Abstract
In the armoury of the immune system developed to combat the various micro-organisms that could invade the host, the neutrophil forms the first line of defence against rapidly dividing bacteria and fungi. However, as humans age they become more susceptible to infection with these microbes and this has been ascribed to a decline in immune status, termed immune senescence. Here we summarize the literature specifically concerning the attenuation of neutrophil function with age and the possible mechanisms underlying their reduced response to infectious agents.
Introduction
As we enter the third millennium, one of the greatest challenges facing health care providers is the consequence of a falling birth rate and the steady increase in adult lifespan. If current trends continue, by the year 2025 one in five of the population in the Western world will be over 65 years of age.1,2 However, whilst life expectancy for people living in the UK is now 79 years for women and 74 years for men, the age at which good health can be expected to continue is ≈ 10 years less. In particular, the elderly suffer a higher morbidity and mortality from infectious diseases and it is now accepted that compromised immune function is a primary cause of increased disease risk in the elderly.3 Much research effort is now focused on identifying age-related changes in immune function3–5 in the hope of developing intervention strategies to delay or prevent immune senescence. To ensure that any changes to the immune system identified are related to normal ageing and are not secondary to illness or chronic disease, only healthy elderly subjects meeting the immunogerontological criteria of the SENIEUR protocol6 should be used. Indeed, much of the early literature concerning ageing and the immune system cannot be readily interpreted because of concerns over the health status of the elderly subjects used.
Defence against infectious disease consists of adaptive immune responses, involving T and B lymphocytes, and innate immunity, mediated by phagocytic cells, cytotoxic natural killer (NK) cells, cytokines and complement. Functional decline in the adaptive immune response with increasing age is already well characterized.3 For example, aged humans have a diminished ability to generate high-affinity antibodies after immunization7 and CD4+ T-cell populations of aged humans show a shift from naïve to memory or primed cells,8 resulting in decreased response to new antigen challenge. There is also an increase in T cells with a T helper 2 (Th2) cytokine profile upon stimulation, relative to T helper 1 (Th1), in the elderly, and the production of proinflammatory cytokines by monocytes is also raised,6,9 both of which will influence the host response to specific infectious agents. However, the innate immune system, more specifically neutrophils, respond most rapidly to infection and play a crucial role in the early days of an infection by phagocytosing and killing invading microbes. Despite the fact that neutrophil function does decline with age and will be a significant factor in immune senescence, there is relatively little known of the molecular basis of this loss of function. This article reviews our current understanding of immune senescence in the neutrophil and suggests areas where further study is now required.
AGE and NEUTROPHIL PRODUCTION
Neutrophils mediate the immediate host response to bacterial and fungal infections, which are largely responsible for the higher rates of mortality and morbidity in the elderly population.10 Vulnerability to infection in the elderly could result from an age-related decline either in neutrophil supply and/or functional efficiency. Neutrophils are short-lived (half-life 12–18 hr), postmitotic granulocytic cells that are produced in vast numbers (1–2 × 1011 per day) in the bone marrow. Haemopoiesis is a tightly regulated process controlled by chemokines,11 growth factors such as interleukin (IL)-3 and lineage-specific cytokines, specifically granulocyte–colony-stimulating factor (G-CSF) and granulocyte–macrophage colony-stimulating factor (GM-CSF) in the case of neutrophils.12 Several studies have shown that neutrophil numbers in the blood12,13 and neutrophil precursors in the marrow12 are not lowered in the healthy elderly, although the proliferative response of neutrophil precursor cells to G-CSF was reduced.12 As responses to GM-CSF and IL-3 were not affected by age,12 the altered response to G-CSF is unlikely to affect the ability of the elderly to maintain normal neutrophil numbers. However, during periods of severe, chronic infection, neutropenia can arise in the elderly14 and this could be caused, in part, by a blunted response to G-CSF.12 If responsiveness of neutrophil progenitors to GM-CSF is, however, retained in the elderly, then this could provide a useful short-term therapy for neutropenia during chronic infection. Whether the reduced responsiveness of neutrophil progenitors to G-CSF is caused by a decrease in receptor number/affinity or altered intracellular signalling is not known and warrants further study.
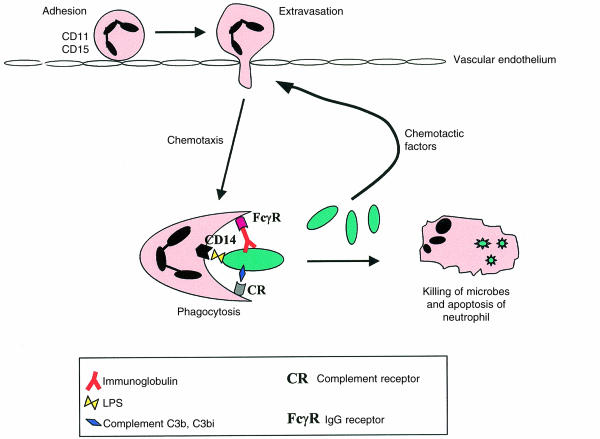
Owing possibly to the difficulty of obtaining bone marrow from healthy elderly subjects, a majority of studies concerning ageing and neutrophil status have considered mature neutrophil function. The initial response of neutrophils to infection (Fig. 1) is initiated by their recruitment from peripheral blood along a gradient of chemotactic factors, including complement components (C5a) and bacterial products (e.g. N-formyl-methionyl-leucyl-phenylalanine [FMLP]), produced at the site of infection. Later on in the immune response, recruitment also involves chemokines and proinflammatory cytokines produced by neutrophils and other inflammatory cells. Binding of chemotactic factors induces adhesion to vascular endothelial cells and migration into the affected tissue, a process known as margination. At the site of infection neutrophils attach to the infecting microbes via receptors for bacterial components (i.e. lipopolysaccharide [LPS]), the opsonizing molecules complement C3b and C3bi, and immunoglobulin G (IgG), leading to phagocytosis. Ingested microbes are killed by processes involving degranulation, which releases lytic enzymes, and the generation of reactive oxygen species, including superoxide. The neutrophil then dies by apoptosis. The effect of ageing on each of these aspects of neutrophil function has been investigated, although in many cases the data are sparse and often contradictory.
Figure 1.
Neutrophil response to infection. The figure shows the adhesion of neutrophils to vascular endothelium, which is mediated by selectins and β2-integrins, leading to extravasation from blood into the infected tissue. Neutrophils then migrate towards the microbe along a chemotactic gradient, composed of bacterial products and activated complement molecules. The pathogenic organism is ingested by phagocytosis, which is stimulated by bacterial membrane components, complement and/or immunoglobulins, that are recognized by receptors on the neutrophil. Once ingested the microbes are killed by lytic enzymes and reactive oxygen species, and the neutrophil dies by apoptosis. LPS, lipopolysaccharide.
Chemotaxis and adhesion
In vitro studies of chemotaxis have found migratory responses of neutrophils from healthy elderly subjects to be either unaltered2,15 or only slightly reduced.16,17 In order to leave the circulation, neutrophils roll along and then adhere to vascular endothelial cells, predominantly at the intersection of three endothelial cells.18 Rolling and adhesion are mediated by the sequential interaction of selectins, which initially tether the neutrophil to endothelium, and β2-integrins, which induce rapid (mediated by lymphocyte function-associated antigen-1 [LFA-1]/CD11a) and sustained (mediated by Mac-1/CD11b) adhesion.18 Adhesion assays with neutrophils from elderly subjects have demonstrated increased adhesion to nylon fibres,16 whereas no alteration in adhesion to endothelium was detected.2,19 Measurements of adhesion molecule expression also suggest the lack of any negative effect of ageing on neutrophil recruitment processes. The level of neutrophil CD15, which binds to E-selectin on endothelium and mediates neutrophil rolling, was slightly increased with donor age,19 CD11a was found to be unaltered19 and CD11b has been reported as either unaltered20 or slightly increased.19 Taken together, these data suggest that reduced extravasation and recruitment of neutrophils are not major factors contributing to increased risk of infection in the elderly.
Phagocytosis
Phagocytosis is initiated by the interaction of specific receptors on the surface of the neutrophil with particulate ligands on the microbe. In the presence of circulating antibodies to microbial antigens, the key receptors inducing phagocytosis are those for the Fc region of immunoglobulin (FcγRIII/CD16 and FcγRII/CD32), and for the complement molecules C3b (CD35/CR1) and C3bi (CD11b/CR3), all of which are bound to the surface of the infecting microbe. CD35 enhances phagocytosis induced by antibody, but cannot act independently of antibody. However, neutrophils can phagocytose bacteria in the absence of antibody, which is crucial in the early phase of infection and if the host has not encountered the pathogen previously. In these situations phagocytosis can be stimulated through CD11b and CD11c, which bind complement but also recognize bacterial surface molecules, such as LPS, and other specific receptors for LPS and lipoprotein such as CD14 and bactericidal/permeability-increasing protein (BPI). BPI is expressed exclusively in neutrophils and binds LPS, thus promoting the uptake and killing of Gram-negative bacteria.21
The data concerning phagocytic ability of neutrophils from the elderly are reasonably consistent. Studies measuring phagocytosis of opsonized bacteria or yeast and opsonized zymosan as the neutrophil target have all shown a significant reduction in phagocytic ability in the elderly.22–24 The reduced response of neutrophils to Staphylococcus aureus24 is of particular clinical importance bearing in mind the increased susceptibility to this pathogen in elderly subjects.10 The few studies that have not found decreased phagocytosis have in the main used targets either not opsonized with complement and antibody23 or coated only with antibody.25,26 Adequate recruitment of neutrophils to sites of infection may thus be offset by reduced phagocytic capacity and result in the poor resolution of infection seen so frequently in the elderly.
The molecular basis of reduced phagocytosis in neutrophils from elderly subjects is not known. However, Emmanuelli et al. showed that neutrophil phagocytosis of unopsonized bacterial targets occurred at the same low level in young and old subjects.23 Their data suggest that the receptors for innate recognition of bacterial components (BPI, CD14) are not affected by ageing and, moreover, confirm that they do not contribute significantly to phagocytosis in the absence of complement and antibody. The reduced response of neutrophils to opsonized targets suggests that the relevant receptors may be present at a reduced level or have compromised signalling function, particularly as serum immunoglobulin and complement levels are within the normal range in the elderly.2 Levels of CD11b and CD11c, as stated above, are essentially unaltered in the elderly, and there are no published data concerning expression of CD35. There are also no reports describing the effect of age on the expression of Fcγ receptors, although our own studies show a significant decline in CD16 levels with age (S. Butcher et al., unpublished). Attenuated Fc-mediated phagocytosis may thus prove to be a significant factor in reduced microbicidal function in the elderly19 and deserves further investigation. Furthermore, there are no published data directly relating to the effect of ageing on Fc receptor signals that induce phagocytosis. Fc receptors are tyrosine kinase linked, with ligation resulting in activation of the src family kinase, Syk.27 Fc-induced phagocytsosis involves subsequent activation of phospholipase D (PLD) leading to the generation of diacylglycerol and activation of protein kinase C-δ and Erk2. Erk2 phosphorylates and activates myosin light-chain kinase leading to the formation of pseudopods and phagocytosis.28 Interestingly, activation of PLD following ligation of FMLP receptors has been shown to be normal in neutrophils from elderly donors.29,30 However, these data cannot be extrapolated to Fc receptors because FMLP receptors recruit and activate signalling enzymes via distinct G protein-linked mechanisms. Further studies are clearly required to establish any effect of normal ageing on Fc-receptor signalling.
Microbicidal mechanisms
Microbial killing following phagocytosis is achieved through two basic processes: the generation of reactive oxygen species with a broad range of antimicrobial properties, including superoxide which is produced by the enzyme NADPH oxidase; and degranulation of cytoplasmic granules, which contain a variety of cytotoxic proteases. In the case of Gram-negative bacteria, BPI also contributes to killing by increasing permeability of the bacterial cell wall and inducing lysis.21 Neutrophil microbicidal activity has been examined by several groups and although data are often conflicting31,32 a majority support a decline in cytotoxicity towards bacteria and yeast with age.16,19,26,33,34 What is not clear from these basic assays is whether the observed reduction in microbicidal function with age is additional to the decline in phagocytic capacity, or occurs secondary to this effect. Analysis of individual microbicidal processes addresses this question and suggests that superoxide responses to Fc ligation are reduced per se with age.26
Several authors have reported normal superoxide production by neutrophils in response to FMLP,19,31 whilst others have shown reduced responses.35,36 Activation of PLD by FMLP receptor ligation leads to production of phosphatidic acid, which is able to activate NADPH oxidase.37 As FMLP-induced activation of PLD is not affected by age,29,30 this supports those studies reporting a normal neutrophil superoxide response in the elderly. In contrast to the data regarding FMLP, studies concerning superoxide generation in response to particulate stimuli consistently report a reduced response to these agents in the elderly. In a recent publication by Wenisch et al.,24 superoxide generation was decreased in response to S. aureus, but not to Escherichia coli, an observation with particular clinical relevance bearing in mind the reduced ability of the elderly to resolve infection by Gram-positive bacteria. It is possible that neutrophil superoxide responses to E. coli involving binding of LPS by CD1438 may be unaffected by age, whilst responses to Gram-positive bacteria such as S. aureus, which are more dependent upon complement and Fc-receptor ligation, are attenuated. Indeed, the Fc receptor-mediated superoxide response has already been shown to be significantly reduced in the elderly,26 again identifying attenuation of Fc-mediated responses as a significant factor in age-related functional decline.
Neutrophil priming and apoptosis
Neutrophil responses to microbes and soluble microbial products (FMLP) at the site of infection are optimized by agents that prime the neutrophil in the circulation and during margination. The major priming agents are proinflammatory cytokines such as IL-8 and GM-CSF, and a reduction in the priming process would significantly affect neutrophil efficiency. Seres et al.39 have shown that neutrophils from elderly volunteers were not primed as efficiently by GM-CSF as those from young subjects. Although only a single report, these data suggest that lack of responsiveness to GM-CSF could affect neutrophil function at sites of infection. In addition, neutrophils are short-lived leucocytes with a half-life in the circulation of ≈ 12–18 hr. Effete neutrophils die by apoptosis40 and extension of their lifespan contributes significantly to the accumulation of functional neutrophils at sites of infection. Neutrophil apoptosis can be delayed by antiapoptotic signals provided by a variety of factors including: proinflammatory cytokines (GM-CSF, IL-8), complement C5a and bacterial components, such as LPS.41 The rates of spontaneous and Fas-induced neutrophil apoptosis are normal in the elderly,42,43 but the ability of GM-CSF, G-CSF and LPS to delay neutrophil apoptosis was found to be significantly reduced.43 These data predict that, compared with their younger counterparts, neutrophils from elderly donors will respond less well to infectious stimuli upon recruitment to the site of infection and will also die more rapidly, blunting the neutrophilia response to infection. Further studies are now required to establish whether neutrophil senescence might be related to their attenuated response to proinflammatory cytokines.
Neutrophil functional decline: nature versus nurture
Neutrophil function is adversely affected by ageing. An important question that remains, the answer to which will influence attempts at therapeutic intervention to prevent functional decline, is whether this arises in the progenitor population during haemopoiesis, or in the circulation, possibly under the influence of cytokines. One of the dominant theories of ageing, the soma theory,44 suggests that replicative cells accumulate genetic damage, resulting in functional decline. Arguing against this proposal, in the case of neutrophil functional decline, are the data showing that the proliferative and differentiation capacity of haemopoietic progenitor cells is largely unaffected by ageing.12,13 However, the ability of neutrophil progenitors to proliferate and differentiate in response to haemopoietic factors does not necessarily guarantee that the resulting neutrophils are competent. Inflammation and infection increase the rate of neutrophil production, shortening the maturation time of progenitors and leading to the release of immature neutrophils in to the circulation. Studies of peripheral blood neutrophils in the elderly during neutrophilia would be informative as they would indicate whether immature neutrophils already exhibit reduced phagocytic and microbicidal functions.
Age-related changes in neutrophil function may, however, develop in the circulation. A variety of cytokines, particularly proinflammatory cytokines, are able to modify neutrophil biology and function. As stated above, GM-CSF is able to prime neutrophils for response to FMLP and extend their lifespan by delaying apoptosis. However, continued exposure to GM-CSF can also induce shedding of CD16 from the surface of neutrophils,45 which could modify their phagocytic and microbicidal ability. If cytokines such as GM-CSF are present in the circulation of elderly subjects, in the absence of infection and chemotactic agents, this could lead to reduced CD16 and concomitant reduction in the response to opsonizing immunoglobulins and immune complexes when infection does occur. To date there is little information on the levels of cytokines in the circulation of healthy elderly subjects, although monocyte function is increased in the elderly, with higher levels of IL-1, IL-6, IL-8 and tumour necrosis factor-α (TNF-α) production in response to LPS (reviewed in 5). In addition, exposure of neutrophils to proinflammatory cytokines in the circulation of healthy elderly subjects has been suggested by studies showing elevated CD11b2,19 and an increase in the fraction of neutrophils responding to FMLP.19 Further studies in this area would help to determine whether the basal level of circulating cytokines is altered with age and might contribute to neutrophil functional decline.
SUMMARY and FUTURE PERSPECTIVES
The data published so far on the decline of neutrophil function in the elderly are conflicting in many instances and lacking in information on the mechanisms of functional decline with age. However, some consistent observations can be made, including a decline in phagocytic capacity and bactericidal mechanisms, particularly those initiated by contact with opsonized yeast and Gram-positive bacteria such as S. aureus. As phagocytosis is the primary mechanism by which elimination of extracellular micro-organisms is initiated, identification of the molecular basis of phagocytic decline is clinically relevant and may lead to intervention strategies to improve immune status in the elderly. Calcium mobilization,4 actin polymerization20 and GM-CSF39 mediated signals are all reduced in neutrophils from the elderly, supporting the proposal that intracellular defects may underlie neutrophil immune senescence. Whether these altered responses arise in neutrophil progenitors, or reflect the influence of cytokines on mature neutrophils in the circulation, is not yet known. This information will be required if we are to develop therapeutic strategies to prevent neutrophil functional senescence in the aged. The ultimate reward of such endeavours will be an improved quality of life, rather than extension of lifespan, for those in the third age of humans – in other words, a good life and a short death!
Acknowledgments
S. B. and H. C. are supported by a grant from the Biotechnology and Biological Science Research Council (BBSRC) as part of the Science of Ageing (SAGE) initiative into normal human ageing.
References
- 1.Ferguson M, editor. Technology Foresight Panel on Health and Life Sciences. Progress Through Partnership Technology Foresight 4. London: HMSO; 1995. [Google Scholar]
- 2.MacGregor RR, Shalit M. Neutrophil function in healthy elderly subjects. J Gerontol. 1990;45:M55. doi: 10.1093/geronj/45.2.m55. [DOI] [PubMed] [Google Scholar]
- 3.Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 4.Fulop T. Signal transduction changes in granulocytes and lymphocytes with ageing. Immunol Lett. 1994;40:259. doi: 10.1016/0165-2478(94)00064-6. [DOI] [PubMed] [Google Scholar]
- 5.Rink L, Cakman I, Kirchner H. Altered cytokine production in the elderly. Mech Ageing Dev. 1998;102:199. doi: 10.1016/s0047-6374(97)00153-x. [DOI] [PubMed] [Google Scholar]
- 6.Ligthart GJ, Corberand JX, Fournier C, Galanaud P, Hijmans W, Kennes B, Muller-Hermelink HK, Steinmann GG. Admission criteria for immunogerontological studies in man: the Senieur-protocol. Mech Ageing Dev. 1984;28:47. doi: 10.1016/0047-6374(84)90152-0. [DOI] [PubMed] [Google Scholar]
- 7.Hodes RJ. Aging and the immune system. Immunol Rev. 1997;160:5. doi: 10.1111/j.1600-065x.1997.tb01022.x. [DOI] [PubMed] [Google Scholar]
- 8.Xu X, Beckman I, Ahern M, Bradley J. A comprehensive analysis of peripheral blood lymphocytes in healthy aged humans by flow cytometry. Immunol Cell Biol. 1993;71:549. doi: 10.1038/icb.1993.61. [DOI] [PubMed] [Google Scholar]
- 9.Paganelli R, Scala E, Rosso R, et al. A shift to Th0 cytokine production by CD4+ cells in human longevity: studies on two healthy centenarians. Eur J Immunol. 1996;26:2030. doi: 10.1002/eji.1830260910. [DOI] [PubMed] [Google Scholar]
- 10.Schneider EC. Infectious disease in the elderly. Ann Int Med. 1983;98:395. doi: 10.7326/0003-4819-98-3-395. [DOI] [PubMed] [Google Scholar]
- 11.Reid S, Ritchie A, Boring L, Gosling J, Cooper S, Hangoc G, Charo I, Broxmeyer HE. Enhanced myeloid progenitor cell cycling and apoptosis in mice lacking the chemokine receptor CCR2. Blood. 1999;93:1524. [PubMed] [Google Scholar]
- 12.Chatta GS, Andrews RG, Rodger E, Schrag M, Hammond WP, Dale DC. Hematopoietic progenitors and aging: alterations in granulocyte precursors and responsiveness to recombinant human G-CSF, GM-CSF and IL-3. J Gerontol. 1993;48:M207. doi: 10.1093/geronj/48.5.m207. [DOI] [PubMed] [Google Scholar]
- 13.Born J, Uthgenannt D, Dodt C, Nunninghoff D, Ringvolt E, Wagner T, Fehm H-L. Cytokine production and lymphocyte subpopulations in aged humans. An assessment during nocturnal sleep. Mech Ageing Dev. 1995;84:113. doi: 10.1016/0047-6374(95)01638-4. [DOI] [PubMed] [Google Scholar]
- 14.Finkelstein M, Petkun W, Friedman M. Pneumococcal bacteremia in the elderly. J Am Geriatr Soc. 1983;31:19. doi: 10.1111/j.1532-5415.1983.tb06283.x. [DOI] [PubMed] [Google Scholar]
- 15.Phair JP, Kauffman CA, Bjornson A, Gallagher J, Adams L, Hess EV. Host defense in the aged: evaluation of components of the inflammatory and immune responses. J Infect Dis. 1978;138:67. doi: 10.1093/infdis/138.1.67. [DOI] [PubMed] [Google Scholar]
- 16.Corberand J, Ngyen F, Laharrague P, Fontanilles AM, Gleyzes B, Gyrard E, Senegas C. Polymorphonuclear functions and aging in humans. J Am Geriatr Soc. 1981;29:391. doi: 10.1111/j.1532-5415.1981.tb02376.x. [DOI] [PubMed] [Google Scholar]
- 17.McLaughlin B, O'Malley K, Cotter TG. Age-related differences in granulocyte chemotaxis and degranulation. Clin Sci. 1986;70:59. doi: 10.1042/cs0700059. [DOI] [PubMed] [Google Scholar]
- 18.Mollinedo F, Borregaard N, Boxer LA. Novel trends in neutrophil structure, function and development. Immunol Today. 1999;20:535. doi: 10.1016/s0167-5699(99)01500-5. [DOI] [PubMed] [Google Scholar]
- 19.Esperaza B, Sanchez M, Ruiz M, Barranquero M, Sabino E, Merino F. Neutrophil function in elderly persons assessed by flow cytometry. Immunol Invest. 1996;25:185. doi: 10.3109/08820139609059301. [DOI] [PubMed] [Google Scholar]
- 20.Rao KMK. Age-related decline in ligand-induced actin polymerisation in human leukocytes and platelets. J Gerentol. 1986;41:561. doi: 10.1093/geronj/41.5.561. [DOI] [PubMed] [Google Scholar]
- 21.Horwitz AH, Williams RE, Liu PS, Nadell R. Bactericidal/permeability increasing protein inhibits growth of a strain of Acholeplasma lidlawii and L forms of gram-positive bacteria Staphylococcus aureus and Staphylococcus pyogenes. Antimicrob Agents Chemother. 1999;43:2314. doi: 10.1128/aac.43.9.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mege JL, Capo C, Michel B, Gastaut JL, Bongrand P. Phagocytic cell function in aged subjects. Neurobiol Aging. 1988;9:217. doi: 10.1016/s0197-4580(88)80054-x. [DOI] [PubMed] [Google Scholar]
- 23.Emmanuelli G, Lanzio M, Anfossi T, Romano S, Anfossi G, Calamuggi G. Influence of age on polymorphonuclear leukocytes in vitro: phagocytic activity in healthy human subjects. Gerentology. 1986;32:308. doi: 10.1159/000212809. [DOI] [PubMed] [Google Scholar]
- 24.Wenisch C, Patruta S, Daxbock F, Krause R, Horl W. Effect of age on human neutrophil function. J Leukoc Biol. 2000;67:40. doi: 10.1002/jlb.67.1.40. [DOI] [PubMed] [Google Scholar]
- 25.Horan MA, Gulati RS, Fox RA. Assessment of neutrophil function in the elderly using antibody coated polyacrylamide gel (immunobeads) and nitroblue tetrazolium (NBT) reduction as a combined test. Mech Ageing Dev. 1985;29:29. doi: 10.1016/0047-6374(85)90044-2. [DOI] [PubMed] [Google Scholar]
- 26.Fulop T, Foris G, Worum I, Leovey A. Age-dependent alterations of Fcγ receptor-mediated effector functions of human polymorphonuclear leucocytes. Mech Ageing Dev. 1985;29:1. [PMC free article] [PubMed] [Google Scholar]
- 27.Daeron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 28.Suchard SJ, Hinkovska-Galcheva V, Mansfield PJ, Boxer LA, Shayman JA. Ceramide inhibits IgG-dependent phagocytosis in human polymorphonuclear leucocytes. Blood. 1997;89:2139. [PubMed] [Google Scholar]
- 29.Lipschitz DA, Udupa KB, Indelicato SR, Das M. Effect of age on second messenger generation in neutrophils. Blood. 1991;78:1347. [PubMed] [Google Scholar]
- 30.Fulop T, Varga ZS, Csongor J, Foris G, Cestaro B. Age related impairment in phosphatidylinositol breakdown of polymorphonuclear granulocytes. FEBS Lett. 1989;245:249. doi: 10.1016/0014-5793(89)80231-5. [DOI] [PubMed] [Google Scholar]
- 31.Corberand JX, Laharrague PF, Fillola G. Neutrophils in healthy aged humans are normal. Mech Ageing Dev. 1986;36:57. doi: 10.1016/0047-6374(86)90138-7. [DOI] [PubMed] [Google Scholar]
- 32.Lipschitz DA, Odupa RB, Milton RY, Thompson CO. effect of age on hematopoiesis in man. Blood. 1984;63:502. [PubMed] [Google Scholar]
- 33.Fulop T, Komaromi I, Foris G, Worum I, Leovey A. Age-dependent variations of intra-lysosomal release from human PMN leukocytes under various stimuli. Immunobiology. 1986;171:302. doi: 10.1016/S0171-2985(86)80011-0. [DOI] [PubMed] [Google Scholar]
- 34.Lipschitz DA, Udupa KB, Boxer LA. The role of calcium in the age-related decline of neutrophil function. Blood. 1988;71:659. [PubMed] [Google Scholar]
- 35.Braga PC, Sala MT, Dal Sasso M, Mancini L, Sandrini MC, Annoni G. Influence of age on oxidative bursts (chemiluminescence) of polymorphonuclear neutrophil leukocytes. Gerontology. 1997;44:192. doi: 10.1159/000022009. [DOI] [PubMed] [Google Scholar]
- 36.Peveri P, Curnutte JT. Phosphatidic acid may be a physiological activator of human neutrophil NADPH oxidase. Blood. 1990;76:190. [Google Scholar]
- 37.Soler-Rodriguez AM, Zhang HW, Lichenstein HS, Qureshi N, Niesel DW, Crowe SE, Peterson JW, Klimpel GR. Neutrophil activation by bacterial lipoprotein versus lipopolysaccharide: differential requirements for serum and CD14. J Immunol. 2000;164:2674. doi: 10.4049/jimmunol.164.5.2674. [DOI] [PubMed] [Google Scholar]
- 38.Seres I, Csongor J, Mohacsi A, Leovey A, Fulop T. Age-dependent alterations of human recombinant GM-CSF effects on human granulocytes. Mech Ageing Dev. 1993;71:143. doi: 10.1016/0047-6374(93)90042-p. [DOI] [PubMed] [Google Scholar]
- 39.Haslett C. Resolution of acute inflammation and the role of apoptosis in the tissue fate of granulocytes. Clin Sci. 1992;83:639. doi: 10.1042/cs0830639. [DOI] [PubMed] [Google Scholar]
- 40.Lee A, Whyte MKB, Haslett C. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J Leukoc Biol. 1993;54:283. [PubMed] [Google Scholar]
- 41.Tortorella C, Piazzolla G, Spaccavento F, Pece S, Jirillo E, Antonaci S. Spontaneous and Fas-induced apoptotic cell death in aged neutrophils. J Clin Immunol. 1998;18:321. doi: 10.1023/a:1023286831246. [DOI] [PubMed] [Google Scholar]
- 42.Fulop T, Fouquet C, Allaire P, et al. Changes in apoptosis of human polymorphonuclear granulocytes with aging. Mech Ageing Dev. 1997;96:15. doi: 10.1016/s0047-6374(96)01881-7. [DOI] [PubMed] [Google Scholar]
- 43.Kirkwood TBL. Human senescence. Bioessays. 1996;18:1009. doi: 10.1002/bies.950181211. [DOI] [PubMed] [Google Scholar]
- 44.Moulding DA, Hart CA, Edwards SW. Regulation of neutrophil Fc gamma RIIIb (CD16) surface expression following delayed apoptosis in response to GM-CSF and sodium butyrate. J Leukoc Biol. 1999;65:875. doi: 10.1002/jlb.65.6.875. [DOI] [PubMed] [Google Scholar]
- 45.Varga Z, Kovacs E, Paragh G, Jacob MP, Robert L, Fulop T. Effect of kappa elastin and fMLP on polymorphonuclear leukocytes of healthy middle-aged and elderly. K-elastin changes induced in intracellular free calcium. Clin Biochem. 1988;21:127. doi: 10.1016/s0009-9120(88)80101-2. [DOI] [PubMed] [Google Scholar]