Abstract
This study was conducted to investigate the role of the acute stress hormone adrenaline on macrophage nitric oxide (NO) production. Murine peritoneal macrophages were stimulated in vitro with lipopolysaccharide (LPS) in the absence or presence of adrenaline. Adrenaline inhibited the LPS-induced nitrite response in a dose-dependent manner. The suppressive effect of adrenaline on NO production was mediated via β1 and β2 adrenergic receptors since isoprenaline (a non-selective β1 and β2 agonist), dobutamine and salbutamol (selective β1 and β2 agonists, respectively) had similar effects on the NO response. In addition, the inhibitory effect of adrenaline on NO was abrogated by both propranolol (a non-specific β blocker) and atenolol (a specific β1 inhibitor). In contrast to β receptor activation, the α adrenergic agonist phenylephrine had no effect on the LPS NO response, and furthermore, phentolamine (an α receptor antagonist) did not ameliorate adrenaline’s inhibitory action.
Introduction
Nitric oxide (NO) is produced from l-arginine in a reaction catalysed by nitric oxide synthetase (NOS). Three mammalian forms of this enzyme have been described. Endothelial and neuronal NOS are generally constitutively expressed and their activities are dependent on elevation of intracellular calcium. Inducible NOS (iNOS) is a high-output pathway for NO production during inflammation and is independent of intracellular calcium increases (reviewed in 1). Cells of the immune system, such as macrophages, contribute to the inflammatory response and are a source of NO. Macrophage iNOS expression increases rapidly following lipopolysaccharide (LPS) and cytokine stimulation. 1, 2 Nitric oxide has microbicidal and cytotoxic activities and contributes to the regulation of cytokine production. 1, 3 In addition, several studies in rodents have demonstrated an important pathophysiological role for NO in endotoxic shock. 2, 4, 5
The identification of receptors for neurotransmitters and hormones on cells of the immune system (reviewed in 6) has stimulated studies to determine the role of neurohormonal agents in regulating immune function. Complex interactions between the neuroendocrine and immune systems have been described, for example, the effects of stress and hypercortisolaemia on immunity. 7, 8 Adrenaline is a catecholamine hormone produced during acute stress. It is derived from the amino acid tyrosine and released from the adrenal medulla following sympathetic nervous system stimulation. Immunological stimuli, for example interleukin-1 (IL-1) and IL-6, activate sympathetic discharge which enhances circulating catecholamine levels. 8 Catecholamines in turn may exert significant immunoregulatory effects, for example CD4+ T-cell cytokine profiles which enhance human immunodeficiency virus (HIV) replication in vitro.9
Numerous studies have examined the roles of adrenaline and noradrenaline in the host response to endotoxaemia. Circulating catecholamine levels increase in response to LPS, 10 and furthermore, catecholamine therapy is beneficial in the management of patients with septic shock. 11 These studies suggest that catecholamines produced at the appropriate time during endotoxaemia may downregulate the inflammatory response. Recently, Szabo et al. demonstrated that a β adrenergic agonist, isoproterenol, given before LPS challenge in mice protects against hypotension and is associated with a reduction in plasma nitrite levels. 12 The cellular sources of NO in this type of in vivo model are likely to be numerous and include macrophages, other leucocytes, cardiac myocytes, pulmonary cells, splenocytes, hepatocytes, endothelial cells and vascular smooth muscle cells. 13–15 Therefore, this model is not suitable for examining the effects of adrenaline on a specific cell type. Macrophages play a role in the pathophysiology of endotoxaemia and in the activation and regulation of innate and specific immunity, therefore it is necessary to determine the effects of catcholamines on these cells. This study examines the effects of adrenaline on the LPS NO response of primary murine peritoneal macrophages using an in vitro model.
Materials and methods
Mice
Male 8–12-week-old BALB/c mice, bred at the Animal House of the University of Zimbabwe, were used in these experiments.
Reagents
Lipopolysaccharide from Salmonella typhosa (Sigma, St Louis, MO) was used to activate macrophages for NO production. Phentolamine, propranolol, atenolol, phenylephrine and corticosterone were all obtained from Sigma. Adrenaline was purchased from Datlabs (Harare, Zimbabwe) and salbutamol from Glaxo (Greenford, UK). Isoprenaline was purchased from Thornton and Ross (Huddersfield, UK). Dobutamine was supplied by Eli Lilly Company (Indianapolis, IN).
Macrophage cultures
Resident peritoneal exudate cells were harvested by lavaging the peritoneal cavities of mice with ice-cold RPMI-1640 medium (Highveld Biological, Lyndhurst, UK), supplemented with 1% heat inactivated foetal calf serum (FCS; Highveld Biological), 100 U/ml penicillin (Sigma), 100 µg/ml streptomycin (Sigma), 25 mmN-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid (HEPES) and 10 mm l-glutamine (Highveld Biological) (R1). Cells were centrifuged at 4° for 7 min at 1100 r.p.m. and resuspended in RPMI-1640 supplemented as for R1 but containing 10% FCS (R10). Cells were counted and viability assessed using trypan blue dye exclusion (Sigma). For in vitro stimulation with LPS, 100 µl of the cell suspension was plated onto 96 well sterile flat-bottomed tissue culture plates (Nunc, Roskilde, Denmark) to produce a final concentration of 3 × 105 cells in each well. Cells were adhered at 37° for 2 hr in a 5% CO2 incubator, then non-adherent cells removed by washing three times in warm R10. Medium alone, LPS, or LPS together with various drugs were added to the wells and the plates incubated at 37° for 48 hr, after which supernatants were harvested for immediate nitrite determination.
Determination of nitrite production
The NO produced by activated macrophages quickly reacts with oxygen to produce nitrite. Therefore, nitrite levels in the supernatants of macrophage cultures were measured using the Griess reaction. 16 The reactions were performed in duplicate by addition of 100 µl fresh supernatant to 100 µl Griess reagent (0·1% naphthlyenediamine dihydrochloride/1% sulphanilamide/2·5% H3PO4) (Sigma). After incubation at room temperature for 15 min, absorbance was read at 562 nm using a Multiscan Plus microplate reader (Labsystems, Helsinki, Finland). The nitrite concentration was determined by comparison to a sodium nitrite standard curve (Hopkins and Williams, Chadwell Heath, UK). The limit of detection of the assay was 0·2 µm.
Statistical analysis
Results are expressed as the mean ± standard error of the mean (SEM). Statistical analysis was performed using Student’st-test. A P-value of < 0·05 was considered significant.
Results
Adrenaline suppresses nitric oxide production
Initial experiments examined the effect of LPS on macrophage NO production. Cells cultured in medium alone produced little nitrite (5 ± 1 µm) and stimulation with LPS at 3, 10, and 30 µg/ml for 48 hr induced a dose-dependent increase in supernatant nitrite levels of 48 ± 2, 71 ± 1 and 121 ± 2 µm, respectively. To examine the kinetics of macrophage NO production, cells were cultured in medium alone, or with LPS (10 µg/ml), and culture supernatants harvested at intervals of between 2 and 48 hr for nitrite determination. Cells cultured in medium alone produced little nitrite and this did not alter over 48 hr. In contrast, nitrite production in response to LPS increased with time from 5 ± 1 µm at 2 hr, to 65 ± 1 µm at 48 hr. Therefore, in all subsequent experiments supernatants were harvested at 48 hr.
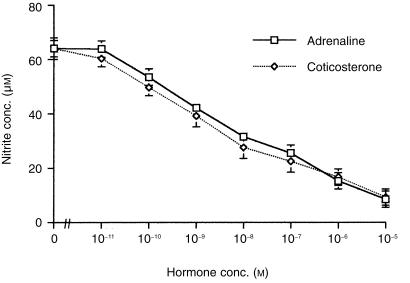
To assess the effects of adrenaline on the LPS NO response, cells were cultured in medium alone or with LPS (10 µg/ml) in the absence or presence of adrenaline. Corticosterone, a steroid hormone known to suppress NO production, 14 was used as a negative control. Cells cultured with either adrenaline alone or corticosterone alone produced 4 ± 1 and 3 ± 1 µm of nitrite, respectively. LPS induced a nitrite response (medium alone 4 ± 1 µm, LPS 64 ± 2 µm), which was inhibited by both adrenaline and corticosterone in a dose-dependent manner (Fig. 1). The IC50 for the two hormones was similar, approximately 10−8 m.
Figure 1.
Comparison of adrenaline and corticosterone effects on the nitric oxide response of macrophages to LPS. Murine peritoneal macrophages (105) were cultured with medium alone, LPS (10 µg/ml), or LPS in the presence or absence of either adrenaline or corticosterone. Additional control cells were cultured with either adrenaline or corticosterone alone. After 48 hr culture supernatants were harvested and nitrite levels determined by the Griess reaction. Results are expressed as mean ±SEM and are representative of three similar experiments.
The inhibitory effect of adrenaline on nitric oxide production is mediated via β1 and β2 receptors and not through α receptors
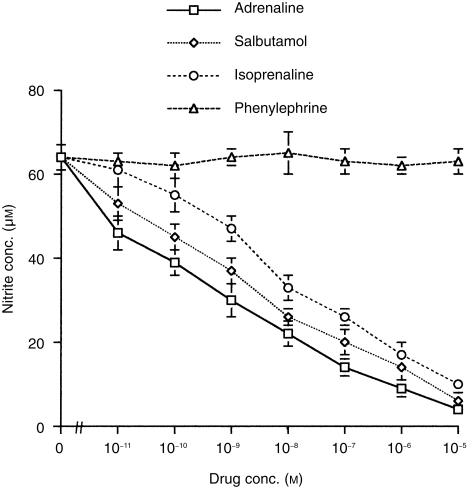
Adrenaline acts by binding to and activating α and β receptors 17 which are present on macrophages. 18, 19 To determine which adrenergic receptors mediate the suppression of NO, macrophages were stimulated with LPS alone or LPS in the absence or presence of adrenaline, or specific α and β receptor agonists. In one set of experiments, the effects of adrenaline on the nitrite response were compared with those of phenylephrine (an α agonist), isoprenaline (a non-selective β1 and β2 agonist), and salbutamol (a β2 agonist). Cells cultured in medium alone or the highest concentrations of either adrenaline, phenylephrine, isoprenaline or salbutamol alone produced little nitrite (< 4 ± 1 µm, respectively). LPS induced a NO response (64 ± 2 µm) which was inhibited by adrenaline, isoprenaline and salbutamol in a similar dose-dependent manner (Fig. 2). In contrast, phenylephrine had no effect on the LPS nitrite response at any concentration tested.
Figure 2.
Comparison of adrenaline and α or β receptor agonist effects on the nitric oxide response of macrophages to LPS. Murine peritoneal macrophages (105) were cultured with either medium alone, LPS alone (10 µg/ml), or LPS in the presence or absence of either adrenaline, isoprenaline, salbutamol, or phenylephrine. After 48 hr culture supernatants were harvested and nitrite levels determined by the Griess reaction. Results are expressed as mean ±SEM and are representative of two similar experiments.
To determine whether β1 specific receptor stimulation alone could also inhibit NO production, the effect of adrenaline was compared with that of either isoprenaline, salbutamol or dobutamine (a selective β1 agonist). Dobutamine had a similar suppressive effect on NO production as the other agents (Table 1). These results suggest that either β1 or β2 receptor activation may independently inhibit the macrophage NO response to LPS.
Table 1.
Comparison of effects of adrenaline with β1 and β2 agonists on the LPS nitric oxide response
| Cell culture conditions | Nitrite concentration (µm) |
|---|---|
| Medium alone | 5 + 1 |
| LPS alone (10 µg/ml) | 63 + 4 |
| Adrenaline 10−7m plus LPS | 25 + 3* |
| Salbutamol 10−7m plus LPS | 28 + 2* |
| Dobutamine 10−7m plus LPS | 27 + 2* |
105 peritoneal macrophages were cultured in medium alone or stimulated with LPS or LPS plus either adrenaline, salbutamol or dobutamine.
Additional control cells were cultured in the presence of either drug alone. After 48 hr culture supernatants were harvested and nitrite levels determined. Results are expressed as the mean ±SEM and are representative of two independent experiments.
P < 0·01 compared to LPS alone value.
β receptor blockade attenuates the inhibitory effect of adrenaline on nitric oxide
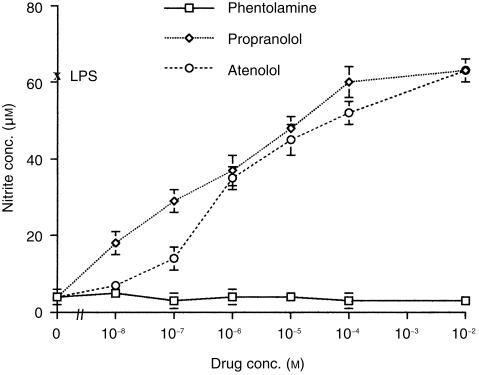
The results described above suggest that adrenaline suppresses the nitrite response through β receptors alone, and that α receptor activation plays little role. To confirm these observations, the effects of α or β receptor antagonists on the action of adrenaline were examined. Cells were cultured in medium alone, LPS alone, and LPS plus adrenaline (10−5 m) in the presence or absence of either propranolol (a non-selective β1 and β2 antagonist), atenolol (a selective β1 antagonist) or phentolamine (an α antagonist). Cells cultured in medium alone or with the antagonists alone produced little nitrite (< 4 + 1 µm, respectively). LPS-induced nitrite production was inhibited by adrenaline (LPS alone 63 ± 2 µm, LPS plus adrenaline 4 ± 1 µm). However, this suppression was completely abrogated by increasing concentrations of either propranolol or atenolol (Fig. 3), with propranolol being more potent than atenolol at lower concentrations. In contrast, phentolamine failed to alleviate the inhibitory effect of adrenaline at any concentration tested.
Figure 3.
Comparison of α and β receptor antagonist effects on the adrenaline mediated suppression of nitric oxide. Murine peritoneal macrophages (105) were cultured with either medium alone, LPS alone (10 µg/ml), LPS plus adrenaline (at 10−5 m), or LPS plus adrenaline together with either phentolamine, propranolol, or atenolol. After 48 hr culture supernatants were harvested and nitrite levels determined by the Griess reaction. Results are expressed as mean ±SEM and are representative of two similar experiments.
Discussion
In this study we used an in vitro model to assess the effect of adrenaline on the macrophage NO response to LPS, and to determine its cellular mechanism of action. Murine peritoneal macrophages were chosen because of they are readily accessible, easily prepared, and have been well characterized. Adrenaline suppressed the NO response at levels that occur physiologically in vivo.20 Adrenaline may also inhibit NO production from macrophage populations other than peritoneal macrophages as we have described here. For example, Persoons et al. have shown that rat alveolar macrophages isolated from acutely stressed animals make a reduced nitrite response to LPS compared to controls. 21 Further studies of this stress model have suggested a role for beta-adrenergic receptor mediated inhibition of NO. 22
The cellular mechanism of corticosterone’s actions is well described. Corticosterone, in common with other members of the steroid hormone family, readily traverses the plasma membrane because of its hydrophobicity. It binds to intracellular receptors present in macrophages, 23 and thereafter the hormone-receptor complex translocates to the nucleus (reviewed in 24). In contrast to corticosterone, adrenaline is hydrophilic and binds extracellular receptors prior to signal transduction. Although α and β receptors are present on macrophages, 18, 19 our data suggests that it is the β receptors alone that play an important role in mediating the inhibitory action of adrenaline.
The present results confirm and extend those of Hasko et al. 25 who showed that isoproterenol inhibits LPS stimulated NO release from the RAW 264.7 macrophage cell line. Our data clearly demonstrates for the first time a role for independent β1 and β2 receptor activation in suppressing NO production. Further investigation of the regulation of macrophage β receptor cell surface expression is also required as this may modulate the effects of adrenaline. It is likely that different physiological or pathological conditions may alter β receptor expression. For example, glucocorticoids increase β expression on respiratory smooth muscle cells, 26 and thyroid hormones have a similar effect on ventricular myocytes. 27 It remains to be determined if and how these hormones could affect β receptors on macrophages.
In the murine system, corticosterone inhibits iNOS transcription through enhancement of Iκ-B which suppresses nuclear factor (NF)-κB activity and thus prevents activation of the iNOS promoter. 28 Further experiments are required to determine the inhibitory mechanism of adrenaline’s effect on NO at the biochemical level. The signal transduction mechanism for β receptors involves activation of adenyl cyclase and an increase in intracellular cAMP. 29 Through the use of forskolin, membrane-permeable cAMP analogues and phosphodiesterase inhibitors, Mustafa and Olson 30 have recently shown that increases in intracellular cAMP suppress LPS-induced NO in rat Kupffer cells. This effect was due to reduction of iNOS mRNA via interference with NF-κB activation and concomitant enhancement of I-κB. Inhibition of adenyl cyclase or protein kinase A could confirm a role for cAMP as a signal transducer of the adrenaline effects observed in our experiments.
In conclusion, we have shown that adrenaline inhibited the macrophage NO response to LPS through β1 and β2 adrenergic receptors, but α receptors played no role. These observations provide further evidence for neurohormonal regulation of immunity and may also have implications for the management of inflammatory conditions where NO contributes to pathology.
Acknowledgments
We are grateful for the support and generosity of Dr Greg Bancroft and his laboratory. We thank Professor Ralph van Furth for critically reading the manuscript and for his helpful suggestions. This study was funded by the Research Board of the University of Zimbabwe.
References
- 1.MacMicking J, Xie Q, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 2.Wei X, Charles IG, Smith A, et al. Altered immune response in mice lacking inducible nitric oxide synthetase. Nature. 1995;375:408. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 3.Kolb H, Kolb-Bachofen V. Nitric oxide in autoimmune disease: cytotoxic or regulatory mediator? Immunol Today. 1998;19:556. doi: 10.1016/s0167-5699(98)01366-8. [DOI] [PubMed] [Google Scholar]
- 4.Rees DD, Monkhouse JE, Cambridge D, Moncada S. Nitric oxide and the haemodynamic profile of endotoxic shock in the conscious mouse. Br J Pharmacol. 1998;124:540. doi: 10.1038/sj.bjp.0701815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rees DD, Celleck S, Palmer RM, Moncada S. Dexamethasone prevents the induction by endotoxin of a nitric oxide synthase and the associated effects on vascular tone: an insight into endotoxic shock. Biochem Biophys Res Commun. 1990;173:541. doi: 10.1016/s0006-291x(05)80068-3. [DOI] [PubMed] [Google Scholar]
- 6.Besedovsky HO, delrey AD. Immune–neuro-endocrine interactions: facts and hypotheses. Endocr Rev. 1996;17:64. doi: 10.1210/edrv-17-1-64. [DOI] [PubMed] [Google Scholar]
- 7.Jefferies WM. Cortisol and immunity. Med Hypotheses. 1991;34:198. doi: 10.1016/0306-9877(91)90212-h. [DOI] [PubMed] [Google Scholar]
- 8.Wilder RL. Neuroendocrine–immune system interactions and autoimmunity. Annu Rev Immunol. 1995;13:307. doi: 10.1146/annurev.iy.13.040195.001515. [DOI] [PubMed] [Google Scholar]
- 9.Cole SW, Korin YD, Fahey JL, Zack JA. Norepinephrine accelerates HIV replication via protein kinase A dependent effects on cytokine production. J Immunol. 1998;161:610. [PubMed] [Google Scholar]
- 10.Benedict CR, Grahame-Smith DG. Plasma noradrenaline and adrenaline concentrations and dopamine-beta-hydroxylase activity in patients with shock due to septicaemia, trauma and haemorrhage. Q J Med. 1978;47:1. [PubMed] [Google Scholar]
- 11.Lipman J, Roux A, Kraus P. Vasoconstrictor effects of adrenaline in human septic shock. Anaesth Intensive Care. 1991;19:61. doi: 10.1177/0310057X9101900111. [DOI] [PubMed] [Google Scholar]
- 12.Szabo C, Hasko OG, Zingarelli B, et al. Isoproterenol regulates tumour necrosis factor, interleukin-10 and nitric oxide production and protects against the development of vascular hyporeactivity in endotoxaemia. Immunology. 1997;90:95. doi: 10.1046/j.1365-2567.1997.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunha FQ, Assreuy J, Moss DW, et al. Differential induction of nitric oxide synthase in various organs of the mouse during endotoxaemia: role of TNF-alpha and IL-1-beta. Immunology. 1994;81:211. [PMC free article] [PubMed] [Google Scholar]
- 14.Hom GT, Grant SK, Wolfe G, Bach TJ, MacIntyre DE, Hutchinson NI. Lipopolysaccharide induced hypotension and vascular hyporeactivity in the rat: tissue analysis of nitric oxide synthase mRNA and protein expression in the presence and absence of dexamethasone, NG-monomethyl-l-arginine or indomethacin. J Pharmacol Exp Ther. 1995;272:452. [PubMed] [Google Scholar]
- 15.Liu SF, Barnes PJ, Evans TW. Time course and cellular localizations of lipopolysaccharide-induced inducible nitric oxide synthase messenger RNA in the rat in vivo. Crit Care Med. 1997;25:512. doi: 10.1097/00003246-199703000-00022. [DOI] [PubMed] [Google Scholar]
- 16.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite and (15) nitrate in biological fluids. Anal Biochem. 1982;126:131. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 17.Van Zwiten PA. Autonomic Failure A Textbook of Clinical Disorders of the Autonomic Nervous System. Oxford: Oxford University Press; 1992. p. 94. [Google Scholar]
- 18.Miles BA, Lafuse WP, Zwilling BS. Binding of alpha-adrenergic receptors stimulates the anti-mycobacterial activity of murine peritoneal macrophages. J Neuroimmunol. 1996;71:19. doi: 10.1016/s0165-5728(96)00113-0. [DOI] [PubMed] [Google Scholar]
- 19.Abrass CK, O'connor SW, Scarpace PJ, Abrass IB. Characterisation of the beta-adrenergic receptor of the rat peritoneal macrophage. J Immunol. 1985;135:1338. [PubMed] [Google Scholar]
- 20.Frayn KN. Hormonal control of metabolism in trauma and sepsis. Clin Endocrinol. 1986;24:577. doi: 10.1111/j.1365-2265.1986.tb03288.x. [DOI] [PubMed] [Google Scholar]
- 21.Persoons JH, Schornagel K, Breve J, Berkenbosch F, Kraal G. Acute stress affects cytokines and nitric oxide production by alveolar macrophages differently. Am J Respir Crit Care Med. 1995;152:619. doi: 10.1164/ajrccm.152.2.7633716. [DOI] [PubMed] [Google Scholar]
- 22.Broug-Holub E, Persoons JH, Schornagel K, Mastbergen SC, Kraal G. Effects of stress on alveolar macrophages: role for the sympathetic nervous system. Am J Respir Cell Biol. 1998;19:842. doi: 10.1165/ajrcmb.19.5.3103. [DOI] [PubMed] [Google Scholar]
- 23.Werb Z, Foley R, Munck A. Interaction of glucocorticoids with macrophages. Identification of glucocorticoid receptors on monocytes and macrophages. J Exp Med. 1978;147:1684. doi: 10.1084/jem.147.6.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnes PJ. Mechanism of action of glucocorticoids in asthma. Am J Respir Crit Care Med. 1996;154:S21. doi: 10.1164/ajrccm/154.2_Pt_2.S21. [DOI] [PubMed] [Google Scholar]
- 25.Hasko G, Nemeth ZH, Szabo C, Zsilla G, Salzman AL, Vizi ES. Isoproterenol inhibits IL-10, TNF alpha and nitric oxide production in RAW 264.7 macrophages. Brain Res Bull. 1998;45:183. doi: 10.1016/s0361-9230(97)00337-7. [DOI] [PubMed] [Google Scholar]
- 26.McGraw DW, Chai SE, Hiller FC, Cornett LE. Regulation of the beta-2-adrenergic receptor and its mRNA in the rat lung by dexamethasone. Exp Lung Res. 1995;21:535. doi: 10.3109/01902149509031757. [DOI] [PubMed] [Google Scholar]
- 27.Bahouth SW. Thyroid hormones transcriptionally regulate the beta-1-adrenergic gene in cultured ventricular myocytes. J Biol Chem. 1991;266:15 863. [PubMed] [Google Scholar]
- 28.De Vera ME, Taylor BS, Wang Q, Shapiro RA, Billiar TR, Geller DA. Dexamethasone suppresses iNOS gene expression by upregulating I-kappa B alpha and inhibiting NF-kappa B. Am J Physiol. 1997;273:G1290. doi: 10.1152/ajpgi.1997.273.6.G1290. [DOI] [PubMed] [Google Scholar]
- 29.Kobilka B. Adrenergic receptors as models for G protein-coupled receptors. Annu Rev Neurosci. 1992;15:87. doi: 10.1146/annurev.ne.15.030192.000511. [DOI] [PubMed] [Google Scholar]
- 30.Mustafa SB, Olson MS. Expression of nitric-oxide synthase in rat Kupffer cells is regulated by cAMP. J Biol Chem. 1998;273:5073. doi: 10.1074/jbc.273.9.5073. [DOI] [PubMed] [Google Scholar]