Abstract
Polyclonal rabbit anti-idiotypic (Ab2) antibodies raised against the antiprogesterone mAb DB3 (Ab1) were used to induce an Ab3 antiprogesterone response in BALB/c mice. While the af®nity of Ab3 sera for progesterone was 10±50-times lower than that of DB3, their steroid-binding speci®city showed considerable similarity to DB3. Two immunoglobulinM(IgM) Ab3 monoclonal antibodies (mAbs), 1A4 and 3B11, were obtained, both of which bound progesterone conjugated to bovine serum albumin (progesterone±BSA). 1A4 also bound free progesterone, although with low af®nity and very broad cross-reactivity. Like DB3, 1A4 is encoded by a heavy-chain variable region (VH) gene segment from the small VGAM3.8 family, a restriction that is characteristic of antibodies raised against progesterone-11α±BSA. In contrast, 3B11 binds progesterone-11α±BSA but not free progesterone and is encoded by an unrelated VH gene from the J558 family. The light chain variable region (VL) of 1A4 lacks the intradomain disulphide bridge owing to replacement of CysL23 by Tyr. Both the 1A4 and 3B11 heavy chains have extremely short complementarity determining region (CDR) H3 loops, comprising three and four amino acids, respectively. Modelling of the combining site of 1A4 from the X-ray crystallographic structure of DB3 indicates that the short H3 loop is a major factor in the loss of affnity and specificity for steroid.
INTRODUCTION
In an anti-idiotypic cascade, 1–3 the reference starting point is an antibody termed Ab1, the combining site of which is reactive with a particular antigen and characterized by a set of idiotopes that collectively comprise its idiotype (Id). Polyclonal or monoclonal anti-Ids constitute an Ab2 population, which is used to induce an anti-anti-idiotypic or Ab3 response. The latter includes antibodies that resemble Ab1 by reacting with the same antigen: they are designated Ab1′ and the Ab2 inducing them is termed the internal image of the antigen.1 Anti-Ids have been used to raise antibodies against, inter alia, various pathogens4–6 and tumour antigens7, 8 and have often been discussed as possible vaccines.2, 9
The relationship between Ab1 and Ab1′ antibodies has been explored in a number of different antigenic systems, including (4-hydroxy-3-nitrophenyl)acetyl (NP), 10p-azophenylarsonate (ARS), 11 poly(Glu60,Ala30,Tyr10) (GAT), 12 angiotensin, 13, 14 human leucocyte antigen (HLA) class II15 and lysozyme, 16, 17 and with either monoclonal10–12 or polyclonal anti-Id reagents.13, 17 One result obtained consistently is that among Ab3 antibodies are some which do indeed closely resemble the Ab1 in that they possess the same idiotopes, bind to the same antigen and derive from similar variable (V) genes. However, whilst the resemblance between Ab1′ and Ab1 can be very close, 13, 15, 17 many permutations of results have been seen. For example, binding of Ab3 to the antigen may not occur even although the same V-gene segments are selected, e.g. where the selection by anti-Id is directed towards the V segment rather than D and J idiotopes;11 or the affinity of Ab1′ antibodies for antigen may be lower than that of the Ab1, corresponding to differences in the complementarity determining region (CDR), particularly H3 sequences;12, 17 or the Ab3 may bind a similar antigen, yet diverge considerably from the Ab1 in V genes and CDRs.8 Thus, depending perhaps on the nature of the Ab2 reagent, an Ab3 population can be more or less heterogeneous and include molecules with a range of fidelities to the Ab1.
Haptens, or other antigens with simple epitopes, may induce antibodies encoded by a limited number of heavy chain variable region/light chain variable region (VH/VL) gene combinations. This is the case for antibodies to NP, 10 ARS, 11 GAT, 18 dextran, 19 phosphorylcholine (PC)20 and 2-phenyl-5-oxazolone (phOx).21 We have shown that the combination of a VH segment from the VGAM3.8 family together with the VK5.1 VL segment occurs repeatedly in antiprogesterone antibodies raised against progesterone linked to bovine serum albumin (progesterone-11α–BSA) in BALB/c mice.22–24 Of 13 antiprogesterone monoclonal antibodies (mAbs) sequenced, all used this combination of V-gene segments and have similar affinities (> 108/m) and cross-reactivities (references 22–24 and M.J. Taussig, unpublished). Moreover, although diverse D and J segments are used, the CDR H3 loops of the mAbs are of similar length and sequence, correlating with the combining site structure for steroid binding revealed by X-ray crystallographic analysis of the monoclonal antiprogesterone antibody DB3 and its steroid complexes.24–26 The residues that make contact with steroid in DB3 are, in general, conserved in all antiprogesterone mAbs studied, while those that are not involved directly in steroid binding, e.g. on H3, may be highly variable.24 Thus, in terms of binding-site architecture, essentially only one high-affinity binding pocket for progesterone appears to be selected in response to progesterone-11α–BSA.
It is possible that this is the only high-affinity site which the repertoire of the BALB/c mouse can generate for progesterone when presented as this conjugate, given the structural requirements for a steroid-binding pocket to accommodate the ligand with good complementarity and in a particular orientation. If so, one might ask whether a similar genetic and structural outcome would be found in antibodies against the same ligand raised by a route not directly involving antigen recognition, in particular an anti-idiotypic cascade. In order to compare antigen-binding members of the Ab3 population and an Ab1 with a structurally well-defined combining site, we induced antiprogesterone antibodies using a polyclonal rabbit anti-idiotype against DB3 (anti-DB3-Id). Previously we reported that immunization with this Ab2 induced antiprogesterone antibodies with serum concentrations of up to 100 µg/ml, sufficient to cause progesterone deficiency and infertility in female BALB/c mice.27, 28 Here we describe the characteristics of serum antiprogesterone Ab3 antibodies and the specificities and sequences of two monoclonal Ab3 antibodies capable of binding progesterone–BSA. The results show that the properties of these anti-anti-Ids deviate significantly from DB3 in specificity, affinity and genetic composition. Modelling the combining site of one of the Ab1′ mAbs provides an explanation for the reduction in affinity and specificity.
MATERIALS AND METHODS
Steroids and steroid–protein conjugates
Progesterone, progesterone-11α–hemisuccinate (HMS), desoxycorticosterone–HMS, aetiocholanolone, testosterone and 11α-hydroxyprogesterone-11-succinyl–BSA (progesterone-11α–BSA) were obtained from Sigma (St. Louis, MO), and progesterone-3-carboxymethyloxime (CMO) and 6α-hydroxyprogesterone–HMS were obtained from Steraloids Ltd. (Croydon, UK). All steroid stock solutions were prepared in glass tubes, at a concentration of 1 mg/ml in 100% ethanol.
Antiprogesterone antibodies
DB3 is a high-affinity mouse antiprogesterone antibody (immunoglobulin G1 [IgG1],κ).22, 29 11/64 is a mouse immunoglobulin M (IgM) antiprogesterone mAb.28, 29
Anti-Id antibodies
The preparation and characterization of affinity-purified polyclonal rabbit anti-DB3-Id was as described previously.30 The salt-precipitated immunoglobulin fraction of the antiserum was absorbed extensively with normal mouse immunoglobulin and purified by affinity chromatography on immobilized DB3 IgG.30 11/7 is a monoclonal rat anti-DB3-Id (IgG1), the specificity of which has been described previously.27
Murine immunizations
Female BALB/c mice were inoculated intraperitoneally (i.p.) with 30 μg of rabbit anti-DB3-Id in Freund's complete adjuvant (FCA) (Difco, Detroit, MI), 2 weeks later with 30 μg of rabbit anti-DB3-Id in Freund's incomplete adjuvant (FIA) and then given three further injections of 20 μg of antigen in phosphate-buffered saline (PBS) at 2-week intervals, the last being 3 days prior to fusion.
Monoclonal Ab3 antibodies
Spleen cells of BALB/c mice immunized with rabbit anti-DB3-Id were fused with the mouse myeloma line NS0. Hybridomas with progesterone-binding activity were identified by using enzyme-linked immunosorbent assay (ELISA) and steroid inhibition (discussed below).
ELISA
For detection of serum progesterone-binding antibodies by ELISA, 96-well microtitre plates were coated with progesterone-11α–BSA (100 µl, 3 µg/ml, 1 hr) and blocked with 10% fetal calf serum (FCS). After titration of the sera (100 µl/well) and appropriate washing, the plates were developed with goat horseradish peroxidase (HRP)-conjugated anti-mouse immunoglobulin (Sigma) and, after addition of substrate, the absorbance was read at 450 nm. For isotype determinations and assay of mAbs, the HRP conjugates used were goat anti-mouse IgG (γ-chain specific), goat anti-mouse IgM (μ-chain specific), goat anti-mouse immunoglobulin A [IgA] (α-chain specific) (Sigma) and sheep anti-mouse light chain (κ specific) (The Binding Site Co., Birmingham, UK). DB3 or 11/64 were used as standards.
Steroid inhibition
For specificity studies, the binding of monoclonal and serum Ab3 antibodies to progesterone-11α–BSA was carried out in the presence of increasing concentrations of different free steroids. Steroids were titrated in 10% FCS/PBS at twice the final assay concentration, and 100 μl was mixed with 100 μl of supernatant/serum and preincubated for 30 min at room temperature. One-hundred microlitres of the mixture was transferred to a progesterone-11α–BSA-coated, blocked microtitre plate and incubated for 2 hr at 4°. Detection and colour reaction was carried out as described above. The absorbance at 450 nm without inhibitor was defined as 100% binding; the IC50 is the steroid concentration producing 50% inhibition of binding.
DNA sequencing
Total RNA was prepared from hybridoma cells by lysis in guanidinium thiocyanate and subsequent ultracentrifugation through a caesium chloride cushion (using a modification of the procedure described in reference 31). For reverse transcription, to 2–3 μg of RNA were added 0·5 mm dNTP, 50 mm Tris-HCl (pH 8·3), 75 mm KCl, 3 mm MgCl2, 10 mm dithiothreitol (DTT) and 5 μm (dT)12−18 in the presence of 20 U of RNAse inhibitor (Boehringer Mannheim, Mannheim, Germany). After denaturation for 5 min at 94° under mineral oil and cooling to 25°, 1 μl of reverse transcriptase (murine-Moloney Leukaemia Virus RT; SuperScript, 200 U/μl; Gibco BRL, Paisley, UK) was added through the mineral oil and incubated for l hr at 37° at a final volume of 20 μl. cDNA was extracted once with phenol–chloroform and twice with chloroform.
For polymerase chain reaction (PCR) amplification of VH and VL sequences of the IgM mAbs, the constant-region primers MCμ (5′-GCTCTAGAGGA(A/G)ATGGTGCTGGGCAGGAAGTC-3′) (XbaI site in bold) and MJκ (5′-ACATCTAGAGGATACAGTTGGTGCAGCATC-3′) were used. The ‘universal’ V-region primers, capable of amplifying VH and VL genes of all families, were MVH (5′-AGGT(C/G)(A/C)A(G/A)CTCGAG(G/C)AGTC-(T/A)GG-3′) (XhoI site in bold) and MVL (5′-ACACTCGAGA(T/C)(G/A)T(T/C)(T/G)TG(A/C)TGACCCAAACT-3′). Digested PCR products were gel purified, ligated into the pBSIISK+ vector (Stratagene, Cambridge, UK) and cloned in Escherichia coli XL1-Blue. DNA sequencing was carried out on both strands of at least two clones using an automatic sequencing system (PE Biosystems, Warrington, UK).
Combining site modelling
The combining site of the 1A4 antibody was modelled using the program insight ii, version 97·0 (Biosym/MSI, San Diego, CA), using the structure of DB3 as the reference for VH and VL (pdb codes 1DBA uncomplexed and 1DBB complexed with progesterone). The antibody 21DH (K. Hotta & D. Hilvert, unpublished) was used as a comparison for the H3 loop.
RESULTS
Characterization of serum antiprogesterone Ab3
Quantification and isotyping
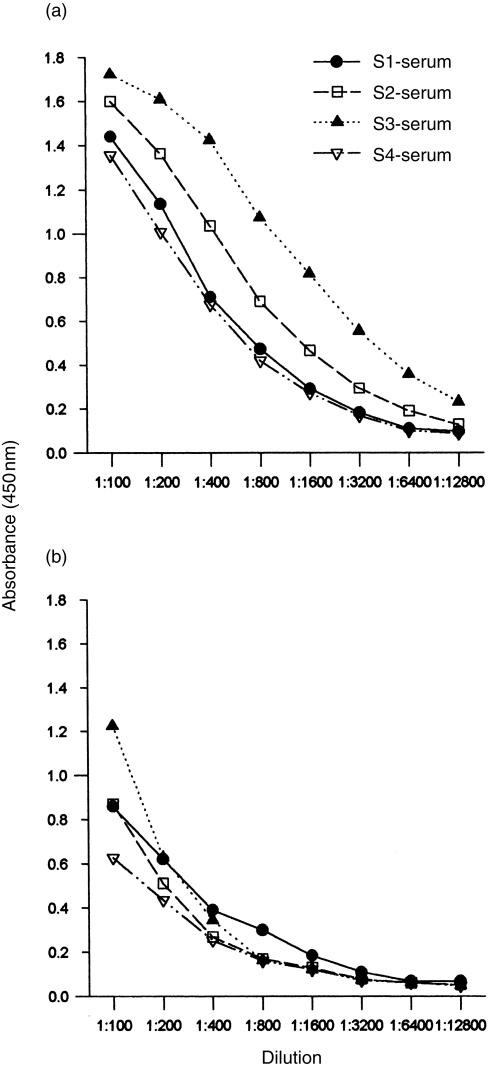
Four mice immunized with rabbit anti-DB3-Id all responded with production of antibodies that bound to progesterone-11α–BSA (Fig. 1). Using DB3 as a standard for quantification, the level of antigen-binding Ab3 IgG was 52 ± 28 μg/ml after five inoculations, with considerable variation between individual mice (range 134–13 μg/ml, sera S3 and S4, respectively). These should probably be regarded as minimum levels, as the determination is influenced by the relative affinities of the standard DB3 and the Ab3 sera (discussed below). As determined by using ELISA, IgG was the predominant isotype, with a minority of IgM (Fig. 1a, 1b).
Figure 1.
Enzyme-linked immunosorbent assay (ELISA) titration of binding to progesterone-11α–bovine serum albumin (BSA) by four Ab3 antibody (Ab3) sera (S1, S2, S3, S4) from mice immunized with the Ab2, rabbit anti-DB3-idiotype. (a) immunoglobulin G (IgG); (b) immunoglobulin M (IgM).
Affinity and cross-reactivity
Serum Ab3 IgG is of lower affinity and more cross-reactive than DB3
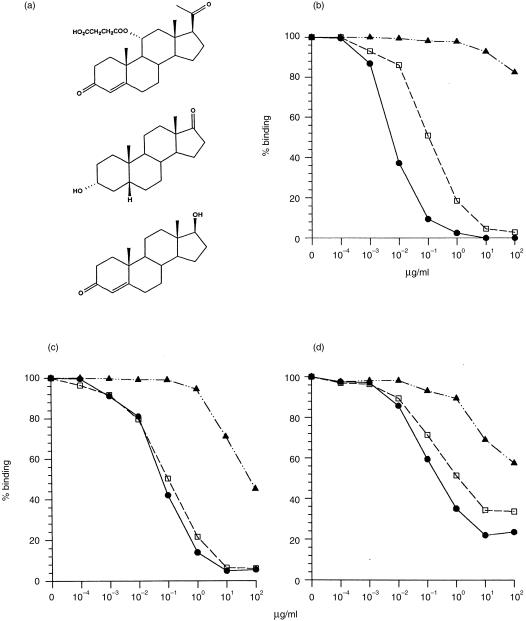
The antiprogesterone IgG antibodies in Ab3 sera were characterized in terms of relative affinity and cross-reactivity by inhibiting their binding to progesterone-11α–BSA by using different steroids. To test for the presence of DB3-like Ab3 antibodies, three different steroids were used as inhibitors, namely progesterone-11α–HMS, aetiocholanolone and testosterone (Fig. 2a). For DB3, the strongest competitor was progesterone-11α–HMS (IC50≈ 5 ng/ml); there was significant cross-reaction with aetiocholanolone (≈ 20-fold lower affinity), while inhibition by testosterone was extremely weak (Fig. 2b). A different pattern was seen with the serum Ab3 antibodies (Fig. 2c, 2d). The IC50 of progesterone-11α–HMS was 10–50 times higher than for DB3, while that for aetiocholanolone was similar and testosterone was found to be a better inhibitor of Ab3 antibodies than of DB3. Thus, the Ab3 pool had a lower affinity for progesterone-11α–HMS and greater cross-reactivity than the Ab1 mAb. These observations applied to both the S3 and S4 sera analysed in Fig. 2(c), 2(d).
Figure 2.
Steroid inhibition of DB3 and Ab3 antiprogesterone-11α–bovine serum albumin (BSA) sera. (a) Structures of steroid inhibitors: (from the top) progesterone-11α–hemisuccinyl (HMS), aetiocholanolone and testosterone. (b) Inhibition of DB3; (c) inhibition of serum S3 immunoglobulin G (IgG); and (d) inhibition of serum S4 immunoglobulin G (IgG) by progesterone-11α–HMS (•), aetiocholanolone (□) and testosterone (▴).
It can also be seen that whereas DB3 was completely inhibitable by free steroids, there are differences in this respect between the two Ab3 sera shown. Binding of serum S3 (Fig. 2c) was 95% inhibitable by progesterone-11α-HMS or aetiocholanolone, whereas S4 was only 65–75% inhibitable by the free steroids. Thus, both Ab3 populations included a minority that bound progesterone-11α–BSA conjugate but not free steroid.
IgM serum Ab3 is not steroid inhibitable
In contrast to the Ab3 IgG antibodies, which were largely inhibitable by free steroid, Ab3 IgM antibodies as a pool were not steroid inhibitable (data not shown). This is unlikely to be a consequence of multivalent IgM binding to the immobilized progesterone-11α–BSA on the ELISA plate, as the high-affinity IgM antiprogesterone mAb 11/64 is sensitively inhibited by steroid in same assay (see Fig. 5a, 5c).
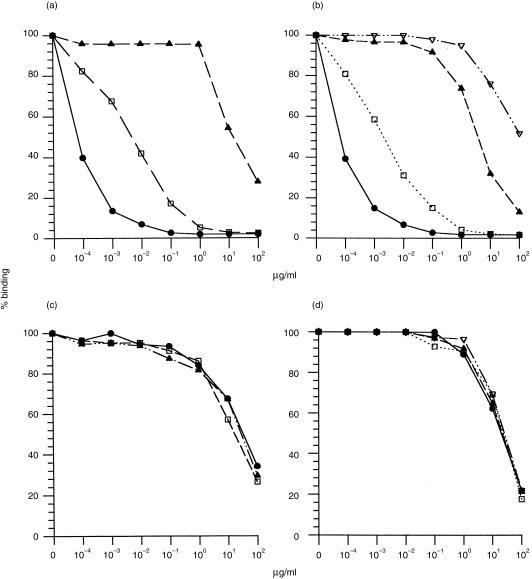
Figure 5.
Steroid specificity of monoclonal Ab3 antibody (mAb3) 1A4 compared with antiprogesterone immunoglobulin M (IgM) 11/64. (a) Inhibition of 11/64 by progesterone-11α–hemisuccinyl (HMS) (•), aetiocholanolone (□) and testosterone (▴). (b) Inhibition of 11/64 by progesterone-11α−HMS (•), progesterone-6β–HMS (▴), progesterone-3–carboxymethyloxime (CMO) (□) and progesterone-21-HMS (▿). (c) and (d) Inhibition of 1A4 by the same steroids as in (a) and (b), respectively. (For the reactivity of Ab1 DB3 with these steroids cf. Figs 2b and 3b.)
Fine specificity of Ab3 for progesterone conjugates
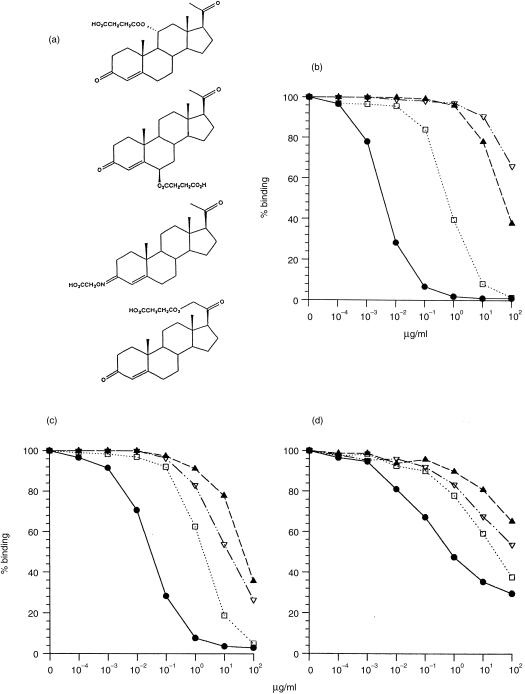
The Ab3 sera were also tested for inhibition of binding to progesterone-11α–BSA by four different progesterone conjugates, in which an HMS or CMO group was linked to the steroid nucleus at positions C3, C6, C11 or C21 (Fig. 3a). The affinity pattern obtained from this inhibition test is related to the orientation of bound steroid in the antibody pocket.24–26 Thus, for DB3 the efficacy of inhibition was progesterone-11α–HMS > progesterone-3–CMO > progesterone-6β–HMS > progesterone-21–HMS (Fig. 3b). This reflects the fact that in the structure of the progesterone–DB3 complex, the steroid atoms C11 and C3 are exposed to solvent while C21 and C6 are buried. Discrimination between these conjugates by the Ab3 sera was less than that of DB3, but the order of reactivity was similar. The inability to inhibit S4 completely with free ligand was again noticeable (Fig. 3d). In both sera S3 and S4, progesterone-11α–HMS was the preferred ligand, although with a lower affinity (high IC50) than DB3, followed by progesterone-3–CMO; however, inhibition by the weakly cross-reactive ligands was reversed (progesterone-21–HMS > progesterone-6β–HMS) compared with DB3, owing to increased reactivity with progesterone-21–HMS. Finally, the range of IC50 values between the ligands, indicative of specificity, was clearly less for the Ab3 than for DB3. The result is consistent with a lower average affinity for progesterone in the Ab3 population than the Ab1 DB3, but with a similar orientation of steroid in the binding site.
Figure 3.
Inhibition of DB3 and Ab3 antiprogesterone–bovine serum albumin (BSA) sera with four progesterone conjugates. (a) Structures of conjugates: (from the top) progesterone-11α–hemisuccinyl (HMS), progesterone-6β–HMS, progesterone-3-carboxymethyloxime (CMO), progesterone-21–HMS. (b) Inhibition of DB3 (c) inhibition of serum 3 IgG and (d) inhibition of serum 4 IgG by progesterone-11α–HMS (•), progesterone-6β–HMS (▴), progesterone-3–CMO (□) and progesterone-21–HMS (▿).
Idiotypy
Inhibition by polyclonal Ab2
Binding of Ab3 to progesterone-11α–BSA was inhibited by the polyclonal Ab2 used for immunization. For the four Ab3 sera, the rabbit anti-DB3-Id showed an IC50 of 1–5 μg/ml. Binding of DB3 itself was inhibited with the same IC50 (data not shown).
Private anti-Id
To examine whether a private DB3 idiotope was present at a significant level in the Ab3 antiprogesterone antibodies, the sera were tested for inhibition by the monoclonal rat anti-DB3-Id 11/7.27 While this efficiently blocks binding of DB3, it did not affect binding of Ab3 antibodies to progesterone–BSA (results not shown).
Monoclonal Ab3 antibodies
As summarized in Table 1, 22 hybridomas producing monoclonal Ab3 antibodies (mAb3) binding to progesterone–BSA were obtained from mouse 4 (donor of serum S4). Of these, 19 were IgM, two IgG and one IgA. Whereas all could be inhibited by progesterone-11α–BSA, only two, both IgM, were inhibitable by free progesterone (i.e. steroid binding). Two Ab3 hybridomas, 1A4 and 3B11, were cloned for further analysis; both mAbs were IgM/κ as determined by ELISA and sequencing.
Table 1.
Hybridomas obtained and their specificity
| % Inhibition by | |||
|---|---|---|---|
| No. of hybridomas | Isotype* | Progesterone | Progesterone– BSA |
| 2 | IgM,κ | 80 | 70 |
| 8 | IgM,κ (7) IgM,λ (1) | 20–30 | 90–95 |
| 12 | IgM,κ (9) IgG,κ (2) IgA,κ (1) | 0–10 | 70–80 |
The value in parenthesis indicates the number of hybridomas found of that particular type.
BSA, bovine serum albumin.
Affinity and specificity
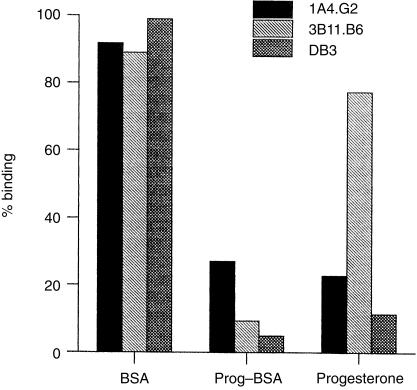
Initial characterization showed that 1A4, but not 3B11, was steroid inhibitable. Figure 4 shows binding of the two mAb3 molecules and DB3 to progesterone–BSA in the presence of 100 µg/ml of free BSA, progesterone-11α–BSA or progesterone. Binding of 1A4 was inhibited by 75% by progesterone whereas 3B11 showed only slight inhibition (20%). Neither mAb3 was inhibited by free BSA, but both were efficiently inhibited by progesterone-11α–BSA. DB3 was inhibited both by progesterone and progesterone-11α–BSA.
Figure 4.
Specificity of antiprogesterone-11α–bovine serum albumin (BSA) monoclonal Ab3 antibody (mAb3). The histogram shows binding to progesterone–BSA of 1A4 (black), 3B11 (light grey cross hatch) and DB3 (dark grey) in the presence of 100 µg/ml of BSA, progesterone-11α–BSA or free progesterone. 1A4.G2 and 3B11.B6 are individual clones of the mAbs used.
The inhibition tests performed on serum Ab3 with different free steroids were repeated on 1A4 (Fig. 5). As standards, antiprogesterone antibodies 11/64 and DB3 were both used; the specificity characteristics and sequences of these Ab1 mAbs are similar, and 11/64 (affinity 9·5 × 109) was used as an IgM reference for assay purposes (Fig. 5a, 5b). Figure 5(c) shows inhibition of 1A4 by progesterone-11α–HMS, aetiocholanolone and testosterone. There was a dramatic loss of specificity, 1A4 being unable to distinguish these steroids, in contrast to DB3 (Fig. 2b) and 11/64 (Fig. 5a). The affinity of 1A4 was also greatly reduced, with an IC50 for progesterone-11α–HMS of 44 μm compared with 12 nm for DB3. Similarly, when the progesterone conjugates coupled at different ring positions were used as inhibitors (Fig. 5d), 1A4 bound all four with the same high IC50, whereas DB3 and 11/64 distinguished them with affinities covering a range of five orders of magnitude (Figs 3b, 5b). The non-specificity of 1A4 also contrasts with the Ab3 serum S4, where there is clear steroid discrimination (Fig. 2d, 3d). As noted, 3B11 essentially bound only progesterone–BSA and was not inhibited by any of the free steroids.
V genes of mAb3
1A4
The VH and VL sequences of 1A4 are shown in Fig. 6(a) 6(b) (and summarized in Table 2). The VH gene segment used, VFM1, is a member of the VGAM3.8 family.23, 32 This gene has also been seen in antibodies to poly Ala-poly Lys, where it was designated VGK7, 32 and in an anti-insulin antibody and related anti-Id.33 However, while a VGAM3.8 gene has been seen in all antiprogesterone Ab1 mAbs we have sequenced to date, 23, 25 no Ab1 antiprogesterone mAb has been reported with the VFM1 segment, DB3 itself being encoded by the VMS9 gene.
Figure 6.
Nucleotide and translated sequences of variable (V) genes of monoclonal Ab3 antibody (mAb3) 1A4 and 3B11 compared with germline gene segments. (a) Heavy chain variable region (VH) of 1A4 aligned with VFM1, 34 DFL16.1 and JH4. (b) Light chain variable region (VL) of 1A4 aligned with V 21E/1.5 kb36 and J 2. (c). VH of 3B11 aligned with VH104B and JH3 (D segment not identified). (d) VL of 3B11 aligned with 17s.539 and Jκ4.
Table 2.
Genes contributing to DB3 and Ab3 antibodies
| mAb | Isotype | VH family | VH gene | D | JH | VL gene | JL |
|---|---|---|---|---|---|---|---|
| DB3 | IgG1 | VGAM3.8 | VMS9 | * | JH1 | VK5.1 | Jκ1 |
| 1A4 | IgM | VGAM3.8 | VFM1 | DFL16.1 | JH4 | Vκ21E | Jκ2 |
| 3B11 | IgM | J558 | VH104B | * | JH3 | Vκ19 | Jκ4 |
Not deducible from the sequence.
IgG, immunoglobulin G; IgM, immunoglobulin M; mAb, monoclonal antibody.
In 1A4, VFM1 was linked to a short D-segment (derived from DFL16.1); position 94, the V–D junction, was ATT (Ile) rather than the germline AGA (Arg), owing to the recombination point with DFL16.1. The JH segment was a JH4, which starts downstream at Trp103, lacking the first six residues compared with the germline.34 As a result, the CDR3 of 1A4 is remarkably short, comprising only three D/N coded amino acids, compared with 10 residues (five D/N, five JH coded) in DB3 (Fig. 7). The 1A4 CDR H3 loop was one of the shortest recorded of any heavy chain; out of 1288 antibodies sequenced, six had a two-residue H3 and 12 had three residues.35
Figure 7.
Amino acid sequences of variable (V) regions of monoclonal Ab3 antibodies (mAb3) 1A4 and 3B11 aligned with those of DB3. (a) Heavy chain variable region (VH) (b) Light chain variable region (VL). Asterisks denote residues that make contact with ligand in the DB3-progesterone-11α–hemisuccinyl (HMS) complex.24
The VH segment of 1A4 appeared to have undergone no somatic mutation; the only divergence from the VFM1 germline gene was that noted at the V–D junction. Similarly, the D segment can be accounted for by the DFL16.1 germline segment with only one base change proximal to the 3′ end, while the JH4 sequence was also germline from the junctional position.
The VL segment of 1A4 had 98% nucleotide and 95% amino acid sequence identity with the Vκ21E/1·5-kb gene36 and was recombined with a Jκ2 segment (Fig. 6b). It was thus distinct from the characteristic light chain of Ab1 antiprogesterone antibodies, which in all cases reported are encoded by the VK5.1 gene, with Jκ1 and Jκ5 as preferred J segments. There was evidence of somatic mutation, with five differences from the putative germline gene Vκ21E; all the apparent mutations encoded substitutions and all but one (L51) were in framework regions. Of particular note is the replacement of CysL23 by Tyr, preventing formation of the internal disulphide bridge; substitution of one of the VL cysteine residues is seen in only 0·6% (18 out of 2985) of VL sequences, 34, 37 Cys to Tyr being the most frequent. It is possible that the absence of the intrachain bridge has an adverse effect on antigen binding by destabilizing the domain.37
3B11
The VH and VL genes of 3B11 were unrelated to those of DB3 or 1A4. The closest VH segment is VH104B, 38 of the J558 family, from which 3B11 differed by seven nucleotides encoding five replacements (Fig. 6c). This was linked to a four amino acid D/N segment (D segment not identified) and a JH3 segment. As in 1A4, the JH was shorter than the germline by six residues and started at TrpH 103. Thus, the H3 loop was again very short, comprising only the four amino acids encoded by the D/N segment. The VL region of 3B11 consisted of a Vκ19 family segment39 linked to the Jκ4 segment.
Structural comparison of Ab3 and Ab1
CDR sequences
The VH and VL sequences of DB3, 1A4 and 3B11 are aligned in Fig. 7. The asterisked residues are those that were in contact with ligand in the complex of DB3 and progesterone-11α–HMS.24 Those with a major role in steroid binding in that structure were AsnH35, TrpH50, TyrH97 and TrpH100 in the heavy chain, and HisL27d, ValL94 and ProL96 in the light chain.
As noted, the only common feature between the mAb3 sequences and DB3 was the use of a VGAM3.8 family VH gene in 1A4 (Fig. 7a). Overall, the homology of the VDJ sequence of 1A4 to DB3 was 82% at the DNA level and 78% at the protein level. Owing to the VGAM3.8 gene, the framework and CDR2 of 1A4 were very similar to those of DB3, including the contact residue TrpH50; there were just two differences out of eight residues in the CDR2 (H53, H54). However, the use of VFM1 rather than VMS9 meant that the CDR1 sequences differed in four of five positions, including the contact residue AsnH35, which was substituted by His in 1A4. The VH of 3B11 was encoded by the J558 family rather than by a VGAM3.8 gene, but there was some sequence overlap in CDR1 with 1A4 (MetH34, HisH35) although none with DB3; the CDR2 of 3B11 was quite different from that of 1A4/DB3 and in particular had Glu at H50 rather than Trp.
The heavy chain CDR3 regions of both the Ab3 antibodies were considerably shorter than that of DB3 (three and four residues for 1A4 and 3B11 compared with 10 for DB3), which clearly will have major structural implications for the combining site. In terms of sequence, there were no common H3 residues between 1A4 and DB3 and, whereas DB3 had four aromatic groups, 1A4 had none. The 3B11 CDR3 was similar to 1A4 in length; while 3B11 has one aromatic residue, like 1A4 it has no significant sequence similarity to DB3.
There was even less homology between Ab3 and Ab1 in the light chains, the V segments of which were all derived from different groups (Fig. 7b). Amino acid sequence identity between the VL segments of 1A4 and DB3 was only 54%. The CDR L1 of 1A4 was one residue shorter than DB3, while that of 3B11 was five shorter, and there was considerable sequence diversity. In particular HisL27d, which in DB3 makes contact to steroid, was not present in either Ab3. The L3 loop, which plays a significant role in DB3 complexes, was also different and neither Ab3 had the Pro,Pro (L95,96) sequence of DB3 and several other antiprogesterone mAbs.
Combining site interactions
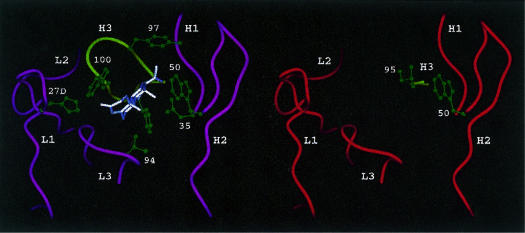
In the DB3–steroid complexes studied by X-ray crystallography, 24–26 contacts were made from five of the six CDRs, all of which contributed significant interactions (Fig. 8a). L2 did not contribute to the steroid pocket, but may be involved in interaction with the carrier protein. In the pocket, tryptophan residues from H2 and H3 (TrpH50, TrpH100) created a binding ‘sandwich’ for the steroid nucleus (A and D rings), while two other H3 aromatic groups (TyrH97, PheH100b) completed the hydrophobic binding of the steroid D ring. Hydrogen bonds donated from AsnH35 and HisL27d formed contacts to the polar extremities of progesterone (O20 and O3, respectively), contributing significantly to binding energy and specificity. The orientation of the steroid, with atoms C11 and C3 exposed and C6 and C20 buried, explains the ability of DB3 to discriminate ligands with substitutions at these positions Fig. 2b, 3b). Cross-reactivity of DB3 with aetiocholanolone occurred through an alternative binding orientation in which the steroid was rotated 180° around its long axis.25 This was made possible by a cavity around the A and B rings, where there is less complementarity with the antibody surface. Thus, the particular architecture of the DB3 site determines the characteristic specificity and cross-reactivity of this antibody, which is shared by all the mAbs raised against progesterone-11α–BSA.24
Figure 8.
(a) Structure of the combining site of the DB3–progesterone complex,24 showing the main chain of the complementarity determining region (CDR) loops and highlighting contact residues in green (AsnH35, TrpH50, TyrH97, TrpH100, PheH100b, HisL27d and ValL94). (Note, the side-chain of PheH100b is shown but not numbered). (b) Model of the combining site of 1A4, showing the short H3 loop and residues IleH95 and TrpH50. No attempt to model the different light chain has been made.
This architecture, or a close approximation of it, would probably be required to replicate these properties in an anti-anti-idiotype. For the Ab3 mAbs studied here this is only partially the case. Although both 1A4 and 3B11 have the ability to bind progesterone-11α–BSA, and also free steroids at low affinity, in the case of 1A4, only one of the characteristic steroid contact residues of DB3 recurred in either of the mAb3 antibodies, namely TrpH50 in 1A4 (Fig. 8b). In DB3, this residue makes contact with four atoms on the β-face of the steroid D ring and contributes ≈ 9% to the area of the combining site.25 AsnH35, which makes an H-bond interaction with the carbonyl oxygen O20 of progesterone, is substituted by His in 1A4 and 3B11. It is possible that histidine could play a similar H-bonding role to asparagine; however, as no Ab1 induced by progesterone–BSA has been observed to use the VFM1 gene, the effect on affinity or specificity of HisH35 is unknown.
Apart from these similarities, the other important interactions of the heavy chain, namely those made by H3, were all absent owing to the extreme shortness of this loop in 1A4 and 3B11 (Fig. 8b). In DB3, three H3 aromatic residues (TyrH97, TrpH100 and PheH100b) interacted with the top, α-face and base of the steroid, respectively. A model of the H3 loop of 1A4 was created in insight II and confirmed by reference to the recently determined crystal structure of a catalytic antibody 21D8 (K. Hotta et al. unpublished); the H3 loop of 21D8 was the same length as in 1A4 and also had an Ile as the first residue (IleH95). Replacing the H3 loop of DB3 with that of the 1A4 model clearly opened a large gap such that interactions to the α-face and top of the steroid would be missing (Fig. 8b). Were a steroid to occupy the same position in 1A4 as in DB3, interactions to the D ring (C15, C16) might be made by IleH95, but the other two H3 residues, and particularly ArgH97, would be orientated away from the steroid. The loss of major interactions and the reduced binding site complementarity consequent on the very short H3 loops readily explain the low affinity of both Ab3 for free steroids (non-detectable in the case of 3B11). The fact that 1A4 binds free steroids but does not discriminate between ligands of distinct steric properties Fig. 5c, 5d) suggests that a number of different steroid orientations may be accommodated equally in the 1A4 site, whereas in DB3 several are excluded. The space produced by the short H3 loop could make such accommodation possible, explaining the poor specificity.
Light chain interactions from L1 and L3, which maintain the orientation and binding affinity of progesterone in DB3, were all different in the Ab3. Thus, HisL27d, which makes an H-bond to the ketone O3 in DB3 (Fig. 8a), was absent in 1A4 and 3B11, both of which had a Thr at the equivalent sequence position. Moreover, as the L1 loops of the Ab3 were both shorter than in DB3, it was not clear whether an equivalent H-bond might be made using Thr as a donor. For L3, the key steroid interactions in DB3 were with ValL94, which forms van der Waals interactions to the A and B rings (C5, C6) and the C19 methyl group (Fig. 8a); it is possible that these could be similarly made from Leu (1A4) or Tyr (3B11) residues. The base of the L3 loop was clearly different in the Ab3; where DB3 had prolines at positions L95 and L96, 1A4 had TyrL95 and a shorter L3 loop, while 3B11 had ProL95 and IleL96.
DISCUSSION
Here we have described the serum Ab3 antiprogesterone-11α-BSA response induced by a polyclonal rabbit Ab2 against the antiprogesterone antibody DB3 and characterized two mAb3 obtained from an Ab2-immunized mouse. The observations show that while the anti-idiotype readily induces progesterone-specific antibodies, they are heterogeneous in their genetic and structural characteristics and in general exhibit lower affinity and greater cross-reactivity than the Ab1. While this was partly evident from serum analysis, it was exemplified in detail by the 1A4 mAb3.
All four Ab2-immunized mice responded with the production of Ab3 IgG antibodies capable of binding free progesterone. Their serum concentrations were relatively low (≈ 50 µg/ml) compared with Abl responses after immunization with progesterone-11α–BSA, consistent with previous observations.27 Using a number of related steroids to analyse the serum Ab3 population, the affinity and fine specificity of the Ab3 IgG antibodies differed from the Ab1 (DB3) pattern, the lower average affinity being correlated with higher cross-reactivity Figs 2, 3). Nevertheless, despite the overall reduction in affinity by one to two orders of magnitude, the fine specificity of the Ab3 population showed remarkable similarities in significant respects to that of DB3; the ability of the Ab3 pool to recognize structurally distinct steroids in a similar order of reactivity to DB3 implies a considerable degree of structural relatedness between Ab1 and the majority of the Ab3. Idiotypically, the Ab3 differed from DB3 in lacking a characteristic private idiotope (11/7). Thus, while the Ab1′ population retained sufficient similarity to suggest considerable representation of DB3-like binding sites, it was an inexact replica of the Ab1. There was also evidence of heterogeneity of response in different mice, e.g. Ab3 in serum S3 were nearly 100% steroid inhibitable, whereas those of serum S4 were only partially so.
A marked isotype-related difference in specificity of the Ab3 antiprogesterone antibodies was noted. After three immunizations, the major component of the ELISA titre was IgG, which bound progesterone-11α–BSA and was inhibited by free steroid; in contrast, the low-titre IgM population binding progesterone-11α–BSA was mostly not inhibitable by steroid at concentrations up to 100 µg/ml (≈ 3 × 10−4m). Thus, affinity for steroid and closer resemblance to Ab1 are associated with class-switching, the expected outcome if better affinity for anti-idiotype leads to increased reactivity with steroid. However, the IgG population also included mAbs that were not sensitive to free steroid, as shown by the two IgG antiprogesterone-11α–BSA mAb3 obtained Table 1).
Although several Ab3 mAbs were obtained from a fusion from mouse S4, only two were steroid inhibitable and most were IgM. Thus, they do not represent the IgG Ab1′ population that is most closely related to DB3. Similarity to DB3 was less pronounced in serum S4 than in other immunized mice; while a population of the S4 Ab3 antibodies was relatively specific for progesterone-11α–HMS, a significant fraction was not inhibited at free steroid concentrations of > 1 μg/ml (Figs 2d, 3d). Both 1A4 and 3B11 belong to this low-affinity population; 1A4 is steroid inhibitable, but with an IC50 of ≈ 5 µg/ml (cf. DB3, ≈ 1 ng/ml), and 3B11 could not be inhibited by progesterone concentrations up to 100 µg/ml. The fact that most of the mAb3 obtained were non-inhibitable by steroid suggests that the low-affinity population is larger than the ELISA titres suggest.
Sequence analysis of the two mAb3 demonstrates an important aspect of the anti-anti-idiotypic response, namely spreading away from the original Ab1 by inducing combining sites with different structural characteristics. The DB3 Ab1 antibody is encoded by VMS9, a member of the small and proportionately little expressed VGAM3.8 family of VH genes, and VK5.1, a frequently expressed light chain gene. The polyclonal Ab2 was rendered selective for DB3 by absorption with normal serum IgG and elution from a DB3 column; ≈ 10% of the reactivity with DB3 was seen with other VGAM3.8 antiprogesterone antibodies, suggesting that the anti-idiotype might act as a VH-specific reagent.30 There is a very close relationship between the VGAM3.8 family and antiprogesterone antibodies, in that the antiprogesterone Ab1 response appears to be entirely VGAM3.8 encoded. However, not all of the eight or nine VGAM3.8 genes23, 32 have been seen among the high-affinity antiprogesterone mAbs isolated from hyperimmunized mice;23, 26 in particular, genes have not been found in which the DB3 steroid-contact residue AsnH35 is replaced by His (VFM1, VMS1) or Ser264. Induction by the polyclonal anti-Id of these gene products is one predictable cause of a lower affinity in the Ab3. This is the case for the mAb3 1A4, for which the encoding VGAM3.8 gene is VFM1, and suggests that the selective process that gave rise to the Ab3 included recognition of epitopes determined by the VGAM3.8 framework.
In addition to the use of a different VH segment, the H3 loop of 1A4 is considerably shorter than that of DB3 and the light chain is not VK5.1. The implications of these differences have been considered in detail in the description of the 1A4 model above and lead to a clear explanation for the low affinity and absence of steroid discrimination by 1A4. Nevertheless, it is impressive that even in a combining site with only one contact residue in common with the Ab1, the selection of a VGAM3.8 gene product still allows some steroid binding to occur.
In the case of mAb 3B11, which also binds progesterone-11α–BSA, the lack of resemblance of the binding pocket to DB3 in practically all respects explains its inability to bind free steroid. It is probable that interaction with the BSA carrier is significant to the binding reaction by 3B11. However, there is very little to suggest how this antibody resembles DB3 in idiotope because all the CDRs are different; there are some shared residues in L2, but otherwise the sequence relatedness is distant. Hence, the basis of selection of an Ab3 that binds the original ligand (progesterone-11α–BSA), but with no evident relationship to Ab1 in terms of CDRs or V-gene usage, is unclear. mAbs that differ completely from the Ab1 can be expected to occur as part of the non-antigen-binding Ab3, which recognizes the Ab2 as idiotype; however, in that case, the ability to bind progesterone-11α–BSA would not be expected.
3B11 also possesses the intriguing structural feature of a very short H3 loop, which as noted must be a major part of the explanation of poor steroid-binding ability. In both 1A4 and 3B11, generation of the CDR H3 requires a rearrangement to occur within the JH segment such that the first six JH residues are excluded, although different JH segments are involved and there is no similarity in D or N region other than length. There is no indication why a short H3 loop was selected by anti-Id; it is not a feature of the Ab3 in other systems, including those where polyclonals have been used.
Others have shown that Ab1′ antibodies can be closely related in genetic origin and serological specificity to the Ab1. Examples of this using polyclonal Ab2 are the Ab3 against angiotensin13, 14 and hen egg lysozyme.16, 17 In the latter, the mAb3 had an affinity for lysozyme that was two orders of magnitude lower than the Ab1 (D1.3), despite being encoded by similar VH and VL genes; this was attributed to one or two critical changes in contact residues on H3 or L2. It was concluded that the Ab3 mAbs had a better affinity for the inducing Ab2 than for the antigen. Our own results demonstrate that the use of a polyclonal Ab2 leads to a heterogeneous Ab3 response, comprising some antibodies that appear to have a rather close resemblance to the Ab1 together with others having a more distant genetic makeup. Whilst we were not able to obtain mAb3 of the relevant IgG population for characterization, the lower affinity of the mAb3 we did obtain clearly relates to the fact that their genetic constitutions (and hence their CDRs) are radically different from that of the Ab1 antibody. The effect of the Ab2 is thus to act as an imprecise mimic of the antigen. A similar ‘spreading’ of an Ab3 response away from the initial Ab1 has also been described in Ab3 to human immunodeficiency virus (HIV), where low fidelity was seen to have the useful effect of broadening viral neutralization.5 However, such diversification of the antibody response by anti-idiotypic immunization is achieved at the expense of affinity and specificity. As might be expected, most if not all alterations from the precise combining site selected against the inducing antigen are deleterious to these basic parameters.
Acknowledgments
We thank Ms Maureen Hamon and Dr Hong Liu for technical assistance and Dr Andrei Popov for excellent technical advice. We thank Dr K. Hotta, Dr D. Hilvert and Dr I. A. Wilson for the opportunity to view the unpublished structure of antibody 21DH. The work was supported by the Biotechnology and Biological Sciences Research Council (UK) and by a travel award from the British Council.
Glossary
Abbreviations
- mAb3
monoclonal Ab3 antibody
- ARS
p-azophenylarsonate
- BSA
bovine serum albumin
- CDR
complementarity determining region
- FCS
fetal calf serum
- GAT
poly(Glu60,Ala30,Tyr10)
- H1
H2, H3, CDRs of the heavy chain
- HMS
hemisuccinyl
- HRP
horseradish peroxidase
- IC50
concentration of inhibitor producing 50% inhibition of a binding reaction
- Id
idiotype
- L1
L2, L3, CDRs of the light chain
- NP
(4-hydroxy-3-nitro-phenyl)acetyl
- PBS
phosphate-buffered saline
- PC
phos-phorylcholine
- PCR
polymerase chain reaction
- phOx
2-phenyl-5-oxazolone
- V
variable
- VH
heavy chain variable region
- VL
light chain variable region.
REFERENCES
- 1.Jerne NK. Idiotypic networks and other preconceived ideas. Immunol Rev. 1984;79:5. doi: 10.1111/j.1600-065x.1984.tb00484.x. [DOI] [PubMed] [Google Scholar]
- 2.Nisonoff A. Idiotypes: concepts and applications. J Immunol. 1991;147:2429. [PubMed] [Google Scholar]
- 3.Bona CA. Internal image concept revisited. Proc Soc Exp Biol Med. 1996;213:32. doi: 10.3181/00379727-213-44033. [DOI] [PubMed] [Google Scholar]
- 4.Anders EM, Kapaklis-Deliyannis GP, White DO. Induction of immune response to influenza virus with anti-idiotypic antibodies. J Virol. 1989;63:2758. doi: 10.1128/jvi.63.6.2758-2767.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boudet F, Keller H, Kieny MP, Theze J. Single peptide and anti-idiotype based immunizations can broaden the antibody response against the variable V3 domain of HIV-1 in mice. Mol Immunol. 1995;32:449. doi: 10.1016/0161-5890(95)00007-2. [DOI] [PubMed] [Google Scholar]
- 6.Su S, Ward MM, Apicella MA, Ward RE. A nontoxic, idiotope vaccine against Gram-negative bacterial infections. J Immunol. 1992;148:234. [PubMed] [Google Scholar]
- 7.Caton AJ, Herlyn D, Ross AH, Koprowski H. Identical D region sequences expressed by murine monoclonal antibodies specific for a human tumor-associated antigen. J Immunol. 1990;144:1965. [PubMed] [Google Scholar]
- 8.Flamini G, Marche PN, Cazenave PA, Natali PG, Siccardi AG, Viale G. Idiotypic replica of an anti-human tumour-associated antigen monoclonal antibody. DNA sequence comparison between Ab1 and Ab3. Scand J Immunol. 1991;34:373. doi: 10.1111/j.1365-3083.1991.tb01559.x. [DOI] [PubMed] [Google Scholar]
- 9.Taussig MJ. Anti-idiotypic antibodies and their uses. In: Animal Cell Biotechnology. In: Spier R E, Griffiths J B, editors. Vol. 4. London: Academic Press; 1990. p. 344. [Google Scholar]
- 10.Takemori T, Tesch H, Reth M, Rajewsky K. The immune response against anti-idiotope antibodies I. Induction of idiotope-bearing antibodies and analysis of the repertoire. Eur J Immunol. 1982;12:1040. doi: 10.1002/eji.1830121210. [DOI] [PubMed] [Google Scholar]
- 11.Margolies MN, Wysocki LJ, Sato VL. Immunoglobulin idiotype and anti-anti-idiotype utilise the same variable region genes irrespective of antigen specificity. J Immunol. 1983;130:515. [PubMed] [Google Scholar]
- 12.Roth C, Somme G, Schiff C, Theze J. Immune response against poly (Glu60,Ala30,Tyr10) pGAT: immunization with monoclonal anti-idiotypic antibodies leads to the predominant stimulation of idiotypically similar immunoglobulins with anti-GAT activity. Eur J Immunol. 1985;15:576. doi: 10.1002/eji.1830150609. [DOI] [PubMed] [Google Scholar]
- 13.Garcia KC, Ronco PM, Verroust PJ, Brunger AT, Amzel LM. Three-dimensional structure of an angiotensin II-Fab complex at 3 Å: Hormone recognition by an anti-idiotypic antibody. Science. 1992;257:502. doi: 10.1126/science.1636085. [DOI] [PubMed] [Google Scholar]
- 14.Garcia KC, Desiderio SV, Ronco PM, Verroust PJ, Amzel LM. Recognition of angiotensin II: antibodies at different levels of an idiotypic network are superimposable. Science. 1992;257:528. doi: 10.1126/science.1636087. [DOI] [PubMed] [Google Scholar]
- 15.Iwasaki Y, Takabatake H, Shinji T, Monestier M, Ferrone S. Structural profile of idiotype, anti-idiotype and anti-anti-idiotype monoclonal antibodies in the HLA-DQ3 antigenic system. Eur J Immunol. 1994;24:2874. doi: 10.1002/eji.1830241144. [DOI] [PubMed] [Google Scholar]
- 16.Goldbaum FA, Velikovsky CA, Dallacqua W, et al. Characterisation of anti-anti-idiotypic antibodies that bind antigen and an anti-idiotype. Proc Natl Acad Sci USA. 1997;94:8697. doi: 10.1073/pnas.94.16.8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldbaum FA, Braden BC, Poljak RJ. The molecular basis of antigen mimicry by anti-idiotypic antibodies. Immunologist. 1998;6:13. [Google Scholar]
- 18.Schiff C, Milili M, Hue I, Rudikoff S, Fougereau M. Genetic basis for expression of the idiotypic network. One unique Ig VH germline gene accounts for the major family of Ab1 and Ab3 (Ab1′) antibodies of the GAT system. J Exp Med. 1986;163:573. doi: 10.1084/jem.163.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lundkvist I, Ivars F, Holmberg D, Coutinho A. The immune response to bacterial dextrans. V. A ‘dominant’ idiotype in IgCHb mice. J Immunol. 1987;138:4395. [PubMed] [Google Scholar]
- 20.Perlmutter RM, Crews ST, Douglas R, et al. The generation of diversity in phosphorylcholine-binding antibodies. Adv Immunol. 1984;35:1. doi: 10.1016/s0065-2776(08)60572-6. [DOI] [PubMed] [Google Scholar]
- 21.Berek C, Milstein C. Mutation drift and repertoire shift in the maturation of the immune response. Immunol Rev. 1987;96:23. doi: 10.1111/j.1600-065x.1987.tb00507.x. [DOI] [PubMed] [Google Scholar]
- 22.Deverson E, Berek C, Taussig MJ, Feinstein A. Monoclonal BALB/c anti-progesterone antibodies use family IX variable region heavy chain genes. Eur J Immunol. 1987;17:9. doi: 10.1002/eji.1830170103. [DOI] [PubMed] [Google Scholar]
- 23.Sims MJ, Krawinkel U, Taussig MJ. Characterisation of germline genes of the VGAM3.8 VH gene family from BALB/c mice. J Immunol. 1992;149:1642. [PubMed] [Google Scholar]
- 24.Arevalo JH, Hassig CA, Stura EA, Sims MJ, Taussig MJ, Wilson IA. Structural analysis of antibody specificity. J Mol Biol. 1994;241:663. doi: 10.1006/jmbi.1994.1543. [DOI] [PubMed] [Google Scholar]
- 25.Arevalo JH, Taussig MJ, Wilson IA. Molecular basis of cross-reactivity and the limits of antigen-antibody complementarity. Nature. 1993;365:859. doi: 10.1038/365859a0. [DOI] [PubMed] [Google Scholar]
- 26.Arevalo JH, Stura EA, Taussig MJ, Wilson IA. Three-dimensional structure of an anti-steroid Fab and progesterone-Fab complex. J Mol Biol. 1993;231:103. doi: 10.1006/jmbi.1993.1260. [DOI] [PubMed] [Google Scholar]
- 27.Wang MW, Heap RB, Taussig MJ. Blocking of pregnancy in mice by immunisation with anti-idiotype directed against monoclonal antiprogesterone antibody. Proc Natl Acad Sci USA. 1989;86:7098. doi: 10.1073/pnas.86.18.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang MW, Sims MJ, Symington PR, Humphreys AS, Taussig MJ. Induction of anti-progesterone immunity and pregnancy blocking by anti-progesterone anti-idiotypes. Variable efficacy of polyclonal Ab2 antibodies directed against a panel of closely related Ab1 antibodies. Immunology. 1991;73:348. [PMC free article] [PubMed] [Google Scholar]
- 29.Ellis ST, Heap RB, Butchart AR, et al. Efficacy and specificity of monoclonal antibodies to progesterone in preventing the establishment of pregnancy in the mouse. J Endocrinol. 1988;118:69. doi: 10.1677/joe.0.1180069. [DOI] [PubMed] [Google Scholar]
- 30.Taussig MJ, Brown N, Ellis S, et al. Anti-idiotypic sera against monoclonal anti-progesterone antibodies: production in rabbits and rats and characterisation of specificity. Immunology. 1986;58:445. [PMC free article] [PubMed] [Google Scholar]
- 31.Chirgwin JM, Przybyla AE, McDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 32.Press JL, Giorgetti CA. Molecular and kinetic analysis of an epitope-specific shift in the B cell memory response to a multideterminant antigen. J Immunol. 1993;151:1998. [PubMed] [Google Scholar]
- 33.Thomas JW. Anti-insulin and regulatory anti-idiotypic antibodies use the same germline VHIX gene. Eur J Immunol. 1992;22:2445. doi: 10.1002/eji.1830220938. [DOI] [PubMed] [Google Scholar]
- 34.Kabat EA, Wu TT, Perry HM, Gottesman KS, Foeller C. Sequences of Proteins of Immunological Interest. 5. Bethesda, MD, USA: National Institutes of Health; 1991. [Google Scholar]
- 35.Wu TT, Johnson G, Kabat EA. Length distribution of CDRH3 in antibodies. Proteins. 1993;16:1. doi: 10.1002/prot.340160102. [DOI] [PubMed] [Google Scholar]
- 36.Heinrich G, Traunecker A, Tonegawa S. Somatic mutation creates diversity in the major group of mouse immunoglobulin light chains. J Exp Med. 1984;159:417. doi: 10.1084/jem.159.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Proba K, Honegger A, PĻckthun A. A natural antibody missing a cysteine in VH: consequences for thermodynamic stability and folding. J Mol Biol. 1997;265:161. doi: 10.1006/jmbi.1996.0726. [DOI] [PubMed] [Google Scholar]
- 38.Cohen JB, Givol D. Allelic immunoglobulin VH genes in two mouse strains: possible germline gene recombination. EMBO J. 1983;2:2013. doi: 10.1002/j.1460-2075.1983.tb01693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tillman DM, Jou N, Hill RJ, Marion TN. Both IgM and IgG anti-DNA antibodies are the products of a clonally selective B cell stimulation in (NZB × NZW) F1 mice. J Exp Med. 1992;176:761. doi: 10.1084/jem.176.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]