Abstract
Problems of logistics, compliance and drug resistance point to an urgent need for immunotherapeutic strategies capable of shortening the current 6-month chemotherapy regimens used to treat tuberculosis, or of supplementing ineffective therapy. In this study we sought to define the mechanism of action of two immunotherapies, both of which have previously been shown to prolong survival. Secondly, we wished to identify any clinically useful synergy between these therapies. In BALB/c mice infected via the trachea with Mycobacterium tuberculosis H37Rv there is an initial phase of partial resistance dominated by type 1 cytokines plus tumour necrosis factor-α (TNF-α) and interleukin-1 (IL-1), followed by a phase of progressive disease. This progressive phase is accompanied by increasing expression of IL-4, and diminished expression of IL-1 and TNF-α. Animals in this late progressive phase of the disease (day 60) were treated with two injections (day 60 and day 90) of 0·1 or 1·0 mg of heat-killed Mycobacterium vaccae, or with 3β,17β-androstenediol (AED; 25 µg subcutaneously three times/week), or with both therapies. We show here using four techniques in parallel (morphometry, immunohistochemistry with automated cell counting, semiquantitative reverse transcription–polymerase chain reaction and enzyme-linked immunosorbent assays of cytokines in lung extracts) that treatment with M. vaccae causes a switch back towards a type 1 cytokine profile, restoration of expression of IL-1α and TNF-α, and a switch from pneumonia to granuloma. This is very similar to the changes previously seen after treatment with AED. However, there was no evidence for synergy between M. vaccae and AED.
Introduction
The current treatment for tuberculosis requires 6 months of chemotherapy so there are problems with logistics and compliance. There has therefore been interest in the possibility that bacterial clearance and cure might be accelerated by therapeutic vaccination, or by other manipulations of the immune system. A plausible approach is promotion of the type 1 response and the suppression of type 2 cytokine production, because immunity to Mycobacterium tuberculosis requires a T helper type 1 (Th1) pattern of cytokine release, accompanied by expression of tumour necrosis factor-α (TNF-α), 1–4 and fails if there is a marked Th2 component.5 Pulmonary infection of the BALB/c mouse provides a suitable model because an initial phase of T-cell infiltration that is dominated by Th1 lymphocytes, and accompanied by high levels of TNF-α and interleukin-1α (IL-1α) is followed, after 3 weeks, by an increase in T cells expressing IL-4.6, 7 At the same time, there is a decrease in cells expressing IL-2, TNF-α, or IL-1α, a decrease in granuloma formation and an increase in the proportion of the lung affected by pneumonia. This pattern of pathology persists until death.
Mycobacterium vaccae is a potent inducer of Th1 responses to cross-reactive mycobacterial antigens and also induces CD8+ cytotoxic T cells.8, 9 It is an effective preventive vaccine against tuberculosis in mice and Cynomolgous monkeys 5, 9, 10 and can non-specifically down-regulate pre-existing Th2 responses in man and mice.11, 12 Moreover, we have reported increased survival following administration of a heat-killed preparation of M. vaccae 60 days after intratracheal infection of BALB/c mice with M. tuberculosis H37Rv, although the mechanism of the effect was not investigated.13 In the present study we have tested the hypothesis that the therapeutic effect of M. vaccae administered on day 60 in the late Th2-biased phase of tuberculosis in BALB/c mice is accompanied by a reversal of the switch to Th2, and increased production of IL-1 and TNF.
In parallel, we have investigated whether M. vaccae can synergize with the immunoregulatory steroid hormone that was recently shown to be therapeutically active in this BALB/c model.14, 15 The hormone therapy was devised because the switch to Th2 in tuberculous BALB/c mice described above coincides with a striking activation of the hypothalamo–pituitary–adrenal axis, 14–16 and appears to be partly attributable to the ability of glucocorticoids (cortisol in man, corticosterone in mice) to deviate newly recruited T cells towards a Th2 cytokine profile.17–19 This represents a physiological feedback pathway that modulates Th1-mediated inflammatory sites.20 The antiglucocorticoid steroids dehydroepiandrosterone (DHEA) and 3β,17β-androstenediol (AED) can delay the switch to Th2 if given from the start of infection, 14 and can partially reverse an already established switch to Th2 if given from day 60.15
We show here, using three different methods in parallel [automated counting of immunohistochemically stained cells using an image analysis system, enzyme-linked immunosorbent assay (ELISA) on extracted proteins, and semiquantitative reverse transcription–polymerase chain reaction (RT-PCR)], that a single injection of M. vaccae caused a partial reversal of the Th1 → Th2 switch, and increased expression of IL-1α and TNF-α. AED had similar effects but there was no convincing evidence for synergistic or additive effects between M. vaccae and AED. We discuss the applicability of these findings to the human disease, where there is a continuing need for novel therapeutic strategies to shorten treatment times and combat multiresistant organisms.
Materials and methods
Experimental model of tuberculosis infection in mice
All animal work was performed in conformity with the Home Office regulations in the UK, or the Local Ethical Committee for Experimentation in Animals in Mexico. The tuberculosis model has been described in detail elsewhere.6, 7 Briefly, male BALB/c mice were used at 6–8 weeks of age. Virulent M. tuberculosis H37Rv was cultured in Youman's modification of the medium of Proskauer and Beck. After 4 weeks of culture the colonies were supended in phosphate-buffered saline (PBS) containing 0·05% Tween-80 by shaking for 10 min with glass beads. The suspension was centrifuged for 1 min at 350 g to remove large clumps of bacilli. Then a preliminary bacterial count was achieved by smearing the supernatant at a known ratio of volume to area, and counting 10 random fields after staining by the Ziehl–Neelsen technique. The suspension was finally diluted to 1 × 106 bacteria in 100 µl of PBS and aliquoted at −70°. Before use bacteria were recounted and viability was checked as described.6, 7
To achieve intratracheal infection, mice were anaesthetized with 18 µl of intraperitoneal pentothal, equivalent to a dose of 56 mg/kg. The trachea was exposed via a small midline incision, and 1 × 106 viable bacteria were injected in 100 µl of PBS. The incision was then sutured with sterile silk, and the mice were maintained vertical until the effects of the anaesthetic had worn off. Infected animals were maintained in groups of five in cages fitted with microisolators. At 60 days, surviving mice were randomly allotted to the required experimental groups, as described below. They were therefore a relatively healthier subset of mice than the average, so there are minor differences between the findings in this study, and those in previous ones where animals were treated from the day of infection so that the results included those that died before 60 days. Mice were exsanguinated at 1, 3, 7, 14, 21, 28 and 60 and (for some parameters only) 120 days after starting steroid treatment or receiving the first vaccination. Data are plotted as days since infection (i.e. days 61, 63, 67, 74, 81, 88, 120 and 180).
Treatment with AED and corticosterone
Both steroids were obtained from Sigma (Poole, Dorset, UK). The selection of appropriate doses is decribed in detail elsewhere.14, 15 AED was dissolved in ultrapure olive oil (Sigma) and 25 µg were administered subcutaneously three times/week in 0·1 ml. This dose regimen was started on day 60, and continued until the animals were killed.
Corticosterone was dissolved in drinking water at 3 µg/ml. This concentration has been shown to result in corticosterone levels in adrenalectomized animals that are within the physiological range.21 Moreover, administration in this way also mimics the correct rodent diurnal rhythm.21 This replacement dose of a low physiological level of corticosterone is essential when mice with late-stage tuberculosis are given AED, because the marked adrenal atrophy seen at this time results in adrenal insufficiency and rapid death when antiglucocorticoid hormones are given without glucocorticoid supplements.15, 16
Treatment with autoclaved M. vaccae
Mycobacterium vaccae NCTC11659 was from a batch manufactured to Good Manufacturing Practice at the Centre for Applied Microbiological Research, Porton Down, UK (Batch MV001). The manufacturing process involved culture on Sauton's medium solidified with 1·5% (w/v) agar. This medium contains no antigenic substances. Organisms were suspended in borate-buffered saline, pH 8·0 at 10 mg/ml (equivalent to approximately 1 × 1010 organisms/ml), and autoclaved at 1·05 N m−2 for 15 min, before storage in glass vials at 4°. Mice were immunized 60 days and 90 days after intratracheal infection with M. tuberculosis. They received a subcutaneous injection at the base of the tail containing either 0·01 mg or 1 mg of autoclaved M. vaccae in 100 µl of pyrogen-free normal saline. Controls received saline only.
Assessment of colony-forming units in infected lungs
Half of each right and half of each left lung was used for colony counting, while the other halves were used for studying other parameters. Lungs were disrupted in a Polytron homogenizer (Kinematica, Luzern, Switzerland) in sterile 50 ml tubes containing 3 ml of isotonic saline. Four dilutions of each homogenate were spread onto duplicate plates containing Bacto Middlebrook 7H10 agar (Difco Lab code 0627-17-4) enriched with OADC also from Difco code 07-22-64-0). The time for incubation was 21 days. Two animals were killed at each time-point, in two different experiments, so the data points are the means of four animals.
Preparation of tissue for histology, morphometry and immunohistochemistry
For histological study, the lungs were perfused via the trachea with 100% ethanol and immersed for 24 hr in the same fixative. Parasaggital sections were taken through the hilus, and these were dehydrated and embedded in paraffin, sectioned at 5 µm and stained with haematoxylin and eosin. The following five parameters were then measured in µm2 with a Zidas Zeiss image analysis system; area of peribronchial infiltration; area of perivascular infiltration; area of granuloma; area of interstitial inflammation; % of lung affected by pneumonia. Measurements were made blind, and data are expressed as the mean of four to six animals ±SD.
For immunohistochemistry, lung sections were mounted on silane-coated slides, deparaffinized and the endogenous peroxidase was quenched with 0·03% H2O2 in absolute methanol. Lung sections were incubated overnight at 4° with biotin-labelled polyclonal goat antibodies against IL-2 or IL-4 (R & D Systems, Minneapolis, MI), or with polyclonal rabbit antibodies to TNF-α or IL-1α (Genzyme, Cambridge MA) diluted 1/50 in PBS. Bound antibodies were detected with avidin–biotin peroxidase (Vector, Burlinghame, CA) and were counterstained with haematoxylin. Immunohistochemistry was quantified blind. In previous studies the stained cells were evaluated and counted manually.6, 22 In the studies reported here the counting of stained cells was automated using the Leica QWin system, as recommended by the manufacturer (Leica, Milton Keynes, UK). Three random fields of each pulmonary compartment were evaluated at ×400 magnification in four to six mice and expressed as mean ±SD. Thus each point is based on 12–18 fields. Immunohistochemically positive and negative cells located in the alveolar–capillary interstitium, perivascular and peribronchial inflammation, granulomas and pneumonic areas were counted and the number of positive cells was expressed as a percentage of the total number of cells present.
Measurement of delayed hypersensitivity (DTH)
Culture filtrate was harvested from M. tuberculosis H37Rv grown as described above for 5–6 weeks. Then culture filtrate antigens were precipitated with 45% (w/v) ammonium sulphate, washed and redissolved in PBS. For evaluation of DTH each mouse received an injection of 20 µg of antigen in 40 µl of PBS into the hind foot-pad. In order to achieve very low non-specific background swelling, the needle perforated the skin near the ‘heel’. Then the needle travelled under the skin so that antigen was injected over the ‘palm’, where the readings of swelling were also made. We have found that ensuring the absence of a skin puncture wound at the site where the DTH is read reduces background and variability. The swelling at the ‘palm’ was measured with an engineer's micrometer before and 24 hr after the injection. Subsequently 1 µg of recombinant TNF-α (Genzyme, Boston, MA) was injected into the same site, which was read again 20 hr later (i.e. 44 hr after the original antigen challenge). This provides a measure of the TNF-sensitivity of the skin-test site.22
RT-PCR analysis of cytokines in lung homogenates
After the mice were killed, the lungs were removed, hilar lymph nodes and thymus were eliminated, and the tissue was immediately frozen by immersion in liquid nitrogen. RNA was isolated using the reagent Trizol as described previously, 23 cDNA was synthesized using murine Moloney leukaemia virus (M-MLV) reverse transcriptase (Gibco BRL, Gaithersburgh, MD) and oligo-dT priming. The mRNA of the cytokines IL-lα, IL-2 and TNF-α were analysed by PCR essentially as described.6, 7. The PCR products were electrophoresed on 6% polyacrylamide gels and analysed by an image densitometer (Bio-Rad, Hercules, CA) coupled to a computer program (molecular analyst, 1·4). The primer sequences to detect these cytokines have been reported previously.24 Glyceraldehyde 3-phosphate dehydrogenase was used as control for RNA content and integrity.
Quantification of cytokines in lung homogenates by ELISA
The lung homogenates used for RNA purification were also used to quantify cytokines by ELISA. After homogenization and centrifugation, the protein phase was extensively dialysed in sodium dodecylsulphate (SDS), and quantified with Bradford reagent (Bio-Rad) using a standard curve with bovine serum albumin (BSA). The ELISA method used for TNF-α and IL-1α was described previously.7 To quantify IL-2 and IL-4, 96-well plates were coated with 0·5 µg/ml of monoclonal rat anti-mouse IL-2 or IL-4 dissolved in 100 µl of 0·05 m carbonate buffer, pH 9·5, overnight at 4°. After washing with PBS–Tween 0·05%, wells were blocked with 1% BSA in PBS–Tween-20 for 2 hr at room temperature. Lung homogenate proteins at 0·5 mg/ml concentration suspended in 100 µl PBS were incubated for 3 hr at 37°. After washing, biotinylated polyclonal rabbit anti-mouse IL-2 or IL-4 at l µg/ml in PBS–Tween were incubated for 3 hr at 37°. After rinsing, plates were incubated with streptavidin–peroxidase diluted 1/1000 in PBS–Tween for l hr at room temperature. To reveal the peroxidase, orthophenylenediamine and H2O2 were used.
Statistical analysis
Student's t-test, two-tailed, for unpaired data was used to compare morphometric, immunohistochemical and delayed hypersensitivity data. The non-parametric Mann–Whitney U-test was used for other parameters. The P-values are indicated by symbols on the graphs, explained in the legends.
Results
Colony-forming units (CFU)
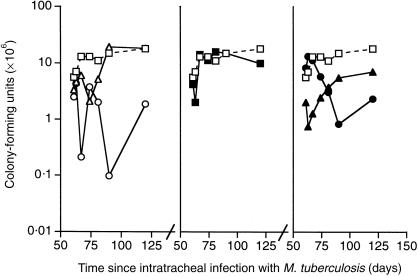
Treatment with 0·01 mg heat-killed M. vaccae on day 60 resulted in a transient reduction in CFU of less than 1 log, which did not recur following the second injection on day 90 (Fig. 1). The larger dose of 1·0 mg M. vaccae caused a larger fall, which was sustained and possibly boosted by the second injection. Treatment with AED + corticosterone had no effect on CFU, and there was no convincing synergy with M. vaccae, although the reduction of CFU following combined treatment with 0·01 mg M. vaccae and AED + corticosterone was > 1 log 3 and 7 days after the first injection.
Figure 1.
CFU from the lungs of BALB/c mice that had been infected via the trachea with M. tuberculosis on day 0, and then entered into the experiment on day 60. (□) control infected mice; (▵) mice treated day 60 and day 90 with M. vaccae 0·1 mg (P < 0·005 on day 74); (○) mice treated day 60 and day 90 with M. vaccae 1·0 mg (P < 0·005 from day 67); (▪) mice treated from day 60, three times per week with AED + corticosterone; (▴) mice given AED + corticosterone and 0·01 mg M. vaccae (P < 0·005 from day 61); (•) mice given AED + corticosterone and 1·0 mg M. vaccae (P < 0·005 from day 81). (The symbols remain the same in all other figures.) Error bars not shown on the log scale.
Morphometric analysis of pneumonia and granuloma
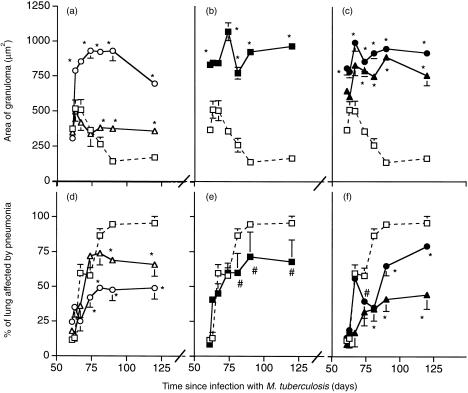
In this model granulomas form during the early phase of resistance, and the appearance of pneumonia correlates with increased expression of IL-4 and disease progression.5, 6 Morphometric analysis of the area of granuloma showed that the larger dose of M. vaccae or AED + corticosterone both caused striking increases in the area of granuloma (Fig. 2a,b), as did the combination of either M. vaccae dose plus the hormones (Fig. 2c). There was a corresponding decrease in the percentage of the lung affected by pneumonia. In the absence of the hormones the effect of M. vaccae was dose-related.
Figure 2.
Morphometric analysis of the area of granuloma in µm2 and the percentage of the lung affected by pneumonia in BALB/c mice infected via the trachea with M. tuberculosis on day 0, and then entered into the experiment on day 60. (□) control infected mice; (▵) mice treated day 60 and day 90 with M. vaccae 0·1 mg; (○) mice treated day 60 and day 90 with M. vaccae 1·0 mg; (▪) mice treated from day 60, three times per week with AED + corticosterone; (▴) mice given AED + corticosterone and 0·01 mg M. vaccae; (•) mice given AED + corticosterone and 1·0 mg M. vaccae. Error bars are SD, and where not apparent, are too small to plot. *P < 0·005, #P < 0·025, relative to controls.
Cytokine expression
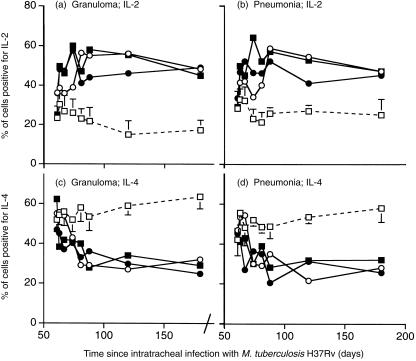
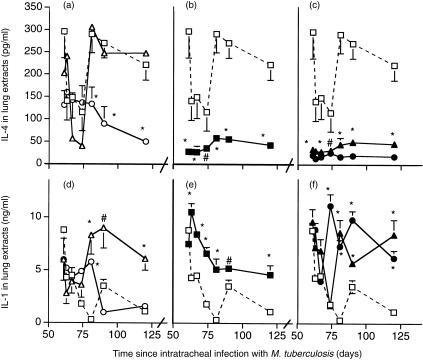
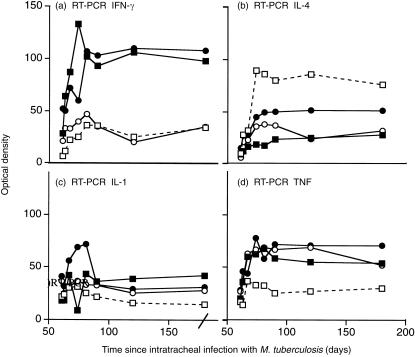
We showed previously using manual counting that at this late stage of pulmonary tuberculosis in BALB/c mice the percentage of cells in the lesions expressing IL-4 was greater than the percentage of cells expressing IL-2. Immunohistochemical analysis with automated counting (Leica QWin system) of the percentage of cells staining positive for IL-4 and IL-2 in the areas of granuloma and pneumonia in the present study confirmed this, and showed that both the hormone treatment and treatment with 1 mg of M. vaccae caused a reversal of this balance (Fig. 3) (the immunohistochemical analysis was not performed in the recipients of the low-dose M. vaccae). This was reflected in similar changes in the quantity of IL-2 (data not shown) and IL-4 detected by ELISA (Fig. 4a,b,c) in lung extracts. Similarly, RT-PCR confirmed that all the treatments decreased expression of IL-4 (Fig. 6b), and AED + corticosterone or AED + corticosterone + M. vaccae increased expression of IFN-γ (Fig. 6a).
Figure 3.
Automated immunocytochemical analysis of cells positive for IL-2 (a and b) and IL-4 (c and d) in the areas of granuloma (a and c) and pneumonia (b and d) of the lungs of BALB/c mice infected with M. tuberculosis H37Rv, and treated with the indicated regimen from day 60. Data are means of 12–18 random fields (three fields from each of four to six mice). Both immunotherapeutic regimens (and the combined regimen) caused an increase in IL-2-positive cells, and a decrease in IL-4-positive cells. (□) control infected mice; (○) mice treated day 60 and day 90 with M. vaccae 1·0 mg; (▪) mice treated from day 60, three times per week with AED + corticosterone; (•) AED + corticosterone and 1·0 mg M. vaccae. Error bars on the control points are SD. They are omitted from the test groups for the sake of clarity, but are of similar size in these groups. All test groups are significantly different from controls from day 88 (i.e. from 28 days after initiating immunotherapy P < 0·01).
Figure 4.
ELISA anaysis of IL-4 (a–c) and IL-1 (d–f) in the lungs of BALB/c mice infected with M. tuberculosis H37Rv, and treated with the indicated regimen from day 60. The higher dose of M. vaccae or AED caused decreased IL-4 content, in agreement with the immunohistochemical data. There was also increased expression of IL-1, particularly in the combined therapy group. (□) control infected mice; (▵) mice treated day 60 and day 90 with M. vaccae 0·1 mg; (○) mice treated day 60 and day 90 with M. vaccae 1·0 mg; (▪) mice treated from day 60, three times per week with AED + corticosterone; (▴) AED + corticosterone and 0·01 mg M. vaccae; (•) AED + corticosterone and 1·0 mg M. vaccae. Error bars are SD. *P < 0·005, #P < 0·025, relative to controls. The trough on days 63, 67 and 74 is unexplained.
Figure 6.
Semiquantitative RT-PCR for IFN-γ (a), IL-4 (b), IL-1 (c) and TNF-α (d) in the lungs of BALB/c mice infected with M. tuberculosis H37Rv, and treated with the indicated regimen from day 60. There were not sufficient duplicates for error bars or statistics. However, the findings help to confirm the ELISA and immunohistochemical data. (□) control infected mice; (○) mice treated day 60 and day 90 with M. vaccae 1·0 mg; (▪) mice treated from day 60, three times per week with AED + corticosterone; (•) AED + corticosterone and 1·0 mg M. vaccae.
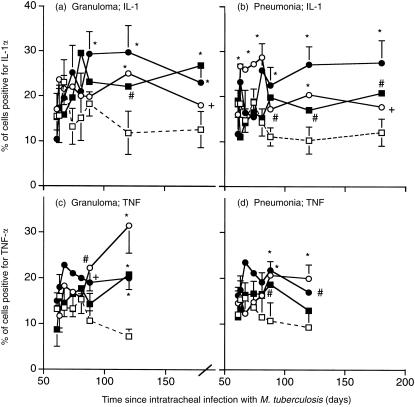
The percentage of cells staining for IL-1α or for TNF-α was also increased in recipients of M. vaccae, AED + corticosterone or both (Fig. 5), and the ELISA assays (Fig. 4,d,e,f) and RT-PCR (Fig. 6c,d) confirmed this.
Figure 5.
Immunocytochemical analysis of cells positive for IL-1α (a and b) and TNF-α (c and d) in the areas of granuloma (a and c) and pneumonia (b and d) of the lungs of BALB/c mice infected with M. tuberculosis H37Rv, and treated with the indicated regimen from day 60. Data are means ±SD of 12–18 random fields (three fields from each of four to six mice). (□) control infected mice; (○) mice treated day 60 and day 90 with M. vaccae 1·0 mg; (▪) mice treated from day 60, three times per week with AED + corticosterone; (•) AED + corticosterone and 1·0 mg M. vaccae. Error bars are SD. *P < 0·005, #P < 0·025, +P < 0·05 relative to controls.
DTH to soluble antigens of M. tuberculosis
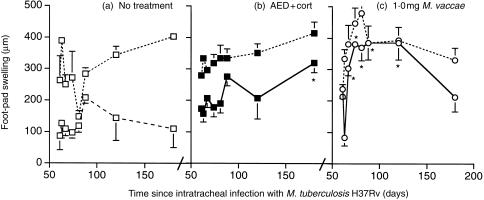
Treatment with 0·01 mg M. vaccae had no significant effect on DTH responses or on the sensitivity of the DTH site to a local injection of TNF-α, whereas treatment with 1·0 mg caused striking restoration of the DTH response. Treatment with AED + corticosterone caused modest increases in DTH responsiveness relative to untreated controls only on day 180 of infection (Fig. 7).
Figure 7.
Delayed hypersensitivity responses (DTH) to soluble antigens of M. tuberculosis in tuberculous mice. Treatment groups are indicated on the graphs, and by the symbols used which are as on the earlier Figs. Swelling was measured 24 hr after challenge (lower curves). Then 1 µg recombinant TNF-α was injected into the same site, and a second reading was taken 20 hr later (i.e. 44 hr after challenge) (upper curves, with dotted lines). Treatment with AED + corticosterone from day 60 enhanced DTH responses at day 180. Treatment with 1·0 mg M. vaccae markedly enhanced the DTH response between day 63 and day 120 (i.e. day 63–120 of the infection). For DTH without addition of TNF-α, *P < 0·005, #P < 0·025, relative to untreated controls [lower curve, dashed lines, (a)].
Discussion
The long duration of conventional therapy for tuberculosis causes problems of compliance. This fact, together with the increasing incidence of drug-resistant strains, points to a need for additional immunotherapeutic regimens. Several previous studies have shown immunotherapeutic effects in murine tuberculosis, using M. vaccae,13 or AED, 14, 15 or DNA vaccines.25 These effects are important because they are seen in the total absence of chemotherapy, and may therefore be relevant to eventual treatments for multidrug-resistant tuberculosis.
Using survival as the end-point it was shown previously that autoclaved M. vaccae is an effective immunotherapeutic in the BALB/c model of pulmonary tuberculosis when administered at 60 days.13 However, at that time no attempt was made to study the underlying immunological changes. Meanwhile, in view of the striking activation of the hypothalamo–pituitary–adrenal axis in murine tuberculosis, 15, 16 a hormone-based therapy was also devised.15 This exploited the antiglucocorticoid effects of AED and DHEA to prevent the glucocorticoid-dependent components of the switch to a type 2 cytokine profile, 17–19 and the switch towards diminished macrophage function 26 and diminished IL-1 and TNF production. Since the mechanism of action of AED is radically different from the mechanism of action of M. vaccae, which evokes a Th1 response to common antigens, 9 primes cytotoxic T cells 8 and non-specifically down-regulates Th2, 11 it was anticipated that there would be synergy between the two types of therapy. The results of this study show that the overall effect of M. vaccae is rather similar to the previously demonstrated effect of AED, 15 Thus M. vaccae caused a switch back towards a type 1 cytokine profile, increased expression of IL-1α and TNF-α, and a return to the development of granuloma rather than pneumonia. However, despite the different underlying ways in which M. vaccae and AED achieve these changes, no synergy was seen.
This study also confirmed the findings in the previous study with AED, this time using automated image analysis and cell counting, rather than a human observer.
The role of Th1 to Th2 deviation in human tuberculosis has been controversial, but this has been largely for technical reasons, and the presence of specific immunoglobulin E (IgE) antibody constitutes powerful evidence.27 A recent study using a sensitive quantitative RT-PCR and unstimulated fresh peripheral blood mononuclear cells has revealed that although mRNA copy numbers for IFN-γ always remain higher than the mRNA copy numbers for IL-4 or IL-13 in the peripheral blood of tuberculosis patients in the UK, there is nevertheless an increase in mRNA copy number for IL-4 and IL-13 of 1–2 logs, relative to that seen in cells from normal donors, 28, 29 whereas expression of IFN-γ falls about two-fold.28, 29 Thus the major change in cytokine expression in human tuberculosis is a very large increase in Th2 mRNA copy number, which correlates significantly with serum levels of IgE and soluble CD30.28, 29 Immunotherapeutic strategies, such as those described here, are therefore likely to be of benefit in the human disease, although proof will require testing in multidrug-resistant disease.
Acknowledgments
Dr R. Hernandez-Pando would like to thank the CONACYT for financially supporting his work, and The Royal Society of Great Britain for supporting a visit to the UK. The authors are also grateful for funding from the INCO-DC programme of the European Community, and from SR Pharma plc.
References
- 1.Cooper AM, Magram J, Ferrante J, Orme IM. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J Exp Med. 1997;186:39. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flynn JL, Goldstein MM, Chan J, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez-Pando R, Pavon L, Arriaga K, Orozco H, Madrid-Marina V, Rook GAW. Pathogenesis of tuberculosis in mice exposed to low and high doses of an environmental mycobacterial saprophyte. Infect Immun. 1997;65:3317. doi: 10.1128/iai.65.8.3317-3327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernandez-Pando R, Orozco H, Sampieri A, et al. Correlation between the kinetics of Th1/Th2 cells and pathology in a murine model of experimental pulmonary tuberculosis. Immunology. 1996;89:26. [PMC free article] [PubMed] [Google Scholar]
- 7.Hernandez-Pando R, Orozco H, Arriaga K, Sampieri A, Larriva SJ, Madrid MV. Analysis of the local kinetics and localisation of interleukin 1α, tumour necrosis factor α and transforming growth factor β during the course of experimental pulmonary tuberculosis. Immunology. 1997;90:507. doi: 10.1046/j.1365-2567.1997.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skinner MA, Yuan S, Prestidge R, Chuk D, Watson JD, Tan PLJ. Immunization with heat-killed Mycobacterium vaccae stimulates CD8+ cytotoxic T cells specific for macrophages infected with Mycobacterium tuberculosis. Infect Immun. 1997;65:4525. doi: 10.1128/iai.65.11.4525-4530.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abou-Zeid C, Gares M-P, Inwald J, et al. Induction of type 1 immune responses to a recombinant antigen from Mycobacterium tuberculosis expressed in Mycobacterium vaccae. Infect Immun. 1997;65:1856. doi: 10.1128/iai.65.5.1856-1862.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watson JD, et al. Genesis Research and Development Corporation Two intradermal immunisations with 0.5mg heat-killed M. vaccae fully protected Cynomolgous monkeys against subsequent challenge with 103 M. tuberculosis Erdman strain. Patent application; International publication Number WO 98/08542. 1998 [Google Scholar]
- 11.Wang CC, Rook GAW. Inhibition of an established allergic response to ovalbumin in Balb/c mice by killed Mycobacterium vaccae. Immunology. 1998;93:307. doi: 10.1046/j.1365-2567.1998.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hopkin JM, Shaldon S, Ferry B, et al. Mycobacterial immunisation in grass pollen asthma and rhinitis. Thorax. 1998;53(Suppl. 4):AbstractS63. [Google Scholar]
- 13.Rook GAW, Hernandez-Pando R. The pathogenesis of tuberculosis. Annu Rev Microbiol. 1996;50:259. doi: 10.1146/annurev.micro.50.1.259. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez-Pando R, de la Luz Streber M, Orozco H, et al. The effects of androstenediol and dehydroepiandrosterone on the course and cytokine profile of tuberculosis in Balb/c mice. Immunology. 1998;95:234. doi: 10.1046/j.1365-2567.1998.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernandez-Pando R, de la Luz Streber M, Orozco H, et al. Emergent therapeutic properties of a combination of glucocorticoid and anti-glucocorticoid steroids in tuberculous Balb/c mice. Q J Med. 1998;91:755. doi: 10.1093/qjmed/91.11.755. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez-Pando R, Orozco H, Honour JP, Silva J, Leyva R, Rook GAW. Adrenal changes in murine pulmonary tuberculosis; a clue to pathogenesis? FEMS Immunol Med Microbiol. 1995;12:63. doi: 10.1111/j.1574-695X.1995.tb00176.x. [DOI] [PubMed] [Google Scholar]
- 17.Ramirez F, Fowell DJ, Puklavec M, Simmonds S, Mason D. Glucocorticoids promote a Th2 cytokine response by CD4+ T cells in vitro. J Immunol. 1996;156:2406. [PubMed] [Google Scholar]
- 18.Visser J, van Boxel-Dezaire A, Methorst D, Brunt T, de Kloet ER, Nagelkerken L. Differential regulation of interleukin 10 (IL-10) and IL-12 by glucocorticoids in vitro. Blood. 1998;91:4255. [PubMed] [Google Scholar]
- 19.Vieira PL, Kalinski P, Wierenga EA, Kapsenberg ML, de Jong E. Glucocorticoids inhibit bioactive IL-12p70 production by in vitro-generated human dendritic cells without affecting their T cell stimulatory potential. J Immunol. 1998;161:5245. [PubMed] [Google Scholar]
- 20.Mason D. Genetic variation in the stress response: susceptibility to experimental allergic encephalomyelitis and implications for human inflammatory disease. Immunol Today. 1991;12:57. doi: 10.1016/0167-5699(91)90158-P. [DOI] [PubMed] [Google Scholar]
- 21.Häusler A, Persoz C, Buser R, Mondadori C, Bhatnagar A. Adrenalectomy, corticosteroid replacement and their importance for drug-induced memory enhancement in mice. J Steroid Biochem Molec Biol. 1992;41:785. doi: 10.1016/0960-0760(92)90425-i. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez-Pando R, Rook GAW. The role of TNFα in T cell-mediated inflammation depends on the Th1/Th2 cytokine balance. Immunology. 1994;82:591. [PMC free article] [PubMed] [Google Scholar]
- 23.Chomczyski PA. Reagent for the single step simultaneous isolation of RNA, DNA and proteins from cells and tissue samples. Biotechniques. 1993;15:532. [PubMed] [Google Scholar]
- 24.Pietch JL, Ehlers S, Jacobs E. Cytokine gene expression in the lung of Balb/c mice during primary and secondary intranasal infection with Mycoplamsa pneumoniae. Microbiology. 1994;14:2043. doi: 10.1099/13500872-140-8-2043. [DOI] [PubMed] [Google Scholar]
- 25.Lowrie DB, Tascon RE, Bonato VL, et al. Therapy of tuberculosis in mice by DNA vaccination. Nature. 1999;400:269. doi: 10.1038/22326. [DOI] [PubMed] [Google Scholar]
- 26.Rook GA, Steele J, Ainsworth M, Leveton C. A direct effect of glucocorticoid hormones on the ability of human and murine macrophages to control the growth of M. tuberculosis. Eur J Respir Dis. 1987;71:286. [PubMed] [Google Scholar]
- 27.Yong AJ, Grange JM, Tee RD, et al. Total and anti-mycobacterial IgE levels in serum from patients with tuberculosis and leprosy. Tubercle. 1989;70:273. doi: 10.1016/0041-3879(89)90021-4. [DOI] [PubMed] [Google Scholar]
- 28.Seah GT, Rook GA. A sensitive, non-radioactive quantitative method for measuring IL-4 and IL-4delta2 mRNA in unstimulated cells from multiple clinical samples, using nested RT-PCR. J Immunol Methods. 1999;228:139. doi: 10.1016/s0022-1759(99)00084-8. [DOI] [PubMed] [Google Scholar]
- 29.Seah GT, Scott GM, Rook GA. Type 2 cytokine gene activation and its relationship to extent of disease in patients with tuberculosis. J Infect Dis. 2000;181:385. doi: 10.1086/315200. [DOI] [PubMed] [Google Scholar]