Abstract
Migration of T cells into extravascular sites of inflammation is mediated by cell–cell and cell–matrix adhesion receptors, including the hyaluronan-binding glycoprotein, CD44. The biochemical nature of CD44 variants and the ligand specificity, function and the regulation of activation of CD44 expressed on various cell types have been extensively studied. However, little is still known about the short-term influence of cytokines and chemokines on the activation of CD44 on human T cells. Therefore, we studied the role of inflammatory mediators in regulating the adhesion of T cells from human peripheral blood to immobilized hyaluronan under static or shear stress conditions. We found that the CD44-dependent adhesion, under static and shear stress (i.e. relative gradual resistance to flow of 150 and 1500 s−1) conditions, of T cells to hyaluronan requires a T-cell activation of 2–3 hr and is regulated by the cross-linking of CD3, cytokines (e.g. interleukin-2 and tumour necrosis factor-α), and chemokines (e.g. MIP-1β, interleukin-8, and RANTES). This T-cell adhesion was manifested by polarization, spreading and co-localization of cell surface CD44 with a rearranged actin cytoskeleton in hyaluronan-bound T cells. Thus, cytokines and chemokines present in the vicinities of blood vessel walls or present intravascularly in tissues where immune reactions take place, can rapidly activate the CD44 molecules expressed on T cells.
Introduction
Migration of T cells across blood vessel walls and tissue barriers, such as the extracellular matrix (ECM), requires T-cell activation, and is primarily mediated by β1-integrins, which, within minutes of activation, bind their glycoprotein ligands.1In vivo, inflammatory mediators, including cytokines and chemokines, appear to activate T cells, which in turn activate integrins and ligate ECM glycoprotein epitopes.2–5 However, besides integrins, migrating T cells use other cell–ECM and cell–cell receptors, such as the CD44 glycoprotein.6, 7 Upon activation, CD44 recognizes and binds hyaluronan (HA), a glycosaminoglycan present on endothelial cells and the ECM. The function and regulation of the ligand-binding activity of CD44 has been studied.7–9 As reported in these studies, the ligation of HA by CD44 appears to depend on both the activation state of the cell and the post-translational modifications of the CD44 molecule. These modifications include phosphorylation of serines and palmitoylation on cytoplasmic and internal domains of the molecule, as well as N- andO-linked glycosylation, the addition of glycosaminoglycan side chains, and sulphation of galactoside residues on the external domains of the molecule.10, 11 Although the biochemical nature of CD44 activation has been studied, it remains unclear whether short-term T-cell activation through pro-inflammatory mediators affects the ligation of HA by CD44 expressed on human T cells. This activation may occur during T-cell migration to inflammatory loci.
In the present study, we analysed the likelihood that human T-cell adhesion to HA is affected by exposure of the cells to pro-inflammatory cytokines and chemokines which can be found in inflamed tissue areas. We found that within a few hours of activation by these mediators, T cells adhere to HA, predominantly via their CD44 receptors and that this binding was resistant to relatively low shear flow force. To understand this process further, we investigated the requirements for intracellular protein synthesis, the rearrangement of cytoskeletal proteins and the sulphation and glycosylation of the activated CD44. We believe that our findings shed additional light on the activatory and pro-migratory roles of cytokines and chemokines that operate in inflamed loci.
Materials and methods
Reagents
The following reagents and chemicals were obtained as noted: recombinant human macrophage inflammatory protein-1β (MIP-1β), regulated on activated T-cell expressed and secreted (RANTES) and IL-8 (Pepro Tech; Rocky Hill, NJ); recombinant human tumour necrosis factor-α (TNF-α; Boehringer; Ingelheim, Germany); interleukin-2 (IL-2; Chiron B.V.; Amsterdam, the Netherlands), bovine serum albumin (BSA), phorbol 12-myristate 13-acetate (PMA), cytochalasin D and HA (Sigma Chemical Co.; St. Louis, MO); and HEPES buffer and RPMI-1640 (Kibbutz Beit-Haemek, Israel). Mouse anti-human monoclonal antibody (mAb) specific for CD44 (clone MCA P89) and CD3 were obtained from Serotec (Oxford, UK).
Purification and labelling of human T cells
Human T cells were obtained from the peripheral blood of healthy human donors and purified as previously described.5 The cell population thus obtained contained > 94% CD3+ T cells (usually 44% were CD8+ and 56% were CD4+ T cells), as determined by fluorescence-activated cell sorter (FACS) analysis. The purified human T cells were labelled with Na251[Cr]O4 (Amersham, Bucks, UK) washed, resuspended in adhesion medium (RPMI-1640 supplemented with 0·1% BSA and HEPES buffer), and seeded in the HA-coated,flat-bottomed, 96-well microtitre plates (Nunc, Roskilde, Denmark).
T-cell adhesion assays
Flat-bottomed microtitre plates were coated with HA by adding HA in phosphate-buffered saline (PBS; 50 µg/ml) to the wells (50 µl PBS per well) and were incubated for 24 hr at 4°. The wells were then extensively washed, the remaining binding sites were blocked (60 min, 22°) with BSA (1% in PBS), and the wells were washed again. The adhesion of labelled T cells to immobilized HA was examined as previously described.5, 12 To evaluate the moieties involved in T-cell adhesion mediated by CD44, we added anti-CD44 mAb or cytochalasin D, an inhibitor of actin rearrangement, to the HA-coated wells along with the T cells. After various time intervals, non-adherent and weakly adherent labelled T cells were removed from the wells by gentle washing, the remaining cells in the wells were lysed, and the radioactivity associated with the matrix, which represented the actual T-cell adhesion, was measured using a γ-counter.
T-cell adhesion to HA under shear stress conditions
The binding to HA of human T cells, exposed to RANTES (40 ng/ml), MIP-1β (40 ng/ml), IL-2 (50 U/ml), or TNF-α (20 ng/ml), was analysed essentially as previously described.13 Briefly, tissue culture grade, four-well plates (Nunc) were precoated (2 hr, 37°) with HA (250 µl containing 50 mg/ml HA), and the unbound material was then washed with PBS. The wells were then blocked with 1% BSA in water (30 min, 37°) After adding the human T cells (1·5 × 106/well), the wells were subjected to increased shear stress (2 min; 150 s−1 or 1500 s−1) using a specially designed rotating Teflon magnetic cone in a cone and plate device.13 The wells were then thoroughly washed, the T cells were stained with May–Grunwald stain, and the morphologies and amounts of matrix-bound T cells were analysed with an inverted light microscope (Olympus; Tokyo, Japan) connected to an image analysis system (Galai; Migdal Haemek, Israel), which assayed the amount of surface-covered, matrix-bound cells.
Staining of the actin cytoskeleton
T cells were activated with IL-2 (50 U/ml) and incubated (for 18 hr at 37° in a 7·5% CO2 humidified atmosphere) in a standard cell culture media. The T cells were then washed and seeded on to HA-covered cover slips in the presence of either PMA (40 ng/ml), IL-2 (100 U/ml), TNF-α (80 ng/ml), IL-8 (40 ng/ml), or TNF-α and cytochalasin D (1 µm). After 3 hr at 37°, the adherent cells were fixed (3 min, 3% paraformaldehyde, 0·05% Triton X-100). Then, the fixed adherent cells were washed and incubated with mAb anti-CD44 and TRITC phalloidin (molecular probes, Eugene, OR). Next, the cells were incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse antibody (Jackson Laboratories; West Grove, PA), and washed again. Changes in the shape of HA-bound cells were studied by exposing (3 hr, tissue culture conditions) HA-bound T cells to various activators and inhibitors. The T cells were then visualized using an inverted phase-contrast Diaphot Microscope (Nikon, Japan) or a fluorescent microscope (MC 80 DX; Zeiss, Germany).
Results and discussion
Induction of T-cell adhesion to HA and the kinetics of the adhesion
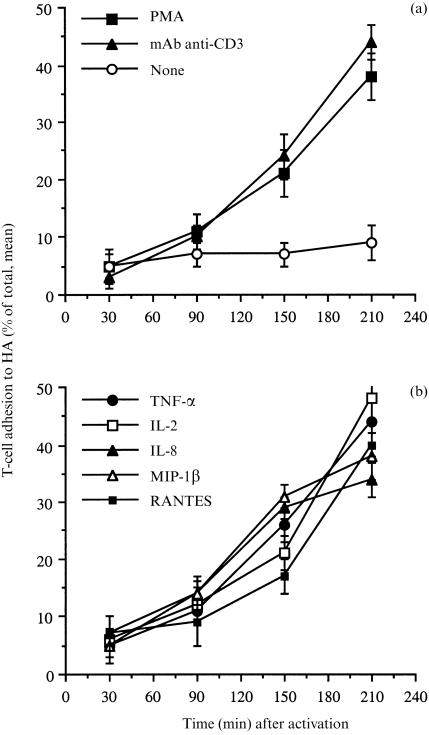
We investigated whether T-cell activation by physiological and non-physiological stimuli induces T-cell adhesion to immobilized HA, and we studied the adhesion-inducing patterns of each activator. The results, shown in Fig. 1(a,b), indicate that PMA, a prototypic protein kinase C activator, 14 induced a marked T-cell adhesion to HA. Moreover, cross-linking of CD3 by specific anti-human CD3 mAb also induced adhesion of the freshly purified human T cells to a significant extent 150 min after activation and seeding (Fig. 1a), as previously shown for T-cell lines.15 Within the time frame of our study, maximal adhesion induced by all activators was apparent 180–210 min after activation with no further increase at later times (data not shown), whereas non-activated T cells almost did not adhere to the HA substrate. The adhesion of unstimulated cells was always between 5 and 15%. These background levels of T-cell adhesion were probably due to the fact that the CD44 molecule is already expressed on the T cells at the beginning of the assays. Nevertheless, for its proper functioning and epitope ligation, the CD44 molecule appears to require activation by pro-inflammatory mediators. A similar phenomenon is observed with β1-integrin binding to ECM glycoproteins.
Figure 1.
Induction of human T-cell adhesion to HA by anti-CD3 mAb (a), PMA (a), various cytokines (b), and chemokines (b). Freshly purified human T cells were radiolabelled with 51Cr, seeded on HA-coated wells, and exposed to the following T-cell activators. (a) PMA (50 ng/ml) and mouse anti-human CD3 mAb (1 µg/ml). (b) the recombinant human cytokines and chemokines: TNF-α (20 ng/ml), IL-2 (50 U/ml), MIP-1β (40 ng/ml), IL-8 (40 ng/ml) and RANTES (40 ng/ml). Mean ±SD of triplicate wells is depicted for one experiment representative of five.
Next, we examined whether exposing human T cells to various chemokines and cytokines also induces adhesion to immobilized HA. The chemokines (RANTES, MIP-1β and IL-8) and the cytokines (IL-2 and TNF-α) induced a marked T-cell adhesion to HA (Fig. 1b), thus demonstrating that human T-cell adhesion to HA can be induced physiologically upon cell activation by various cytokines and chemokines. Interestingly, increased levels of chemokine- and cytokine-induced T-cell adhesion to HA were observed only 150–210 min after activation, whereas β1-integrin-dependent, pro-inflammatory mediator-induced T-cell adhesion to immobilized ECM glycoproteins peaked within 30–45 min of T-cell activation.5, 16, 17 The activation-dependent increase in T-cell adhesion to HA, observed with specific activators and for given time intervals, was neither accompanied by increased expression of CD44, nor by increased binding of soluble FITC-conjugated HA to the T-cell surfaces, as determined by FACS analysis (data not shown). Thus, adhesion of freshly purified human T cells to immobilized HA requires a relatively prolonged exposure to the physiological stimuli, unlike the relatively rapid kinetic nature of T-cell adhesion to immobilized ECM glycoproteins, which is dependent on integrin activation.
Dose–response analysis of cytokine- and chemokine-inducedT-cell adhesion to HA
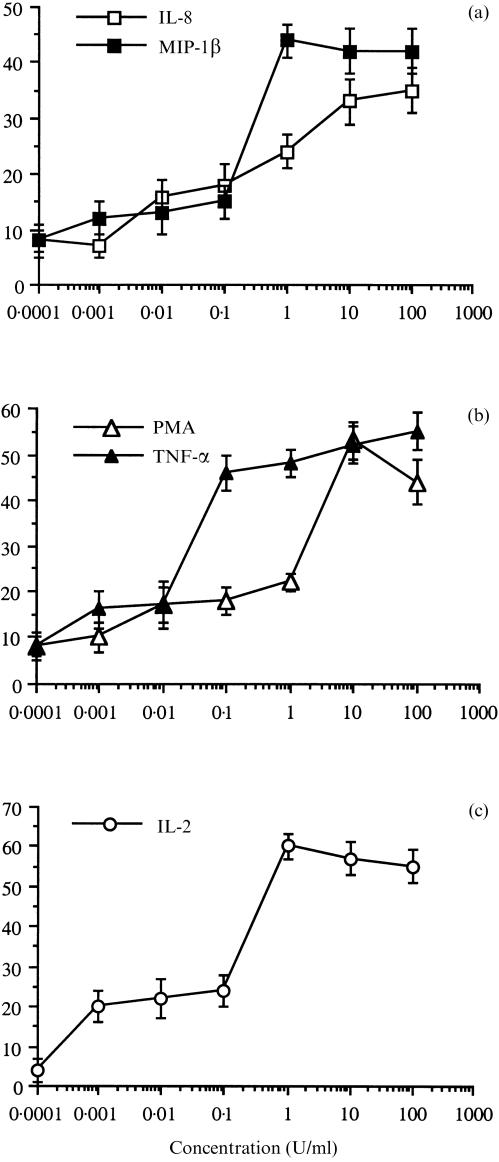
The optimal amount of various pro-inflammatory mediators required for optimal T-cell adhesion to HA after a prolonged activation time was determined. The results are shown in Fig. 2(a–c). The optimal amount of the chemokines IL-8 and MIP-1β required for maximal adhesion was 1–10 ng/ml. The optimal amounts of TNF-α and PMA were 0·1–10 ng/ml, and that of IL-2 was 1–10 U/ml. Similar amounts of PMA, cytokines and chemokines were required for the optimal induction of β1-integrin-mediated adhesion of T lymphocytes to fibronectin, a major cell-adhesive ECM glycoprotein.2–5
Figure 2.
Dose-dependence analysis of PMA-, cytokine- and chemokine-induced human T-cell adhesion to HA. Human T cells were seeded on HA-coated wells, in the presence of IL-8 or MIP-1β (a), PMA or TNF-α (b), or IL-2 (c). Cell adhesion was determined after 3 hr. Mean ±SD of triplicate wells is depicted for one experiment representative of four.
Inhibition of cytokine and chemokine-induced adhesion of T cells to HA by mAb against CD44 and cytochalasin D
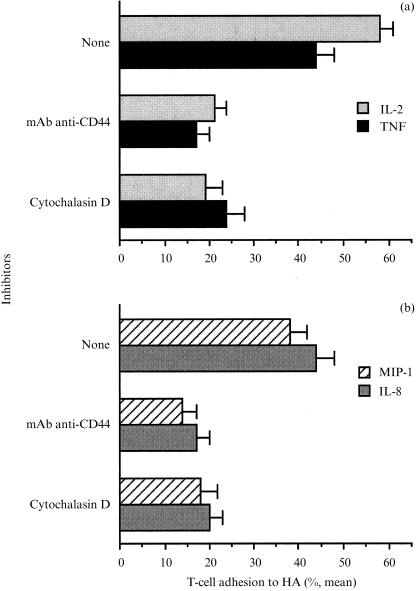
Previous studies have shown that the activation of T cells for long time periods (i.e. days) elevates CD44 expression on the cell surfaces and thereby the binding of fluorescent HA to the cells.15, 18 However, we found that these processes do not play a role in the short-term adhesion induced by the different pro-inflammatory mediators. To examine the mechanisms that control T-cell adhesion to HA, the involvement of the CD44 molecule itself and the need for an intact actin cytoskeleton in cytokine- and chemokine-dependent T-cell adhesion to immobilized HA was examined. T cells were activated by exposure to various pro-inflammatory cytokines, chemokines, and in the presence of various inhibitors. The results are shown in Fig. 3. The adhesion of T cells to immobilized HA induced by IL-2-, TNF-α-, MIP-1β- and IL-8-induced T-cell adhesion was markedly inhibited (70–85%) by anti-human CD44 mAb. The presence of cytochalasin D (0·1 µm), an inhibitor of actin polymerization, 14, 19 inhibited the TNF-α-, IL-2-, MIP-1β- and IL-8-induced T-cell adhesion (Fig. 3a,b). Thus, the rearranging of the T-cell cytoskeleton appears to be involved in T-cell adhesion to HA.
Figure 3.
Inhibition of IL-2-, TNF-α- (a), MIP-1β- and IL-8-induced (b) adhesion of T cells to HA by anti-CD44 mAb and cytochalasin D. The 51Cr-labelled T cells were seeded onto HA-coated wells in the presence of IL-2 (100 U/ml), TNF-α (20 ng/ml), MIP-1β (10 ng/ml), IL-8 (10 ng/ml) and the inhibitors: anti-CD44 mAb (1 µg/ml) and cytochalasin D (0·1 µm). After 3 hr at 37° in a 7·5% CO2 humidified atmosphere, unbound cells were removed, and the adhesion of T cells to HA was determined. Mean ±SD of triplicate wells is depicted for one experiment representative of four.
Cytoskeleton-dependent, morphological changes of cytokine- and chemokine-activated T cells that adhered to HA
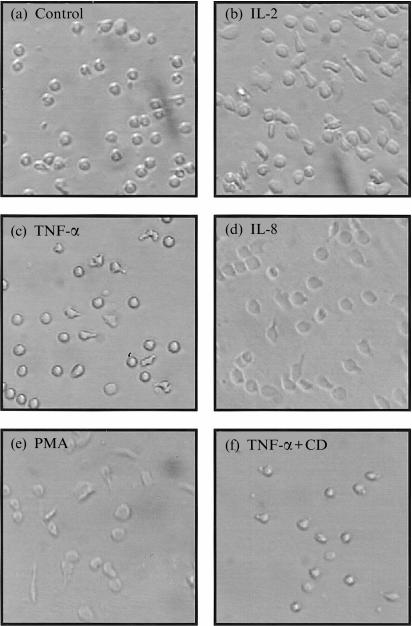
We investigated whether the HA-specific, pro-adhesive effects of IL-8, TNF-α and IL-2 can be verified visually. Specifically, we studied whether these mediators actually influence the morphology of HA-adherent T cells. Activation of resting peripheral blood lymphocytes did not induce significance morphological changes in T cells upon adhesion to HA. However, light microscopy revealed that the morphologies and shapes of IL-2-preactivated T cells whose adherence to HA was induced by IL-2, TNF-α and IL-8, which appeared to induce cell spreading, cellular elongation and pseudopod formation, were similar to those exposed to PMA, a non-physiological activator of protein kinase C used as a control, but were markedly different from the regular, rounded shapes of T cells exposed to the medium alone or to TNF-α plus cytochalasin D, an inhibitor of cytoskeletal rearrangement (Fig. 4). The IL-2-preactivated T cells were found to be non-adhesive to HA once the cytokine was washed away. However, the T cells resumed their adhesive characteristics after the cells were activated by the indicated mediators (not shown). Thus, the shape and spreading of HA-adherent T cells, which are pivotal in T-cell migration, are indeed influenced and altered by various inflammatory mediators.
Figure 4.
Induction by IL-2, TNF-α, MIP-1β, IL-8 and PMA of T-cell spreading and polarization on HA-coated surfaces. Freshly purified T cells were activated (37°, 18 hr) with IL-2 (50 U/ml), washed and seeded on HA-coated wells in the presence of adhesion medium alone (a), IL-2 (100 U/ml; b), TNF-α (80 ng/ml; c), IL-8 (40 ng/ml; d), PMA (40 ng/ml; e) and TNF-α (80 ng/ml) plus cytochalasin D (CD; 1 µm; f). After 3 hr, the bound cells were fixed with 3% PFA, and photographed. Original magnification ×200.
Next, we studied whether the actin cytoskeleton and CD44 molecules actually co-localize during cytokine- and chemokine-induced T-cell adhesion to and spreading on immobilized HA.20 IL-2-preactivated T cells were placed on coverslips coated with HA, subjected to various pro-inflammatory mediators, and then stained with TRITC-phalloidin and mAb anti-CD44. The results are in Fig. 5. T cells stimulated by IL-2, TNF-α, and MIP-1β not only underwent significant morphological alterations (their size and spreading increased), but also, their cell surface CD44 molecules appeared toco-localize morpologically with the actin cytoskeleton. The observed altered cell shapes probably result from cell activation and CD44-mediated ligand occupancy, since non-activated cells seeded on HA, as well as T cells simultaneously exposed to both TNF-α and cytochalasin D, exhibited shapes typical of resting lymphocytes. Thus, the CD44 molecules expressed on cytokine and chemokine-activated T cells appear not only to mediate T-cell binding to HA, but also to be involved in the regulation of T-cell spreading. Moreover, IL-2-, TNF-α- and MIP-1β-induced CD44 ligation of immobilized HA by T cells may be accompanied by post-receptor occupancy events, such as changes in cell shape, re-localization of CD44 receptors, and cell spreading. As recently suggested, 21 the co-localization of CD44 with the actin cytoskeleton suggests that CD44 associates with the cell's cytoskeleton through a linking molecule.
Figure 5.
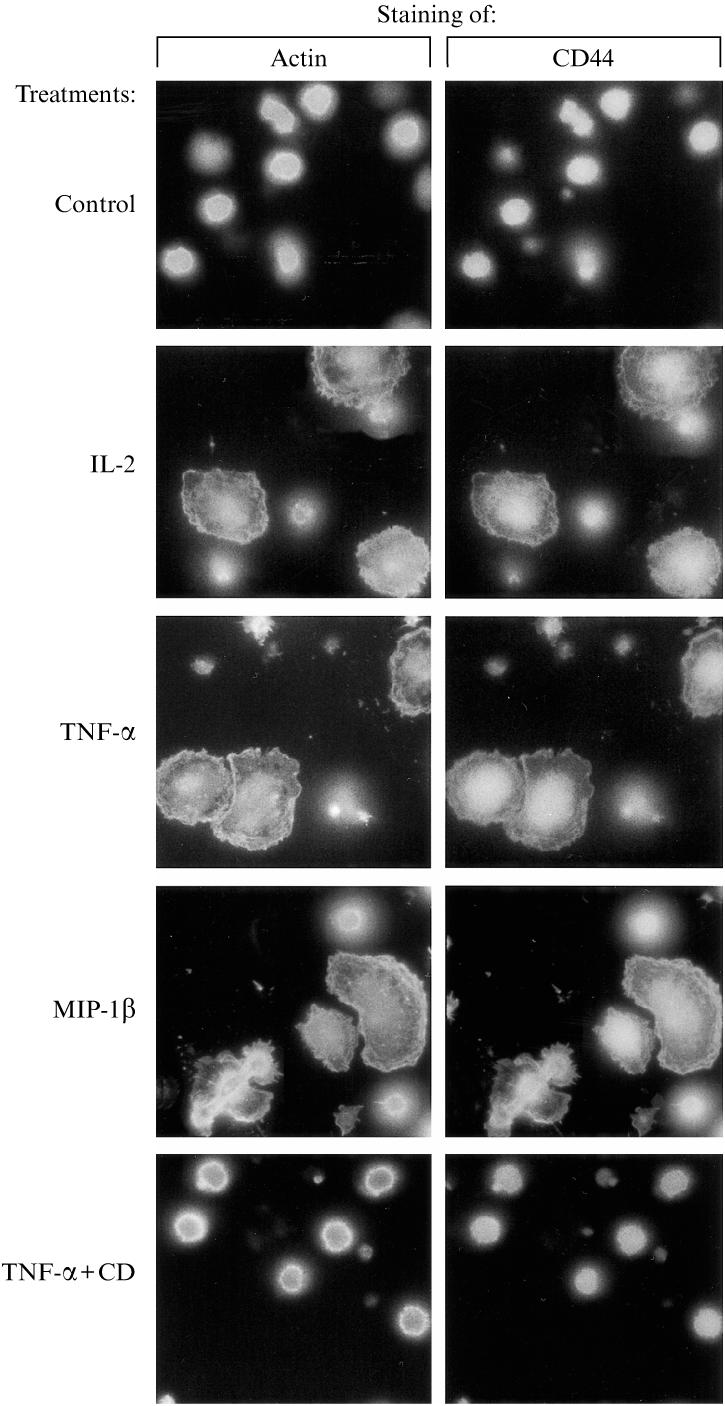
Reorganization of the actin cytoskeleton and re-localization of membrane CD44 receptors upon induction, by PMA, IL-2, TNF-α and MIP-1β, of T-cell binding to HA. IL-2-activated (50 U/ml; 18 hr; 37°, 7·5% CO2, humidified atmosphere) human T cells were seeded on cover slips that were coated with HA and exposed to various cytokines and chemokines (see the legend of Fig. 4 for quantities). After 3 hr, the bound T cells were fixed and stained with TRITC-phalloidin and anti-CD44 mAb, followed by staining with a FITC-conjugated secondary antibody. Original magnification ×1000.
Analysis of the resistance of IL-2-, TNF-α-, RANTES- and MIP-1β-induced T-cell adhesion to HA under shear stress
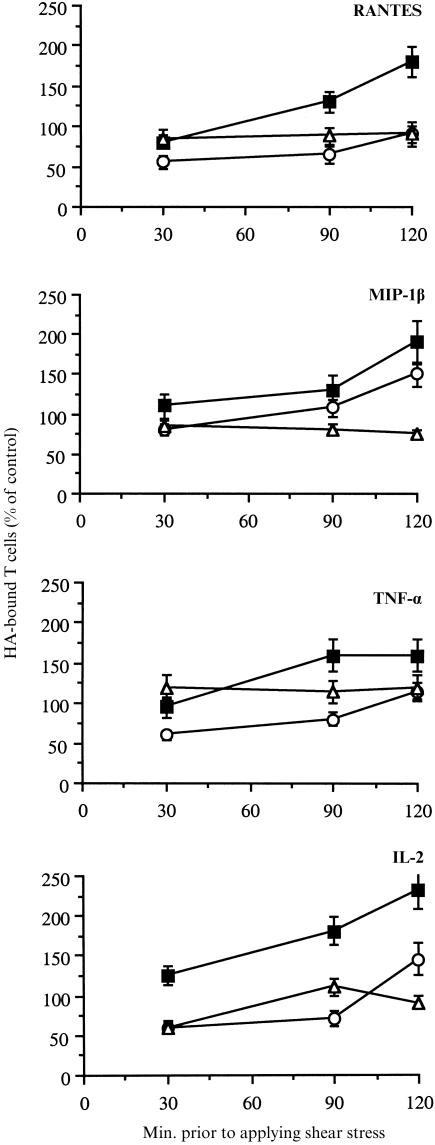
Cytokine or chemokine stimulation of peripheral T cells may occur not only in extravascular sites of the ECM present in extravascular tissues, but also within blood vessels, where shear stress conditions are present. Hence, we studied the resistance to moderate and high shear flow conditions of T-cell–HA adhesion induced by the pro-inflammatory mediators. Specifically, we delineated the effects of cytokines and chemokines on the binding of human T cells to HA under static and defined shear rate conditions using a cone and plate device.13 T cells treated with various stimulators were placed on the HA-coated 4-well plates for the indicated time periods. The cells were then subjected to flow conditions applied in the device for 2 min at shear rates of 150 or 1500 s−1. The amount of cells that were bound to the HA-coated surfaces after these conditions was measured by light microscopy. The results, presented in Fig. 6, show the relative amounts of cytokine- or chemokine-treated T cells bound to HA as compared to the control cells that adhered to the substrate under static conditions with no activator. Both the cytokines IL-2 and TNF-α, as well as the chemokines RANTES and MIP-1β, induced T-cell adhesion to the immobilized HA under static, as well as under the shear force conditions tested. However, the stability of the adhesive interactions between the T cells and immobilized HA under shear stress conditions varied according to the activatory agent. Thus, T-cell adhesion induced by IL-2 and MIP-1β was resistant to 150 s−1, though with reduced amplitude, whereas at these conditions, RANTES- and TNF-α-induced binding to HA was almost completely abrogated. A shear stress of 1500 s−1 prevented T-cell adhesion induced by all the activators tested.
Figure 6.
T-cell adhesion to HA is resistant to shear stress flow conditions. Purified human T cells were treated with RANTES or MIP-1β (each at 40 ng/ml), TNF-α (20 ng/ml) or IL-2 (50 U/ml), seeded onto HA-coated surfaces, and exposed to shear stress (2 min). The shear stresses tested were 150 s−1, 1500 s−1, or none (static conditions). The cells that still remained attached after these treatments were visualized and counted. Cell adhesion to HA in the absence of activation and shear flow was considered as control. These results are the average (and SD) of four experiments. ▪, static conditions; ○, 150 s−1; ▵, 1500 s−1.
The ligand-binding capacity of CD44 expressed on immune cells was recently shown to be regulated by various physio-logical and non-physiological activators. However, the in vivo relevance of these findings, which were tested by long-term in vitro activation (i.e. several days) of clones of lymphocytes, leucocytes and tumour cells, 22–24 is not yet known. We postulate that migrating T cells are capable of responding quickly by manipulation of their adhesive features toalterations in their environment and to the presence of pro-inflammatory mediators that are either ECM-bound or soluble.25, 26 We have demonstrated that a relatively short-term exposure of peripheral human T cells to several pro-inflammatory cytokines and chemokines results in a CD44- dependent recognition of HA and a rapid and specific adhesion to the immobilized HA. The time scale for integrin- (30–90 min) and CD44- (120–180 min) mediated T-cell adhesion implies that the interactions with these different blood vessel wall ligands are consecutive processes in the migration of T cells within the ECM. Migrating T cells may first exploit their integrin recognition and binding properties, and then, utilize their CD44–HA interactions, which may become substantial for adhesion, and thus, dominate migration. Our results provide additional insight into the role of cytokines and chemokines in context; these mediators activate not only β1-integrins, but also CD44 molecules on T cells, both of which are known to be involved in immune cell adhesion, extravasation, and co-stimulation.6–8, 27
Acknowledgments
This study was supported by research grants from The Israeli Cancer Research Funds, The Robert Koch-Minerva Center for Research in Autoimmune Diseases and The Center for the Study of Emerging Diseases. O. Lider is the incumbent of the Weizmann League Career Development Chair in Children's Diseases. The authors wish to thank Dr B. Shenkman (Tel Hashomer Hospital, Tel Aviv) for his technical assistance.
References
- 1.Butcher EJ, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 2.Moseries B, Loetscher M, Piali L, Loetscher P. Lymphocyte responses to chemokines. Int Rev Immunol. 1998;16:323. doi: 10.3109/08830189809043000. [DOI] [PubMed] [Google Scholar]
- 3.Schall TJ, Bacon KB, Camp RD, Caspari JW, Goeddel DV. Human macrophage inflammatory protein α (MIP-1α) and MIP-1β chemokines attract distinct population of lymphocytes. J Exp Med. 1993;177:1821. doi: 10.1084/jem.177.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taub DD, Conlon K, Lloyd AR, Oppenheim JJ, Kelvin DJ. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1α and MIP-1β. Science. 1993;20:355. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- 5.Gilat D, Hershkoviz R, Mekori YA, Vlodavsky I, Lider O. Regulation of adhesion of CD4+ T lymphocytes to intact or heparinase-treated subendothelial extracellular matrix by diffusible or anchored MIP-1β and RANTES. J Immunol. 1994;153:4899. [PubMed] [Google Scholar]
- 6.Degrendele HC, Estess P, Siegelman MH. Requirement for CD44 in activated T cell extravasation in an inflammatory site. Science. 1997;278:672. doi: 10.1126/science.278.5338.672. [DOI] [PubMed] [Google Scholar]
- 7.Estess P, Degrendele HC, Pacual V, Siegelman MH. Functional activation of lymphocyte CD44 in peripheral blood is a marker of autoimmune disease activity. J Clin Invest. 1998;102:1173. doi: 10.1172/JCI4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lesley JR, Hyman R, English N, Catterall JB, Turner GA. CD44 in inflammation and metastasis. Glycoconjugate J. 1997;14:611. doi: 10.1023/a:1018540610858. [DOI] [PubMed] [Google Scholar]
- 9.Liu D, Liu T, Li R, Sy M-S. Mechanisms regulating the binding activity of CD44 to hyaluronic acid. Front Biosci. 1998;3:631. doi: 10.2741/a307. [DOI] [PubMed] [Google Scholar]
- 10.Skelton TP, Zeng C, Nocks A, Stamenkovic I. Glycosylation provides both stimulatory and inhibitory effects on cell surface and soluble CD44 binding to hyaluronan. J Cell Biol. 1998;140:431. doi: 10.1083/jcb.140.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dasgupta A, Takahashi K, Cutler M, Tanabe KK. O-linked glycosylation modifies CD44 adhesion to hyaluronate in colon carcinoma cells. Biochem Biophys Res Commun. 1996;227:110. doi: 10.1006/bbrc.1996.1475. [DOI] [PubMed] [Google Scholar]
- 12.Levite M, Cahalon L, Hershkoviz R, Netzer L, Steinman L, Lider O. Neuropeptides, via specific receptors, regulate T cell adhesion to fibronectin. J Immunol. 1998;160:993. [PubMed] [Google Scholar]
- 13.Varon D, Dardik R, Shenkman B, et al. A new method for quantitative analysis of whole blood platelet interaction with extracellular matrix under flow conditions. Thromb Res. 1997;85:283. doi: 10.1016/s0049-3848(97)00014-5. [DOI] [PubMed] [Google Scholar]
- 14.Wilkinson PC, Lackie JM, Haston WS, Islam LN. Effects of phorbol esters on shape and locomotion of human blood lymphocytes. J Cell Sci. 1988;90:645. doi: 10.1242/jcs.90.4.645. [DOI] [PubMed] [Google Scholar]
- 15.Degrendele HC, Kosfiszer M, Estess P, Siegelman MH. CD44 activation and associated primary adhesion is inducible via T cell receptor stimulation. J Immunol. 1997;159:2549. [PubMed] [Google Scholar]
- 16.Miron S, Kachalsky SG, Hershkoviz R, Lider O. The induction of CD4+ T cell adhesion to fibronectin by activation of tyrosine kinases is associated with phosphorylation of the cytoskeletal protein paxillin. J Leuk Biol. 1997;62:405. doi: 10.1002/jlb.62.3.405. [DOI] [PubMed] [Google Scholar]
- 17.Esford LE, Maiti A, Bader SA, Tufaro F, Johnson P. Analysis of CD44 interactions with hyaluronan in murine L cell fibroblasts deficient in glycosaminoglycan synthesis: a role for chondroitin sulfate. J Cell Sci. 1998;111:1021. doi: 10.1242/jcs.111.7.1021. [DOI] [PubMed] [Google Scholar]
- 18.Maiti A, Maki G, Johnson P. TNFα-induction of CD44-mediated leukocyte adhesion by sulfation. Science. 1998;282:941. doi: 10.1126/science.282.5390.941. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez-Mateos PA, Arroyo AG, Balboa MA, Sanchez-Madrid F. Post-receptor occupancy events in leukocytes during β1 integrin–ligand interactions. Eur J Immunol. 1993;23:2642. doi: 10.1002/eji.1830231038. [DOI] [PubMed] [Google Scholar]
- 20.Murakami S, Shimabukuro Y, Miki Y, et al. Inducible binding of human lymphocytes to hyaluronate via CD44 does not require cytoskeletal association but does require new protein synthesis. J Immunol. 1994;152:467. [PubMed] [Google Scholar]
- 21.Tsukita SA, Iish K, Sato N, Sagara J, Kawai A, Tsukita SH. ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeleton. J Biol Chem. 1994;108:31. doi: 10.1083/jcb.126.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levesque MC, Haynes BF. Cytokine induction of the ability of human monocyte CD44 to bind hyaluronan is mediated primarily by TNFα and is inhibited by IL-4 and IL-13. J Immunol. 1997;15:6184. [PubMed] [Google Scholar]
- 23.Foster LC, Arkonac BM, Sibinga NE, Shi C, Perrella MA, Haber E. Regulation of CD44 gene expression by the proinflammatory cytokine IL-1β in vascular smooth muscle cells. J Biol Chem. 1998;273:203417. doi: 10.1074/jbc.273.32.20341. [DOI] [PubMed] [Google Scholar]
- 24.Vogt-Simonov R, Naor D. Hyaluronan-independent lodgment of CD44+ lymphoma cells in lymphoid organs. Int J Cancer. 1997;71:462. doi: 10.1002/(sici)1097-0215(19970502)71:3<462::aid-ijc26>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 25.Ben-Baruch A, Michiel DF, Oppenheim JJ. Signals and receptors involved in recruitment of inflammatory cells. J Biol Chem. 1995;20:117036. doi: 10.1074/jbc.270.20.11703. [DOI] [PubMed] [Google Scholar]
- 26.Gilat D, Cahalon L, Hershkoviz R, Lider O. Counter-interactions between tissue-infiltrating T lymphocytes, pro-inflammatory mediators, and enzymatically-modified extracellular matrix. Immunol Today. 1996;17:16. doi: 10.1016/0167-5699(96)80563-9. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu Y, Van Seventer GA, Siraganian R, Wahl L, Shaw S. Dual role of the CD44 molecule in T cell adhesion and activation. J Immunol. 1989;143:2457. [PubMed] [Google Scholar]