Abstract
2B4 is a surface molecule found on all human natural killer (NK) cells, a subset of CD8+ T cells, monocytes and basophils. It was originally identified on mouse NK cells and the subset of T cells that mediate non‐major histocompatibility complex (MHC)‐restricted killing. Recently, 9 we have cloned the human homologue of 2B4 (h2B4) and found h2B4 to also mediate non‐MHC‐restricted cytotoxicity. In this study, we examine h2B4 in regulating various functions of NK cells using a human NK cell line YT, with monoclonal antibody (mAb) C1.7, an antibody that specifically recognizes h2B4. Ligation of surface 2B4 with mAb C1.7 increases YT's ability to destroy tumour cells. In the presence of mAb C1.7, the production of interferon‐γ (IFN‐γ) by YT cells is greatly enhanced. Engagement of surface 2B4 by mAb C1.7 downregulates the expression of h2B4 at the cell surface as well as the expression of h2B4 mRNA. Also, signalling through h2B4 causes the increased expression of matrix metalloproteinase‐2, a member of the matrix degrading proteinase family. Thus, in addition to modulating cytolytic function and cytokine production of NK cells, activation through surface 2B4 may play a role in upregulating the machinery for degradation of extracellular matrices to promote invasion of the tumour by NK cells.
Introduction
Natural killer (NK) cells have the ability to recognize and target certain tumour and virally infected cells in the absence of prior stimulation and without major histocompatibility complex (MHC) restriction. 1–3 NK cells provide important mechanisms of primary defence against virus‐infected cells and tumour metastases through cytotoxic activities and the production of various cytokines such as interferon‐γ (IFN‐γ), tumour necrosis factor‐α (TNF‐α) and granulocyte–macrophage colony‐stimulating factor (GM‐CSF).4, 5 NK cells express several surface molecules that regulate NK cell function both positively and negatively. It is the sum of these signals that ultimately determines NK cell function and activation.1, 6 Thus, it is vitally important that these surface molecules on NK cells are identified and that their roles in regulating NK cell functions are characterized in order to gain a better understanding of NK cells and their role in the immune response.
We have previously identified a surface molecule designated 2B4, which is expressed on all murine NK cells and a subset of T cells that mediate NK‐like killing.7, 8 In addition to defining cells capable of non‐MHC‐restricted killing, the 2B4 molecule is also involved in modulating their function. The lytic activity of cultured NK cells and non‐MHC‐restricted T cells is greatly enhanced in the presence of a monoclonal antibody (mAb) against 2B4. We have also characterized the human homologue of 2B4, h2B4. 9 In both mice and humans, 2B4 is the counter‐receptor for CD48.10, 11 It has been reported that mAb C1.7, initially characterized by its ability to activate human cytotoxic lymphocytes, is able to recognize h2B4. 12 Human 2B4 has been found to be expressed on CD8+ T cells and to associate with signalling adapter molecule signalling lymphocyte‐activation molecule (SLAM, also known as CDw150)‐associated protein (SAP).12, 13 The defect in X‐linked lymphoproliferative syndrome patients is caused by mutations in SAP which may also interfere with 2B4 signalling.12, 14 Recently, it has been reported that the expression of 2B4 on CD8+ T cells is a better predictor of disease progression in aquired immune deficiency (AIDS) patients than CD4+ T‐cell levels. 15
In addition to NK cell cytolytic functions, our laboratories have also investigated other NK cell activities such as secretion of degradative enzymes including matrix metalloproteinases (MMPs). NK cells also produce various proteolytic enzymes including MMPs. Rat A‐NK cells have been shown to produce two gelatinases, MMP‐2 and MMP‐9; 16 and RNK‐16 cells, a rat NK tumour cell line, produce MMP‐3 and MMP‐13. 17 MMPs are a family of enzymes, which consists of at least 17 members. Of these 17, 12 are soluble secreted enzymes, while the other five are membrane bound. 18–21 MMP activities are induced by co‐ordinated increases in transcription, secretion, proteolytic activation, and, in some instances, association of the activated form with cell surfaces. These enzymes also play a role in the migration and extravasation of lymphocytes. They are capable of degrading extracellular matrix components and cleaving several cytokines such as pro‐TNF‐α and Fas ligand leading to their release from the cell surface. 22–24 It has been reported that MMP‐2 was induced upon binding of T cells to vascular cell adhesion molecule‐1 (VCAM‐1)‐positive endothelial cells but not to VCAM‐1‐negative ones, suggesting the role of VCAM‐1 in MMP‐2 induction.
Here, we have examined h2B4 with mAb C1.7 and its ability to affect NK cell functions including its influence on MMP expression. In this study, we used YT cells, as a human NK cell model. The YT cells have a surface phenotype similar to human NK cells and have NK‐like killing activity. 25 YT cells have a high expression of h2B4 and show increased cytolytic activity upon stimulation with mAb C1.7. Stimulation of YT cells by mAb C1.7 increases production of IFN‐γ and modulates the expression of h2B4. Herein, we also report that h2B4 stimulation up regulates the expression of MMP‐2. In sum, our results suggest that the NK cell receptor 2B4 exhibits an array of regulatory functions.
Materials and methods
Cell lines and antibodies
YT (human NK cell line), K562 (human erythroleukaemia cell line), Jurkat (human T‐cell leukaemic cell line), DB (human B‐cell line), 722.221 (human MHC class I‐deficient B‐cell line) P815 (mouse lymphoma cell line), and RMA (mouse lymphoma), were cultured in culture media (RPMI‐1640 supplemented with 10% fetal bovine serum (FBS, Hyclone, Logan, UT), 2 m m glutamine, 100 U/ml penicillin, 100 U/ml streptomycin, 10 m m HEPES and 10 m m non‐essential amino acids). Growth was at 37° in a humidified 5% CO2/95% air incubator. Cell culture reagents were obtained from Life Technologies (Gaithersburg, MD) unless otherwise noted. C1.7 antibody, which recognizes h2B4, 12 was purchased from Coulter (Miami, FL). Isotype mAb control was kindly donated by Dr V. Kumar, UT South‐western, Dallas, TX).
Flow cytometric analysis
YT, DB and Jurkat cells resuspended at 5 × 105 cells/100 µl in blocking buffer (phosphate‐buffered saline (PBS), 1% BSA) were incubated with 2 µg/ml of C1.7 mAb for 40 min on ice. Cells were then washed and resuspended in blocking buffer. Secondary goat F(ab′)2 fluoroscein isothiocyanate (FITC)‐ anti-mouse immunoglobulin (αmIg) (Coulter, Miami, FL) was added at a final dilution of 1/200 (v/v). After 30 min incubation on ice in the dark, the cells were washed two times in blocking buffer and resuspended in 1 ml blocking buffer. Routine analysis was performed using a Coulter EPICS XL flow cytometer.
Cell‐mediated cytotoxicity assay
Target cells were labelled by incubating 1 × 106 cells with 2 MBq of Na251CrO4 (NEN Research Products, Boston, MA) for 90 min at 37° under 5% CO2 in air. The target cells were then washed three times in culture media. 1 × 104 labelled target cells (100 µl) were incubated with effector YT cell suspension (100 µl) under various conditions of interleukin‐2 (IL‐2, 20 U/ml conc.), mAb C1.7 (200 ng/ml conc.) and/or isotype mAb control (200 ng/ml conc.). Effector YT cells were resuspended at 1, 2, 5, 10 and 20 times the number of labelled target cells. After incubation for 4 hr at 37° under 5% CO2 in air, the cells were pelleted at 250 g for 5 min, 100 µl of the supernatants were removed and their radioactivity was measured. The percentage of specific lysis was calculated by the following equation: (a − b/c − b) × 100, where a is the radioactivity of the supernatant of target cells mixed with effector cells, b is that in the supernatant of target cells incubated alone, and c is that in the supernatant after lysis of target cells with 1% Nonidet P‐40.
Human NK cell isolation
Heparinized peripheral blood from healthy donors was diluted with four volumes of PBS and then layered over Ficoll Paque (Pharmacia, Piscataway, NJ). It was then subjected to centrifugation at 400 g for 30 min. Peripheral blood mononuclear cells (PBMC) were then extracted from the interface and washed with PBS twice. The NK cells were then purified using NK cell isolation kit from Miltenyi Biotec (Auburn, CA) as per manufacturer's instructions.
Cell stimulation and semiquantitative reverse transcriptase (RT)–polymerase chain reaction (PCR)
YT cells were centrifuged and resuspended at 1 × 106 per sample in 1 ml culture media. The cells were then incubated with mAb C1.7 at a final concentration of 200 ng/ml. At the end of each time point, cells were spun down at 250 g for 5 min and the supernatant decanted. Total RNA was isolated from YT cells using RNeasy columns (Qiagen, Chatsworth, CA). cDNA synthesis was performed using murine Moloney leukaemia virus (M‐MLV) reverse transcriptase (Promega, Madison, WI). For each cDNA synthesis, total RNA from 1 × 106 cells were reverse transcribed using random hexamer or oligo (dT)16 primer in a volume of 50 µl each. The two reactions were combined after heat inactivation of reverse transcriptase, and 2 µl of the cDNA was used for each PCR amplification. Gene‐specific primers used for PCR amplification are given in Table 1. PCR was performed for 25–30 cycles in a Perkin Elmer thermocycler 2400 (Perkin Elmer, Norwalk, CT). The PCR products were separated on 6% polyacrylamide gels or 1% agarose gels and stained with ethidium bromide. For internal controls, primers used to amplify β‐actin or glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) were also used in PCR. PCR products of the genes in question were then normalized with the PCR control products.
Table 1.
Oligonucleotides used in this study
| Name | Gene | Sequence | Position* | Reference |
|---|---|---|---|---|
| MMP‐2F | MMP‐2 | 5′‐GAGTTGGCAGTGCAATACCT‐3′ | +95 to +114 | 26 |
| MMP‐2R | MMP‐2 | 5′‐GGGCAGAATCCATACTTCTT‐3′ | +951 to +932 | 26 |
| MT‐1F | MT1‐MMP | 5′‐GTGCCCTATGCCTACATCCG‐3′ | +597 to +616 | 27 |
| MT‐1R | MT1‐MMP | 5′‐TTGGGTATCCGTCCATCACT‐3′ | +1176 to +1157 | 27 |
| GAPDH‐F | GAPDH | 5′‐TAGACGGGAAGCTCACTGGC‐3′ | +731 to +750 | 28 |
| GAPDH‐R | GAPDH | 5′‐AGGTCCACCACCCTGTTGCT‐3′ | +1040 to +1021 | 28 |
| H5F | H2B4 | 5′‐GAAATGCTGGGGCAAGTGGTCAC‐3′ | +117 to +139 | 9 |
| H432R | H2B4 | 5′‐AGAGGCCACTGTCCTGCTGCTGAG‐3′ | +432 to +409 | 9 |
| βF | β‐actin | 5′‐TACCACTGGCATCGTGATGGACT‐3′ | +482 to +504 | 29 |
| βR | β‐actin | 5′‐TCCTTCTGCATCCTGTCGGCAAT‐3′ | +997 to +975 | 29 |
Position of nucleotide from the first base in the cDNA sequence.
Interferon‐γ release assay
YT cells were incubated in the presence of K562 cells in 1 ml culture media and in various conditions including IL‐2, C1.7 mAb and isotype mAb control for 16 hr at 37° under 5% CO2 in air. Where indicated, IL‐2, mAb C1.7 and isotype mAb control, was added to final concentrations of 20 U/ml, 200 ng/ml and 200 ng/ml, respectively. The cells were then spun down at 250 g for 5 min at 4°. One hundred µl of the supernatant was then extracted and tested for the presence of IFN‐γ. IFN‐γ protein secretion was quantitated immunologically by IFN‐γ human enzyme‐linked immunosorbent assay (ELISA) system (Amersham, UK), as per manufacturer's instructions.
Results
Expression of h2B4 is found on YT cells but not DB and Jurkat cells
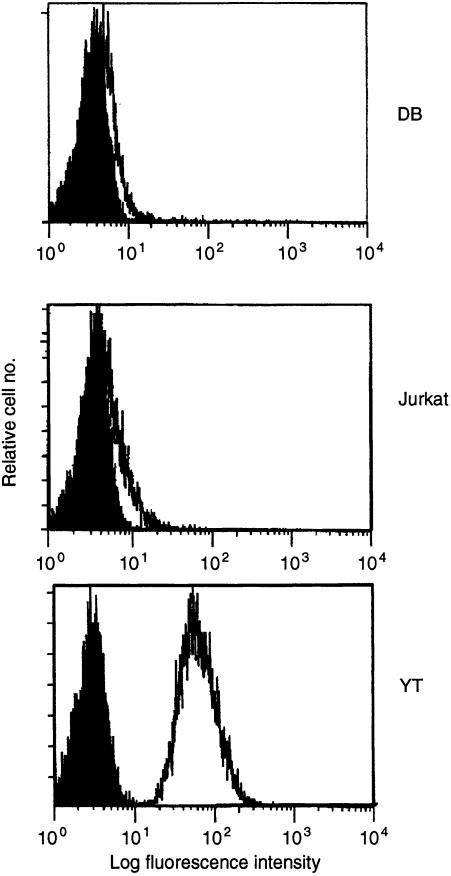
To examine h2B4's ability to activate NK cell functions, we used YT cells, a human NK cell line. mAb C1.7 has been shown to recognize the human homologue of 2B4, h2B4 on NK cells. 12 We tested whether YT, DB and Jurkat cell lines expressed h2B4 (Fig. 1). We found that YT cells expressed h2B4 while the mAb C1.7 failed to detect surface expression of h2B4 on either DB or Jurkat cells. This indicates YT cells are suitable to examine the functional implications of cell signalling through the h2B4 receptor.
Figure 1.
Expression of h2B4 on YT, DB and Jurkat cells. Cells were incubated with mAb C1.7 (open curve), or isotype control (closed curve) as described in Materials and methods. A secondary FITC–anti‐mouse immunoglobulin antibody was then used to detect C1.7 staining.
Effect of mAb C1.7 on YT cells increases cytotoxic activity
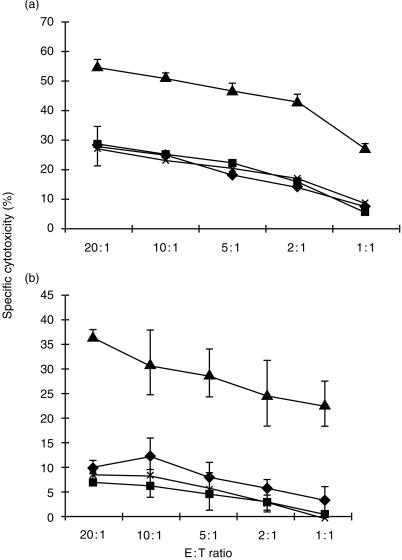
Previously, we have shown the engagement of h2B4 by a monoclonal antibody causes increased cytolytic activity of purified human polyclonal NK cells. 9 When mAb C1.7 was initially characterized, the antibody is also able to increase cytolytic activity of polyclonal cultured NK cells. 30 Thus it is likely that mAb C1.7 would activate YT cell cytolytic function. To study this, we tested the ability of mAb C1.7 to induce NK cell‐mediated antibody redirected lysis against 51Cr‐labelled FcγR+ K562 and P815 cells. In this assay, lysis of 51Cr‐labelled target cells occurs when the antibody is bound to both the FcγR on target cells and an activating receptor on NK cells, thus imitating the receptor ligand. mAb C1.7 was able to significantly raise lytic activity of YT cells against target K562 cells (Fig. 2a). Similarly, mAb C1.7 also enhanced cell‐mediated killing of target P815 cells (Fig. 2b). These results correlate well with our previous results which showed increased cytolytic activity of human PBMCs incubated with anti‐2B4 mAb. 9 However, when FcγR– target 721.221, RMA and Jurkat cells were used in similar assays, YT cell lysis of these target cells was not significantly changed (data not shown). These data suggests that h2B4 can transduce activation signals on YT cells. Previous studies have found that h2B4 can be found on γδ T cells and on subsets of CD8+ T cells and CD4+ T cells. 13 However, antibodies directed against h2B4 on these cells failed to elicit T‐cell‐mediated lysis.
Figure 2.
mAb C1.7‐induced, NK cell‐mediated cytotoxicity. YT cells were used as effector cells in standard 4 hr 51Cr release assays against (a) 51Cr‐labelled FcγR+ K562 target cells and (b) 51Cr‐labelled FcγR+ P815 target cells. Assays were performed in the presence of culture media alone (♦), or culture media with 20 U/ml IL‐2 (▪), or culture media with 20 U/ml IL‐2 and 200 ng/ml mAb C1.7 (▴) or culture media with 20 U/ml IL‐2 and 200 ng/ml isotype control mAb (×). All data points represent the mean of a minimum of three independent trials. Vertical bars represent the standard deviation.
h2B4 upregulates IFN‐γ production
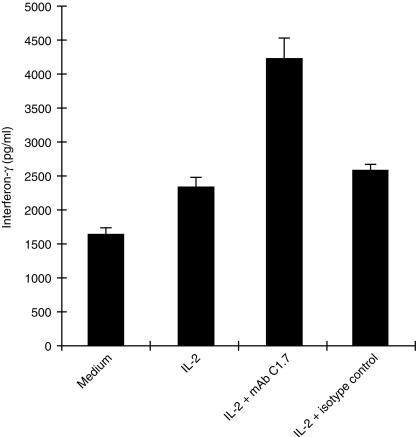
IFN‐γ is a cytokine shown to stimulate signalling cascades resulting in activation of gene transcription. This activity is important in antiviral, antiproliferative, and immunomodulatory processes. 31 We wanted to test the effect of h2B4 stimulation on IFN‐γ production by YT cells. One million cells were incubated under varying conditions alone, IL‐2, IL‐2 plus mAb C1.7 or isotype antibody control for 16 hr and then tested for IFN‐γ production by IFN‐γ ELISA. While the presence of IL‐2 increased the production of IFN‐γ by YT cells, IFN‐γ release was significantly augmented by the additional presence of mAb C1.7 (Fig. 3). The addition of isotype antibody control to IL‐2‐stimulated cells failed to show any significant change in IFN‐γ production. The addition of mAb C1.7 to YT cells alone caused IFN‐γ secretion at levels similar to YT cell secretion of IFN‐γ when stimulated by IL‐2 alone (data not shown).
Figure 3.
IFN‐γ production by YT cells. YT cells (1 × 106) were incubated in 1 ml of culture media alone, or culture media with 20 U/ml IL‐2, or culture media with 20 U/ml IL‐2 and 200 ng/ml mAb C1.7 or culture media with 20 U/ml IL‐2 and 200 ng/ml isotype control mAb for 16 hr. The cells were then spun down and 100 µl of supernatant was assayed for the presence of IFN‐γ by ELISA.
Engagement of h2B4 alters YT expression of h2B4
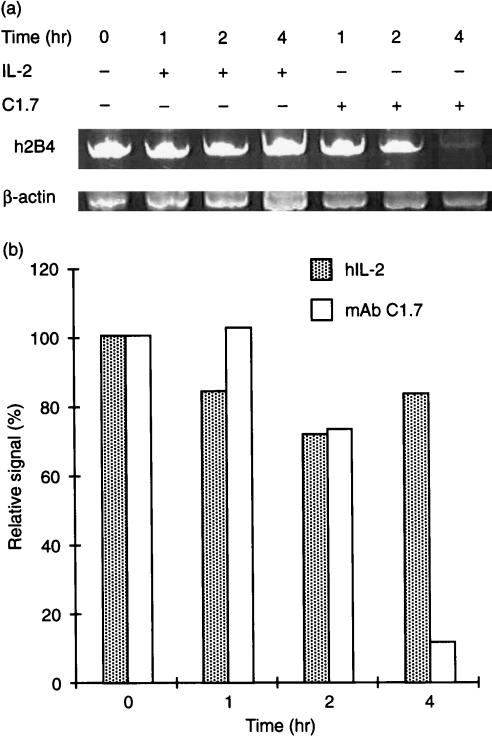
Stimulation of YT cells with mAb C1.7 results in increased cytolytic activity and IFN‐γ secretion. In order to determine whether h2B4 stimulation affected its own expression, we analysed mRNA levels of h2B4 in human PBMC stimulated with mAb C1.7. We isolated PBMC from healthy human donors using Ficoll Paque. NK cells were then isolated from the PBMC using NK cell isolation kit (Miltenyi Biotec, Auburn, CA). Fluorescence‐activated cell sorting (FACS) analysis showed NK cells were isolated > 96% purity (data not shown). One million purified human NK cells were cultured with mAb C1.7 for 1, 2 and 4 hr at 37° before their RNA was extracted. RT–PCR revealed a drastic decrease in mRNA levels of h2B4 after 4 hr (Fig. 4). We examined the expression of h2B4 after mAb C1.7 incubation to see whether surface expression of h2B4 correlated with changes in its mRNA production. We found h2B4 decreased by almost 20% within 1 hr of mAb C1.7 incubation with YT cells (data not shown). h2B4 expression remained depressed for up to 12 hr after mAb C1.7 stimulation. Therefore, as a result of mAb stimulation, h2B4 downregulates its own expression quickly after stimulation.
Figure 4.
h2B4 modulates its own RNA expression. Purified human NK cells (1 × 106) were incubated in the presence of mAb C1.7 (200 ng/ml) or hIL‐2 (20 U/ml) for various time intervals. RNA was then extracted and subjected to RT–PCR with primers amplifying sequences of the h2B4 gene and the β‐actin gene (Table 1). (a) RT–PCR analysis of h2B4. PCR products were run on a 6% polyacrylamide gel and then stained with ethidium bromide. PCR reactions were performed within the linear range of both primer sets. (b) Band intensities from (a) are normalized to β‐actin.
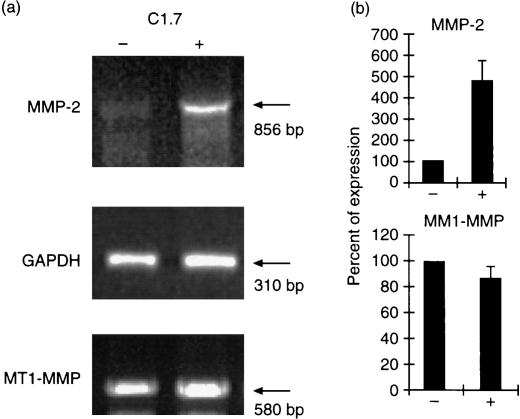
Stimulation of YT cells through h2B4 increases expression of MMP‐2
In our preliminary results, we observed that h2B4 stimulation increased the migration of YT cells through Matrigel invasion chamber (M. H. Kim and R. H. Goldfarb, unpublished observation). To determine the possible mechanism of increased migration through Matrigel, we investigated the expression of matrix metalloproteinases, a family of extracellular matrix degrading enzymes. Indeed, 2B4 stimulation increased the expression of MMP‐2 by four‐ to fivefold over control level, while the expression of membrane‐type (MT)‐MMP‐1 was not changed significantly (Fig. 5). To determine the kinetics of MMP‐2 expression, we performed RT–PCR analysis using total RNA prepared from YT cells stimulated with mAb C1.7 at various time points between 0 hr and 4 hr. The results show that the expression of MMP‐2 peaked between 30 min and 1 hr time points, while the level of GAPDH expression remained unchanged (data not shown). These results suggested that stimulation of 2B4 could lead to other functions of NK cells different from cytolytic activity. Such functions include extracellular matrix degradation by induction of MMP-2 expression.
Figure 5.
Induction of MMP‐2 expression by h2B4 stimulation. Total RNA was prepared from control (–) and C1.7 stimulated (+) YT cells. RT‐PCR was performed using primers described in Table 1. (a) Semiquantitative RT–PCR of MMP‐2 and MT1‐MMP. PCR for MMP‐2, MT1‐MMP, and GAPDH were done at 36, 35, and 20 cycles of PCR amplification, respectively, which were determined as in linear range of amplification for each gene. Each PCR was a representative of three separate PCR reactions. (b) Quantitation of PCR products. After electrophoresis, the ethidium bromide‐stained gel was scanned and densitometric analysis was performed using AlphaImager 2000 (Alpha Innotech Corp., San Leandro, CA). The MMP‐2 or MT1‐MMP PCR values were normalized to the average of GAPDH PCR value and expressed as percentage of expression. PCR value of control sample (–) was set to 100%. Each bar represents the average of triplicate determinations (± SD).
Discussion
The 2B4 receptor is a member of the immunoglobulin superfamily and belongs to the CD2 subgroup which includes SLAM, CD48, CD58, CD84 and Ly9. We have recently identified CD48 as the high affinity counter‐receptor of 2B4 in both mice and humans. 10 Early studies found that CD48 binds CD2 with low affinity in the mouse, 32 whereas CD58 is the low affinity counter‐receptor for CD2 in the human. 33 We have found the 2B4–CD48 interaction is six‐ to ninefold times stronger than that of CD2–CD48 interaction. The 2B4–CD48 interaction may be more physiologically significant and shed new light on conflicting observations surrounding CD48 ligand studies. Moreover, it has also been reported that CD48–2B4 interactions enhance the lytic function of human NK cells. 13 Our study shows that engagement of surface 2B4 on YT cells induces NK cytolytic function, cytokine production and production of MMP-2.
It has been reported that MMP‐2 expression can be transcriptionally regulated. 34 In human tumour cell lines, as well as untransformed cells, MMP‐2 expression is modulated in a cell‐type and stimulus‐specific manner by various immunological agents such as transforming growth factor‐β (TGF‐β), IFN‐γ, IL‐1β, TNF‐α, phorbol 12‐myristate 13‐acetate (PMA), and prostaglandin E2 (PGE2).s34 Here, we demonstrated that h2B4 stimulation up regulated the expression of MMP‐2, a member of matrix‐degrading proteinases, but not the expression of MT1‐MMP. More interestingly, engagement of 2B4 with mAb C1.7 downregulated the expression of surface 2B4 as well as 2B4 mRNA. Thus, signalling through surface 2B4 may involve transcriptional regulation of several genes including MMP‐2, IFN‐γ and 2B4, that play a role in various NK cell functions.
The signalling pathway of h2B4 is still unknown. h2B4 contains four tyrosine‐based motifs that resemble immuno-receptor tyrosine‐based inhibitory motifs on its cytoplasmic tail. 9 These motifs are similar to SLAM, and have been shown to interact with protein tyrosine phosphatase, SHP‐2 and the SH‐2 containing adaptor molecule, SAP.14, 35 A defect in SAP in patients suffering from X‐linked lymphoproliferative‐disease leads to the inability to control B‐cell proliferation caused by Epstein–Barr virus infections. However, it is still unclear what signal transduction pathway h2B4 utilizes to send its activating message. h2B4 may associate with another signal transduction molecule, as suggested by its activating signal.
Our study reveals that h2B4 leads to the induction of NK cell functions including both cytotoxicity and upregulation of invasive, degradative potential. These findings therefore argue well for further investigation of 2B4 and related molecular structures in the regulation of NK cell function.
Acknowledgments
This work was supported by National Institutes of Health Grant AI38938. The authors thank Dr Vinay Kumar (UT Southwestern, Dallas, Texas) for a kind gift of mAb 22B5 and Dr Eric Long (NIH, Bethesda, MD) for providing YT cells.
Abbreviations
- h2B4
human 2B4
- SLAM
signalling lymphocyte‐activation molecule
- SAP
SLAM‐associated protein
- MMP
matrix metalloproteinase
- GAPDH
glyceraldehyde 3‐phosphate dehydrogenase
- PBMC
peripheral blood mononuclear cells
- MT
membrane type
References
- 1.Lanier LL. NK cell receptors. Annu Rev Immunol. 1998;16:359. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- 2.Scott P, Trinchieri G. The role of natural killer cells in host–parasite interactions. Curr Opin Immunol. 1995;7:34. doi: 10.1016/0952-7915(95)80026-3. [DOI] [PubMed] [Google Scholar]
- 3.Spits H, Lanier LL, Phillips JH. Development of human T and natural killer cells. Blood. 1995;85:2654. [PubMed] [Google Scholar]
- 4.Biron CA. Activation and function of natural killer cell responses during viral infections. Curr Opin Immunol. 1997;9:24. doi: 10.1016/s0952-7915(97)80155-0. [DOI] [PubMed] [Google Scholar]
- 5.Naume B, Espevik T. Immunoregulatory effects of cytokines on natural killer cells. J Immunol. 1994;40:128. doi: 10.1111/j.1365-3083.1994.tb03441.x. [DOI] [PubMed] [Google Scholar]
- 6.Long EO, Burshtyn DN, Clark WP, et al. Killer cell inhibitory receptors: diversity, specificity, and function. Immunol Rev. 1997;155:135. doi: 10.1111/j.1600-065x.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 7.Mathew PA, Garni‐wagner BA, Land K, et al. Cloning and characterization of the 2B4 gene encoding a molecule associated with non‐MHC‐restricted killing mediated by activated natural killer cells and T cells. J Immunol. 1993;151:5328. [PubMed] [Google Scholar]
- 8.Garni‐wagner BA, Mathew PA, Bennett M, Kumar V. A novel function associated molecule related to non‐MHC‐restricted cytotoxicity mediated by activated natural killer cells and T cells. J Immunol. 1993;151:1117. [PubMed] [Google Scholar]
- 9.Boles KS, Nakajima H, Colonna M, et al. Molecular characterization of a novel human natural killer cell receptor homologous to mouse 2B4. Tissue Antigens. 1999;54:27. doi: 10.1034/j.1399-0039.1999.540103.x. [DOI] [PubMed] [Google Scholar]
- 10.Brown MH, Boles KS, van der Merwe PA, Kumar V, Mathew PA, Barclay AN. 2B4, the NK and T cell immunoglobulin superfamily surface protein, is a ligand for CD48. J Exp Med. 1998;188:2083. doi: 10.1084/jem.188.11.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Latchman Y, McKay PF, Reiser H. Identification of the 2B4 molecule as a counter‐receptor for CD48. J Immunol. 1998;161:5809. [PubMed] [Google Scholar]
- 12.Tangye SG, Lazetic S, Woollatt E, Sutherland GR, Lanier LL, Phillips JH. Human 2B4, an activating NK cell receptor, recruits the protein tyrosine phosphatase SHP‐2 and the adaptor signaling protein SAP. J Immunol. 1999;162:6981. [PubMed] [Google Scholar]
- 13.Nakajima H, Cella M, Langen H, Friedlein A, Colonna M. Activating interactions in human NK cell recognition: the role of 2B4‐CD48. Eur J Immunol. 1999;29:1676. doi: 10.1002/(SICI)1521-4141(199905)29:05<1676::AID-IMMU1676>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 14.Sayos J, Wu C, Morra M, et al. The X‐linked lymphoproliferative‐disease gene product SAP regulates signals induced through the co‐receptor SLAM. Nature. 1998;395:462. doi: 10.1038/26683. [DOI] [PubMed] [Google Scholar]
- 15.Peritt D, Sesok‐pizzini A, Schretzenmair R, et al. C1.7 antigen expression on CD8+ T cells is activation dependent: increased proportion of C1.7+ CD8+ T cells in HIV‐1‐infected patients with progressing disease. J Immunol. 1999;162:7563. [PubMed] [Google Scholar]
- 16.Kitson RP, Appasamy PM, Nannmark U, Albertsson P, Gabauer MK, Goldfarb RH. Matrix metalloproteinases produced by rat IL‐2 activated natural killer cells. J Immunol. 1998;160:4248. [PubMed] [Google Scholar]
- 17.Zeng LS, Goetzl EJ. Selective regulation of RNK‐16 cell matrix metalloproteinases by the EP4 subtype of prostaglandin E2 receptor. Biochemistry. 1996;35:7159. doi: 10.1021/bi960036x. [DOI] [PubMed] [Google Scholar]
- 18.Westermarck J, Kahari VM. Regulation of matrix metalloproteinase expression in tumor invasion. Faseb J. 1999;13:781. [PubMed] [Google Scholar]
- 19.Hagmann WK, Lark MW, Becker JW. Inhibition of matrix metalloproteinases. Annu Rep Med Chem. 1996;34:231. [Google Scholar]
- 20.Ennis BW, Matrisian LM. Matrix degrading metalloproteinases. In: Goldfarb RH, editor. Brain Tumor Invasiveness. Dordrecht: Kluwer; 1994. pp. 17–22. [Google Scholar]
- 21.Matrisian LM. The matrix‐degrading metalloproteinases. Bio Essays. 1992;14:455. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- 22.Kayagaki N, Kawasaki A, Ebata T, et al. Metalloproteinase‐mediated release of human Fas ligand. J Exp Med. 1995;182:1777. doi: 10.1084/jem.182.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gearing AJ, Beckett P, Christodoulou M, et al. Processing of tumour necrosis factor‐alpha precursor by metalloproteinases. Nature. 1994;370:555. doi: 10.1038/370555a0. [DOI] [PubMed] [Google Scholar]
- 24.McGeehan GM, Becherer JD, Bast RC, Jr, et al. Regulation of tumour necrosis factor‐alpha processing by a metalloproteinase inhibitor. Nature. 1994;370:558. doi: 10.1038/370558a0. [DOI] [PubMed] [Google Scholar]
- 25.Yodoi J, Teshigawara K, Nikaido T, et al. TCGF (IL‐2) ‐receptor inducing factor(s) I. Regulation of IL‐2 receptor on a natural killer‐like cell line (YT cells) J Immunol. 1985;134:1623. [PubMed] [Google Scholar]
- 26.Collier IE, Wilhelm SM, Eisen AZ, et al. H‐ras oncogene‐transformed human bronchial epithelial cells (TBE‐1) secrete a single metalloprotease capable of degrading basement membrane collagen. J Biol Chem. 1988;263:6579. [PubMed] [Google Scholar]
- 27.Will H, Hinzmann B. cDNA sequence and mRNA tissue distribution of a novel human matrix metalloproteinase with a potential transmembrane segment. Eur J Biochem. 1995;231:602. doi: 10.1111/j.1432-1033.1995.tb20738.x. [DOI] [PubMed] [Google Scholar]
- 28.Tso JY, Sun XH, Kao TH, Reece KS, Wu R. Isolation and characterization of rat and human glyceraldehyde‐3‐phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nuc Acids Res. 1985;12:9179. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernard K, Cambiaggi A, Guia S, et al. Engagement of natural cytoxicity programs regulates AP‐1 Expression in the NKL human NK cell line. J Immunol. 1999;162:4062. [PubMed] [Google Scholar]
- 30.Valiante NM, Trinchieri G. Identification of a novel signal transduction surface molecule on human cytotoxic lymphocytes. J Exp Med. 1993;178:1397. doi: 10.1084/jem.178.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park C, Schindler C. Protein–DNA interactions in interferon‐γ signaling. Methods Enzymol. 1998;15:175. doi: 10.1006/meth.1998.0622. [DOI] [PubMed] [Google Scholar]
- 32.Kato K, Koyanagi M, Okada H, et al. CD48 is a counter‐receptor for mouse CD2 and is involved in T cell activation. J Exp Med. 1992;176:1241. doi: 10.1084/jem.176.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis SJ, van der Merwe PA. The structure and ligand interactions of CD2; implications for T cell function. Immunol Today. 1996;17:177. doi: 10.1016/0167-5699(96)80617-7. [DOI] [PubMed] [Google Scholar]
- 34.Qin H, Moellinger JD, Wells A, Widsor LJ, Sun Y, Benveniste EN. Transcriptional suppression of matrix metalloproteinase‐2 gene expression in human astroglioma cells by TNF‐α and IFN‐γ. J Immunol. 1998;161:6664. [PubMed] [Google Scholar]
- 35.Cocks BG, Chang CC, Carbaiido JM, Yssel H, de Vries JE, Aversa G. A novel receptor involved in T cell activation. Nature. 1998;376:260. doi: 10.1038/376260a0. [DOI] [PubMed] [Google Scholar]