Distinct signal thresholds for the unique antigen receptor-linked gene expression programmes in mature and immature B cells. J Exp Med. 1999;190:749. doi: 10.1084/jem.190.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]