Abstract
γδ T lymphocytes recognize non-peptidic microbial antigens without antigen processing and major histocompatibility complex (MHC) restriction, representing an early defence mechanism against invading pathogens. As a defective response to non-peptidic antigens was observed in human immunodeficiency virus-positive (HIV+) persons, the aims of this study were twofold: to analyse the incidence of γδ T-cell anergy in HIV+ patients with opportunistic infections/co-infections (HIV-OIC), and to investigate the role of highly active antiretroviral therapy (HAART) on γδ T-cell functions. Peripheral γδ T-cell distribution and in vitro reactivity to a non-peptidic mycobacterial antigen, isopentenyl pyrophosphate (IPP), were analysed. γδ T-cell subset distribution was altered more in HIV-OIC patients than in asymptomatic HIV+ subjects (HIV-ASY). Specifically, the Vδ2/Vδ1 ratio was inverted as a consequence of a decrease in Vδ2 T-cell number. Moreover, IPP-stimulated Vδ2 T cells from the HIV-OIC group displayed a major defect in interferon-γ (IFN-γ) production. Interestingly, HAART induced a sustained recovery of naive CD45RA+ and CD62L+ T cells and restored γδ T-cell function. Accordingly, in vitro CD45RA depletion resulted in γδ T-cell hyporesponsiveness. Altogether, the incidence of γδ T-cell anergy was increased in HIV-OIC patients and dependent on CD45RA helper function. Moreover, HAART was able to restore γδ T-cell reactivity, extending the immune recovery to non-peptidic microbial antigens.
Introduction
T lymphocytes bearing the γδ T-cell receptor (TCR) recognize non-peptidic microbial antigens in a major histocompatibility complex (MHC)-unrestricted manner, without antigen processing by professional antigen-presenting cells (APC) (reviewed in ref. 1). This subset displays an apparent homogeneity in the peripheral blood, mainly expressing the Vδ2 associated with Vγ9 variable segments.1,2 Vγ9Vδ2 T cells produce tumour necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) in response to bacterial ligands3,4 and are also broadly reactive against different viruses, including human immunodeficiency virus (HIV).4–7 Thus, it is probable that Vγ9Vδ2 T cells do not respond to specific viral antigens, but rather to cellular ligands, such as phosphoantigens, and/or MHC products induced or modified by viral infections.1
Alterations of γδ T-cell distribution have been previously reported in the peripheral blood of HIV-infected persons, including a polyclonal decrease in absolute number of Vγ9Vδ2 T cells.8–13 Moreover, the remaining Vγ9Vδ2 T cells are frequently unable to proliferate12–16 and/or to express the interleukin (IL)-2 receptor.12 The most important consequence of the Vδ2 deletion and anergy in HIV-infected persons may be an increased susceptibility to opportunistic pathogens.16 In this study, we examined the functional reactivity of γδ T lymphocytes in HIV-infected persons with opportunistic infections and co-infections (HIV-OIC) and the recovery of γδ T-cell functions after highly active antiretroviral therapy (HAART).
Materials and methods
Blood samples
HIV-infected persons (n = 47) from the ‘L. Spallanzani’ Institute Hospital were at various disease stages (23 CDC-A, 18 CDC-B, six CDC-C). HIV-OIC patients (n = 19) were as follows: seven with candidiasis, four with tuberculosis, three with hepatitis C virus (HCV), three with herpes zoster and two with salmonellosis. Informed consent was obtained from all persons prior to enrolment. HAART consisted of combined therapy with two reverse transcriptase inhibitors (lamivudine, stavudine, zidovudine or didanosine) and one protease inhibitor (indinavir, nelfinavir, sequinavir or ritonavir). Blood samples were collected before HAART treatment and after 3 and 6 months of therapy. HIV-seronegative healthy donors (n = 15) were used as controls. Plasma HIV-1 RNA levels were determined using the NASBA QT assay (Organon Teknika, Boxtel, the Netherlands). Only HAART-treated patients whose HIV RNA levels dropped below the detection threshold (80 copies/ml) were studied for immune recovery.
Monoclonal antibodies
Surface staining was performed using: anti-CD4 (immuno-globulin G1 [IgG1], clone 13B8.2), anti-CD3 (IgG1, clone UCHT1), anti-CD45RA (IgG1, clone 2H4), anti-CD62L (IgG1, clone DREG56) and anti-TCR-Vδ2 (IgG1, clone IMMU389) monoclonal antibodies (mAbs). All mAbs were directly coupled to fluorochromes – fluorescein isothiocyanate (FITC), phycoerythrin (PE) or phycoerythrin-cyanin 5.1 (PE-Cy5) – and purchased from ImmunoTech (Coulter, Miami, FL). FITC-coupled anti-TCR-Vδ1 (IgG1, clone TS8.2) was purchased from Endogen (Woburn, MA).
Cell preparation and stimulation
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood by Ficoll–Hypaque (Pharmacia, Uppsala, Sweden) and cultured at 1·5 × 106 cells/ml in complete medium (RPMI-1640, 10% v/v heat-inactivated fetal calf serum [FCS] 2 mm l-glutamine, 10 U/ml of penicillin/streptomycin). PBMC from control donors or HIV patients were stimulated in vitro for 10 days in the presence of 120 µm isopentenyl pyrophosphate (IPP) (Sigma Chemical Co., St. Louis, MO) and 100 U/ml of recombinant IL-2 (rIL-2) (Boehringer Mannheim Corp., Mannheim, Germany). After 1 week of culture, a volume corresponding to 50% of the culture supernatant was replaced with complete medium containing IL-2. The expansion of Vγ9Vδ2 T cells was determined by cytometric analysis using double staining with anti-CD3 (PE) and anti-TCR-Vδ2 (FITC) mAbs. Vδ2 expansion index was calculated as follows: the absolute number of Vδ2 T cells in stimulated cultures divided by the absolute number of Vδ2 T cells in unstimulated cultures.4,12 The IPP-driven PBMC proliferation assay was performed in triplicate in 96-well flat-bottomed plates containing 2·5 × 105 PBMC/ml in a 0·2-ml volume with 120 µm IPP and 5 U/ml of rIL-2. Proliferation was measured after 5 days by pulsing cultures for 6 hr with [3H]thymidine (Amersham) (1 µCi/well), then harvesting and counting the cells using a liquid scintillation counter (Wallac 1450 Microbeta, EG & G, Wellesley, MA). Stimulation index was calculated as follows: counts per minute (c.p.m.) from IPP-stimulated cultures divided by c.p.m. from unstimulated cultures.
Flow cytometry analysis
Phenotypic analysis of γδ T cells was performed by incubating 50 µl of whole blood for 20 min at 4° with mAbs (specific for surface Ags) at titred concentrations. After lysis/fixation using a Multi-Q-Prep (Coulter, Miami, FL), samples were analysed in an Epics-XL cytometer (Coulter). Each sample comprised 20 000 viable lymphocytes, gated following size/granularity criteria and analysed using the system ii software (Coulter). Negative controls were irrelevant isotype-matched antibodies.
CD45RA cell depletion
To assess the CD45RA+ T-cell role in ‘helping’ the Vγ9Vδ2 response to non-peptidic antigens, the γδ T-cell response to IPP was analysed in asymptomatic anergic patients after HAART, in cultures set up both with total PBMC and with CD45RA-depleted PBMC. Briefly, after reaction with purified unlabelled anti-CD45RA mAb (Immunotech clone 2H4), cells were incubated with Pan Mouse IgG Dynabeads (Dynal A.S & Nordic, Oslo, Norway) at 4° for 30 min and depleted by magnetic separation following the manufacturer's instructions. After-depletion CD45RA contamination, assessed by flow cytometry, was always lower than 1%. Undepleted and CD45RA-depleted cultures were then set up to assess IPP responsiveness, at both an optimal IL-2 concentration (100 U/ml) and at a limiting IL-2 concentration (5 U/ml).
Enzyme-linked immunosorbent assay for cytokines production
Supernatants from 24-hr IPP-stimulated cultures were analysed for IFN-γ, TNF-α and IL-4 production using enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions (Endogen, Woburn, MA). The non-specific cytokine synthesis induced by IL-2 alone was always subtracted from the results.
Statistical analysis
Multiple group comparison was performed by the Kruskal–Wallis H-test; when necessary, Bonferroni correction was used to adjust P-values for the effects of multiple comparisons. In HAART treatment comparisons and in depletion experiments, differences among group means were evaluated using the Mann–Whitney U-test. P-values < 0·05 were considered significant.
Results
Altered γδ T-cell subset distribution in the peripheral blood of HIV-OIC subjects
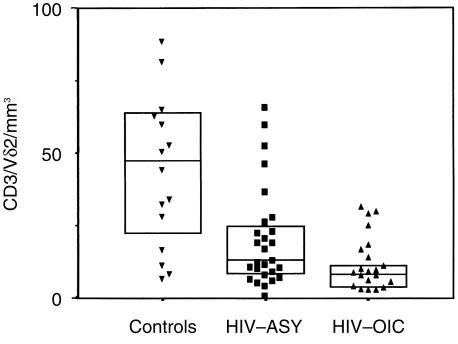
PBMC from healthy donors (n = 15), HIV-OIC (n = 19) or asymptomatic subjects with HIV (HIV-ASY) (n = 28), were analysed by flow cytometry for γδ T-cell subset distribution. Figure 1 shows that the absolute number of Vδ2 T cells was decreased in the HIV-ASY group, as previously reported.8–13 Interestingly, the reduction was more pronounced in HIV-OIC patients. The Vδ2 T-cell reduction was concomitant with a significant increase of circulating Vδ1 T cells in HIV-infected persons (Table 1), which are probably recruited from tissues by chronic inflammation.11 These data reveal that a marked Vδ2 T-cell deletion may be associated with the presence of opportunistic infections in HIV-infected persons.
Figure 1.
γδ T-cell subset distribution in the peripheral blood of asymptomatic subjects infected with human immunodeficiency virus (HIV-ASY) and in HIV-infected patients with opportunistic infections/co-infections (HIV-OIC). γδ T-cell subset distribution was analysed ex vivo by flow cytometry in peripheral blood mononuclear cells (PBMC) from healthy donors (controls, n = 15) and HIV-positive subjects (n = 47: HIV-ASY, n = 28; HIV-OIC, n = 19). Vδ2 T-cell absolute numbers are shown. Lines represent group medians and boxes represent interquartile ranges. Kruskal–Wallis H-test results with Bonferroni correction: χ2 = 15·020, d.f. = 2, P = 0·005.
Table 1.
γδ T-cell subset distribution in the peripheral blood of asymptomatic subjects infected with human immunodeficiency virus (HIV-ASY) and in HIV-infected patients with opportunistic infections/co-infections (HIV-OIC)
| Groups | n | Vδ1/mm3 | Vδ2/mm3 |
|---|---|---|---|
| Controls | 15 | 19·1 (7·2–17·4) | 47·4 (22·2–63·9) |
| HIV-ASY | 29 | 37·4 (26·6–52·8) | 13·2* (8·6–24·7) |
| HIV-OIC | 25 | 36·2 (24·0–73·8) | 9·2* (5·6–14·0) |
Median values are shown with interquartile ranges in brackets.
Mann–Whitney U-test after Bonferroni correction: P<0·005 versus controls.
γδ T-cell anergy to non-peptidic microbial antigens inHIV-infected persons
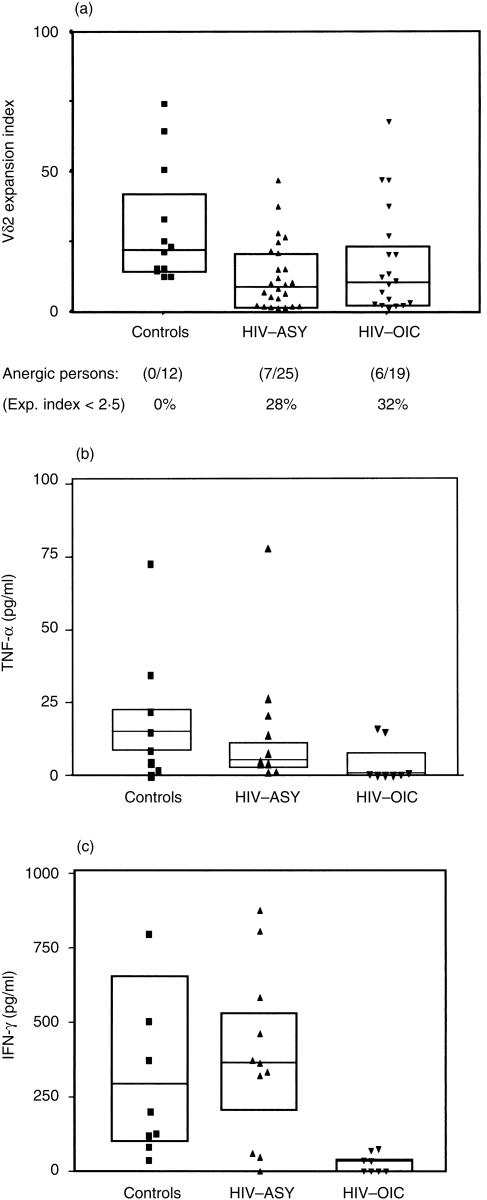
PBMC from controls and HIV-infected persons were stimulated with IPP, a prenyl pyrophosphate from mycobacteria known to be recognized by human Vγ9Vδ2 T cells.17Figure 2(a) shows the Vδ2 expansion indexes of control, HIV-ASY and HIV-OIC groups. A consistent number of HIV-infected subjects were unable to proliferate to antigenic stimulation, independently of the presence of opportunistic infections (28% HIV-ASY; 32% HIV-OIC). Culture supernatants were collected after 24 hr of IPP stimulation. TNF-α, IFN-γ and IL-4 levels were measured using ELISA. No IL-4 production was observed (data not shown). In Fig. 2(b), 2(c), the T helper 1 (Th1) cytokine levels are shown. A lower level of IFN-γ (Fig. 2c) was produced by HIV-OIC patients than by HIV-ASY or control patients. Altogether, a major reduction in IFN-γ production was found in HIV-OIC patients.
Figure 2.
γδ T-cell anergy to non-peptidic microbial antigens in human immunodeficiency virus (HIV)-infected persons. (a) In vitro isopentenyl pyrophosphate (IPP)-driven expansion indexes obtained by cytometric analysis of peripheral blood mononuclear cell (PBMC) Vγ9Vδ2 T-cell cultures are shown for controls (n = 12) and HIV-infected persons (n = 44): asymptomatic subjects infected with HIV (HIV-ASY) n = 25; HIV-infected patients with opportunistic infections/co-infections (HIV-OIC) n = 19. Lines represent group medians, and boxes represent interquartile ranges. Data represents Kruskal–Wallis H-test results with Bonferroni correction: χ2 = 9·223, d.f. = 2, P = 0·05 (NS); for each group, the percentage of anergic (expansion index < 2·5) subjects is reported. The 24-hr IPP-driven tumour necrosis factor-α (TNF-α) production levels are shown in (b) and 24-hr IPP-driven interferon-γ (IFN-γ) levels in (c) for controls (n = 8) and HIV-infected persons (n = 19: HIV-ASY, n = 11; HIV-OIC, n = 8). Lines represent group medians, and boxes represent interquartile ranges. Data represents Kruskal–Wallis H-test results with Bonferroni correction: TNF-α (pg/ml): χ2 = 9·262, d.f. = 2, P = 0·05 (ns); IFN-γ (pg/ml): χ2 = 16·315, d.f. = 2, P = 0·005. EI, expansion index.
Recovery of γδ T-cell functions by HAART: role of CD45RA helper cells
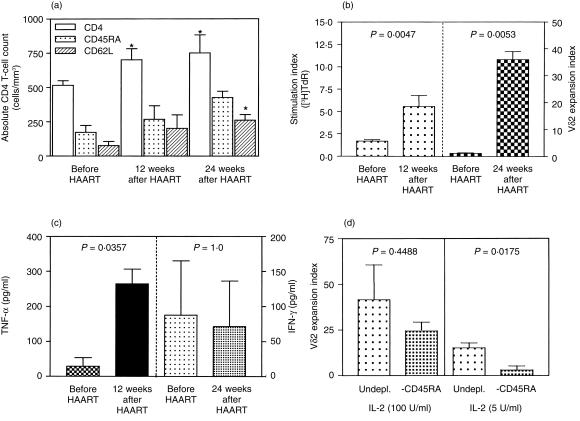
One of the most marked effects of HAART was a sustained increase in naive CD4+ T-lymphocyte counts, evident after 12 weeks of therapy (Fig. 3a). The IPP-driven Vγ9Vδ2 proliferative response was also compared before and after HAART. γδ T-cell reactivity was markedly increased after only 3 months of HAART (Fig. 3b). The IPP-induced cytokine levels were compared before and after 6 months of HAART. IFN-γ production was essentially unchanged in the HIV-ASY group before and after HAART (Fig. 3c). However, a potentially harmful increase in TNF-α production was observed. To assess the role of CD45RA cells in the IPP response by anergic patients after HAART, CD45RA-depleted cultures were stimulated with IPP at optimal and suboptimal IL-2 concentrations (100 and 5 U/ml, respectively). Vγ9Vδ2 expansion was assessed after 10 days, as described above, and the expansion index distribution was determined (Fig. 3d). A significant reduction of IPP reactivity was found in CD45RA-depleted cultures at the suboptimal IL-2 concentration (5 U/ml). Altogether, these observations suggest that HAART improves not only αβ T-cell function but also γδ T-cell reactivity, and demonstrate that γδ T-cell activation depends on IL-2 provided by CD45RA helper cells.
Figure 3.
Recovery of γδ T-cell functions by highly active antiretroviral therapy (HAART). (a) Increase in the number of naive CD4+ T cells, induced by HAART, from 12 human immunodeficiency virus (HIV) patients. *P < 0·05 versus pretherapy (Mann–Whitney U-test). (b) The isopentenyl pyrophosphate (IPP)-driven [3H]thymidine ([3H]TdR) stimulation indexes (3 months after HAART) or expansion indexes (6 months after HAART) were analysed in eight asymptomatic HIV+ persons previously IPP anergic (expansion index < 2·5). (c) The IPP-driven tumour necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) production levels are shown for eight asymptomatic IPP-anergic HIV+ persons (expansion index < 2·5) before and after 6 months of HAART. (d) The effect of CD45RA depletion on six HIV patients undergoing HAART is shown both at optimal (100 U/ml) and at limiting (5 U/ml) interleukin-2 (IL-2) concentrations. Error bars refer to standard errors. Significances refer to Mann–Whitney U-test results. Undpl., undepleted.
Discussion
The generation of an effective immune response depends on the cross-talk between adaptive and innate pathways. Among natural effector cells, Vγ9Vδ2 T lymphocytes are believed to play a major role in promoting the cell-mediated response against intracellular pathogens.1 We observed in this study that γδ T-cell distribution and reactivity is particularly affected in HIV-OIC patients.
Several groups8–13 reported that γδ T-cell subset distribution is altered in a proportion of HIV-infected persons. Our results confirm that this was caused by a decrease in Vδ2 T-cell number. Interestingly, the Vδ2 T-cell deletion was more evident in the HIV-OIC group, suggesting an involvement of this T-cell subset in the protection from opportunistic pathogens. As HIV patients were all treated with HAART, it was not possible to perform a longitudinal study to determine a direct link between a deficit in Vδ2 T cells and the development of opportunistic infections. Thus, it is also possible that opportunistic infections themselves may cause γδ T-cell deletion. As previously observed,12,13,15 the remaining Vγ9Vδ2 T cells were unable to proliferate to their constitutive antigens. This is consistent with the functional defects previously described in untreated HIV-infected persons for the αβ T-cell compartment.18 No CDR3-dependent antigenic selection was previously reported for Vδ212 or Vδ1 T lymphocytes,11 although Vδ1 cells show a preactivated state. In healthy donors, Vδ1 T cells are mainly distributed throughout the human intestinal epithelium (70–90% of intestinal γδ T cells)19,20 and recognize stress-induced antigens on intestinal epithelial cells,20 suggesting that these cells may function as sentinels that respond to self-antigens. Thus, Vδ1 T-cell expansion in the peripheral blood of HIV-infected persons may be the consequence of cell migration from tissues to the bloodstream as a result of chronic inflammation.
A protective response to intracellular pathogens requires potent activation of cell-mediated immunity, promoted by a Th1 cytokine profile. A major role as a Th1-inducing factor is played by IFN-γ, stimulating the activation of natural killer cells and recruiting cytotoxic T lymphocytes. Under normal conditions, Vγ9Vδ2 T cells respond to antigen challenge by secreting large quantities of TNF-α and IFN-γ,3–4 which contributes to the activation of both specific and aspecific immune responses.1 Moreover, γδ T cells were shown to be important IFN-γ producers during tuberculosis infection, and knockout mice lacking γδ T cells develop a more exacerbated disease.21 We showed a significant reduction of IFN-γ production by Vδ2 T cells in the HIV-OIC group. Thus, Vδ2 T-cell anergy may also contribute to the Th1 hyporesponsiveness to microbial antigens in HIV-infected persons.
The mechanisms of Vγ9Vδ2 T-cell anergy are still poorly understood. This subset expresses the inhibitory CD94/NKG2 receptor that recognizes human leucocyte antigen-E (HLA-E)1 and is stimulated by HIV-infected cells through CD94–HLA interactions.4 Moreover, activated lymphocytes are potent stimulators of autoreactive cytotoxic Vδ2 T cells,22 and HIV infection is known to induce a persistent activation of the immune system. As Vγ9Vδ2 T-cell activation is followed by the programmed cell death of the cytotoxic Vγ9Vδ2 T-cell effectors,23 the persistent stimulation of Vδ2 T cells in vivo during chronic HIV infection may be responsible for Vδ2 T-cell deletion/anergy. Preliminary data (C. D. Pauza, personal communication) indeed show differences between anergic and responder HIV patients at the level of Vγ9 CDR3 sequences, known to be involved in the recognition of non-peptidic antigens.24 Thus, an altered Vγ9 repertoire may also cause γδ T-cell anergy. Finally, a major role in γδ T-cell anergy may be played by cytokine dysfunction. As IL-15 can act in synergy with IL-12 and promotes IFN-γ production by γδ T cells,25 Vδ2 T-cell anergy may be caused by defective IL-15 and/or IL-12 production by accessory cells.
The overall course of HIV disease dramatically changed after the introduction of combined antiretroviral therapy.18,26 In lymphoid tissue damaged by HIV infection, CD4+ T-cell repopulation occurs during treatment with HAART.18,26 This treatment can induce sustained recovery of CD4 T-cell reactivity against opportunistic pathogens,18,26 reducing the incidence of opportunistic infections. As γδ T-cell anergy was found to be independent from plasma viral load in HIV patients, the recovery of γδ T-cell function by HAART may be consequent to the overall immune restoration. It has been previously shown that γδ T-cell anergy in HIV-infected patients may depend on CD4 T-cell functions. Specifically, allogeneic CD4 T cells from healthy donors were shown to restore γδ T-cell functions in HIV-infected persons.13 Accordingly, we observed that in vitro CD45RA depletion results in γδ T-cell hyporesponsiveness, showing that IL-2 must be provided by CD45RA helper cells for γδ T-cell activation. Thus, γδ T-cell restoration after HAART may be consequent to the recovery of helper functions mediated by naive T cells.
Altogether, γδ T-cell distribution and functional reactivity was specifically affected in HIV-OIC subjects, resulting in the functional inability to secrete IFN-γ upon specific stimulation with non-peptidic microbial antigens. These observations indicate that γδ T cells are significantly affected during symptomatic HIV infection. Interestingly, the HAART-induced recovery of naive T cells can extend its effects on γδ T-cell responsiveness to non-peptidic microbial antigens. Thus, analysis of γδ T-cell reactivity may be useful for evaluating changes in immune function during HAART.
Acknowledgments
This work was supported by grants from the Current Research Project of the Italian Ministry of Health, from the CNR-UNESCO Project on AIDS and the EU Copernicus Project. A. A. was supported by an ISS-AIDS fellowship and by MURST COFIN98. We thank Dr G. D'Offizi and Dr E. Girardi for helpful discussions and F. Polucci for technical assistance.
References
- 1.Poccia F, Gougeon ML, Bonneville M, et al. Innate T-cell immunity to nonpeptidic antigens. Immunol Today. 1998;19:253. doi: 10.1016/s0167-5699(98)01266-3. [DOI] [PubMed] [Google Scholar]
- 2.Parker CM, Groh V, Band H, et al. Evidence for extrathymic changes in the T cell receptor gamma/delta repertoire. J Exp Med. 1990;171:1597. doi: 10.1084/jem.171.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Follows GA, Munk ME, Gatrill AJ, Conradt P, Kaufmann SH. Gamma interferon and interleukin 2, but not interleukin 4, are detectable in gamma/delta T-cell cultures after activation with bacteria. Infect Immun. 1992;60:1229. doi: 10.1128/iai.60.3.1229-1231.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poccia F, Cipriani B, Vendetti S, et al. CD94/NKG2 inhibitory receptor complex modulates both anti-viral and anti-tumoral responses of polyclonal phosphoantigen-reactive V gamma V delta 2 T lymphocytes. J Immunol. 1997;159:6009. [PubMed] [Google Scholar]
- 5.Bukowski JF, Morita CT, Brenner MB. Recognition and destruction of virus-infected cells by human gamma delta CTL. J Immunol. 1994;153:5133. [PubMed] [Google Scholar]
- 6.Wallace M, Malkovsky M, Carding SR. Gamma/delta T lymphocytes in viral infections. J Leukoc Biol. 1995;58:277. doi: 10.1002/jlb.58.3.277. [DOI] [PubMed] [Google Scholar]
- 7.Poccia F, Malkovsky M, Gougeon ML, et al. Gammadelta T cell activation or anergy during infections: the role of nonpeptidic TCR ligands and HLA class I molecules. J Leukoc Biol. 1997;62:287. doi: 10.1002/jlb.62.3.287. [DOI] [PubMed] [Google Scholar]
- 8.Autran B, Triebel F, Katlama C, Rozenbaum W, Hercend T, Debre P. T cell receptor gamma/delta+ lymphocyte subsets during HIV infection. Clin Exp Immunol. 1989;75:206. [PMC free article] [PubMed] [Google Scholar]
- 9.De Paoli P, Gennari D, Martelli P, et al. A subset of gamma delta lymphocytes is increased during HIV-1 infection. Clin Exp Immunol. 1991;83:187. doi: 10.1111/j.1365-2249.1991.tb05612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Maria A, Ferrazin A, Ferrini S, Ciccone E, Terragna A, Moretta L. Selective increase of a subset of T cell receptor gamma delta T lymphocytes in the peripheral blood of patients with human immunodeficiency virus type 1 infection. J Infect Dis. 1992;165:917. doi: 10.1093/infdis/165.5.917. [DOI] [PubMed] [Google Scholar]
- 11.Boullier S, Cochet M, Poccia F, Gougeon ML. CDR3-independent gamma delta V delta 1+ T cell expansion in the peripheral blood of HIV-infected persons. J Immunol. 1995;154:1418. [PubMed] [Google Scholar]
- 12.Poccia F, Boullier S, Lecoeur H, et al. Peripheral V gamma 9/V delta 2 T cell deletion and anergy to nonpeptidic mycobacterial antigens in asymptomatic HIV-1-infected persons. J Immunol. 1996;157:449. [PubMed] [Google Scholar]
- 13.Weshch D, Kabelitz D, Friese K, Pechhold K. Mycobacteria-reactive gamma delta T cells in HIV-infected individuals: lack of V gamma 9 cell responsiveness is due to deficiency of antigen-specific CD4 T helper type 1 cells. Eur J Immunol. 1996;26:557. doi: 10.1002/eji.1830260309. [DOI] [PubMed] [Google Scholar]
- 14.Wallace M, Bartz SR, Chang WL, Mackenzie DA, Pauza CD, Malkovsky M. Gamma delta T lymphocyte responses to HIV. Clin Exp Immunol. 1996;103:177. doi: 10.1046/j.1365-2249.1996.d01-625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace M, Scharko AM, Pauza CD, et al. Functional gamma delta T-lymphocyte defect associated with human immunodeficiency virus infections. Mol Med. 1997;3:60. [PMC free article] [PubMed] [Google Scholar]
- 16.Poccia F, Wallace M, Colizzi V, Malkovsky M. Possible protective and pathogenic roles of gamma delta T lymphocytes in HIV-infections. Int J Mol Med. 1998;1:409. doi: 10.3892/ijmm.1.2.409. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka Y, Morita CT, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature. 1995;375:155. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 18.Li TS, Tubiana R, Katlama C, Calvez V, Ait Mohand H, Autran B. Long-lasting recovery in CD4 T-cell function and viral-load reduction after highly active antiretroviral therapy in advanced HIV-1 disease. Lancet. 1998;351:1682. doi: 10.1016/s0140-6736(97)10291-4. [DOI] [PubMed] [Google Scholar]
- 19.Groh V, Porcelli S, Fabbi M, et al. Human lymphocytes bearing T cell receptor gamma/delta are phenotypically diverse and evenly distributed throughout the lymphoid system. J Exp Med. 1989;169:1277. doi: 10.1084/jem.169.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science. 1998;279:1737. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 21.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halary F, Peyrat MA, Champagne E, et al. Control of self-reactive cytotoxic T lymphocytes expressing gamma delta T cell receptors by natural killer inhibitory receptors. Eur J Immunol. 1997;27:2812. doi: 10.1002/eji.1830271111. [DOI] [PubMed] [Google Scholar]
- 23.Ferrarini M, Heltai S, Toninelli E, Sabbadini MG, Pellicciari C, Manfredi AA. Daudi lymphoma killing triggers the programmed death of cytotoxic V gamma 9/V delta 2 T lymphocytes. J Immunol. 1995;154:3704. [PubMed] [Google Scholar]
- 24.Bukowski JF, Morita CT, Band H, Brenner MB. Crucial role of TCR gamma chain junctional region in prenyl pyrophosphate antigen recognition by gamma delta T cells. J Immunol. 1998;161:286. [PubMed] [Google Scholar]
- 25.Garcia VE, Jullien D, Song M, et al. IL-15 enhances the response of human gamma delta T cells to nonpeptide microbial antigens. J Immunol. 1998;160:4322. [PubMed] [Google Scholar]
- 26.Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]