Abstract
We have investigated methods for modulating immune responses, against herpes simplex virus (HSV), generated from DNA vaccination by co-delivery of genes encoding costimulatory molecules.A strong delayed-type hypersensitivity (DTH) reaction was induced in mice co-injected via the intradermal (i.d.) route with a eukaryotic expression plasmid encoding the CD80 molecule (pCD80) and a plasmid encoding the glycoprotein D of the HSV-2 (pgD). Furthermore, when spleen cells from these mice were cultured in the presence of inactivated HSV, a significant increase in the expression of interleukin-2 receptor (IL-2R) was observed in the CD4 subset compared with mice immunized only with pgD. Analysis of cytokine synthesis at the single-cell level indicated that CD80 genes induce a significant increase in the number of interferon-γ (IFN-γ)-, IL-2- and IL-4-secreting cells in the spleen. On the other hand, co-administration of the CD80 gene via the intramuscular (i.m.) route did not induce an increase in the cell-mediated immune response. When a plasmid carrying the CD86 gene (pCD86) was co-injected via the i.m. route with the pgD plasmid, a small decrease in the number of IFN-γ-secreting cells was observed. This down-regulation of the immune response was also observed when eukaryotic expression cassettes for CD80 and for CD86 were coadministered with the pgD plasmid via the i.d. route. However, co-injection of pCD86 via the i.m. route produced a small increase in the number of IL-4-secreting cells. When immunized mice were challenged intravaginally with 100 plaque-forming units of virus, only co-injection of the CD80 gene by the i.d. route provoked an adjuvant effect compared with mice immunized with pgD alone. A reduction in the titres of HSV in vaginal washings was observed together with a decrease in the lesion score
INTRODUCTION
Nucleic acid immunization is a new vaccination technology that delivers DNA constructs encoding a specific immunogen into host cells.1, 2 In addition to the ability of DNA vaccine to induce both antigen-specific cellular and humoral immune responses, this technique has the potential to manipulate the immune responses generated through the co-delivery of plasmids coding for immunologically important molecules. Recently it has been reported that specific immune responses generated by DNA vaccines could be modulated with the co-injection of gene expression cassettes for the costimulatory molecules CD80 and CD86.3, 4 In all of these studies, costimulatory molecules were injected by the intramuscular (i.m.) route. The basis of this strategy is that whilst muscle cells express or can be induced to express adhesion molecules, cytokines and major histocompatibility complex (MHC) class I and II molecules, they do not seem to express the costimulatory molecules required for efficient antigen presentation. The results obtained in these studies seem to indicate that the co-injection of CD86 genes by the i.m. route results in a greater enhancement of T-cell mediated immune responses than that of CD80 genes. In addition to muscle, the skin is a possible target tissue for genetic immunization. Gene expression in the skin was observed after bombardment with gold microparticles coated with plasmid DNA, 5 and also after the use of needle injection of plasmid DNA.6, 7 The skin-associated lymphoid tissues contain specialized cells such as keratinocytes, macrophages (Mφ) and Langerhans' cells that are involved in the initiation and further augmentation of immune responses. Langerhans' cells carry the antigen from the skin to draining lymph nodes where they function as professional antigen-presenting cells (APCs) for priming naïve T lymphocytes. Although the costimulatory molecules CD80 and CD86 are naturally present on APCs, a differential expression was observed. Dendritic cells, B cells and Langerhans' cells express, before activation, quantitatively higher levels of CD86 than CD80.8–10 It seems therefore reasonable to consider that co-delivery of costimulatory molecules by the intradermal (i.d.) route could change the kinetics of expression of these molecules, altering the immune response induced by DNA vaccination. In the present study we found that the co-injection of costimulatory molecules modulated the immune response against the glycoprotein D (gD) of the herpes simplex virus-2 (HSV-2) in a route-dependent manner. While the co-administration of CD86 genes by the i.m. route resulted in a weak increase of the T helper 2 (Th2)-mediated immune responses together with a down-regulation of the T helper 1 (Th1) response, no effect was observed with the CD80 gene. On the other hand, co-injection of CD80 genes by the i.d. route led to a strong increase in cell-mediated immune responses against gD of the HSV and an increase in the protection after an intravaginal challenge.
MATERIALS AND METHODS
Mice
Female BALB/c mice, 6–8 weeks of age, were purchased from Harlan (Milan, Italy) and maintained at the International Centre for Genetic Engineering and Biotechnology under standard conditions, according to Institutional Guidelines.
Virus
HSV-2 (strain MS) was grown in BHK cells and stored in aliquots at −80° until used. Titres were measured in Vero cells and expressed as plaque-forming units (PFU)/ml.
Media and reagents
Cells were cultured in RPMI 10% fetal bovine serum (Seromed, Berlin, Germany). Solid and liquid Luria–Bertani media (LB) were used to grow Escherichia coli. Media were supplemented, when required, with 100 µg/ml of ampicillin.
Bacterial strains and plasmids
The E. coli strain DH5α was used as host during the cloning experiments and for propagation of plasmids. Bacterial strains were routinely grown at 37° in LB broth or agar, supplemented when required with 100 µg/ml of ampicillin. The eukaryotic expression vector pCDNA3 (Invitrogen, Groningen, The Netherlands) was used for cloning gD of the HSV under the control of the immediate early promoter of cytomegalovirus. For immunization, plasmid was purified using ‘mega prep’ plasmid isolation columns (Qiagen, Hilder, Germany). Endotoxin levels of the plasmid preparations were determined using a Limulus Amebocyte Lysate Analysis kit (Sigma, St. Louis, MO).
Recombinant DNA techniques
DNA preparation, genetic manipulations, polymerase chain reaction (PCR) and transformation of bacteria were carried out according to standard protocols.11
Cloning of gD, CD80, CD86 and Fc cytotoxic T-lymphocyte antigen-4 (FcCTLA-4) into the eukaryotic expression vector pCDNA
HSV-2 MS strain (American Type Culture Collection [ATCC] no. VR-540; ATCC, Rockville, MD) DNA, used as template for the PCR, was prepared from nucleocapsids isolated from BHK cells. For construction of the eukaryotic expression vector pCDNAgD, a 1·2-kb fragment encoding the gD precursor gene was amplified by PCR using the following primers: forward, 5′-TCGGTCATAAACTGCATTGCGAACCACTAGTCG-3′; reverse, 5′-CCTAGTTTCCCTCCTTCTAGACTCCCTTTATGCGG-3′. The PCR product was cloned in the HindIII site of the eukaryotic expression vector pCDNA3 and completely sequenced.
To clone CD80 and CD86 cDNA, total RNA was purifed from peritoneal Mφ using the RNAzol reagent (Biotecx Laboratories, Inc., Friendwood, TX) and then reverse transcribed to cDNA using oligo-dT12–18 primers and M-MLV reverse transcriptase (Life Technology Inc., Rockville, MD). The CD80 and CD86 cDNAs were then amplified by PCR, cloned in the BamHI site of the pCDNA3 vector and completely sequenced. Primers used for CD80 amplification were: forward, 5′-TCCCCATCGGATCCTCCAAAGCATCTGAAGCTATGGCT-3′; and reverse, 5′-ACATAATACCATGAATTCCACATGGACAGAGAAGAA-3′. Primers used for CD86 amplification were: forward, 5′-GTAGACGGGTACCAGAACTTACGGA-3′; and reverse, 5′-TCAGCTGAGAACATTTTTGAATTCTGAGA-3′.
For the construction of FcCTLA-4, the region of the Fcγ2a encoding the hinge, CH2 and CH3 domains of the heavy chain were amplified from spleen cells by using reverse transcription (RT)–PCR. The cDNA was then amplified by PCR using region oligonucleotides designed to append unique BamHI and EcoRV restriction sites onto the 5′ and 3′ ends, respectively. The cDNA encoding the sequences for the leader and extracellular domains of CTLA-4 was amplified by PCR using oligonucletoides designed to append unique HindIII and BamHI restriction sites onto the 5′ and 3′ ends of this cDNA, respectively. The Fcγ2a and the ΔCTLA-4 fragments were digested with the appropriate enzymes and cloned into the pCDNA vector restricted at the cloning site with HindIII and EcoRV. The correct sequence was confirmed by complete DNA sequencing.
In vitro evaluation
Plasmid pCDNAgD was transfected in COS cells using lipofectin (Boehringer Mannheim, Mannheim, Germany) according to the manufacturer's instructions. The expression of gD was analysed by immunoblotting. Briefly, after 48 hr, cell lysates and cell supernatants were resolved by electrophoresis and then transferred to nitrocellulose membranes. Immunoblots were processed with an anti-gpD monoclonal antibody (ViroStat, Portland, ME) and developed using an enhanced chemiluminescence (ECL) detection kit (Amersham, Little Chalfont, Bucks, UK). The expression of CD80 and CD86 constructs was analysed by transfecting them into COS cells. Cells were harvested 48 hr after transfection and tested for expression using fluorescence-activated cell sorter (FACS) analysis with fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies (mAbs) for mouse CD80 and CD86 (PharMingen, San Diego, CA). The binding of CD80 and D86 to the counter-receptor CTLA-4 was determined by FACS analysis using the FcCTLA-4 molecule. COS cells transfected with pCD80 or pCD86 were incubated at 4° for 30 min with different dilutions of the supernatant of COS cells transiently transfected with the pCDNAFcCTLA-4 plasmid. After washing, cells were incubated with an appropriate dilution of an FITC-conjugated rabbit anti-mouse immunoglobulin G (IgG) and the binding was detected using a FACScalibur (Becton-Dickinson, Mountain View, CA).
Immunization and fluid collection
Mice were immunized on days 0, 15 and 30 with 50 µg of pCDNAgpD (or pCDNA) and 30 µg of pCDNACD80 or pCDNACD86 in a final volume of 100 µl of phosphate-buffered saline (PBS). The animals were immunized i.m. in the quadriceps muscle or i.d. in the footpads. Blood samples were obtained from the submaxillary sinus of the mice. Vaginal fluids were collected by pipetting 50 µl of PBS into and out of the vagina several times. The vaginal washings were then centrifuged to remove particulate matter and the supernatants were stored at −20°.
Challenge
In order to synchronize the oestrus cycle, the immunized mice were injected subcutaneously (s.c.) with Depo-Provera (DP) (Upjohn Co., Kalamazoo, MI) at a concentration of 3 mg per mouse in 100 µl of distilled water.12 Five days after the administration of DP, the animals were anaesthetized with Avertin and the vaginal cavity was washed with PBS before the instillation of 5 × 106 PFU of HSV in 20 µl. The mice were examined daily for vaginal inflammation, neurological illness and death. The severity of disease was scored on a scale of 0–5 (0, no symptoms; 1, mild inflammation; 2, moderate swelling; 3, severe inflammation; 4, paralysis; 5, death).13 Vaginal washings were collected at different time-points after intravaginal challenge by pipetting 200 µl of PBS into the vaginal cavity. The samples were filtered through 0·45-µm filters and stored at −80° until titred.
Enzyme-linked immunosorbent assay (ELISA)
To evaluate the levels of specific total IgG or IgG subclasses in serum and in vaginal washings, standard indirect ELISA was employed. Levels of total IgG, IgG1 and IgG2a were determined using affinity-purified rabbit anti-mouse Abs specific for γ, γ1 or γ2a, respectively, and as a second antibody an affinity-purified goat anti-rabbit horseradish peroxidase (HRP)-conjugated IgG (Zymed Lab. Inc. San Francisco, CA) was used. The two synthetic peptides (amino acids 8–23 and amino acids 222–252), corresponding to the main neutralizing in vitro epitopes,14, 15 were used for attachment to the plates.
Determination of cytokines after in vitro stimulation
Cytokines were assayed in supernatants of spleen cells (107 cells/ml) cultured for 24 or 96 hr in the presence of inactivated HSV or mock antigen. Cytokines were measured by sandwich ELISA using mAb pairs 1D11/BVD6-24G2, JES6-1A12/JES6-5H4 and R4-6A2/XMG1.2 (PharMingen) for interleukin (IL)-4, IL-2 and interferon-γ (IFN-γ) determination, respectively.
Cytokine ELISPOT assay
Fifteen days after the last immunization, mice were killed and spleens were aseptically removed. Single-cell suspensions were made, and eythrocytes were lysed with ammonium chloride. Cells were cultured for 24 hr at 107 cells/ml in the presence of an appropriate concentration of UV-inactivated HSV or mock antigen. Subsequently, cells were added in twofold dilutions to coated and blocked 96-well nitrocellulose bottom plates (Millipore, Bedford, MA). ELISPOT plates were precoated with anti-cytokine antibodies anti-(IL-2), anti-IL-4 and anti-IFN-γ (Pharmingen) at a concentration of 8 µg/ml in 50 µl of sterile 0·1 m sodium phosphate, pH 9·0, at 4° overnight, and the plates were then blocked with RPMI containing 10% fetal calf serum (FCS) for 30 min at 37°. After a 24-hr incubation, the ELISPOT plates were washed 10 times with PBS-Tween (PBS-T) and twice with H2O-Tween. Fifty microlitres each of biotinylated anti-IL-2, biotinylated anti-IL-4 and biotinylated anti-IFN-γ (PharMingen), diluted to 2 µg/ml in PBS-T, were added and the plates were incubated for 4 hr at room temperature. After six washes with PBS-T, 50 µl of 1:500-diluted peroxidase-conjugated streptavidin was added and the plates were incubated at room temperature for 1 hr. The spots were developed with the 3-amino-9-ethyl carbazole (AEC) substrate and counted using a dissecting microscope.
Determination of CD25 expression after in vitro stimulation
Expression of CD25 (IL-2 receptor [IL-2R]) responding to antigen stimulation in vitro was determined by FACS analysis. Spleen cells (1 × 107/ml) cultured for 96 hr in the presence of a 1 : 20 dilution of UV-inactivated HSV or mock antigen, were collected and double-stained with phycoerythrin (PE)-labelled anti mouse CD4 and with FITC-labelled anti-mouse IL-2R (PharMingen) for 30 min at 4°. Cells were washed twice with medium and a two-colour immunofluorescence analysis was performed using a FACScalibur (Becton-Dickinson), with gates set by forward angle light and side scatter. Gates were adjusted to include the discrete CD4+ mononuclear population, and 20 × 103 cells were analysed per sample.
Delayed-type hypersensitivity (DTH)
The DTH response to HSV was tested 15 days after immunization. The antigens were injected in 20-µl volumes into the right ear pinna and ear thickness was measured 24 hr later using a micrometer calliper (Oditest, Mitutoyo, Japan). The test antigen was the UV-inactivated HSV-2 with a titre of 108 prior to the inactivation. Mock antigen (BHK cell extract) was injected in the left ear pinna as a negative control. The ear thickness was measured in a blinded fashion before and 24 hr after the injection. The DTH reaction was expressed as the increase in ear thickness following ear pinna injection over the prechallenged thickness
Statistical analysis
The Student's t-test was used for comparison between two means. The normality of samples and homogeneity of variance was confirmed by the Kolmogorov–Smirnov test and the Scheffe–Box test, respectively. For non-parametric samples, the Mann–Whitney U-test was used.
RESULTS
Expression and binding ability of CD80 and CD86
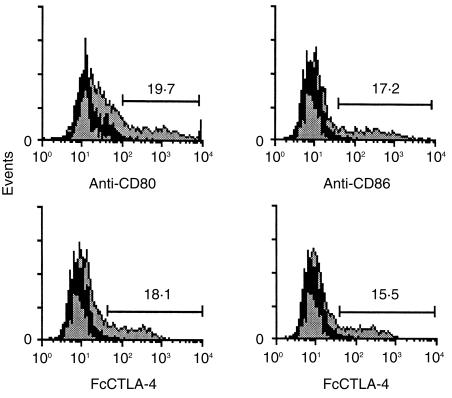
To study the expression and functionality of CD80 and CD86 constructs, COS cells were transfected with pCD80 and pCD86 plasmids. Transfected COS cells were incubated with specific FITC-conjugated mAb against CD80 and CD86 or with different dilutions of the supernatant from COS cells transfected with the pFcCTLA-4 plasmid, followed by incubation with an FITC-conjugated antibody against mouse immunoglobulin. FACS analysis indicated that both molecules were expressed with a similar efficiency. Furthermore, an equivalent proportion of cells expressing CD80 or CD86 molecules were detected when the cells were incubated with the soluble counter-receptor FcCTLA-4 or after incubation with the specific mAbs (Fig. 1). From these results it can be concluded that the binding ability of both molecules was conserved.
Figure 1.
Flow cytometric analysis of CD80 and CD86 expression in transfected COS cells. COS cells transfected with expression vector pCD80 or pCD86 (grey histograms) or with pCDNA3 (black histograms) were stained with fluorescein isothiocyanate (FITC)-conjugated specific monoclonal antibodies (mAbs) against CD80 or CD86 (upper panel). Binding to their counter receptor, cytotoxic T-lymphocyte antigen-4 (CTLA-4), was determined using fluorescence-activated cell sorter (FACS) analysis by incubating transfected COS cells with an appropriate dilution of FcCTLA-4 followed by incubation with a specific FITC-conjugated anti-mouse immunoglobulin G (IgG) (lower panel).
Humoral immune response
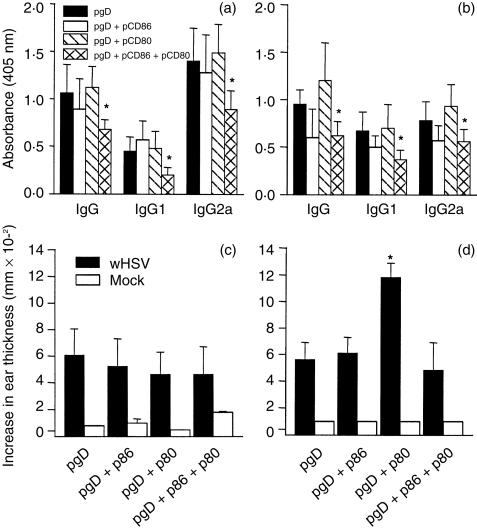
Fifteen days after the last immunization, sera samples were analysed by ELISA for reactivity against epitope 8–23 of the gpD. Co-immunization of the mice with pgpD and pCD80 or pCD86 either by the i.m. or the i.d. routes appeared to have no effect on the specific antibody immune response. However, co-injection of pCD80 with pCD86 resulted in a slight decrease in antibody titres (Fig. 2a).
Figure 2.
Subclass distribution of specific antibody response in serum (upper panel). Mice were immunized three times with 50 µg of expression vector pCDNAgpD or pCDNA, together with 30 µg of pCD80 or pCD86, by the intramuscular (i.m.) route (a) or intradermal (i.d.) route (b), as described in the Materials and methods. Fifteen days after the last dose, the level of antibodies in serum (dilution 1:100) against the epitope 8–23 was determined by using enzyme-linked immunosorbent assay (ELISA). *P < 0·05. Development of a delayed-type hypersensitivity (DTH) reaction in immunized mice (lower panel). Each group of six mice was immunized by the i.m. route (c) or the i.d. route (d) as described in the Materials and methods. For the DTH assay, each mouse was injected with either 108 UV-inactivated herpes simplex virus (HSV) (titrated before inactivation) in the right ear pinna or BHK extract in the left ear pinna. *P < 0·01. For statistical determinations, an analysis of variance (anova) was performed.
Cell-mediated immune response
The HSV-specific DTH reaction was analysed, 15 days after immunization, using the ear pinna swelling response. Co-injection of CD86 or CD80 genes with the pgpD plasmid by the i.m. route did not result in an increase in the ear thickness compared with injection of pgpD alone (Fig. 2c). On the other hand, co-injection of pCD80 by the i.d. route led to a significant increase in the DTH reaction (Fig. 2d) whereas no effect was observed by inoculation of the pCD86 plasmid. Interestingly, co-administration of the pCD86 plasmid by the i.d. route abolished the stimulatory effect observed following the inoculation of pCD80 when the genes for both costimulatory molecules were delivered together (Fig. 2d).
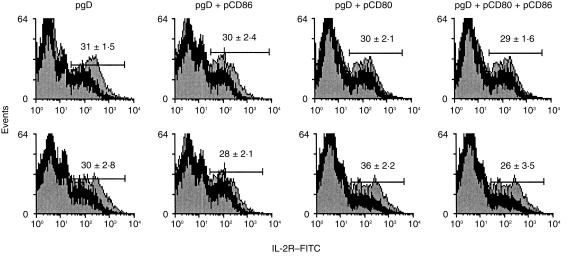
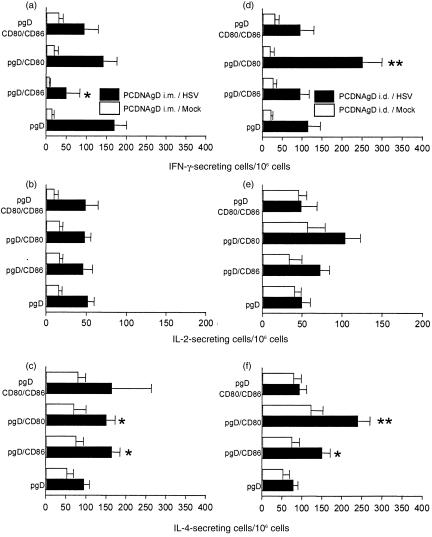
The effect of the co-administration of costimulatory molecules was also evaluated in vitro. Spleen cells from mice immunized by the i.m. or i.d. routes were cultured in the presence of UV-inactivated HSV or mock antigen. After 3 days of culture the expression of IL-2R on CD4+ T cells was analysed using FACS. Co-injection of pCD86 or pCD80 with pgpD by the i.m. route did not induce any effect on the expression of IL-2R compared with spleen cells from mice immunized with pgpD alone (Fig. 3). However, the results were different after i.d. immunization. An increase in the expression of IL-2R was observed in CD4+ T cells from mice co-immunized with pCD80, but not with pCD86. Furthermore, co-injection of both costimulatory molecules resulted in abolition of the effect observed by immunization with pCD80 (Fig. 3). Analysis of the cellular immune response at single-cell level was performed on spleen cells, using ELISPOT, after secondary stimulation in vitro. Co-injection of pgpD with pCD86 by the i.m. route resulted in an increase of the specific IL-4-secreting, but not of the IL-2-secreting, cells. Furthermore, a decrease in the number of IFN-γ secreting cells was observed in these mice. Co-injection of pCD80 did not have any effect on the number of specific, IL-2-and IFN-γ-secreting cells, but provoked an increase in the number of IL-4-secreting cells. When both costimulatory molecules were co-injected with pgpD, no effect was observed on the number of IL-4-, IL-2- or IFN-γ-secreting cells. (Fig. 4a, 4b, 4c). On the other hand, when CD80 genes were co-administered with pgpD by the i.d. route, a strong increase in the number of specific IFN-γ-, IL-4- and IL-2-secreting cells was observed. Co-inoculation of pgpD with pCD86 had no effect whereas co-administration of pCD86 with pCD80 abolished the stimulatory effect induced by pCD80 (Fig. 4d, 4e, 4f).
Figure 3.
Expression of the interleukin-2 receptor (IL-2R) by CD4+ spleen cells from mice immunized by the intramuscular (i.m.) route (upper panels) or by the intradermal (i.d.) route (lower panels). After in vitro stimulation with UV-inactivated herpes simplex virus (HSV) (grey histograms) or with mock antigen (black histograms) a cytofluorometric analysis was performed. A gate on CD4+ T cells was performed and 20 000 events were collected. Values represent the mean ±SD from five animals per group.
Figure 4.
Mice were immunized with the pCDNAgpD expression vector by the intramuscular (i.m.) route (panels a, b and c) or by the intradermal (i.d.) route (panels d, e and f), as described in the Materials and methods. Fifteen days after the last immunization, spleen cell suspensions were stimulated in vitro for 24 hr with UV-inactivated herpes simplex virus (HSV) or mock antigen. Frequencies of cytokine-producing cells were measured by ELISPOT assay. Pooled data from two experiments (three mice per group in each experiment) are presented. Bars represent the mean ±SE.
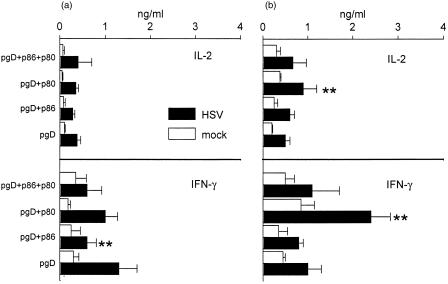
When the pattern of cytokines was analysed in the supernatant of cultured lymphocytes after in vitro stimulation, the results were generally in agreement with those described above (Fig. 5). However, a non-significant reduction in the levels of IFN-γ was observed after i.m. injection of pgpD together with pCD80 (Fig. 5a) and after i.d. inoculation of pgpD together with pCD86 (Fig. 5b).
Figure 5.
Profile of cytokines released by herpes simplex virus (HSV) or mock-stimulated spleen cells from mice immunized by the intramuscular (i.m.) route (left panel) or the intradermal (i.d.) route (right panel). Cells (107/ml) were cultured in quadruplicate in 24-well plates and after 24 hr of culture, supernatants were harvested and the level of cytokines was determined by using sandwich enzyme-linked immunosorbent assay (ELISA). Bars represent the mean ±SE from six mice per group. *P < 0·01.
Protection after an intravaginal challenge with HSV-2
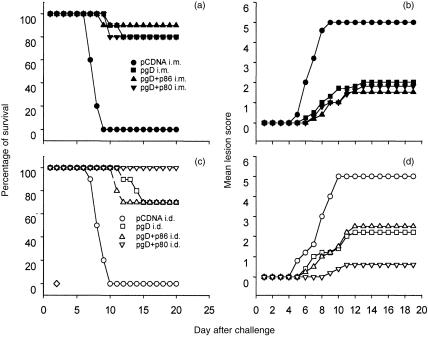
To analyse in vivo the effect induced by the co-delivery of costimulatory molecules, the different groups of mice were challenged intravaginally with 5 × 106 PFU of HSV-2. Animals were controlled for signs of disease, and vaginal washings were collected for virus titration. No effect was observed following the co-delivery of stimulatory molecules by the i.m. route. When the signs of disease were analysed, a similar lesion score was observed in mice co-immunized with pCD80 or pCD86 when compared to mice immunized with pgpD alone (Fig. 6). Furthermore, no differences were observed among the different groups in the virus titres determined in vaginal washings. (Table 1). When the groups of mice immunized by the i.d. route were challenged, a reduced lesion score was observed in mice that had received the pCD80 plasmid compared to mice immunized with pgpD alone (Fig. 6). Furthermore, this reduction in the clinical symptoms was accompanied by a significant decrease in the levels of virus measured in the vaginal washings (Table 1).
Figure 6.
Protective immune response after DNA immunization. Groups of eight mice were immunized as described in the Materials and methods. Fifteen days after the last immunization, mice were injected with 3 mg of Depo Provera (DP). Five days following the administration of DP, mice were challenged intravaginally with 5 × 106 plaque-forming units (PFU) of herpes simplex virus-2 (HSV-2) MS strain. Survival rates of controls and animals immunized with the expression vector pCDNAgpD alone or together with pCD80 or pCD86 by the intramuscular (i.m.) or intradermal (i.d.) route was calculated (left panel). Numerical scores were assigned to specific disease signs using the following scale: 0, no symptoms; 1, mild inflammation; 2, moderate swelling; 3, severe inflammation; 4, paralysis; 5, death. Daily mean lesion score was calculated by dividing the sum of a group's lesion scores by the number of observations (right panel).
Table 1.
Resistance to herpes simplex virus (HSV) challenge after DNA immunization†
| Log10 of viral titre at day postchallenge | ||||||
|---|---|---|---|---|---|---|
| Immunization | Route of immunization | No. of mice survived/no. of mice challenged | 1 | 3 | 5 | Higher mean lesion score |
| pCDNA | i.m. | 0/8 | 0·9 ± 0·2 (7/8) | 4·1 ± 1·2 (8/8) | 4 ± 0·8 (8/8) | 5 |
| pgpD | i.m. | 6/8 | 1 ± 0·3 (3/8) | 2·1 ± 0·7 (6/8) | 1·7 ± 0·6 (6/8) | 2·2 |
| pgpD + pCD80 | i.m | 6/8 | 1·1 ± 0·3 (1/8) | 2·2 ± 0·5 (5/8) | ND | 2 |
| pgpD + pCD86 | i.m. | 5/8 | ND | 2·5 ± 0·4 (5/6) | 2·3 ± 0·4 (5/6) | 1·8 |
| pgpD + pCD80 + pCD86 | i.m. | 5/8 | ND | 1·9 ± 0·6 (5/6) | 1·8 ± 0·4 (5/6) | 2 |
| pCDNA | i.d. | 0/8 | ND | 4·5 ± 0·6 (6/6) | 4·2 ± 0·8 (6/6) | 5 |
| pgpD | i.d. | 5/8 | ND | 1·8 ± 0·5 (4/6) | 1·9 ± 0·4 (4/6) | 2·3 |
| pgpD + pCD80 | i.d. | 8/8 | ND | 1·2 ± 0·2* (3/6) | 1·3 ± 0·2* (3/6) | 0·9* |
| pgpD + pCD86 | i.d. | 6/8 | ND | 2·3 ± 0·8 5/6 | 2·1 ± 0·9 5/6 | 2·7 |
| pgpD + pCD80 + pCD86 | i.d. | 5/8 | ND | 2·3 ± 1·2 6/6 | 2·0 ± 1·0 6/6 | 2·5 |
BALB/c mice were immunized via the intramuscular (i.m.) or intradermal (i.d.) route with expression vector pgpD alone or together with pCD80 or pCD86. The immunization was repeated three times at 15-day intervals. Fifteen days after the last dose, the mice were injected with 3 mg of Depo Provera (DP) per mouse. Five days following the administration of DP, the mice were challenged intravaginally with 100 lethal doses of herpes simplex virus-2 (HSV-2) MS strain. Vaginal viral titres were determined for each individual mouse.
P < 0·05 when compared with pgpD-immunized mice.
DISCUSSION
In the present study an analysis was performed of the immunomodulatory effect of CD80 and CD86 on the HSV-2-specific immune response induced by an HSV-2 DNA vaccine. After an i.m. DNA vaccination, muscle is the primary site of antigen expression. Although little is known about the mechanisms involved in antigen presentation after i.m. immunization with naked DNA, myocytes could have the ability to present antigens, as these cells weakly express MHC class I and class II antigens. For effective presentation of antigen, a second, antigen-non-specific stimulatory signal is essential for T-cell activation following the initial, antigen-specific T-cell receptor (TCR)/MHC signalling.16 Myocytes not expressing CD80 or CD86 could have a limited capacity to act as APCs. Subsequently, the transfection of myocytes with costimulatory molecules could result in these cells acting as professional APCs. Recently it has been reported that co-administration of CD86 with human immunodeficiency virus-1 (HIV-1) DNA vaccine cassettes by the i.m. route results in a significant increase of antigen-specific T-helper cell proliferation and of the antigen-specific CTL response.3, 4 On the other hand, co-immunization with CD80 seems to have a mimimal effect on the level of cellular immune response. Surprisingly, however, in this study it was found that co-injection of CD86 genes produced a decrease in the number of specific IFN-γ-secreting cells together with an increase in the number of IL-4-secreting cells in the spleen after antigen stimulation in vitro. Furthermore, when mice co-immunized with CD86 genes and pgpD were challenged intravaginally with 100 PFU of virus, no increase in the protection was observed. The differences between these results and those published by others could be owing to the fact that in the present report the T helper immune response was analysed whereas in previous reports the CTL response was mainly evaluated. As few MHC class II antigens are probably expressed, while high levels of MHC class I antigen should be expressed by muscle cells, it is logical that the immune response is directed towards MHC class I-restricted CD8+ cells. The results obtained after the co-injection of costimulatory molecules by the i.d. route were totally different. The i.d. co-injection of CD80 resulted in a strong enhancement of the cell-mediated immune response against the gpD of HSV, leading to an increase in protection after intravaginal challenge with 100 PFU of HSV-2. On the other hand, co-injection of CD86 led to a minor decrease in the cell-mediated immune response. Furthermore, co-injection of CD86 and CD80 genes abolished the stimulatory effect observed after co-injection of CD80 genes alone. The injection of plasmid DNA by the i.d. route results mainly in gene transfer to dendritic cells.17 Professional APCs naturally express costimulatory molecules. However, dramatic differences exist in the kinetics and the signals that control CD80 and CD86 expression.18, 19 In B cells and dendritic cells, the induction of CD86 occurs within hours after stimulation. In contrast, in CD80, expression is not detected until 24 hr poststimulation and only reaches maximal levels 48–72 hr later.20, 21 Furthermore, activated B cells and dendritic cells express quantitatively higher levels of CD86 than CD80.8
One interpretation of the results reported in the present study is that subsequent to the co-injection of pCD80 and pgpD, APCs in the skin were able to express CD80 molecules at the first stage of the induction of the immune response, leading to a more complete activation of T cells. On the other hand, there are several possible explanations as to why co-injection of CD86 did not result in an increase in the immune response to gpD. It is possible to reason that the levels of CD86 naturally expressed by the APCs were sufficient to effectively initiate the immune response. However, this does not explain the negative effect observed when the genes encoding both CD80 and CD86 were coinjected. In studies in which animals were immunized with irradiated tumour cells, only the CD80-transfected tumours generated tumour-specific CTLs and subsequently protected against tumour challenge.22, 23 Interestingly, the combined expression of CD80 and CD86 on the tumour cells was less effective in stimulating an antitumour response than the expression of CD80 alone.22, 24 These results are in agreement with those presented in this report and may reflect a competition between CD80 and CD86 for binding to CD28. A more suitable explanation for our results could be that the over-expression of CD86 by the transfectant (mainly dendritic cells) may hyperstimulate T cells, leading it to express high levels of CTLA-4 that could result in the transmission of a negative signal. Recently, it was reported that B7-2 expressed on EL-4 cells preferentially bound CTLA-4 receptor.25 It is therefore possible that CD86 expressed after DNA vaccination could bind CTLA-4 but not CD28 molecules. In fact, it was reported that CD86 was the main CTLA-4 ligand on mouse dendritic cells.8
In the past few years evidence has been presented indicating that CD28 co-stimulation may play distinct roles in the differentiation of T helper cells.26 Treatment with anti-CD80 resulted in the generation of effector T cells that had a Th2 phenotype. In contrast, treatment with anti-CD86 resulted in the production of effector cells of a Th1 phenotype.27–29 In the present work we present results indicating that the co-injection of CD80 by the i.d. route induces an increase in the number of specific IFN-γ-, IL-2- and IL-4-secreting cells. On the other hand, co-injection with the pCD86 plasmid resulted in an increase of Th2 cells and in a slight decrease of Th1 cells. These results taken together seem to support the idea that costimulatory molecules play an important role in Th1 and Th2 differentiation. However, the increased number of IL-4-secreting cells observed in mice co-immunized with genes for both costimulatory molecules renders it difficult to reconcile our results with a model where only CD80 and CD86 are involved in Th1 and Th2 differentiation, respectively. There are studies that do not support the preferential usage of CD80 versus CD86 in the induction of Th1 versus Th2 cells.30 In fact, IL-4 production by costimulation with murine CD80 and CD86 molecules has been described.31 Although the mechanisms of action and the interactions involved are not clear, all these results, including those presented herein, strongly suggest that costimulation plays a role in the differentiation of Th cells.
In conclusion, our data demonstrate that it is possible to modulate the immune response in a differential fashion by the co-administration of costimulatory molecules via different routes. Furthermore, our results strongly support the differential usage of CD80 and CD86 in T-cell activation.
Acknowledgments
We are grateful to Andres Muro for valuable discussion, Mauro Sturnega for animal care and Fondo Para el Hejoramiento de la Calidad Universitaria (FOMEC) for the fellowship awarded to Juan Fló.
REFERENCES
- 1.Fynan EF, Webster RG, Fuller DH, Haynes JR, Santoro JC, Robinson HL. DNA vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc Natl Acad Sci USA. 1993;90:11478. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ulmer JB, Donnelly JJ, Parker SE, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 3.Kim JJ, Bagarazzi ML, Trivedi N, et al. Engineering of in vivo immune responses to DNA immunization via codelivery of costimulatory molecule genes. Nat Biotechnol. 1997;15:641. doi: 10.1038/nbt0797-641. [DOI] [PubMed] [Google Scholar]
- 4.Tsuji T, Hamajima K, Ishii N, et al. Immunomodulatory effects of a plasmid expressing B7-2 on human immunodeficiency virus-1-specific cell-mediated immunity induced by a plasmid encoding the viral antigen. Eur J Immunol. 1997;27:782. doi: 10.1002/eji.1830270329. [DOI] [PubMed] [Google Scholar]
- 5.Tang DC, De Vit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356:152. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 6.Rax E, Carson DA, Parker SE, et al. Intradermal gene immunization: the possible role of DNA uptake in the induction of cellular immunity to viruses. Proc Natl Acad Sci USA. 1994;91:9519. doi: 10.1073/pnas.91.20.9519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hengge UR, Walker PS, Vogel JC. Expression of naked DNA in human, pig, and mouse skin. J Clin Invest. 1996;97:2911. doi: 10.1172/JCI118750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inaba K, Witmer-Pack M, Inaba M, et al. The tissue distribution of the B7-2 costimulator in mice: abundant expression on dendritic cells in situ and during maturation in vitro. J Exp Med. 1994;180:1849. doi: 10.1084/jem.180.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caux C, Vanbervliet B, Massacrier C, et al. B70/B7-2 is identical to CD86 and is the major functional ligand for CD28 expressed on human dendritic cells. J Exp Med. 1994;180:1841. doi: 10.1084/jem.180.5.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engel P, Gribben J, Freeman G, et al. The B7-2 (B70) costimulatory molecule expressed by monocytes and activated B lymphocytes is the CD86 differentiation antigen. Blood. 1994;84:1402. [PubMed] [Google Scholar]
- 11.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- 12.Parr MB, Kepple L, McDermott MR, Drew MD, Bonzzola JJ, Parr EL. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex type 2. Lab Invest. 1994;70:369. [PubMed] [Google Scholar]
- 13.Overall JC, Kern ER, Schlitzer RL, Friedman SB, Glasgow LA. Genital herpes virus infection in mice. I. Development of an experimental model. Infect Immun. 1975;11:476. doi: 10.1128/iai.11.3.476-480.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen GH, Dietzschold B, de Ponce Leon M, et al. Localization and synthesis of an antigenic determinant of herpes simplex virus glycoprotein D that stimulates the production of neutralizing antibody. J Virol. 1984;49:102. doi: 10.1128/jvi.49.1.102-108.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicola AV, de Ponce Leon M, Xu R, et al. Monoclonal antibodies to distinct sites on herpes simplex virus (HSV) glycoprotein D block HSV binding to HVEM. J Virol. 1998;72:3595. doi: 10.1128/jvi.72.5.3595-3601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bluestone J. New perspective of CD28–B7 mediated T cell costimulation. Immunity. 1995;2:555. doi: 10.1016/1074-7613(95)90000-4. [DOI] [PubMed] [Google Scholar]
- 17.Condon C, Watkins SC, Celluzzi CM, Thompson K, Falo LD., Jr DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2:1122. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 18.Linsley P, Greene JBW, Bajorath J, Ledbetter J, Peach R. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1:793. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 19.Van der Merwe P, Bodian D, Daenke S, Linsley P, Davis S. CD80 (B7-1) binds both CD28 and CTLA-4 with low affinity and very fast kinetics. J Exp Med. 1997;185:2515. doi: 10.1084/jem.185.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lenschow D, Su GH-T, Zuckerman L, et al. Expression and functional significance of an additional ligand for CTLA-4. Proc Natl Acad Sci USA. 1993;90:11054. doi: 10.1073/pnas.90.23.11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hathcock K, Laszlo G, Pucillo C, Linsley P, Hodes R. Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J Exp Med. 1994;180:631. doi: 10.1084/jem.180.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gajewsky T, Fallarino F, Uyttenhove C, Boon T. Tumor rejection requires a CTLA-4 ligand provided by the host or expressed on the tumor: superiority of B7-1 over B7-2 for active tumor immunization. J Immunol. 1996;156:2909. [PubMed] [Google Scholar]
- 23.Matulonis U, Dosiou C, Freeman G, et al. B7-1 is superior to B7-2 costimulation in the induction and maintenance of T cell-mediated antileukemia immunity. Further evidence that B7-1 and B7-2 are functionally distinct. J Immunol. 1996;156:1126. [PubMed] [Google Scholar]
- 24.Lenschow D, Walunas T, Bluestone J. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 25.Greenfield E, Howard E, Paradis T, et al. B7-2 expressed by T cells does not induce CD28-mediated costimulatory activity but retains CTLA-4 binding: implications for induction of antitumor immunity to T cell tumors. J Immunol. 1997;158:2025. [PubMed] [Google Scholar]
- 26.Thompson C. Distinct roles for the costimulatory ligands B7-1 and B7-2 in T helper cell differentiation? Cell. 1995;81:979. doi: 10.1016/s0092-8674(05)80001-7. [DOI] [PubMed] [Google Scholar]
- 27.Kuchroo V, Das M, Brown JRA, et al. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathway: application to autoimmune disease therapy. Cell. 1995;80:707. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 28.Freeman GBV, Anumanthan A, Bernstein G, et al. B7-1 and B7-2 do not deliver identical costimulatory signals, as B7-2 but not B7-1 preferentially costimulates the initial production of IL-4. Immunity. 1995;2:523. doi: 10.1016/1074-7613(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 29.Petro T, Chen S, Panther R. Effect of CD80 and CD86 on T cell cytokine production. Immunol Invest. 1995;24:965. doi: 10.3109/08820139509060721. [DOI] [PubMed] [Google Scholar]
- 30.Lanier L, O'fallon S, Somoza C, et al. CD80 (B7) and CD86 (B70) provide similar costimulatory signals for T cell proliferation, cytokine production, and generation of CTL. J Immunol. 1995;154:97. [PubMed] [Google Scholar]
- 31.Natesan M, Razi-Wolf Z, Reiser H. Costimulation of IL-4 production by murine B7-1 and B7-2 molecules. J Immunol. 1996;156:2783. [PubMed] [Google Scholar]