Abstract
Despite the capacity for antigen-specific activation and rapid clonal expansion, homeostatic mechanisms ensure that the mature immune system contains a relatively stable number of T cells. In recent years, it has become apparent that this stability is a consequence of apoptotic death of most of the specific T cells generated during an immune response. Clearly this process must be tightly regulated in order to retain sufficient T-cell progeny to mediate an effective response, whilst allowing the rapid deletion of these cells at the end of the response to prevent lymphadenopathy and cross-reactive autoimmunity. In this study, the factors that regulate the sensitivity of T cells to apoptosis were investigated in vitro after the induction of primary T-cell activation within a mixed lymphocyte reaction (MLR). It was found that activated T cells rapidly acquire the expression of both Fas and Fas ligand (FasL) on their surface and contain high levels of the precursor form of the pro-apoptotic enzyme, caspase 8 (FLICE). However, these T cells were resistant for up to 5 days to apoptosis following the stimulation of Fas; a maximal apoptotic response was observed after 7 days. This time point coincided with a marked reduction in expression of the FLICE inhibitory protein (FLIP) and maximal activity of caspase 8. At time points beyond day 7, the number of viable cells in the MLR decreased further despite a reduction in the expression of FasL. However, the expression of interleukin-2 (IL-2) at these late time points was low, resulting in a decrease in expression of the anti-apoptotic protein Bcl-2. This can produce apoptosis by allowing leakage of cytochrome-c from mitochondria resulting in direct activation of the caspase cascade. In this study, it is shown that T cells are resistant to apoptosis for the first 5 days after activation as a consequence of insensitivity of the Fas pathway and the presence of intracellular Bcl-2. After between 5 and 7 days, the cells become sensitive to Fas-mediated apoptosis while retaining Bcl-2 expression. At later time points, Fas ligation is reduced but the cells respond to a decreased availability of IL-2 by reducing Bcl-2 expression; this encourages further apoptosis by allowing the direct activation of caspase enzymes.
Introduction
Developed almost 40 years ago, 1 the mixed lymphocyte reaction (MLR) has become an integral tool in the field of immunological research. Following definition of the structures of the major histocompatibility complex (MHC) antigen–peptide complex and the T-cell receptor (TCR), it became clear that the basis of T-cell alloreactivity in MLR was essentially similar to the recognition of nominal peptide epitopes presented by self-MHC molecules. 2 Thus, the MLR provides a convenient in vitro method to study the processes of normal T-cell activation and proliferation in primary culture. More recently, researchers have utilized the MLR to evaluate the critical process of lymphocyte apoptosis in homeostatic regulation of the immune system.
Lymphocytes have a remarkable ability to maintain a state of numerical equilibrium, despite responding to a diverse array of pathogen-derived antigens by proliferation. Once an infection has been overcome by an immune response, most of the expanded subpopulation of specific lymphocytes is eliminated; this restores equilibrium and, more importantly, reduces the potential for cross-reactive autoimmune damage. Indeed, in mature T cells, programmed cell death following TCR stimulation is considered a mechanism for termination of an immune response and for the maintenance of peripheral tolerance. In recent years, the regulatory mechanisms involved in this process of apoptotic cell death have been increasingly understood.
It is now apparent that activated T cells may be cleared by two distinct death pathways following an immune response. Lymphocytes that are deprived of survival stimuli, such as cytokines, down-regulate anti-apoptotic proteins such asBcl-2. 3 This results in what is termed ‘passive cell death’. 4 However, activated lymphocytes can also be induced to die at the peak of their response by a process known as ‘activation-induced cell death’ (AICD). 5 This is caused by ligation of the Fas (CD95) cell-surface receptor, 6 and is not prevented by constitutive expression of Bcl-2 or Bcl-xL. 3
The Fas antigen is a 45 000 MW transmembrane protein which has sequence homology with other members of the tumour necrosis factor receptor family; 7 it is widely distributed on normal body cells. The importance of Fas for immune homeostasis is illustrated by study of mice with the lpr mutation or humans suffering from Canale–Smith syndrome. In both cases, defective Fas results in lymphadenopathy and the expression of autoimmune lesions in the liver and other vital organs. During the past few years the biochemical processes that link Fas to the enzymes that mediate apoptosis have been defined.8, 9
It has been demonstrated that cross-linking Fas results in the assembly of a death-inducing signalling complex (DISC), which results in the activation of caspase enzymes and the induction of apoptotic cell death. 10 Caspases are a family of proteases that can be activated within the cell; following activation, they cleave their cellular substrates after aspartic acid residues and produce the changes associated with apoptosis. 11 Caspase 8 (FLICE) plays a major role in mediating Fas-induced apoptosis 12 as it can interact with Fas and is therefore the most receptor proximal caspase in the pathway. Following activation, caspase 8 can provide a pro-apoptotic signal by initiating a proteolytic cascade of activation of other members of the caspase family that ultimately results in cell death. 13
It has been shown that T cells only acquire sensitivity to Fas-mediated apoptosis some days after antigen-specific activation.14, 15 The current study was designed to investigate the molecular events that regulate apoptosis by application of an MLR system, which allows the processes of primary T-cell activation to be modelled in vitro. During this series of experiments, particular emphasis was placed on definition of the contribution of AICD and passive cell death to regulation of the number of viable, antigen-specific T cells present within the MLR at various time points after the initiation of culture.
Materials and methods
Cell culture
Epstein–Barr virus-transformed B-cell lines (EBV) were generated by culture of tonsil-derived lymphocytes in medium conditioned by the EBV-secreting B95-8 cell line (ECACC 8501419), in the presence of cyclosporin A at 1 µg/ml (Sigma, Poole, UK) to prevent overgrowth by antigen-specific T cells. 16
All cell cultures were routinely tested for the common species of Mycoplasma by enzyme-linked immunoassay (Boehringer Mannheim, Lewes, UK); no infection was observed.
PBL preparation and culture
Peripheral blood mononuclear cells (PBMC) were prepared from heparinized venous blood taken from healthy adult volunteers. This blood was diluted 1:1 with RPMI-1640 medium (Life Technologies, Paisley, UK), underlayed with Ficoll–metrizoate at 1·077 g/ml (Lymphoprep; Nycomed, Birmingham, UK) and centrifuged at 400 g for 25 min. The interfacial cells were then harvested, washed twice and resuspended at a concentration of 1 × 106 cells/ml in lymphocyte medium consisting of RPMI-1640 supplemented with 10% fetal bovine serum (FBS) and antibiotics.
Peripheral blood lymphocytes (PBL) were purified from the PBMC preparation by the removal of plastic-adherent cells during culture at 37° for 1 hr in a horizontal 25 cm2 flask (Corning, Stone, UK).
Mixed lymphocyte reaction
One-way MLR was carried out by seeding 2 × 105 PBL into each of triplicate wells of a round-profile 96-well plate (Corning) and adding 1 × 105 irradiated allogeneic EBV-B cells to each well in a final volume of 200 µl of lymphocyte medium. Lymphoproliferation was measured daily for 11 days, by addition of 1 µCi of [3H]thymidine (5 Ci/mmol; ICN Pharmaceuticals, Thame, UK) to each well on a separate plate at each time point. After 6 hr the cells were harvested onto glass-fibre filters (Tomtec; Wallac, Milton Keynes, UK) and the incorporated [3H]thymidine was measured by β-scintillation counting (Microbeta Plus; Wallac).
Large-scale, one-way MLR was performed by coculturing 1 × 107 PBL and 1 × 107 irradiated allogeneic EBV-B cells in 30 ml of media in a canted 75-cm2 tissue culture flask (Corning).
Interleukin-2 (IL-2) bioassay
Culture supernatants were assayed for IL-2 using the CTLL-2 line (ECACC 87032605) maintained in RPMI-1640 culture medium supplemented with recombinant IL-2 at a concentration of 2 U/ml (R & D Systems, Abingdon, UK). Supernatants were thawed and added in 100 µl aliquots to five replicate wells of a round profile 96-well plate; CTLL-2 cells that had been cultured in IL-2-free medium for 16–20 hr were then added at 5 × 104 cell/well to a make a final volume of 200 µl. After incubation for 24 hr, cell proliferation was estimated by measurement of [3H]thymidine incorporation as described above. The IL-2 concentration in the supernatants was determined by interpolation using the equation (Prism, Version 3·01; GraphPad Software, San Deigo, CA) describing a curve generated using known concentrations of a standard IL-2 preparation (NIBSC, South Mimms, UK).
Antibodies and flow cytometric analysis
The murine and rat antibodies used for immunofluorescence flow cytometric analysis were 124 (anti-Bcl-2; Oncogene Research Products, Nottingham, UK), UCHL-1 (anti-CD45RO, Sigma), DX2 (anti-Fas; PharMingen, San Diego, CA) and H11 (anti-Fas ligand; Alexis Corporation, Nottingham, UK). Cells for intracellular Bcl-2 and FasL staining were fixed in 1% paraformaldehyde in phosphate-buffered saline (PBS) for 10 min at room temperature prior to staining. The fixed cells were then permeablized by the addition of 1% saponin to the staining buffer. Cells were labelled with predetermined optimal concentrations of these primary antibodies at 4° for 30 min in 50 µl of staining buffer (PBS supplemented with 5% FBS) and then washed in cold PBS. After further washing the stained cells were analysed by flow cytometry (FACsort; Becton Dickinson, Cowley, UK) with acquisition of 10 000 events; data analysis was performed using Lysis II software (Becton Dickinson). The irrelevant, isotype-matched antibodies X931 (mouse immunoglobulin G1 (IgG1); Dako, High Wycombe, UK) and LO-DNP-16 (rat IgG2a; Serotec, Oxford, UK) were used for negative control in each labelling experiment.
Cell-counting experiments were performed by harvesting 200 µl aliquots of lymphocytes from a bulk-one way MLR at regular intervals. Cells were incubated with 10 µl of fluorescent spheres with high side-scatter and fluorescence (Flow-Count; BeckmanCoulter, High Wycombe, UK) and propidium iodide (2·5 mg/ml) prior to flow cytometric analysis.
Fluorescence standard curves were routinely generated using a mixture of standardized fluorescent microspheres (Fluorosphere; Dako) to quantify the logarithmic photomultiplier signals. All flow cytometric results were expressed as linear median equivalents of fluorescence (MEF) values, which may be compared directly across a time-course experiment.
Tdt-mediated dUTP nick-end labelling (TUNEL) staining
A lymphocyte sample was harvested at daily intervals from a large-scale one-way MLR and subcultured into two wells of a 48-well plate at a concentration of 2–2·5 × 106 cells/ml in time-matched MLR-conditioned medium. One well was treated with (5 µg/ml) DX2 monoclonal antibody (mAb, anti-Fas; Pharmingen) and the other with an irrelevant control mouse IgG1 mAb (5 µg/ml). The plates were incubated for 8 hr at 37°. After this time, the cells were harvested, washed by centrifugation, and fixed by incubation in 5 ml of 1% paraformaldehyde in PBS at 4° for 15 min. The suspensions were centrifuged again and washed twice with PBS. 5 ml of cold 70% (v/v) ethanol was then added to each sample, which was then stored at −20°.
The commercially available APO-Direct kit (PharMingen) was used to measure apoptosis. This kit supplies all the reagents necessary for the assay, including suspensions of fixed apoptotic and non-apoptotic control cells. Suspensions of the control and anti-Fas-treated cells were washed twice by centrifugation in PBS, and 50 µl of a staining solution containing terminal deoxynucleotidyl transferase and fluorochrome-tagged deoxyuridine triphosphate nucleotides was added to each pellet. The suspensions were mixed and incubated at 37° for 3 hr.
The suspensions were then washed twice by centrifugation in PBS. The cell pellets were resuspended in 0·5 ml of a solution of propidium iodide (0·01 mg/ml; Sigma) and RNase A (0·05 mg/ml; Sigma) to remove extraneous FL-2 signal caused by RNA in the cells. The cells were incubated at room temperature for a further 30 min before flow cytometric analysis.
Measurement of Fas, FLIP and caspase 8 expression by Western blotting
A lymphocyte sample was harvested at regular intervals from a large-scale one-way MLR for 11 days of culture. The culture medium and debris was removed by washing twice with PBS and cells were lysed with a buffer containing 10% sodium dodecyl sulphate (Sigma), 10% glycerol (Sigma), 10% 2- mercaptoethanol (Sigma) 0·001% bromophenol blue (Sigma), 500 mm ethylenediamine tetra-acetic acid (EDTA; Sigma), and 1 m Tris-HCL. (Sigma). Lysates were denatured by incubation at 95° for 5 min. The protein content was measured using a bovine serum albumin (BSA) Bradford protein assay reagent (Biorad, Hemel Hempstead, UK). The cell lysates (15 µg for each sample) were analysed by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) using a 10% polyacrylamide gel and were transferred to a nitrocellulose membrane (Hybond-C; Amersham International, Amersham, UK) by electroblotting. After blocking with 5% non-fat dry milk (Marvel; Premier Beverages, Stafford) in Tris-buffered saline (TBS), the membrane was incubated with G254-274 (anti-Fas; Pharmingen), B92 (anti-caspase 8; PharMingen), anti-FLIP rabbit serum (Alexis Corporation) or DM 1 A (anti-α-tubulin; Sigma). Membranes were washed with TBS supplemented with 0·05% Tween-20 and incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse or goat anti-rabbit polyclonal antibody (Transduction Laboratories, Mamhead, UK) as appropriate. After washing with TBS, antibody binding was detected using enhanced chemi-luminescence (Amersham).
After primary development, membranes were frequently stripped for secondary antibody labelling. In these cases the membranes were incubated for 30 min in a buffer containing 62·5 mm Tris/HCl, pH 6·8, 2% SDS, and 100 mmβ-mercaptoethanol at 60°. The blots were then washed six times for 10 min in PBS/Tween and blocked again in 5% non-fat dry milk.
Measurement of caspase 8 activity by fluorescent assay
A lymphocyte sample was harvested at regular intervals from a large-scale one-way MLR for 11 days of culture. The culture medium was removed by washing twice with PBS and cells (1 × 106/well) were seeded into duplicate wells of a round-profile 96-well plate. The commercially available ApoAlert Caspase 8 Fluorescent Assay kit (Clontech Laboratories, Basingstoke, UK) was used to measure caspase 8 activity; this supplies all the reagents necessary for the assay. Briefly, cells were centrifuged at 400 g for 6 min. The cell supernatants were discarded and the cell pellets were resuspended with lysis buffer (50 µl/well). The plate was incubated on ice for 10 min. Following centrifugation at 400 g for 6 min, the cell supernatants were transferred to a fresh 96-well plate. 10 mm dithiothreitol (DTT)-supplemented reaction buffer was added to each well (50 µl/well). The fluorogenic substrate IETD-AFC was added to a final concentration of 50 µm. Fluorescence was measured using a Millipore Cytofluor 96-well reader (Hertfordshire, UK) with a filter setting of 400 nm (excitation) and 505 nm (emission). Changes in fluorescence were standardized using free AFC (7-amino-4-trifluormethylcoumarin). Units of caspase 8 activity were calculated using the equation:
 |
The factor of 1000 converts nmol AFC/min to activity units.
Statistical analysis
All experimental data are presented as the mean ± standard error (SEM). Statistical comparison of sample data was performed by application of Student's t-test (Minitab Software; Clecom, Birmingham, UK) and Prism Software (San Deigo, CA).
Results
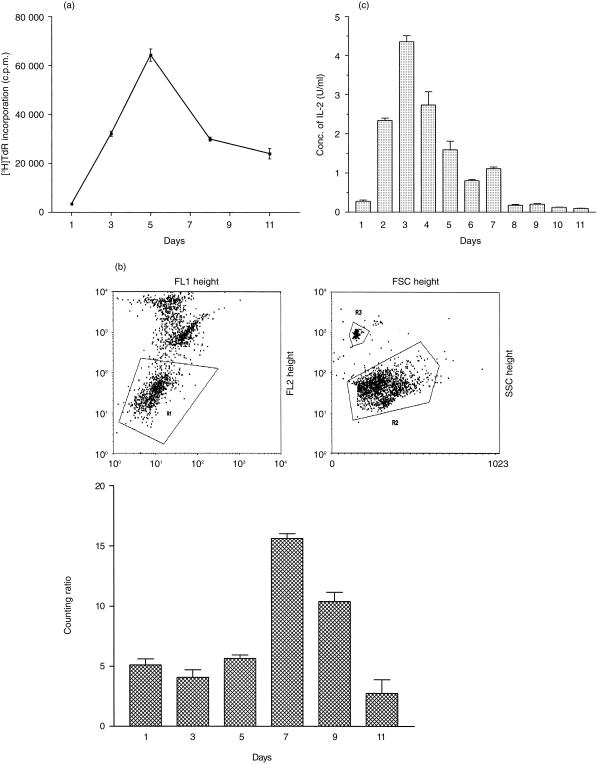
In order to assess the kinetics of the MLR response, proliferation assays were performed. Serial measurement of [3H]thymidine incorporation demonstrated maximal lymphoproliferation after culture for 5 days (Fig. 1a); after this time the number of proliferating cells in the culture declined rapidly. The number of viable cells in the culture (Fig. 1b) remained constant for the first 5 days, peaked after 7 days and then declined rapidly. Serial measurement of the concentration of free IL-2 in the culture medium demonstrated a maximum value of 4·35 ± 0·15 U/ml on the third day of culture; after culture for 7 days, the concentration of IL-2 had declined to 1·13 ± 0·041 U/ml (Fig. 1c). Phenotypic analysis of the cell population during MLR culture, showed that intact irradiated EBV-B cells, identified by CD19-staining, were absent after the first day in culture. By day 7, the ratio of activated CD4+:CD8+ T cells was 0·7:1 (data not shown).
Figure 1.
Kinetic examination of the MLR system. (a) Lymphocyte proliferation during MLR for 11 days at an original stimulator:responder cell ratio of 2:1. Proliferation was determined each day by measurement of [3H]thymidine incorporation; the results are shown as the mean c.p.m. ± SEM (n = 3). (b) Cell counts during a MLR. Dead cells were excluded from this analysis by propidium iodide exclusion (R1). Relative cell counts were expressed as a cell (R2):bead (R3) ratio. Each point represents the mean of three replicate determinations; the error bars show the SEM. (c) Representative results showing the production of IL-2 at daily time points during MLR as determined by a CTLL-2 bioassay. Each point represents the mean of three replicate determinations; the error bars show the SEM.
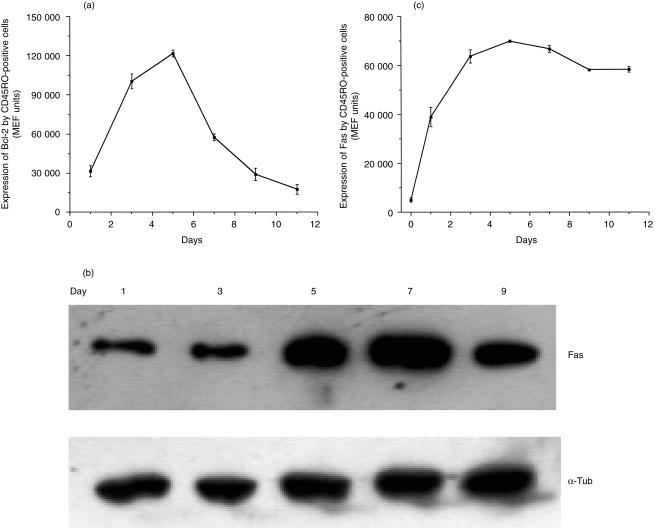
The intracellular expression of the anti-apoptotic protein Bcl-2 by T cells responding during MLR was examined by semiquantitative immunofluorescence flow cytometry at regular intervals following the initiation of culture. It was found that Bcl-2 expression by large, CD45RO positive cells was upregulated quickly after initiation of the culture and reached maximal levels after incubation for 5 days (Fig. 2a) and remained elevated for 7 days. However by day 9, the expression of Bcl-2 by these cells was low and fell further by day 11. This time point coincided with the measurement of a very low concentration of IL-2 in the reaction medium (Fig. 1c). Further experiments have shown that addition of exogenous IL-2 (or the IL-2R γ-chain dependent cytokine, IL-15) to activated T cells that have been starved of this cytokine produces a rapid upregulation in Bcl-2 expression (data not shown).
Figure 2.
(a) Representative flow cytometric data evaluating changes in Bcl-2 expression at various time points during an MLR. Cells were dual-labelled to detect Bcl-2 and CD45RO antibodies and gated to allow analysis of the expression of Bcl-2 by the activated CD45RO+ cells. (b) Representative Western blot demonstrating expression of the 45 000 MW Fas protein by peripheral blood lymphocytes harvested at various time intervals from a MLR assay; α-tubulin was also visualized to demonstrate constant protein loading. (c) Representative flow cytometric time course demonstrating Fas expression on the surface of activated lymphocytes during the course of a MLR. These results are expressed as MEF values and show the mean of duplicate determinations; error bars show the SEM.
Given the important role of the Fas receptor for transduction of pro-apoptotic signals, the amount of this protein within the culture was examined by Western blot analysis. It was found that freshly isolated cells showed very low levels of this protein (data not shown). Indeed, the level of Fas was not increased greatly during the first 3 days during MLR. However, Fas expression was greatly upregulated after culture for 5 days; this increased expression was maintained at least until day 9 (Fig. 2b). In the blotting experiments, equal gel loading was demonstrated by stripping the blot and re-probing for the housekeeping protein α-tubulin. A parallel series of measurements was performed by flow cytometry to quantify cell-surface Fas expression by activated lymphocytes within the MLR (Fig. 2c); in this case, it was found that Fas expression was upregulated rapidly, and peaked 5 days after the initiation of culture. At this time more than 95% of the activated lymphocytes expressed the Fas antigen (data not shown).
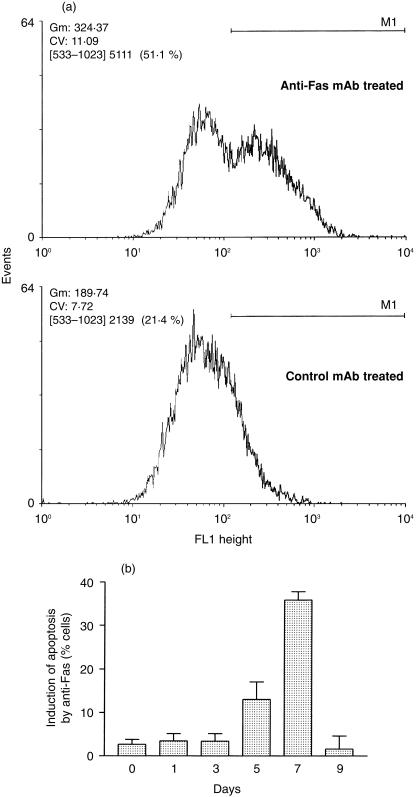
Measurement of DNA fragmentation by flow cytometric TUNEL analysis was used to assess the sensitivity of T cells harvested from MLR to the induction of apoptosis through stimulation of the Fas receptor. In these experiments, the apoptosis occurring spontaneously in cultures treated with an irrelevant control antibody (which produced no additional apoptosis) was subtracted from that measured after the stimulation of Fas with an agonistic antibody. An example of the flow cytometric data is shown in Fig. 3(a) for cells harvested 7 days after the initiation of MLR. Daily examination of the cells showed that an anti-Fas antibody did not induce DNA fragmentation above baseline levels until the cells had been in culture for at least 5 days (Fig. 3b). After 7 days, a maximal 36 ± 2% of the cells were responsive to Fas ligation; however, by day 9 the response had returned to baseline levels.
Figure 3.
Representative flow cytometric TUNEL results showing the susceptibility of lymphocytes from a MLR to apoptosis induced by treatment with anti-Fas antibodies. (a) Fluorescence histograms of lymphocytes harvested from a day 7 MLR treated with anti-Fas or control antibodies and stained by the TUNEL procedure. (b) Time course indicating the susceptibility to Fas-induced apoptosis of lymphocytes harvested at various time points during the MLR. The results are expressed as the percentage of cells induced to undergo apoptosis following ligation of the Fas antigen and represent the mean of duplicate determinations; error bars show the SEM.
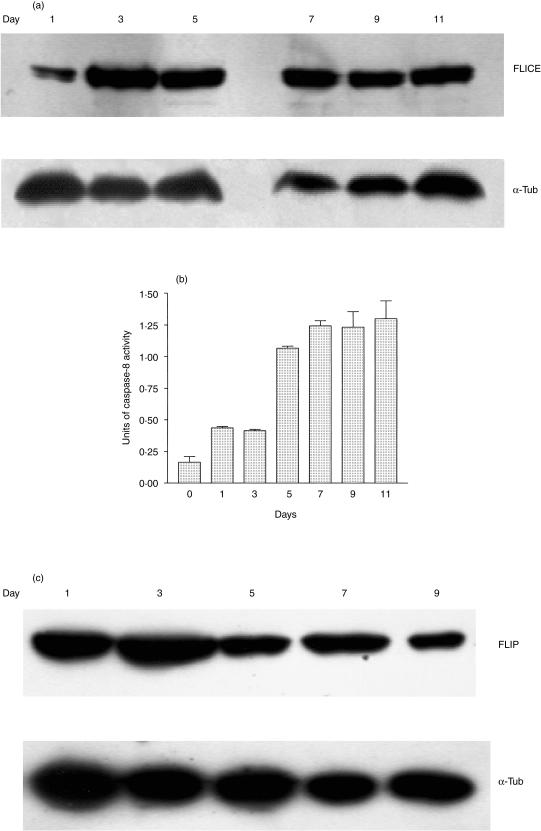
The expression of caspase 8 (FLICE) by cells in the MLR was assessed by Western blotting (Fig. 4a). It was found that the amount of this protein was relatively low at the earliest time point but was high by day 3 and then remained at this elevated level for the duration of the experiment. However, the activity of this enzyme only showed a significant increase (P < 0·02) on day 5; after this time the activity of caspase 8 remained at a high level (Fig. 4b). In addition, levels of cellular FLIP were assessed by Western blotting (Fig. 4c). It was found that this protein was expressed at high levels during the early stages of the MLR, but showed a decreased expression by day 5.
Figure 4.
(a) Western blot demonstrating expression of the 55 000 MW pro-caspase 8 (FLICE) protein by peripheral blood lymphocytes harvested at various time intervals from a MLR assay; α-tubulin was also visualized to demonstrate constant protein loading. (b) Results from a fluorimetric assay of the enzymatic activity of caspase 8. Each column represents the mean of duplicate determinations; the error bars show the SEM. (c) Western blot demonstrating expression of the 55 000 MW FLIP protein by peripheral blood lymphocytes harvested at various time intervals from a MLR assay; α-tubulin was also visualized to demonstrate constant protein loading.
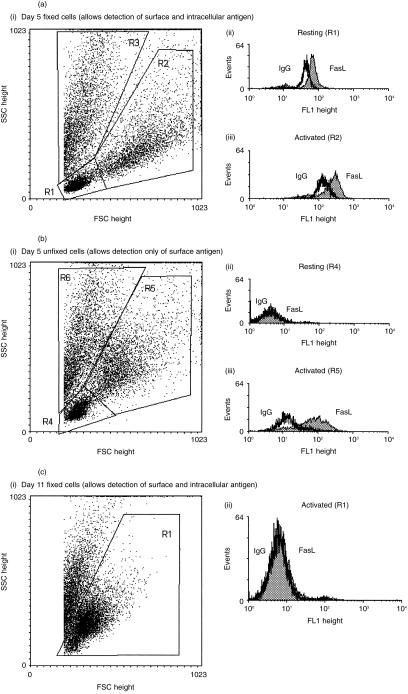
The expression of FasL by the cells in MLR was measured by semiquantitative immunofluorescence flow cytometry at day 5 (Fig. 5a, b) and day 11 (Fig. 5c). Appropriate analysis allowed small resting cells (R1 and R4) to be distinguished from larger and more granular activated lymphocytes (R2 and R5) on the basis of their forward and side-scatter characteristics (Fig. 5aI, bI). Small granular cells (R3 and R6) were excluded from the analysis, as these represent the remains of the irradiated stimulator cell line and other non-viable cells. At the time of maximal proliferation during the MLR (day 5; Fig. 1b), it was found that resting cells, although containing intracellular stores of FasL (Fig. 5aII), failed to express detectable levels of FasL on their surface (Fig. 5bII). By contrast, it was found that activated lymphocytes showed both intracellular (Fig. 5aIII) and cell-surface FasL (Fig. 5bIII). Viable cells (R1) harvested from a MLR after 11 days (Fig. 5cI) were negative for FasL expression (Fig. 5cII).
Figure 5.
(a) Representative flow cytometric profiles showing the expression of total FasL (both surface and intracellular) in lymphocytes harvested from MLR at day 5. (I) Dot plot showing gated populations in the paraformaldehyde-fixed MLR sample. (II) Histogram showing total FasL in resting lymphocytes. (III) Histogram showing total FasL in activated lymphocytes. (Note: when comparing this with Fig. 5b, cell fixation can cause alterations in light scatter properties and an apparent reduction in surface FasL staining.) (b) Representative flow cytometric profiles showing the expression of FasL on the surface of lymphocytes harvested from an MLR at day 5. (I) Dot plot showing the gated populations from an unfixed MLR sample. (II) Histogram showing the expression of FasL on the surface of resting lymphocytes. (III) Histogram showing the expression of FasL on the surface of activated lymphocytes. (c) Representative flow cytometric profiles showing the expression of total FasL on lymphocytes harvested from a MLR at day 11. (I) Dot plot showing the gated populations from a paraformaldehyde-fixed MLR sample. (II) Histogram showing the expression of FasL by activated lymphocytes.
Discussion
Immunological, cellular and molecular evidence indicates that apoptosis may be induced at several points within the life of a T cell to produce and preserve a healthy and balanced immune system. 17 The sensitivity of T cells to apoptosis following antigen-specific activation varies with time, and cell death can be produced actively by stimulation of cell-surface receptors such as Fas, or passively by depletion of cytokines such as IL-2. It is clear that lymphocytes are resistant to apoptosis early after activation. Indeed, there are excellent physiological reasons why it is not advantageous for T cells to be vulnerable to apoptotic clearance before they have completed a productive immune response to antigen. However, after successful antigen eradication most of the activated T cells are removed by apoptosis, leaving a small memory population intact. Stimulation of Fas is believed to play a major role in T-cell clearance since mice and humans with defects in the expression of this antigen show a pathological accumulation of pre-apoptotic T cells.18, 19 However, it is clear that cytokine deprivation may also play a role in T-cell deletion during the later stages of an immune response.
Examination of the kinetic parameters of a primary MLR in vitro provides evidence of the mechanism responsible for maintaining homeostasis of activated T cells in vivo. Following primary antigen-presentation at initiation of an MLR, the concentration of the T-cell growth factor IL-2 increases to peak after 3 days. The kinetics of the T-cell proliferative response are slightly slower, showing a peak rate of DNA synthesis after 5 days followed by a gradual decrease. Despite this evidence of immune cell activation, the number of viable lymphocytes remained relatively constant during the first 5 days suggesting a balance between the formation of new, allospecific cells by proliferation and the death of non-specific lymphocytes, which at time 0 constitute the majority of the lymphocyte population. 20 On day 7, an increase in cell numbers is observed; this parallels the emergence of new allospecific T cells formed during the rapid proliferative phase of the response. However, the number of T cells then shows a marked reduction despite some continued proliferation within the activated cell population. This paradoxical reduction in cell numbers is indicative of T-cell death that must occur at, or shortly after, the period of maximal, alloantigen-specific T-cell proliferation.
In this study, the concentration of IL-2 was measured by application of a CTLL-2 bioassay that is also responsive to other T-cell growth factors, such as IL-15, which stimulate receptors bearing a common IL-2R γ chain. 21 However, medium from a mixed culture containing pure lymphocyte populations only develops IL-2 (data not shown). The reduction in the concentration of free IL-2 at the peak of lymphocyte proliferation (day 5) may be indicative of cytokine consumption during receptor internalization. However, after day 8 it is more likely that the low concentration occurs as a consequence of decreased cytokine production, as the rate of T-cell proliferation was relatively low.
At initiation, the MLR culture contained irradiated EBV-B cells and a mixed population of PBL. Serial flow cytometric analysis demonstrated that CD19-expressing B cells were absent after the first day, which is consistent with rapid apoptotic deletion of the irradiated stimulator cells. For this reason, Western blot analysis of protein expression by the cultures was not performed at time points earlier than 24 hr. However, flow cytometry allowed the increasing population of large CD45RO+ cells to be defined for investigation of the phenotypic and apoptotic changes that occur at all stages during the activation of alloreactive T cells. After day 7, it was found that these cells expressed little Bcl-2, which paralleled a reduction in the concentration of IL-2 within the reaction medium. This is consistent with a previous study that showed a causal link between stimulation of γ-chain receptors and the level of Bcl-2 expression. 22
In contrast to the decrease in Bcl-2 expression, the expression of Fas increases rapidly during the MLR. Flow cytometric analysis of the activated T cell subpopulation shows increased cell-surface expression within 1 day of the initiation of culture. However, the overall expression of Fas within the culture shows a marked increase by day 5, presumably reflecting expansion of the small proportion of alloreactive cells that become activated initially within an MLR. 20 As the expression of cell-surface Fas precedes the acquisition of sensitivity to apoptosis induced by ligation of this molecule, it is clear that Fas-mediated apoptosis is regulated within the activated T cell. However, this is not explained by the presence of an inadequate level of caspase 8 as the inactive precursor of this enzyme is also present from an early time point in the MLR.
It is clear that early expression of cell-surface FasL is a feature of T cells following activation in MLR. Small, resting T cells also expressed FasL but, in these cells, the antigen was restricted to an intracellular location. This observation is entirely consistent with a recent demonstration that FasL is stored in specialized secretory granules. 23 In this study, it was shown that granule fusion with the plasma membrane might control the appearance of FasL on the surface of T cells following activation. Although the activated lymphocyte population at this time consisted of both CD4+ and CD8+ cells, the fluorescence-activated cell sorting (FACS) histogram showed a single peak for FasL expression, suggesting similar regulation of this antigen in both subpopulations.
Given the cell-surface expression of Fas and FasL by activated lymphocytes in a day 7 MLR, and the sensitivity of these cells to the induction of apoptosis by treatment with anti-Fas antibodies, it is likely that stimulation of the Fas pathway is responsible for apoptotic clearance of cells at the height of an immune response in vivo.24 The induction of this AICD is probably mediated by a trans-pathway, termed fratricide, in which FasL on one cell can stimulate Fas on an adjacent activated T cell. The fact that relatively little spontaneous apoptosis was detected in the MLR culture at this time is suggestive of a poor interaction between Fas- and FasL-expressing cells in this in vitro model; this may be the result of steric hindrance by the remains of stimulator cells and non-specific lymphocytes. This observation also indicates that a suicidal stimulation of Fas by co-expressed FasL is unlikely to mediate significant death of activated T cells in vitro.25
It is clear that the activation of caspase 8 plays a central role in the induction of Fas-mediated apoptosis. It has been suggested that the resistance to apoptosis of Fas-positive cells at early time points is caused by the failure to form functional DISC and, hence, to immobilize caspase 8. 26 The actual activation of caspase 8 in mature, activated cells is explained by an ‘induced-proximity’ model. In this model, pro-caspase 8 generates the active molecule by autocatalytic cleavage. 8 Following activation, caspase 8 can activate caspases 3 and 7. This leads to the cleavage of a multitude of cellular substrates, resulting in the morphological changes associated with apoptosis and DNA cleavage. 11 For example, caspase 3 can activate the protein acinus which, in turn, plays an important role in apoptotic chromatin condensation. 27
Recently, a family of FLICE inhibitory proteins (FLIPs) has been characterized which prevents binding of caspase 8 to the adapter molecule Fas-associated death domain protein (FADD) and subsequent assembly of the DISC after Fas cross-linking. 28 FLIP is structurally similar to caspase 8 but lacks the cysteine residue important for catalytic activity. However, the role of cellular FLIP in producing resistance to Fas-mediated apoptosis remains controversial. A recent report suggests that mitogen-activated T cells can become sensitive to apoptosis despite expressing high levels of cellular FLIP. 29 However, in the present study it was shown that the level of FLIP expression, although high during the early stages of an MLR, is markedly reduced after 7 days in culture; this coincides with the acquisition of maximal sensitivity to Fas-mediated apoptosis. A similar reduction in the expression of FLIP has been reported for OKT3-stimulated T cells after prolonged culture. 30
Recent studies have suggested that cells can be classified as type I or type II with respect to their sensitivity to Fas-mediated apoptosis; the basis for this division is the efficiency with which the cells can form a functional DISC. 31 Type I cells can form DISC, resulting in direct activation of caspase 8, whilst stimulation of Fas on type II cells results neither in the generation of functional DISC nor direct activation of caspase 8. It has been shown recently that the death of activated peripheral T cells involves switching from type II to a type I phenotype, resulting in the acquisition of sensitivity to Fas cross-linking. 29 In addition to the FLIPs, a potential candidate for regulation of the transition between type I and type II sensitivity has been designated FLASH (FLICE-associated huge protein).32, 33 However, the precise functional significance of this molecule has not been determined.
During the early stages of an MLR, it is likely that the activated T cells are predominantly of type II. At this time the cells are resistant to Fas cross-linking and maintain a expression of anti-apoptotic Bcl-2 because of the high ambient IL-2 concentration. However, after culture for between 5 and 7 days these T cells change to a type I phenotype and, despite the continued presence of IL-2 and intracellular Bcl-2, become sensitive to Fas-mediated apoptosis.
The induction of passive cell death by cytokine withdrawal is likely to account for the decrease in cell numbers during the later stages of the reaction when FasL expression is minimal. By day 11, the availability of IL-2 (and, in this in vitro system, other IL-2R γ-chain dependent cytokines) is low, resulting in the loss of Bcl-2 expression. This causes the leakage of apoptogenic molecules such as cytochrome-c from mitochondria that undergo permeability transition.34, 35 It is known that cytoplasmic cytochrome-c can activate directly both caspases 8 and 9, 36 resulting in downstream processing of caspase 3 and other pro-apoptotic effector molecules. This process does not involve the DISC and is therefore likely to be independent of any regulatory activity of FLIP, which is still detectable during the later stages of the reaction.
It is possible to view the in vitro MLR as a useful model of the processes involved in T-cell activation, proliferation and ultimate clonal downsizing in vivo. Furthermore, it is likely that this system provides a valuable insight into the allospecific immune response generated after organ transplantation. Further investigation of the mechanisms that regulate the sensitivity of activated T cells to the induction of apoptosis might enable therapeutic manipulation of the immune response. For example, decreasing the period required for acquisition of sensitivity to Fas ligation may cause a beneficial premature death of the specific T cells involved in graft rejection and certain autoimmune diseases.
Acknowledgments
The authors are grateful to the Dr WE Harker Bequest and the Northern Counties Kidney Research Fund for supporting this work. We would also like to thank Dr Dave Cook and Dr Brian K Shenton for valuable technical assistance.
References
- 1.Bach F, Hirschhorn K. Lymphocyte interaction: a potential histocompatibility test in vitro. Science. 1964;143:813. doi: 10.1126/science.143.3608.813. [DOI] [PubMed] [Google Scholar]
- 2.Sherman LA, Chattopadhay S. The molecular basis of allorecognition. Annu Rev Immunol. 1993;11:385. doi: 10.1146/annurev.iy.11.040193.002125. [DOI] [PubMed] [Google Scholar]
- 3.Akbar AN, Salmon M. Cellular environments and apoptosis: tissue microenvironments control activated T-cell death. Immunol Today. 1997;18:72. doi: 10.1016/s0167-5699(97)01003-7. [DOI] [PubMed] [Google Scholar]
- 4.Vanparijs L, Abbas AK. Homeostasis and self-tolerance in the immune system: turning lymphocytes off. Science. 1998;280:243. doi: 10.1126/science.280.5361.243. [DOI] [PubMed] [Google Scholar]
- 5.Brunner T, Mogil RJ, Laface D, et al. Cell-autonomous Fas (CD95) Fas–ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 6.Lynch DH, Ramsdell F, Alderson MR. Fas and FasL in the homeostatic regulation of immune responses. Immunol Today. 1995;16:569. doi: 10.1016/0167-5699(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 7.Itoh N, Yonehara S, Ishii A, et al. The polypeptide encoded by the cDNA for human cell-surface antigen Fas can mediate apoptosis. Cell. 1991;66:233. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 8.Muzio M, Stockwell BR, Stennicke HR, Salvesen GS, Dixit VM. An induced proximity model for caspase-8 activation. J Biol Chem. 1998;273:2926. doi: 10.1074/jbc.273.5.2926. [DOI] [PubMed] [Google Scholar]
- 9.Medema JP, Scaffidi C, Kischkel FC, et al. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC) EMBO J. 1997;16:2794. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Debatin KM, Beltinger C, Bohler T, et al. Regulation of apoptosis through CD95 (APO-I/Fas) receptor–ligand interaction. Biochem Soc Trans. 1997;25:405. doi: 10.1042/bst0250405. [DOI] [PubMed] [Google Scholar]
- 11.Nicholson DW, Thornberry NA. Caspases: killer proteases. Trends Biochem Sci. 1997;22:299. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 12.Li HL, Zhu H, Xu CJ, Yuan JY. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 13.Hirata H, Takahashi A, Kobayashi S, et al. Caspases are activated in a branched protease cascade and control distinct downstream processes in Fas-induced apoptosis. J Exp Med. 1998;187:587. doi: 10.1084/jem.187.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'flaherty E, Ali S, Pettit SJ, Kirby JA. Examination of the sensitivity of T cells to Fas ligation. Transplantation. 1998;66:1. doi: 10.1097/00007890-199810270-00017. [DOI] [PubMed] [Google Scholar]
- 15.Owenschaub LB, Yonehara S, Crump WL, Grimm EA. DNA fragmentation and cell death is selectively triggered In activated human lymphocytes by Fas antigen engagement. Cell Immunol. 1992;140:197. doi: 10.1016/0008-8749(92)90187-t. [DOI] [PubMed] [Google Scholar]
- 16.Wilson JL, Cunningham AC, Kirby JA. Alloantigen presentation by B-cells – analysis of the requirement for B-cell activation. Immunology. 1995;86:325. [PMC free article] [PubMed] [Google Scholar]
- 17.Lenardo MJ. Fas and the art of lymphocyte maintenance. J Exp Med. 1996;183:721. doi: 10.1084/jem.183.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rieuxlaucat F, Ledeist F, Hivroz C, et al. Mutations In Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268:1347. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- 19.Singer GG, Carrera AC, Marshakrothstein A, Martineza C, Abbas AK. Apoptosis, Fas and systemic autoimmunity – the MRL-Lpr/Lpr model. Curr Opin Immunol. 1994;6:913. doi: 10.1016/0952-7915(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 20.Cattell EL, Cunningham AC, Bal W, Taylor RMR, Dark JH, Kirby JA. Limiting dilution analysis: quantification of IL-2 producing allospecific lymphocytes after renal and cardiac transplantation. Transplant Immunol. 1994;2:300. doi: 10.1016/0966-3274(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 21.Giri JG, Anderson DM, Kumaki S, Park LS, Grabstein KH, Cosman D. IL-15, a novel T-cell growth-factor that shares activities and receptor components with IL-2. J Leukocyte Biol. 1995;57:763. doi: 10.1002/jlb.57.5.763. [DOI] [PubMed] [Google Scholar]
- 22.Akbar AN, Borthwick NJ, Pilling D, Salmon M. Interleukin-2 receptor common gamma-chain signalling cytokines regulate activated T cell apoptosis by selectively inducing anti-apoptotic (bcl-2, bcl-xL) but not pro-apoptotic gene (bax, bcl-xS) expression. FASEB J. 1996;10:414. doi: 10.1002/eji.1830260204. [DOI] [PubMed] [Google Scholar]
- 23.Bossi G, Griffiths GM. Degranulation plays an essential part in regulating cell surface expression of Fas ligand in T cells and natural killer cells. Nature Med. 1999;5:90. doi: 10.1038/4779. [DOI] [PubMed] [Google Scholar]
- 24.Orteu CH, Poulter LW, Rustin MHA, Sabin CA, Salmon M, Akbar AN. The role of apoptosis in the resolution of T cell-mediated cutaneous inflammation. J Immunol. 1998;161:1619. [PubMed] [Google Scholar]
- 25.Marrack P, Mitchell T, Bender J, et al. T-cell survival. Immunol Rev. 1998;165:279. doi: 10.1111/j.1600-065x.1998.tb01245.x. [DOI] [PubMed] [Google Scholar]
- 26.Peter ME, Kischkel FC, Scheuerpflug CG, Medema JP, Debatin KM, Krammer PH. Resistance of cultured peripheral T cells towards activation-induced cell death involves a lack of recruitment of FLICE (MACH/caspase 8) to the CD95 death-inducing signaling complex. Eur J Immunol. 1997;27:1207. doi: 10.1002/eji.1830270523. [DOI] [PubMed] [Google Scholar]
- 27.Sahara S, Mamoru A, Eguchi Y, Imamoto N, Yoshihiro Y, Tsujimoto Y. Acinus is a caspase-3-activated protein required for apoptotic chromatin condensation. Nature. 1999;401:168. doi: 10.1038/43678. [DOI] [PubMed] [Google Scholar]
- 28.Thome M, Schneider P, Hofmann K, et al. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 29.Scaffidi C, Schmitz I, Krammer PH, Peter ME. The role of c-FLIP in modulation of CD95-induced apoptosis. J Biol Chem. 1999;274:1541. doi: 10.1074/jbc.274.3.1541. [DOI] [PubMed] [Google Scholar]
- 30.Algeciras-Schimnich A, Griffith TS, Lynch DH, Paya CV. Cell cycle-dependent regulation of FLIP levels and susceptibility to Fas-mediated apoptosis. J Immunol. 1999;162:5205. [PubMed] [Google Scholar]
- 31.Scaffidi C, Fulda S, Srinivasan A, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imai Y, Kimura T, Murakami A, Yajima N, Sakamaki K, Yonehara S. The CED-4-homologous protein FLASH is involved in Fas-mediated activation of caspase-8 during apoptosis. Nature. 1999;398:777. doi: 10.1038/19709. [DOI] [PubMed] [Google Scholar]
- 33.Medema JP. Apoptosis – life and death in a FLASH. Nature. 1999;398:756. doi: 10.1038/19638. [DOI] [PubMed] [Google Scholar]
- 34.Lenardo M, Chan FKM, Hornung F, et al. Mature T lymphocyte apoptosis – immune regulation in a dynamic and unpredictable antigenic environment. Ann Rev Immunol. 1999;17:221. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 35.Kluck RM, Bossywetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 36.Slee EA, Harte MT, Kluck RM, et al. Ordering the cytochrome c-initiated caspase cascade: Hierarchical activation of caspases-2-3-6-7-8, and -10 in a caspase-9- dependent manner. J Cell Biol. 1999;144:281. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]