Abstract
Mucosal cell-mediated immunity (CMI) by CD4+ T cells is postulated to be important for host defence against several vaginal pathogens. In addition to the recognized phenotypic distinctions of resident vaginal T lymphocytes, we recently provided evidence by fluorescence-activated cell sorter (FACS) that murine vaginal CD4+ T lymphocytes, are differentially recognized by two epitope-distinct anti-CD4 antibodies, suggesting that the CD4 protein on vaginal CD4+ cells is atypically expressed. In the present study, we confirm this by FACS and immunohistochemistry under non-denaturing conditions using two additional anti-CD4 antibodies. However, positive immunohistochemical staining of vaginal CD4+ cells under denaturing conditions revealed that the CD4 epitope in question is indeed present within the CD4 protein. Using reverse transcription polymerase chain reaction, amplification of CD3, T-cell receptor-β (TCR-β), and TCR-δ mRNA from lymph node and vaginal tissue, and CD4 mRNA from lymph node tissue was demonstrable. In contrast, amplification of CD4 mRNA from vaginal tissue, vaginal enriched lymphoid cells, or a purified (FACS-sorted) population of vaginal-specific CD4+ cells using two distinct primer sets was not demonstrable. Altogether, our results provide evidence that the CD4 protein on vaginal CD4+ T cells is conformationally distinct compared with its systemic counterpart, either as a result of a unique CD4 mRNA sequence or from a stable interaction of soluble CD4 with the surface of vaginal T cells.
Introduction
There is a paucity of information on host defence mechanisms at the vaginal mucosa. The expression of immunoglobulin A (IgA) and IgG antibodies in vaginal secretions has certainly been the mainstay of immune reactivity at mucosal surfaces, and is intimately involved in both immune exclusion (secretory IgA) and immune elimination (IgG > IgA) at the genital tract. 1–3 Cell-mediated immunity (CMI) and the role of T cells in host defence at the vaginal mucosa, however, are largely unknown. This knowledge is critical for developing strategies to control or prevent vaginal infections caused by a vast number of pathogens, including bacteria, fungi, protozoans and viruses that require CMI host defences for eradication. Furthermore, although deaths due to acquired immune deficiency syndrome (AIDS) have generally declined in the recent past, deaths in women due to AIDS continue to rise.4 In fact, women infected with human immunodeficiency virus (HIV) by heterosexual transmission is the fastest rising group of HIV-infected individuals, 4 stressing the urgency of elucidating host defence mechanisms at the vaginal mucosa.
For the past several years, our laboratory has studied host defence mechanisms important for protection against infections caused by the opportunistic fungal pathogen, Candida albicans. Candida albicans is the causative agent in the majority of mucosal fungal infections in AIDS patients, 5 and the organism most often diagnosed in women with acute or recurrent vulvovaginitis. 6 CMI is the predominant host defence mechanism against C. albicans at mucosal surfaces, 7 and this is evidenced clinically by the high incidence of mucosal candidiasis in individuals with reduced CMI (i.e. AIDS patients, 8, 9 transplantation recipients 10 and individuals on corticosteroid therapy). 11 Studies of women with recurrent vaginitis and an experimental murine model of vaginal candidiasis have suggested little, if any, role for systemic CMI in protection against infection. 12–15 In contrast, murine studies have provided evidence for a locally acquired mucosal immune response that partially protects mice against vaginitis.13 Together, these data suggest that immunity against C. albicans at the vaginal mucosa is compartmentalized with some level of immunological independence, and that local CMI may play a significant role in vaginal host defence.
The vaginal mucosa contains resident T cells, the majority of which are present in the lamina propria.16 We and others have shown that vaginal-associated T cells are phenotypically distinct from those in the periphery. 17–20 While α/β T-cell receptor positive (TCR+) cells predominate, γ/δ TCR+ cells are at a higher percentage (20–50%) and express invariant TCR genes (Vγ4/Vδ1), 18 possibly representing a specialized and distinct T-cell population.17, 21 The majority of α/β TCR+ cells present at the vaginal mucosa are CD4+, with few if any detectable CD8+ cells.19, 20 Finally, both murine vaginal α/β and γ/δ TCR+ cells express an effector phenotype.19, 21 In our studies, we have additionally shown that vaginal lymphocytes are differentially recognized by two epitope-distinct anti-CD4 antibodies. Specifically, we identified a population of vaginal T cells by fluorescence-activated cell sorter (FACS) that stained positive with RM-4.4 (C-terminus-specific), but not RM-4.5 (N-terminus-specific), anti-CD4 antibodies. In contrast, systemic lymph node T cells, peripheral blood T lymphocytes and lymph node cells added to the vaginal tissue during the digestion procedure stained positive with both anti-CD4 antibodies. These vaginal CD4 (RM-4.4)+ cells were considered T cells based on their dual staining characteristics with either anti-CD3 or anti-α/β TCR antibodies. 20 These results suggested that the CD4 protein on vaginal CD4+ T cells was atypically expressed compared to systemic CD4+ T cells. This was confirmed in vivo by the inability of GK1.5 anti-CD4 complement-fixing antibodies to reduce vaginal CD4+ cells when given intravaginally, whereas similar treatment with anti-Thy-1 antibodies reduced Thy-1+ T cells. 20 The present study was designed to further examine the atypical expression of the CD4 protein on vaginal CD4+ cells at the protein and molecular levels.
Materials and methods
Mice
Female CBA/J (H-2k), BALB/c (H-2d), C3H/HeN (H-2k), and C57BL/6 (H-2b) mice, 8–10 weeks of age purchased from the National Cancer Institute, Frederick, MD, were used throughout these studies. All animals were housed and handled according to institutionally recommended guidelines.
Antibodies
Four purified or fluorochrome/biotin-conjugated antibodies specific for the mouse CD4 protein were employed (PharMingen Corp., San Diego, CA). Each was produced from different clones with two epitope specificities. The GK1.5 [immunoglobulin G2b (IgG2b)], RM-4.5 (rat IgG2a) and the H129.19 (rat IgG2a) antibodies bind to an epitope located in domain 1 of the CD4 protein. The RM-4.4 antibodies (rat IgG2b) bind to an epitope located at the membrane-proximal region in domain 3 of the CD4 protein. The isotype control antibodies included rat IgG2a or IgG2b (PharMingen).
Isolation of lymph node cells
Lymph nodes (inguinal, mesenteric and lumbar) were used throughout these studies; either as whole tissue or as single-cell suspensions prepared as previously described.20, 22 Briefly, lymph node cells were made into single-cell suspensions by passage through a sterile mesh screen. The cells were then washed, resuspended in Hanks' balanced salt solution (HBSS), and the concentration and viability were determined using trypan blue dye exclusion.
Isolation of vaginal lymphocytes
Vaginas were excised and subjected to collagenase digestion as previously described. 20 Briefly, the vagina was excised from 10 to 40 animals, the cervix was removed and disgarded and the vagina was minced into small pieces using a sterile scalpel in complete tissue culture medium consisting of RPMI-1640 medium supplemented with penicillin (100 U/ml), streptomycin (100 µg/ml), l-glutamine (2 mm), 2-mercaptoethanol (5 × 10−5 m), sodium pyruvate (2 mm), HEPES buffer (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; 20 mm), and 5% heat-inactivated fetal bovine serum (FBS) (Life Technologies, Grand Island, NY). The tissues then were digested in complete tissue culture medium with 0·25% collagenase Type IV (Sigma Chemical Co., St. Louis, MO.) for 30 min at 37° in a shaking water bath with intermittent (15 seconds) Stomacher (Tekmar, Cincinnati, OH) homogenization during the incubation period. The resulting suspension was then filtered through a gauze mesh, washed in RPMI-1640, and subjected to slow speed centrifugation (200 g) for 1 min to eliminate large tissue debris. Lymphoid-enriched cells intended for polymerase chain reaction (PCR) experiments were collected from the supernatant by high-speed centrifugation (800 g) for 10 min, resuspended in HBSS and enumerated by trypan blue dye exclusion. Lymphoid-enriched cells intended for flow cytometry experiments were layered over a Ficoll–Paque® (Pharmacia Biotech AB, Uppsala, Sweden) density gradient. The resulting vaginal lymphoid cells collected from the interface between the medium and Ficoll were counted and the viability was determined by trypan blue dye exclusion. Previous studies showed that single-cell suspensions of lymph node cells added to the vaginal tissue during the digestion process did not affect T-cell surface markers (CD4, CD8). 20
Flow cytometry
Standard methodology was employed for direct and indirect immunofluorescence of vaginal and lymph node lymphocytes. Briefly, 1 × 106 lymph node cells or approximately 1 × 105 vaginal lymphocytes were pelleted in 1·5 ml Eppendorf tubes and incubated on ice for 30 min with RM-4.4, RM-4.5, or H129.19 fluorochrome or biotin-conjugated anti-CD4 antibodies (20 µg/ml), or purified GK1.5 anti-CD4 antibody (20 µg/ml) diluted in 100 µl of phosphate-buffered saline (PBS)−2% FBS (PBS-FBS). Thereafter, the cells were washed twice with 500 µl of PBS-FBS. Biotin-labelled cells were incubated for an additional 30 min on ice with cy-chrome-conjugated streptavidin (2 µg/ml) and washed twice with PBS-FBS while purified antibody was incubated with fluorochrome conjugated anti-rat IgG antibody and similarly washed thereafter. Cells incubated with either PBS-FBS alone or rat fluorochrome-conjugated IgG2a or IgG2b isotype control antibodies were used as negative controls to determine background fluorescence. The samples were analysed using two-colour analysis software on a Coulter Epochs Elite flow cytometer (Coulter Inc., Miami, FL). Compensation for each fluorochrome was determined by parallel single-colour analysis of cells labelled with each fluorochrome-conjugated antibody. Fluorescence limits (gates) were determined by isotype antibody staining. Results were expressed as the per-cent specific fluorescence of each stained population of cells.
Cell sorting
For sorting experiments, vaginal cells from 40 mice were collected as above and stained with RM-4.4 and RM-4.5 anti-CD4 antibodies as above, adjusting the concentration of antibodies proportionately to the number of cells. Sorted RM-4.5– RM-4.4+ cells were collected into PBS-10% FBS, transferred into 1·5-ml Eppendorf tubes, pelleted with 5 × 105 HeLa cells (to increase RNA recovery), and used for RNA extraction. Preliminary experiments showed that while cDNA derived from HeLa cells was amplified with the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) primers (due to the high sequence homology between human, rat and murine GAPDH) 23 no amplification using murine T-cell marker primer sets was observed. Moreover, cDNA derived from a pool of HeLa and lymph node cells or lymph node cells alone was equally amplified for T-cell-associated surface markers confirming that the HeLa cells did not inhibit the reverse transcription–PCR (RT-PCR).
Immunohistochemistry
For staining under denaturing conditions lymph node cells or vaginal cells isolated as above were cytocentrifuged onto slides, fixed with Poly/Lem fixative (denaturing agent) (Polysciences, Inc., Warrington, PA) and washed in PBS. The cells were then incubated in Peroxo-Bloc (Zymed, San Francisco, CA) for 45 seconds to quench any endogenous peroxidase activity, washed and blocked with normal rabbit serum. The cells were then incubated with either purified RM-4.5 or RM-4.4 anti-CD4 antibodies (10 µg/ml) (PharMingen Corp.) for 1 hr at room temperature in a humidified chamber and then washed. Negative controls consisted of cells incubated with rat IgG isotype control antibodies (Zymed). Following incubation with primary antibodies, the cells were incubated with biotinylated rabbit anti-rat IgG (10 µg/ml) (Accurate Chemical & Scientific Corp., Westbury, NY) for 1 hr at room temperature. Washed cells were then incubated with avidin–biotin–peroxidase (Vector Laboratories, Burlingame, CA) followed by the substrate 3-amino-9-ethylcarbazole (AEC) (Vector Laboratories). Cells were counterstained with haematoxylin (FisherDiagnostics, Fair Lawn, NJ) and preserved using Crystal Mount (biomeda, Foster City, LA). For staining under non-denaturing conditions extracted cells were stained with the primary antibody in test tubes at 4° (similar to FACS) and then cytocentrifuged onto slides. The cells were then washed in PBS, and fixed with Poly/Lem fixative. The remaining staining procedure was identical to that above. Immunohistochemical staining was also performed on tissue sections. For this, lymph nodes and vaginas were frozen in optimum cutting temperature (OCT) medium (Sakura Finetek USA, Inc., Torrance, CA) using Tissue Tek cryomolds (Miles Corp., Elkhart, IN). The tissue was sectioned (10 µm) and stained under denaturing conditions as outlined above.
RT-PCR
Whole tissue (lymph node and vagina) was homogenized with an Omni Tissue Homogenizer (Omni International, Gainesville, VA) in UltraSpec™ RNA isolation reagent (BioTecx, Inc., Houston, TX). In some experiments, isolated cell populations (lymph node cells, vaginal lymphocytes and HeLa cells alone or combined) were lysed in UltraSpec™ RNA isolation reagent. Total RNA was isolated according to the manufacturer's instructions and quantified spectrophotometrically (DU® Series 500, Beckman Instruments, Inc., Fullerton, CA) using the Warburg–Christian equation. First-strand cDNA was synthesized from 1 µg of total RNA using a reverse transcription system according to the manufacturer's instructions (Promega, Madison, WI) and stored at −20°. PCR reactions were performed in a 50-µl reaction containing a final concentration of 10x reaction buffer (50 mm KCl, 10 mm Tris–HCl, 1·5 mm MgCl2 and 0·1% Triton® X-100; Promega), 50 µ m dNTPs, 400 ng of each primer, and 2·5 U of Taq thermostable polymerase (Promega) and 3·5 µl of cDNA. A high efficiency platinum Taq DNA polymerase (Life Technologies, Gaithersburg, MD) was used in place of the former DNA polymerase in PCR experiments utilizing cDNA derived from a FACS-sorted cell population. If a second amplification was warranted, unused primers and deoxynucleotides were removed from the original PCR product using MicroSpin™ S-400 HR columns (Pharmacia Biotech, Piscataway, NJ) prior to reamplification. The forward (f) and reverse (r) oligonucleotide primers for the RT-PCR reaction were: CD3δ[f: 5′-ATG-GAG-CAG-AGG-AAG-GGT-CTG-3′, r: 5′-TCA-CTT-CTT-CCT-CAG-TTG-GTT-3′; 549 base pairs (bp)], 24 CD8 (f: 5′-ATG-CAG-CCA-TGG-CTC-TGG-CTG-3′, r: 5′-GCA-TGT-CAG-GCC-CTT-CTG-GGT-3′; 513 bp) (Clontech Laboratories Inc., Palo Alto, CA) CD4 (f: 5′-TGT-GCC-GAG-CCA-TCT-CTC-TTA-GG-3′, r: 5′-GCA-CTG-AGA-GTG-TCA-TGC-CGA-AC-3′; 615 bp) (Clontech), CD4B (f: 5′-AGG-AGG-TGG-AGT-TAT-GGG-TGT-TC-3′, r: 5′-CAC-TGG-CAG-GTC-TTC-TTC-TCA-CTG-A-3′; (983 bp), 25 TCR β-chain constant region (f: 5′-AGG-CTA-CCC-TCG-TGT-GCT-TG-3′, r: 5′-TGC-ACT-TGG-CAG-CGG-AAG-TG-3′; 268 bp), 26 TCR δ-chain constant region (f: 5′-AAA-AGC-CAG-CCT-CCG-GCC-AAA-3′, r: 5′-AAC-TGA-ACA-TGT-CAC-TGA-ATT-3′; 222 bp), 27 and GAPDH (f: 5′-GAA-TCT-ACT-GGC-GTC-TTC-ACC-3′, r: 5′-GTC-ATG-AGC-CCT-TCC-ACG-ATG-C-3′; 239 bp). 28 All primers were synthesized at the Louisiana State University Medical Center Core Laboratories, New Orleans, LA. PCR programmes included denaturization at 94° for 45 seconds, annealing at 60° (57° for CD3, and TCR-β and -δ) for 45 seconds, and extension at 72° for 1·25 min for 35 cycles in an automated thermocycler (Ericomp, San Diego, CA). The GAPDH oligonucleotide set was used as an internal control for the reverse transcription procedure. Amplification reactions receiving no template or product from reverse transcription reactions that did not receive reverse transcriptase were used to control for reagent or DNA contamination, respectively. Furthermore, the CD4 and CD4B primer sets were designed to span introns to ensure that amplification products of the expected size were derived from the amplification of cDNA. PCR products were analysed by electrophoresis on 2% agarose gels and visualized by ethidium bromide staining using a BioRad Video Capture Gel Documentation System 1000 (BioRad, Richmond, CA).
Southern blot analysis
Southern blots were used as a more sensitive assay to verify the presence or absence of CD4 amplification products. Briefly, amplified DNA products were analysed on 1·5% agarose gels and blotted onto Zeta-Probe® GT Genomic Tested Blotting membranes (BioRad). Murine CD4 oligonucleotide probes (5′-CTT-CCA-TCT-TAA-GTT-TAT-TGA-TGA-TGA-GAG- 3′) and (5′-GCA-CTG-AGA-GTG-TCA-TGC-CGA-AC-3′)25 specific for sequences internal of the predicted amplified products of CD4 and CD4B, respectively, were end-labelled with [γ-32P]dATP using T4 polynucleotide kinase (Stratagene Inc., La Jolla, CA). The blotted membranes were subsequently prehybridized at 37° in ExpressHyb hybridization solution (Clontech) for 30 min followed by hybridization of the membrane in fresh hybridization solution containing the labelled probe for 8–12 hr at 37°. Excess probe was removed from membranes by sequential washes in solutions of 2× saline sodium citrate (SSC)/0·1% sodium dodecyl sulphate (SDS) and 0·2×SSC/0·5% SDS. The blots were visualized by autoradiography after exposure to BioMax film (Eastman Kodak Co., Rochester, NY).
Results
CD4 expression on vaginal lymphocytes by flow cytometry
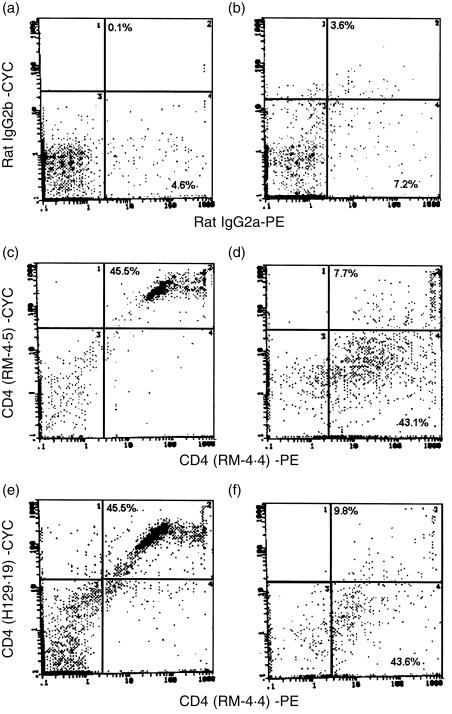
To confirm the putative atypical expression of CD4 on naive vaginal lymphocytes, and naive lymph node cells, dual flow cytometric staining employing RM-4.4 anti-CD4 antibodies together with two additional monoclonal antibodies that recognize the N-terminus of the CD4 protein (RM-4.5, H129.29) 29 was performed. The results illustrated in Fig. 1 show that within lymphoid cell limits, 45·5% of lymph node cells were recognized simultaneously by RM-4.4 and either RM-4.5 or H129.29 anti-CD4 antibodies. In contrast, whereas RM-4.4 anti-CD4 antibodies recognized over 43% of vaginal lymphoid cells, only 7·7 and 9·8% of vaginal lymphoid cells, respectively, were dually recognized by RM-4.4 and RM-4.5 or H129.29 anti-CD4 antibodies. Isotype staining showed negligible fluorescence. Similar findings were also observed when experiments were performed using GK1.5 anti-CD4 antibodies (L3T4) collected from the established hybridoma cell line (American Type Culture Collection, Rockville, MD) (data not shown).
Figure 1.
CD4 protein on vaginal lymphoid cells by flow cytometry. Lymph node cell (106) and collagenase-digested vaginal lymphoid-like cells (105) from CBA/J mice were labelled with biotinylated (plus cychrome [CYC]-conjugated streptavidin) RM-4.5 or H129.19 and phycoerythrin (PE)-conjugated RM-4.4 epitope-distinct anti-CD4 antibodies. RM-4.5 and H129.19 anti-CD4 antibodies recognize an epitope in domain 1 of the CD4 protein, whereas RM-4.4 antibodies recognize an epitope in domain 3. Flow cytometric dual staining of lymph node cells (a, c and e) or vaginal lymphoid cells (b, d and f) with RM-4.4 and RM-4.5 anti-CD4 antibodies (c and d) and RM-4.4 and H129.19 anti-CD4 antibodies (e and f) is shown together with isotype control antibodies (a,b). The percentage in each quadrant represents the fluorescent positive cells within the lymphoid-like cell limits. Data shown are a representative of two experiments using 10 mice per experiment.
Vaginal CD4+ T cells express the RM-4.5 epitope
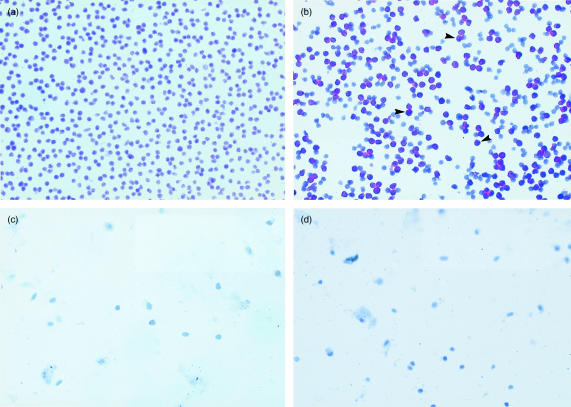
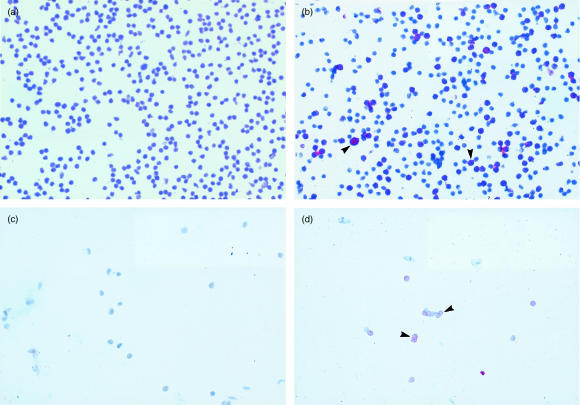
To investigate the differential staining of vaginal lymphoid cells by the epitope-distinct anti-CD4 antibodies, immunohistochemical staining was performed on lymph node cells or vaginal lymphoid cells under non-denaturing (unfixed) or denaturing (fixation) conditions using RM-4.5 anti-CD4 antibodies. The results of staining under non-denaturing conditions are shown in Fig. 2. Lymph node, but not vaginal, lymphocytes stained positively with RM-4.5 anti-CD4 antibodies (Fig. 2b,d, respectively). Negative controls using isotype matched antibodies on each cell preparation demonstrated no positive staining (Fig. 2a,c). In contrast, both lymph node and vaginal lymphocytes stained positively with RM-4.5 anti-CD4 antibodies under denaturing conditions (Fig. 3b,d, respectively). Negative controls using isotype-matched antibodies again demonstrated no positive staining on either cell preparation (Fig. 3a,c). Positive staining for RM-4.5 anti-CD4 antibodies was also detected when frozen whole lymph node or vaginal tissue sections were stained under denaturing conditions (data not shown). The low integrity of tissue sections in the absence of fixation precluded experiments to examine staining under non-denaturing conditions.
Figure 2.
Immunohistochemical analysis of CD4+ lymph node and vaginal lymphoid cells under non-denaturing conditions. Lymphoid cells isolated from whole lymph nodes or collagenase-treated vaginal tissue from CBA/J mice were incubated with purified RM-4.5 anti-CD4 antibodies or with anti-rat IgG isotype-matched antibodies at 10 µg/ml prior to cytocentrifugation onto slides, and fixation. (a) and (b) show staining of lymph node cells with anti-rat IgG isotype matched antibodies, and RM-4.5 anti-CD4 antibodies, respectively. (c) and (d) show staining of vaginal lymphoid cells with anti-rat IgG isotype-matched antibodies, and RM-4.5 anti-CD4 antibodies, respectively. Arrows on each frame show representative stained cells. The figure is representative of three experiments using different mice.
Figure 3.
Immunohistochemical analysis of CD4+ lymph node and vaginal lymphoid cells under denaturing conditions. Lymph nodes cells or cells collected from collagenase-treated vaginal tissue from CBA/J mice were cytocentrifuged onto slides, fixed and stained with RM-4.5 anti-CD4 antibodies or anti-rat IgG isotype-matched antibodies. (a) and (b) show staining of lymph node cells with anti-rat IgG isotype-matched antibodies, and RM-4.5 anti-CD4 antibodies, respectively. (c) and (d) show staining of vaginal lymphoid cells with anti-rat IgG isotype-matched antibodies, and RM-4.5 anti-CD4 antibodies, respectively. Arrows show representative areas of stained cells. The figure is representative of three experiments using different mice.
RT-PCR of vaginal tissue for T-cell-associated surface antigen mRNA expression
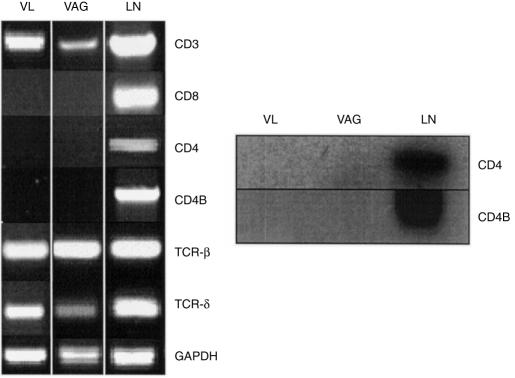
To investigate the altered expression of the CD4 protein on vaginal lymphoid cells at the molecular level, T-cell surface marker mRNA expression in whole vaginal tissue and vaginal lymphoid cells from naive mice was evaluated using primer sets specific for systemic-derived T cells. As illustrated in Fig. 4(a), cDNA derived from lymph node, vaginal tissue and vaginal lymphoid cells were amplified using primer sets specific for CD3, and TCR-β and TCR-δ constant-chain regions. 24 However, no CD4 amplification products were observed in either vaginal tissue- or vaginal lymphoid cell-derived cDNA using two CD4 primer sets that together span 97% of the predicted CD4 translated region despite amplification of cDNA derived from lymph node tissue (Fig. 4a). This lack of amplification of vaginal CD4 mRNA was similarly observed in several additional mouse strains (BALB/c, C3H/HeN, and C57BL/6) (data not shown). Numerous modifications to the RT-PCR parameters including increasing the number of cycles, the addition of more template, varying the Mg2+ concentration, and testing different annealling temperatures did not result in the amplification of vaginal CD4 cDNA using either primer set (data not shown). Consistent with previous flow cytometry results, 20 lymph node-derived, but not vaginal-derived, cDNA was amplified with a primer set specific for CD8 (Fig. 4a). Southern blot hybridization confirmed the absence of CD4 amplification products in both vaginal tissue and in vaginal lymphoid cells, and the presence of the CD4 product in lymph nodes (Fig. 4b).
Figure 4.
RT-PCR analysis of vaginal T-cell surface marker expression. (a) RT-PCR of total RNA extracted from vaginal lymphoid cells (VL) (5 × 106), whole vaginal tissue (VAG), and lymph node tissue (LN) of CBA/J mice was performed using Taq DNA polymerase and primers designed to amplify CD3 (549 bp), CD8 (513 bp), CD4 (615 bp), CD4B 983 bp, TCR-β (268 bp) and TCR-δ (222 bp) chain constant regions of murine systemic T lymphocytes. Amplification with primers for GAPDH (239 bp) was used as an internal control. The figure is representative of at least five experiments using different mice. (b) Southern blot analysis of CD4 and CD4B amplification products derived from vaginal and lymph node tissue cDNA using a γ-32P-end-labelled probe internal of the predicted amplification product.
Lack of CD4 mRNA expression in RM-4.5– RM-4.4+ vaginal cells
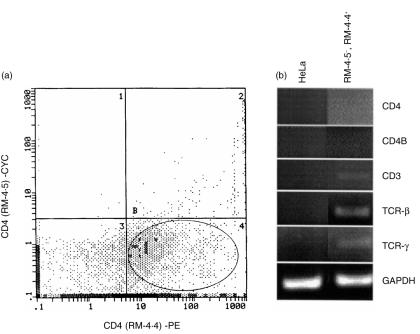
To evaluate this peculiar RT-PCR result in vaginal lymphoid cells on a purer population of CD4+ cells, RT-PCR using a high-efficiency DNA Taq polymerase was performed on cDNA derived from a subpopulation (FACS-sorted) of vaginal lymphoid cells that were recognized by RM-4.4, but not RM-4.5, anti-CD4 antibodies (Fig. 5a). The population of RM-4.5– RM-4.4+ sorted cells was found to be greater than 96% pure (data not shown). As illustrated in Fig. 5(b), amplification products for CD3, and TCR β- and δ-chain constant regions were observed from cDNA isolated from RM-4.5– RM-4.4+ vaginal lymphoid cells. In contrast, CD4 amplification products were absent from cDNA derived from the RM-4.5– RM-4.4+ sorted cells in both first- and second-round amplifications (second-round amplification products shown). Southern blot analysis confirmed these results (data not shown). Control RT-PCR with HeLa cell cDNA was negative for CD4 and all T-cell surface markers (Fig. 5b) confirming in these experiments the specificity of the murine primer sets for their intended targets.
Figure 5.
lack of CD4 mRNA expression in RM-4.5– RM-4.4+ CD4+ vaginal cells. (a) Vaginal lymphoid cells extracted from CBA/J mice by collagenase-digestion and further purified by Ficoll–Paque® density gradient centrifugation were dual labelled with RM-4.4 and RM-4.5 anti-CD4 antibodies. Those cells that stained positively with RM-4.4 but not RM-4.5 monoclonal antibodies (RM-4.5– RM-4.4+) were FACS sorted. The RM-4.5– RM-4.4+ CD4+ cell-sorted population is represented in the encircled gate of quadrant B4. HeLa cells (5 × 105) were added to the sorted cell population and pelleted. Total RNA was extracted from RM-4.5– RM-4.4+-plus HeLa cells or HeLa cells alone, reverse transcribed and subjected to PCR using a high-efficiency Taq DNA polymerase and primers designed to amplify CD3, CD4, CD4B, TCR β-, and TCR δ chain constant regions of murine systemic T lymphocytes (b). The CD4 and CD4B amplification products shown are from column-purified primary amplification products subjected to a second round of amplification. Amplification with primers for GAPDH was used as an internal control. The figure is representative of two experiments using 40 mice in each experiment.
Discussion
The results of this study provide evidence at the protein and molecular levels for a unique CD4 protein on murine vaginal CD4+ cells. The lack of recognition of the CD4 protein on vaginal CD4+ cells by H129.19, GK1.5 and RM-4.5 anti-CD4 antibodies is in agreement with previous flow cytometry experiments. 20 Together these results suggested that either the N-terminus (RM-4.5) epitope is absent from the CD4 protein on vaginal CD4+ cells, or alternatively, is present but masked under non-denaturing conditions. Evidence for the latter came from positive immunohistochemical staining of vaginal lymphoid cells under denaturing, but not non-denaturing, conditions using RM-4.5 antibodies. Upon microscopic examination, the staining was specific for lymphoid-like cells and not for any other cell type that might express the CD4 protein (i.e. Langerhans' cells). This is consistent with our previous observation that vaginal CD4+ cells stain positive with anti-α/β TCR antibodies. 20 Futhermore, in the mouse, unlike human and rat CD4, expression of the CD4 marker has only been demonstrated on lymphoid cells. 30
Since the putative atypical/altered conformation of the vaginal CD4 protein may be the result of changes extending to the mRNA level, subsequent studies focused on examining vaginal and systemic tissue/cell-derived mRNA by RT-PCR. Initial studies showed that consistent with previous flow cytometry analysis, 20 vaginal lymphoid cells and vaginal tissue expressed mRNA whose cDNA could be amplified by several systemic T-cell-specific primer sets, including CD3, TCR β-chain, and TCR δ-chain, but not CD8. Surprisingly however, despite evidence for the presence of vaginal CD4+ cells by flow cytometry and immunohistochemistry, amplification of vaginal tissue-derived cDNA, lymphoid-enriched vaginal cells, or FACS-sorted RM-4.5– RM-4.4+ vaginal cells using two systemic cell-derived CD4 primer sets, that span 97% of the predicted translated portion of the cDNA, sequence, was unsuccessful. Positive amplification products were demonstrable using the same CD4 primer sets with cDNA derived from lymph node tissue. Southern blot analysis confirmed these results. The lack of amplification for CD4 in vaginal tissue did not appear to result from inhibitors in the RT-PCR or degrading enzymes since amplification of cDNA derived from lymph node cells added to vaginal tissue was equivalent to the amplification of lymph node tissue cDNA alone (data not shown). Neither modifications to the PCR protocol designed to enhance amplification, nor the use of RNA derived from lymphoid-enriched vaginal cells affected the results. Furthermore, these results were not specific to only CBA/J mice as similar results were observed with vaginal tissue derived from other strains of mice covering several haplotypes. In fact, the only time a CD4 amplification product was detected in vaginal tissue-derived cDNA was when the high-efficiency Taq DNA polymerase was employed (data not shown). However, since these products were not detected in RM-4.5– RM-4.4+ vaginal cell-derived cDNA using the same polymerase, they are believed to have resulted from small numbers of systemic-derived CD4+ T cells present in the vaginal preparations. The small population of vaginal RM-4.5+ RM-4.4+ cells (Figs 1 and 5a) (3–9%) detected by flow cytometry supports this.
The present results suggest one of two possibilities. One is that the vaginal CD4 mRNA sequence is unique such that the systemic cell-derived primer sets are not able to hybridize to the vaginal CD4 cDNA. In mice, CD4 mRNA species other than the full-length 3·7- kilobase (kb) transcript have been found in brain (2·7 kb 31 and 2·3 kb 32) and fetal liver (3·5 kb). 33 Additionally, the 3·5-kb and the 2·3-kb CD4 transcript, unlike the 2·7-kb transcript, encode a mature CD4 protein that is possibly derived from a differential splicing event.32, 33 The CD4 protein product derived from the 2·3-kb transcript lacks the N-terminal immunoglobulin-like domain that contains the RM-4.5 epitope present on vaginal CD4+ cells, 32 while the expression of the CD4 protein from the 3·5-kb transcript is restricted to a subset of lympho-haematopoietic cells in the murine fetal liver. 33 Although both transcripts are unlikely to have a role at the vaginal mucosa, a similar differential splicing event may occur at the vaginal mucosa. In any event, the differences in the vaginal CD4 mRNA are not expected to be dramatic since epitope integrity is maintained in the vaginal CD4 protein as evidenced by immunohistochemistry. Nevertheless, a unique mRNA has the potential to affect the efficiency of the primer hybridization resulting in a lack of demonstrable amplification products. Alternatively, vaginal cells may have become CD4+ as a result of the passive acquisition of soluble CD4 molecules. In this scenario, the observed flow cytometry and immunohistochemistry results may have been due to the positioning of soluble CD4 molecules on the vaginal cell surface such that the epitopes recognized by the GK1.5, RM-4.5, and H129.19 antibodies were masked under non-denaturing, but not under denaturing, conditions. Indeed the recent finding that CD4– early progenitor thymocytes can passively acquire low-level CD4 surface expression from CD4+ cells 34 adds credibility to this hypothesis. If the CD4 molecules were indeed acquired passively, the results suggest that the interaction of the soluble CD4 persists when denatured and the RM-4.5 epitope is unmasked. An interaction with soluble CD4 would also explain the high CD4 expression on vaginal T cells including γ/δ TCR+ cells 20 which are not usually known to express CD4. Regardless of the mechanism, the conformationally distinct CD4 protein on vaginal T cells should be viewed as a vaginal-specific property and urges caution in the use of RT-PCR and N-terminus-specific anti-CD4 antibodies to analyse CD4+ T cells in the vaginal mucosa under naive conditions.
Whether passively acquired or the result of a unique CD4 mRNA, a conformationally distinct CD4 protein present on vaginal T cells may have a significant impact on vaginal immune responses. The close association of CD4 and TCR tyrosine kinases as a result of the independent binding of CD4 and the TCR to the same major histocompatibility complex (MHC) class II molecule leads to optimal T-cell activation.35, 36 Furthermore, 100-fold more antigen is required for T-cell activation when CD4 is absent. 36 In addition, the N-terminal domain of the CD4 protein that contains the GK1.5, RM-4.5 and the H129.19 epitope predominantly mediates CD4 binding to the β2 domain of MHC class II. A conformational change that effects the N-terminus epitopes on vaginal CD4+ cells may therefore also reduce T-cell activation and perhaps render them relatively anergic to conventional antigenic stimuli. Since the vaginal mucosa is colonized with many saprophytic commensal organisms, a reduced level of local T-cell activation may be a mechanism to allow persistent colonization in the absence of unnecessary inflammation. Due to the lack of effects by systemic T cells in protection against vaginal infection by C. albicans12 the burden for immune responsiveness is placed on resident vaginal immune cells. If local CD4+ T cells are not capable of generating fully competent immune reactivity against C. albicans, infections would be difficult to resolve. Indeed, experimental primary vaginal C. albicans infections in mice are known to persist for several weeks 37 and clinical cases of vaginitis rarely resolve spontaneously and therefore usually require some form of antimycotic therapy. 38
In conclusion, the differential recognition of epitope-distinct anti-CD4 antibodies on vaginal CD4+ cells under non-denaturing conditions, together with the inability of systemic cell-derived CD4 primer sets to amplify cDNA derived from a purified population of vaginal CD4+ cells, suggests that a conformationally distinct CD4 protein is present on the vaginal cells, potentially due to a unique mRNA or the passive acquisition of soluble CD4 molecules. Together these results support our concept of immune compartmentalization at the vaginal mucosa and extend the evidence that T cells in the vaginal mucosa are distinct and may function with some level of immunological independence. The ultimate significance of our finding will be to determine whether or not a similarly unique CD4 expression exists on human vaginal CD4+ cells. A human counterpart would not only be significant for immune reactivity, but may also be important for issues relative to HIV transmission in the genital tract.
Acknowledgments
This work was supported by Public Health Service Grant AI-32556, National Institute of Allergy and Infectious Diseases
References
- 1.Hocini H, Barra A, Belec L, et al. Systemic and secretory humoral immunity in the normal human vaginal tract. Scand J Immunol. 1995;42:269. doi: 10.1111/j.1365-3083.1995.tb03653.x. [DOI] [PubMed] [Google Scholar]
- 2.Brandtzaeg P. Mucosal immunity in the female genital tract. J Reprod Immunol. 1997;36:23. doi: 10.1016/s0165-0378(97)00061-2. [DOI] [PubMed] [Google Scholar]
- 3.Wira CR, Richardson J, Prabhala R. Endocrine regulation of mucosal immunity: effect of sex hormones and cytokines on the afferent and efferent arms of the immune system in the female reproductive tract. In: Ogra PL, Mestecky JJ, Lamm ME, editors. Handbook of Mucosal Immunity. San Diego: Academic Press, Inc.; 1994. p. 705. [Google Scholar]
- 4.Report. Update: Trends in AIDS incidence, deaths and prevalence, United States. MMWR. 46(8):165. [PubMed] [Google Scholar]
- 5.Spinillo A, Michelone G, Cavanna C, Colonna L, Capuzzo E, Nicola S. Clinical and microbiological characteristics of symptomatic vulvovaginal candidiasis in HIV-seropositive women. Genitourin Med. 1994;70:268. doi: 10.1136/sti.70.4.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sobel JD. Pathogenesis and epidemiology of vulvovaginal candidiasis. Ann N Y Acad Sci. 1988;544:547. doi: 10.1111/j.1749-6632.1988.tb40450.x. [DOI] [PubMed] [Google Scholar]
- 7.Odds FC. Candida and Candidosis. Baltimore, MD: University Park Press; 1988. Chronic mucocutaneous candidiasis; p. 104. [Google Scholar]
- 8.Klein RS, Harris CA, Small CB, Moll B, Lesser M, Friedland GH. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N Engl J Med. 1984;311:354. doi: 10.1056/NEJM198408093110602. [DOI] [PubMed] [Google Scholar]
- 9.Macher AM. The pathology of AIDS. Public Health Report. 1988;103:246. [PMC free article] [PubMed] [Google Scholar]
- 10.Clift RA. Candidiasis in the transplant patient. Am J Med. 1984;77(Suppl. 4D):34. [PubMed] [Google Scholar]
- 11.Knight L, Fletcher J. Growth of Candida albicans in saliva: stimulation by glucose associated with antibiotics, corticosteriods and diabetes mellitus. J Infect Dis. 1971;123:371. doi: 10.1093/infdis/123.4.371. [DOI] [PubMed] [Google Scholar]
- 12.Fidel PL, Jr, Lynch ME, Sobel JD. Effects of preinduced Candida-specific systemic cell-mediated immunity on experimental vaginal candidiasis. Infect Immun. 1994;62:1032. doi: 10.1128/iai.62.3.1032-1038.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fidel PL, Jr, Lynch ME, Conaway DH, Tait L, Sobel JD. Mice immunized by primary vaginal C. albicans infection develop acquired vaginal mucosal immunity. Infect Immun. 1995;63:547. doi: 10.1128/iai.63.2.547-553.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fidel PL, Jr, Lynch ME, Sobel JD. Circulating CD4 and CD8 T cells have little impact on host defense against experimental vaginal candidiasis. Infect Immun. 1995;63:2403. doi: 10.1128/iai.63.7.2403-2408.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fidel PL, Jr, Cutright JL, Sobel JD. Effects of systemic cell-mediated immunity on vaginal candidiasis in mice resistant and susceptible to Candida albicans infections. Infect Immun. 1995;63:4191. doi: 10.1128/iai.63.10.4191-4194.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parr MB, Parr EL. Langerhans cells and T lymphocyte subsets in the murine vagina and cervix. Biol Reprod. 1991;44:491. doi: 10.1095/biolreprod44.3.491. [DOI] [PubMed] [Google Scholar]
- 17.Nandi D, Allison JP. Phenotypic analysis and gamma/delta-T cell receptor repertoire of murine T cells associated with the vaginal epithelium. J Immunol. 1991;147:1773. [PubMed] [Google Scholar]
- 18.Itohara S, Farr AG, Lafaille JJ, et al. Homing of a gamma/delta thymocyte subset with homogeneous T-cell receptors to mucosal epithelia. Nature. 1990;343:754. doi: 10.1038/343754a0. [DOI] [PubMed] [Google Scholar]
- 19.Ibraghimov AR, Sacco RE, Sandor M, Iakoubov Z, Lynch RG. Resident CD4+αβ T cells of the murine female genital tract: a phenotypically distinct T cell lineage that rapidly proliferates in response to systemic T cell activation stimuli. Int Immunol. 1995;7:1763. doi: 10.1093/intimm/7.11.1763. [DOI] [PubMed] [Google Scholar]
- 20.Fidel PL, Jr, Wolf NA, KuKuruga MA. T lymphocytes in the murine vaginal mucosa are phenotypically distinct from those in the periphery. Infect Immun. 1996;64:3793. doi: 10.1128/iai.64.9.3793-3799.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rakasz E, Hagen M, Sandor M, Lynch RG. γδ T cells of the murine vagina: T cell response in vivo in the absence of the expression of CD2 and CD28 molecules. Int Immunol. 1997;9:161. doi: 10.1093/intimm/9.1.161. [DOI] [PubMed] [Google Scholar]
- 22.Fidel PL, Jr, Lynch ME, Sobel JD. Candida-specific Th1-type responsiveness in mice with experimental vaginal candidiasis. Infect Immun. 1993;61:4202. doi: 10.1128/iai.61.10.4202-4207.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tso JY, Sun XH, Kao T, Reece KS, Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucl Acids Res. 1985;13:2485. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petitto JM, Huang Z. Molecular cloning of the coding sequence of an interleukin-2 receptor alpha subunit cDNA in murine brain. J Neuroimmunol. 1995;59:135. doi: 10.1016/0165-5728(95)00035-z. [DOI] [PubMed] [Google Scholar]
- 25.Littman DR, Gettner SN. Unusual intron in the immunoglobulin domain of the newly isolated murine CD4 (L3T4) gene. Nature. 1987;325:453. doi: 10.1038/325453a0. [DOI] [PubMed] [Google Scholar]
- 26.Arrunategui-Correa V, Dutt J, Foster CS. The role of B lymphocytes in experimental herpes simplex viral retinitis. Scand J Immunol. 1994;40:299. doi: 10.1111/j.1365-3083.1994.tb03466.x. [DOI] [PubMed] [Google Scholar]
- 27.Komano H, Fugiura Y, Kawaguchi M, et al. Homeostatic regulation of intestinal epithelia by intraepithelial gamma-delta T cells. Proc Natl Acad Sci USA. 1995;92:6147. doi: 10.1073/pnas.92.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwai Y, Bickel M, Pluznik DH, Cohen RB. Identification of sequences within the murine granulocyte-macrophage colony stimulating factor mRNA 3′ untranslated region that mediate mRNA stabilization induced by mitogen treatment of EL-4 thymoma cells. J Biol Chem. 1991;266:17959. [PubMed] [Google Scholar]
- 29.Dianzani U, Shaw A, Al-Ramadi BK, Kubo RT, Janeway CA., Jr Physical association of CD4 with the T cell receptor. J Immunol. 1992;148:678. [PubMed] [Google Scholar]
- 30.Crocker PR, Jeffries WA, Clerk SJ, Chung LP, Gordon S. Species heterogeneity in macrophage expression of the CD4 antigen. J Exp Med. 1987;166:613. doi: 10.1084/jem.166.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tourvieille B, Gorman SD, Field EH, Hunkapiller T, Parnes JR. Isolation and sequence of L3T4 complementary DNA clones: expression in T cells and brain. Science. 1986;234:610. doi: 10.1126/science.3094146. [DOI] [PubMed] [Google Scholar]
- 32.Lonberg N, Gettner SN, Lacy E, Littman DR. Mouse brain CD4 transcripts encode only the COOH-terminal half of the protein. Mol Cell Biol. 1988;8:2224. doi: 10.1128/mcb.8.5.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haars R, Conradt P, Miltner I, Wagner H. A novel form of CD4 (L3T4) mRNA in the murine fetal liver results in cell-surface expression of the L3T4 antigen. Scand J Immunol. 1991;34:253. doi: 10.1111/j.1365-3083.1991.tb01544.x. [DOI] [PubMed] [Google Scholar]
- 34.Michie AM, Carlyle JR, Zuniga-Pflucker JC. Early intrathymic precursor cells acquire a CD4low phenotype. J Immunol. 1998;160:1735. [PubMed] [Google Scholar]
- 35.Janeway CA, Haque S, Smith LA, Saizawa K. The role of the murine L3T4 molecule in T cell activation: differential effects of anti-L3T4 on activation by monoclonal anti-receptor antibodies. J Mol Cell Immunol. 1987;3:121. [PubMed] [Google Scholar]
- 36.Rojo JM, Saizawa K, Janeway CA., Jr Physical association of CD4 and the T-cell receptor can be induced by anti-T-cell receptor antibodies. Proc Natl Acad Sci USA. 1989;86:3311. doi: 10.1073/pnas.86.9.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fidel PL, Jr, Lynch ME, Sobel JD. Candida-specific cell-mediated immunity is demonstrable in mice with experimental vaginal candidiasis. Infect Immun. 1993;61:1990. doi: 10.1128/iai.61.5.1990-1995.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sobel JD. Pathogenesis and treatment of recurrent vulvovaginal candidiasis. Clin Infect Dis. 1992;14:S148. doi: 10.1093/clinids/14.supplement_1.s148. [DOI] [PubMed] [Google Scholar]