Abstract
This work examines the correlation between serum levels of oestrogen, progesterone and dehydroepiandrosterone sulphate (DHEA-S) and the number of human peripheral blood cells actively secreting interleukin (IL)-2, IL-4, IL-6, IL-10, tumour necrosis factor-α (TNF-α) or interferon-γ (IFN-γ) in vivo. Simultaneous assessment of serum hormone levels and cytokine-secreting cell activity throughout the menstrual cycle showed that the number of peripheral blood mononuclear cells (PBMC) able to secrete IL-4 in response to stimulation correlated significantly (P < 0·0001) with oestrogen levels and fluctuated with the menstrual cycle in pre-menopausal women. The activity of IFN-γ-secreting cells, on the other hand, varied as a function of serum DHEA-S levels in pre-menopausal women (P < 0·0001). Similarly, the number of cells secreting IFN-γ in men correlated with serum DHEA-S levels (P < 0·001). In contrast, post-menopausal women had fewer cells actively secreting cytokines and the activity of these cells did not correlate with sex hormone levels. These results suggest that sex hormones may modulate cytokine production in vivo and contribute to gender-related differences in normal and pathological immune responses.
Introduction
Cytokines influence the host's response to vaccination, infection, and their likelihood of developing autoimmune disease. 1–3 Cytokines were initially categorized in mice into three broad categories: pro-inflammatory [such as tumour necrosis factor-α (TNF-α), interleukin (IL)-1 and IL-6), type 1 (such as IL-2 and interferon-γ (IFN-γ)] which promote the development of antigen-specific cell-mediated immunity, and type 2 (such as IL-4 and IL-10) which facilitate the development of antibody-mediated immunity (reviewed in 3 and 4). The same basic characterization has also been observed in humans (reviewed in 3).
Epidemiological, clinical, and laboratory data suggest that women generally have higher serum immunoglobulin levels and mount stronger antibody responses following vaccination or infection than do males. 5–7 This heightened humoral immune responsiveness may contribute to the greater susceptibility of women to autoimmune diseases such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and Sjögren's syndrome (SS) (reviewed in 8–11). There is some evidence that sex hormones can affect the number and/or activation state of lymphocytes. For example, disease severity in patients with immune-mediated disorders is influenced by serum oestrogen, progesterone and/or androgen levels. 12–14
Although other investigators have examined the effect of extreme hormonal changes on the immune function (induced by oophorectomy or hormonal treatment), the impact of normal physiologic fluctuations of sex hormones associated with the menstrual cycles is poorly understood. 12, 15 Evidence suggests that serum IL-1 increases and serum IL-6 decreases during the luteal phase, whereas serum IL-10 levels do not fluctuate with the menstrual cycle. 16–19 The effect of oestrogen levels on TNF-α production is controversial, partly because it appears to have diametrically opposed activity at different concentrations. 14, 20 Moreover, there is no information on whether changes in serum hormone levels correlate with changes in the activity of type 1 or type 2 cytokine-secreting cells.
We used sensitive enzyme-linked immunospot (ELIspot) assays to monitor the number of PBMC secreting IL-2, IL-4, IL-6, IL-10, TNF-α or IFN-γ (spontaneous and in response to phytohaemagglutinin (PHA)) as a function of oestrogen, progesterone and dehydroepiandrosterone sulphate (DHEA-S) levels in pre-menopausal and post-menopausal women. Our findings suggest that physiological fluctuation in sex hormone levels may influence the activity of cytokine producing cells.
Materials and methods
Subjects and cell preparation
Healthy volunteers completed a self-administered questionnaire regarding their menstrual status, reproductive history and use of oral contraceptives. Subjects taking hormone-containing medication were excluded from the study. Peripheral blood was obtained by venepuncture from healthy volunteers at the National Institutes of Health after informed consent. The mean age of pre-menopause subjects was 36 ± 8 (range 21–46; N = 38), post-menopause were 53 ± 6 years old (range 44–64; N = 22) and male subjects were 32 ± 11 years old (range 18–50; N = 28). All subjects were bled in the morning. Mononuclear cells were separated by density gradient centrifugation over Ficoll–Hypaque [Lymphoprep (Nycomed Pharma), Oslo, Norway] as previously described. 21 Cells were washed five times and resuspended in RPMI-1640 (Life Technologies, Grand Island, NY) supplemented with 10% heat inactivated fetal calf serum (FCS; Gibco BRL, Life Technologies), 1·5 mm l-glutamine and 100 U/ml of penicillin/streptomycin. The same batch of FCS was used throughout the study. All samples were processed in the same manner. Where indicated, the cells were stimulated with a 1:100 dilution PHA (Gibco, Grand Island, NY) during the ELIspot assay.
Cytokine ELIspot assays
All samples were tested for a panel of cytokine-secreting cells including IL-2, IL-4, IL-6, IL-10, TNF-α and IFN-γ. As previously described, 21, 22 96-well Immunolon 2 microtitre plates were coated overnight at 4° with 10 μg/ml of anti-human cytokine antibodies in phosphate-buffered saline (PBS) (anti-IL-2, anti-IL-6, anti-TNF-α (R & D systems, Minneapolis, MN), anti-IL-4 (PharMingen, San Diego, CA), anti-IL-10 and anti-IFN-γ (Endogen, Boston, MA), and then blocked with PBS−5% BSA for 2 hr as previously described. 21, 22 The plates were overlaid with eight serial dilutions (1:3) of single-cell suspensions starting with 5 × 105 cells/well (one or two series/subject). These were incubated at 37° in a humidified 5% CO2 in air incubator for 6 (IL-6 and TNF-α) or 18 hr (IL-2, 4, 10 and IFN-γ). The plates were then washed with water−0·025% Tween and overlaid with biotin-conjugated anti-human cytokine antibodies (anti-IL-2, anti-IL6, and anti-TNF-α (Biosource, Camarillo, CA); anti-IL-10 and anti-IFN-γ (Endogen); or anti-IL-4 (Genzyme, Cambridge, MA). After 2 hr the plates were washed again with PBS–Tween and then overlaid with alkaline phosphatase-conjugated avidin. After a final wash, spots were visualized by the addition of 5-bromo-4-chloro-3-indolyl phosphate (Kirkegaard and Perry Laboratories, Gaithersburg, MD) in low-melt agarose (Sigma, St Louis, MO). Spots generated by individual cytokine-secreting cells were counted manually under 40× magnification. The number of cytokine-secreting cells was determined by a single-blinded reader, who analysed the number of spots in three separate wells per sample. Intra-assay variability was less than 10%.
Sex hormone levels
Sex hormone levels were determined by radioimmune analysis using commercially available kits (ICN Pharmaceuticals, Costa Mesa, CA) as per manufacturers instructions. Briefly, serum samples (25–100 ml) and I125-labelled hormone were added to anti-hormone-coated tubes, incubated at 37° for 1–2 hr, washed, and then analysed for radioactivity using a gamma counter. Intra-assay coefficient of variation was less than 10%.
Statistical analysis
Normality of the distribution was determined using the Kolomogorov–Smirnov test. Accordingly, a t-test was used for normally distributed data and Mann–Whitney U test for non-normally distributed data to assess differences between groups. Correlations were determined using Spearman's Rank Order analysis and linear regression analysis.
Results
Frequency of cytokine-secreting cells in the peripheral blood of normal women
Peripheral blood was collected from 60 healthy women, including 38 individuals with regular menstrual cycles and 22 who were post-menopausal. None of the donors was taking hormone-containing drugs. Serum sex hormone levels were examined by radioimmunoassay (RIA), and the number of cells actively secreting cytokines was determined by ELIspot assay. Consistent with previous reports, the serum of women with normal menstrual cycles contained significantly higher levels of all three sex hormones than their post-menopausal counterparts; oestrogen levels were 107 ± 11 versus 33 ± 5 pg/ml, P < 0·001, DHEA-S levels were 1164 ± 105 versus 589 ± 98 ng/ml, P < 0·001 and progesterone levels were 4 ± 1 versus 0·3 ± 0·1 pg/ml, P < 0·05.
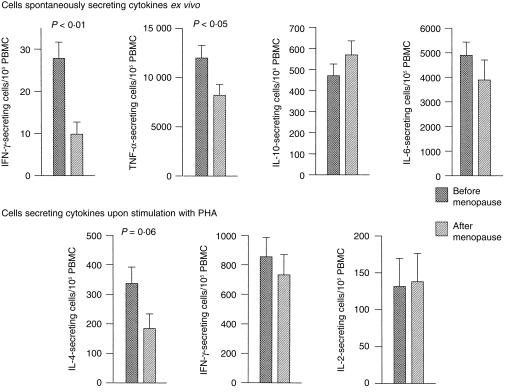
The number of cells secreting cytokines was characterized by high intersubject variability extending over two orders of magnitude. Despite this variability, pre-menopausal women on average had significantly more cells actively secreting IFN-γ (P < 0·01) and TNF-α (P < 0·05) than did post-menopausal women (Fig. 1). The number of cells secreting IL-6 and IL-10 were similar in these two groups. Unfortunately, too few cells (< 5/106) were spontaneously secreting IL-2 or IL-4 in most subjects for their numbers to be accurately determined. To monitor the number of cells in the peripheral blood capable of secreting these cytokines, PBMC were stimulated with PHA. As seen in Fig. 1, pre-menopausal women had more PHA-responsive cells secreting IL-4 than did the post-menopausal group, although the difference did not quite reach statistical significance (P < 0·06).
Figure 1.
Frequency of cytokine-secreting PBMC in women before and after menopause. The number cells secreting cytokines per 105 PBMC, spontaneously or in response to stimulation with PHA, in 38 pre-menopause and 22 post-menopause normal volunteers was determined by ELIspot. Results represent the mean ±SEM of 34 individual experiments. All samples were tested blind and decoded retrospectively. Mann–Whitney U-test.
To determine whether age rather than hormonal status accounted for the differences observed between pre and post-menopausal women, we performed a regression analysis of cytokine levels as a function of age in each subpopulation. No association with age was observed within each group, suggesting that differences between populations were most likely a reflection of sex hormone levels. A similar analysis was run to determine whether parity influenced the activity of cytokine-secreting cells. No significant differences in the number of cytokine-secreting cells were evident between nulliparous and multiparous pre-menopause (n = 14 and 24, respectively) and post-menopause women (n = 7 and 10, respectively).
Correlation between sex hormone levels and cytokine producing cells in pre-menopausal women
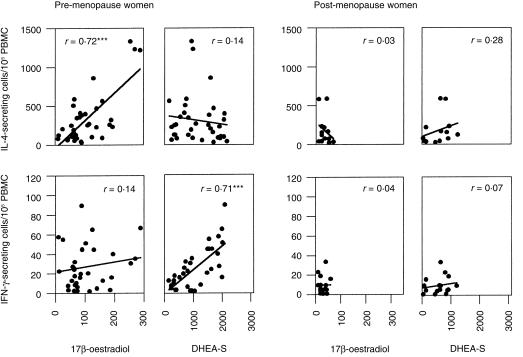
To assess whether sex hormones contributed to the differences in cytokine production noted above, the number of cells secreting IL-2, IL4, IL-6, IL-10, IFN-γ and TNF-α was analysed as a function of serum steroid hormone levels in each donor. A significant correlation was observed between serum levels of DHEA-S and the number of cells spontaneously secreting IFN-γ in pre-menopausal women (r = 0·71, P < 0·0001, Fig. 2). In the same population, 17β-oestradiol levels correlated with the number of cells responding to PHA stimulation by secreting IL-4 (r = 0·72, P < 0·0001). Oestradiol and DHEA-S levels did not correlate with the production of any of other cytokine examined, nor did serum progesterone levels correlate with the activation of cytokine-secreting cells. There was no correlation between the age of menstruating donors and the number of cells actively secreting cytokine in peripheral blood.
Figure 2.
Correlation between hormone levels and frequency of cytokine-secreting cells in pre-menopausal (N = 38) and post-menopause women (N = 22). The number of cytokine-secreting cells was correlated with serum hormone levels in each individual. Pearson rank order analysis ***Statistically significant correlation P < 0·001.
Both oestrogen and DHEA-S levels were significantly reduced among post-menopausal women (P < 0·005), as were the number of cells spontaneously secreting IFN-γ (Fig. 1). Yet no correlation was observed between sex hormone levels and number of cells producing these cytokines in post-menopausal women (Fig. 2).
Synchronous variation in IL-4 producing cell numbers and 17β-oestradiol levels during the menstrual cycle
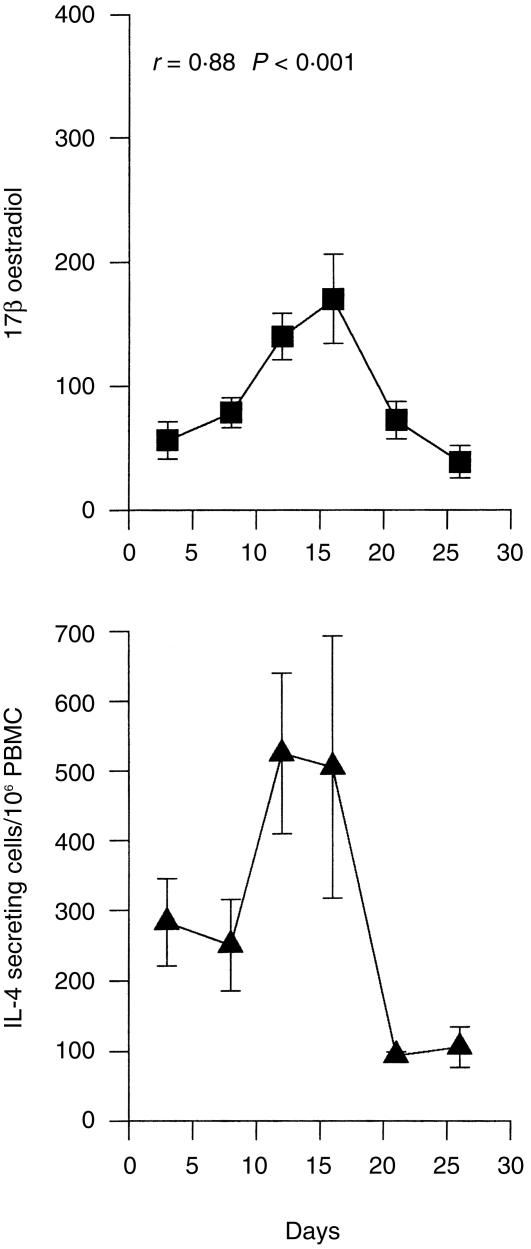
Serum oestrogen levels follow a well-defined monthly cycle. Building on the finding that serum oestrogen levels correlated with PHA-induced IL-4 production, we examined whether the activity of cytokine-secreting cells changed over the course of the menstrual cycle. Although cycle length varied among women, self-reported staging of the menstrual cycle closely approximated the expected changes in serum oestrogen levels (Fig. 3). These changes in 17β-estradiol levels correlated significantly with the number of cells available to secrete IL-4 (as reflected by PHA induced IL-4 production, Fig. 3) (r = 0·88, P < 0·001). The number of cells secreting IL-6 and TNF-α were generally higher during the follicular phase than the luteal phase, but these differences did not achieve statistical significance. No other correlations between menstrual cycle and cytokine production were observed.
Figure 3.
Serum level of 17β-oestradiol and frequency of IL-4 secreting PBMC in women with regular menstrual cycles (N = 38). The samples were taken randomly and then retrospectively assigned a day in the menstrual cycle according to the data provided in their questionnaire. Results represent mean ±SEM; r-value denotes the correlation between the two curves. P < 0·001.
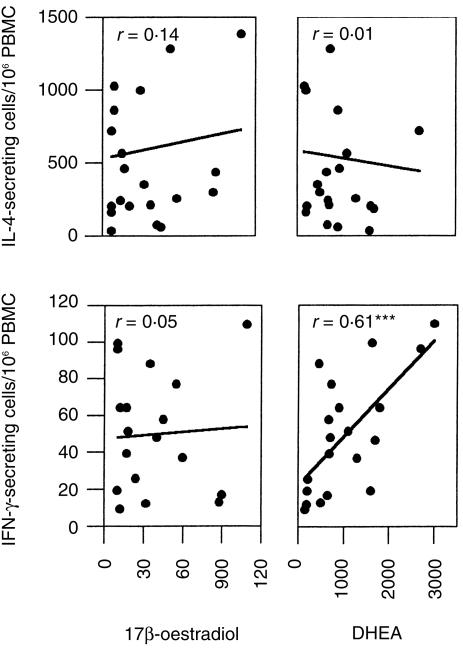
IFN-γ-secreting cell numbers correlate with DHEA-S levels in men
The role of sex hormones on the activity of cytokine-secreting cells in men was also examined. Twenty-eight men aged 18–50 were examined. As expected, males had low serum levels of oestrogen and progesterone, and there was no correlation between these hormones and the number of PBMC secreting any of the cytokines examined. However, as was the case in pre-menopausal women, serum DHEA-S levels correlated with the number of cells spontaneously secreting IFN-γ in men (r = 0·61, P < 0·001; Fig. 4). A correlation that approached statistical significance was noted between DHEA-S and the number of cells induced to secrete IL-2 following PHA stimulation (r = 0·4, P = 0·06), but not with any of the other cytokines tested.
Figure 4.
Correlation between hormone levels and frequency of active cytokine-secreting cells in male volunteers. The number of cytokine-secreting cells and serum hormone levels of individual subjects were correlated. ***Statistically significant correlation, P < 0·001.
Discussion
The greater susceptibility of women to a variety of autoimmune diseases, combined with their stronger humoral responses to foreign antigenic challenge, suggest that sex hormones may influence immune function. 6, 8, 23, 24 We evaluated whether changes in sex hormone levels were associated with altered cytokine production in vivo. Results indicate that the activity of IFN-γ-secreting cells varies as a function of DHEA-S levels in both pre-menopausal women and men, while PHA-induced IL-4 production correlates with serum oestrogen levels (and fluctuates with the menstrual cycle).
Previous studies investigating the interaction between the neuroendocrine and immune systems generally focused on subjects with autoimmune disease 5, 6, 13 or undergoing profound changes in oestrogen levels (due to oophorectomy, discontinuation of oestrogen replacement therapy or menopause). 24, 25 Few studies addressed whether cytokine levels change during the menstrual cycle, and those were almost entirely restricted to an analysis of serum IL-10, IL-6 and/or TNF-α levels. Interpretation of earlier work was hampered by the low concentration, short half-life and rapid uptake/utilization of cytokines in vivo. 26 We used ELIspot assays to quantitate PBMC actively secreting cytokines ex vivo, a technique that accurately reflects the activity of cytokine-secreting cells in vivo. 21, 27 This allowed us to detect ongoing changes in the cytokine milieu with greater sensitivity and accuracy than was previously possible, improving our ability to detect correlations between cytokine production and serum hormones levels.
The number of cells secreting cytokines spontaneously or in response to PHA were similar to those previously reported using the ELIspot assay. 21, 28 Our studies confirm that < 1/103 cells spontaneously secretes IL-2, IL-4 or IFN-γin vivo. 21, 28, 29 As previously reported, a large fraction of PBMC (range 2–24%) secrete IL-6 and TNF-α. 21, 28 These cytokines are difficult to detect in normal serum or in supernatant from PBMC, but intracellular staining of PBMC yielded similar results 30 (M. Gursel, personal communication). The large number of cells secreting IL-6 and TNF-α may reflect basal levels of cytokine secretion or the inadvertent stimulation of cells during the isolation process. Attempts to avoid activation, by collecting samples in glass tubes rather than plastic, using citrate rather than heparin, eliminating the Ficoll gradient step and using nitrocellulose-backed plates rather than the regular Immunolon plates, had no effect. It should be noted that the number of cells secreting IL-6 and TNF-α doubled in response to lipopolysaccharide (LPS) stimulating in vitro, suggesting that endotoxin is not the cause for the high number of cells secreting these cytokines. It is also possible that substances present in culture medium (such as phenol red or FCS) mimicked the effects of sex hormones. Regardless of such possibilities, the observed differences between pre- and post-menopausal women are interpretable since all samples were isolated and treated identically. Indeed, if some form of non-specific stimulation were taking place, it would have blunted our ability to detect an effect of sex hormone levels on immune activation.
Our results show that pre-menopausal women had significantly more cells actively secreting IFN-γ (P < 0·01) and TNF-α (P < 0·05) than did post-menopausal women. The number of cells secreting IL-6 and IL-10 were similar in these two groups. By comparison, serum DHEA-S levels correlated with the spontaneous activation of IFN-γ-secreting cells in men and pre-menopausal women. The latter findings are consistent with that of Daynes et al. who reported that DHEA-S promotes the production of another type 1 cytokine, IL-2, 31 and with reports that DHEA-S stimulates IFN-γ production by murine lymphocytes. 32 We also found that the number of cells spontaneously secreting IFN-γ was significantly lower in post- rather than in pre-menopausal women. However, the smaller changes in serum oestrogen levels occurring during the normal menstrual cycle failed to correlate with fluctuations in IFN-γ-secreting cell numbers.
We observed that changes in serum oestrogen levels during the menstrual cycle correlated with variations in the number of PBMC that responded to PHA stimulation by secreting IL-4. Unfortunately, there were too few PBMC spontaneously secreting IL-4 in vivo to detect such an effect in the absence of mitogen activation. Since IL-4 plus antigen are co-inducers of B-cell activation and IgG production, this increased IL-4 production may contribute to the ability of pre-menopausal women to mount stronger humoral immune responses than their post-menopausal counterparts. This interpretation is supported by evidence that oestrogen treatment increases IL-4 secreting cell numbers, B cell activation, and immunoglobulin G (IgG) antibody levels in mice (D. Verthelyi, unpublished observations). 33 The finding that IL-4 production was maximal during the periovulatory phase of the menstrual cycle suggests that the menstrual cycle may influence the type and magnitude of a woman's response to immune challenge (such as infection or vaccination).
Progesterone is thought to play an important role modulating cytokine production during pregnancy. 34 Our data suggest that physiological changes in progesterone levels in non-pregnant women fail to correlate with the activity of cytokine-secreting cells, including IL-4.
Post-menopausal women had lower serum oestrogen and DHEA-S levels, and also had significantly fewer cells spontaneously secreting IFN-γ and TNF-α than men or pre-menopausal women. This raises the possibility that hormonal changes might contribute to the age-associated decline in immune reactivity of older women. 35 In our study the number of PBMC in post-menopausal women that responded to PHA stimulation by secreting IFN-γ and IL-2 was not reduced. Thus, ageing may be associated with a decline in the activation state rather than frequency of available cytokine-producing cells. Supporting a role for sex hormones in mediating this decline are preliminary studies in our lab suggesting that the number of cells spontaneously secreting IFN-γ in post-menopausal women receiving hormone replacement therapy is comparable to that of pre-menopausal women. Together, these findings suggest that a basal level of oestrogen facilitates the production of IFN-γ, an observation consistent with the findings of Fox et al. that oestrogen can stimulate IFN-γ secretion, 36 but that after menopause lymphocytes may be unable to sense small changes in hormone levels.
The precise mechanism(s) underlying the interaction between sex hormones and the immune system has not been delineated. Previous studies showing that oestrogen and progesterone modulate cytokine secretion in vitro, in addition to murine studies in vivo showing that DHEA-S increases the secretion of IFN-γ and IL-2 while oestrogen promotes the production of IL-4 suggest that sex hormones may influence the production of cytokines. 20, 31, 32, 37 Sex hormone receptors are present on immunologically relevant cells, including CD4+ and CD8+ T cells, 38, 39 monocytes 40 and macrophages. 41 However, our results do not establish whether changes in cytokine production are due to the direct activation of immune cells by hormones, or rather than by some indirect or secondary effect.
This is the first report on the levels of immune activation and cytokine secretion in vivo as a function of physiologic changes in sex hormone levels in healthy subjects. Millions of people take sex hormones in the form of oral contraceptives or hormone replacement, and a number of widely used pesticides and insecticides have hormone-disrupting effects. Our results suggest that sex hormone levels may influence the activation state of the human immune system. This has implications for the planning and the interpretation of clinical studies examining responses to vaccines and immunotherapeutic agents. Moreover, understanding the influence of steroid hormones on immune function may clarify the physiological basis underlying the gender bias observed in many autoimmune states.
Acknowledgments
This work was supported by a grant from the Office of Women's Health. We wish to thank Dr Susan Leitman and the staff of the Blood Bank at the National Institutes of Health for collecting the blood samples. We also wish to thank Drs Alfred Steinberg and Fred Miller for kindly reviewing the manuscript. The assertions herein are the private ones of the authors and are not to be construed as official or as reflecting the views of the Food and Drug Administration.
References
- 1.Paul WE. Pleiotropy and redundancy: T cell-derived lymphokines in the immune response. Cell. 1989;57:521. doi: 10.1016/0092-8674(89)90121-9. [DOI] [PubMed] [Google Scholar]
- 2.Balkwill FR, Burke F. The cytokine network. Immunol Today. 1989;10:299. doi: 10.1016/0167-5699(89)90085-6. [DOI] [PubMed] [Google Scholar]
- 3.Lucey DR. type 1 nad type 2 cytokine dysregulation in human infectious, neoplastic and inflammatory diseases. Clin Microboiol Rev. 1996;9:532. doi: 10.1128/cmr.9.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosmann TR, Coffman RL. Two types of mouse helper T-cell clone. Immunol Today. 1987;8:223. doi: 10.1016/0167-5699(87)90171-X. [DOI] [PubMed] [Google Scholar]
- 5.Cutolo M, Sulli A, Seriolo B, Accardo S, Masi AT. Estrogens, immune response and autoimmunity. Clin and Exp Rheum. 1995;13:217. [PubMed] [Google Scholar]
- 6.Da Silva JAP. Sex hormones, glucocorticoids and autoimmunity: facts and hypotheses. Ann Rheum Dis. 1995;54:6. doi: 10.1136/ard.54.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuurs AHWM, Verheul HAM. Effects of gender and sex hormones on the immune response. Steroid Biochem. 1990;35:157. doi: 10.1016/0022-4731(90)90270-3. [DOI] [PubMed] [Google Scholar]
- 8.Grossman CJ. Interactions between gonadal steroids and the immune system. Science. 1985;227:257. doi: 10.1126/science.3871252. [DOI] [PubMed] [Google Scholar]
- 9.Alt FW, Blackwell TK, Yancopoulos GD. Development of the primary antibody repertoire. Science. 1987;238:1079. doi: 10.1126/science.3317825. [DOI] [PubMed] [Google Scholar]
- 10.Ansar Ahmed S, Talal N. Importance of sex hormones in systemic lupus erythematosus. In: Wallace DJ, Hahn BH, editors. Duboi's Lupus Erythematosus. Philadelphia: Lea and Febiger; 1993. p. 148. [Google Scholar]
- 11.Germain R, Ju ST, Kipps TJ, Benecerraf B, Dorf ME. Shared idiotypic determinants on antibodies and T cell derived suppressor factor specific for the random terpolymer TGAL. J Exp Med. 1979;149:613. doi: 10.1084/jem.149.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinberg AD, Steinberg BJ. Lupus disease activity ssociated with menstrual cycle. J Rheumatol. 1985;12:816. [PubMed] [Google Scholar]
- 13.Lahita RG. Sex hormones and the immune system – part 1. Human data. Baillière's Clin Rheumatol. 1990;4:1. doi: 10.1016/s0950-3579(05)80240-7. [DOI] [PubMed] [Google Scholar]
- 14.Lahita R. The basis for gender effects in the connective tissue diseases. Ann Med Interne. 1996;147:241. [PubMed] [Google Scholar]
- 15.White HD, Crassi KM, Givan AL, et al. CD3+ CD8+ CTL activity within the human female reproductive tract: influence of stage of menstrual cycle and menopause. J Immunol. 1997;158:3017. [PubMed] [Google Scholar]
- 16.Cannon JG, Dinarello CA. Increased plasma interleukin-1 activity in women after ovulation. Science. 1985;227:1247. doi: 10.1126/science.3871966. [DOI] [PubMed] [Google Scholar]
- 17.Polan ML. Gonadal steroids modulate human monocyte interleukin-1 (IL-1) activity. Fertil Steril. 1988;49:964. [PubMed] [Google Scholar]
- 18.Angstwurm MWA, Gartner R, Loms Ziegler-Heitbrock HW. Cyclic plasma IL-6 levels during normal menstrual cycle. Cytokine. 1997;9:370. doi: 10.1006/cyto.1996.0178. [DOI] [PubMed] [Google Scholar]
- 19.Maskill JK, Laird SM, Okon M, Li TC, Blakemore AIF. Stability of serum IL-10 levels during the menstrual cycle. AJRI. 1997;38:339. doi: 10.1111/j.1600-0897.1997.tb00309.x. [DOI] [PubMed] [Google Scholar]
- 20.Gilmore W, Weiner LP, Correale J. Effect of estradiol on cytokine secretion by proteolipid protein specific T cell clones isolated from multiple sclerosis patients and normal control subjects. J Immunol. 1997;158:446. [PubMed] [Google Scholar]
- 21.Hagiwara E, Abbasi F, Mor G, Ishigatsubo Y, Klinman DM. Phenotype and frequency of cells secreting IL-2, IL-4, IL-6, IL-10, IFNγ and TNFα in human peripheral blood. Cytokine. 1995;7:815. doi: 10.1006/cyto.1995.0098. [DOI] [PubMed] [Google Scholar]
- 22.Hagiwara E, Gourley M, Lee S, Klinman DM. Disease severity in patients with systemic lupus erythematosus correlates with an increased ratio of IL-10: IFN-gamma secreting cells in the peripheral blood. Arthritis Rheum. 1996;39:379. doi: 10.1002/art.1780390305. [DOI] [PubMed] [Google Scholar]
- 23.Ansar Ahmed S, Penhale WP, Talal N. Sex hormones, immune responses and autoimmune diseases: Mechanisms of sex hormone action. Am J Pathol. 1985;121:531. [PMC free article] [PubMed] [Google Scholar]
- 24.Wilder RL. Neuroendocrine–immune system interaction and autoimmunity. Annu Rev Immunol. 1994;13:307. doi: 10.1146/annurev.iy.13.040195.001515. [DOI] [PubMed] [Google Scholar]
- 25.Pacifici R. Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. J Bone Miner Res. 1996;11:1043. doi: 10.1002/jbmr.5650110802. [DOI] [PubMed] [Google Scholar]
- 26.Howard M, Miyajima A, Coffman R. T-cell derived cytokines and their receptors. In: Paul WE, editor. Fundamental Immunology. New York: Raven Press; 1993. p. 763. [Google Scholar]
- 27.Klinman DM, Nutman TB. ELIspot assay to detect cytokine-secreting murine and human cells. In: Coligan JE, et al., editors. Current Protocols in Immunology. Brooklyn NY: Greene Publishing Associates; 1994. pp. 6.19.1–6.19.8. [Google Scholar]
- 28.Kanik KS, Hagiwara E, Schumacher R, Wilder RL, Klinman DM. Distinct patterns of cytokine secretion characterize new onset synovitis versus chronic rheumatoid arthritis. J Rheum. 1998;25:16. [PubMed] [Google Scholar]
- 29.Lagoo A, Lagoo-Deenadayalan S, Lorenz H, Byrne J, Barber W, Hardy K. IL-2, IL-4, and IFN-gamma gene expression versus secretion in superantigen-activated T cells. J Immunol. 1994;152:1641. [PubMed] [Google Scholar]
- 30.Nguyen LT, Ramanathan M, Munschauer F, et al. Flow cytometric analysis of in vitro proinflammatory cytokine secretion in peripheral blood from multiple sclerosis patients. J Clin Immunol. 1999;19:179. doi: 10.1023/a:1020555711228. [DOI] [PubMed] [Google Scholar]
- 31.Daynes RA, Dudley DJ, Araneo BA. Regulation of murine lymphokine production in vivo. II Dehydroepiandrosterone is a natural enhancer of interleukin 2 synthesis by helper T cells. Eur J Immunol. 1990;20:793. doi: 10.1002/eji.1830200413. [DOI] [PubMed] [Google Scholar]
- 32.Kim HR, Ryu SY, Kim HS, et al. Administration of dehydroepiandrosterone reverses the immune suppression induced by high doses of antigen in mice. Immunol Invest. 1995;24:583. doi: 10.3109/08820139509066859. [DOI] [PubMed] [Google Scholar]
- 33.Verthelyi D, Ansar Ahmed S. Estrogen increases the number of plasma cells and enhances their autoantibody production in nonautoimmune C57BL/6 mice. Cell Immunol. 1998;189:125. doi: 10.1006/cimm.1998.1372. [DOI] [PubMed] [Google Scholar]
- 34.Szekeres-Bartho J, Faust Z, Varga P, Szereday L, Kelemen K. The immunological pregnancy protective effect of progesterone is manifested via controlling cytokine production. Am J Reprod Immunol. 1996;35:348. doi: 10.1111/j.1600-0897.1996.tb00492.x. [DOI] [PubMed] [Google Scholar]
- 35.Fattal-German M. Immunocompetence in the elderly. Ann Pharm Fr. 1992;50:13. [PubMed] [Google Scholar]
- 36.Fox HS, Bond BL, Parslow TG. Estrogen regulates the IFNγ promotor. J Immunol. 1991;146:4362. [PubMed] [Google Scholar]
- 37.Correale J, Arias M, Gilmore W. steroid hormone regulation of cytokine secretion by proteolipid protein specific CD4+ T cell clones isolated from multiple sclerosis patients and normal controls. J Immunol. 1998;161:3365. [PubMed] [Google Scholar]
- 38.Meikle AW, Dorchuck RW, Araneo BA, et al. The presence of a dehydroepiandrosterone-specific receptor binding complex in murine T cells. J Steroid Biochem Molec Biol. 1992;42:293. doi: 10.1016/0960-0760(92)90132-3. [DOI] [PubMed] [Google Scholar]
- 39.Cohen JHM, Danel L, Cordier G, Saez S, Revillard J. Sex steroid receptors in peropheral T cells: absence of androgen receptorsmand restriction of estrogen receptors to OKT8-positive cells. J Immunol. 1983;131:2767. [PubMed] [Google Scholar]
- 40.Mor G, Amir-Zaltsman Y, Barnard G, Ben-Hur H, Kohen F. Evidence for the presence of estrogen receptors in monocytes. Endocr J. 1993;1:387. [Google Scholar]
- 41.Kovacs WJ, Olsen NJ. Androgen receptors in human thymocytes. J Immunol. 1987;139:490. [PubMed] [Google Scholar]