Abstract
Homologous complement activation is restricted on cells by the complement regulators, decay-accelerating factor (DAF), membrane cofactor protein (MCP) and CD59. These proteins act in concert with other membrane structures to protect cells from homologous complement attack. In contrast, cells are usually sensitive to heterologous complement attack. It has been suggested that species-specific restriction of complement activation can be attributed to the inability of regulators to inhibit across species. We have investigated the capacities of human, rat and mouse analogues of DAF to regulate homologous and heterologous complement. Cells transfected with cDNA encoding these analogues were protected from heterologous complement attack. C3b-deposition experiments indicated that whilst cells were best protected by DAF from the same species, all three analogues inhibited human, rat and mouse complement. Comparable results were obtained in haemolysis assays using soluble, recombinant forms of the proteins. Inhibition of the classical pathway (CP) was best achieved with homologous DAF, although human DAF also inhibited rat complement, rat DAF also inhibited human complement and mouse DAF inhibited complement from all species. Human DAF was the best inhibitor of alternative pathway (AP)-mediated attack, inhibiting complement from all species. Mouse DAF inhibited mouse and rat AP, whilst rat DAF inhibited only rat AP. These data indicate that human and rodent analogues of DAF are not species restricted and highlights interesting differences in the capacity to regulate AP and CP. This has implications in broader fields of research, such as xenotransplantation, where cross-species regulation of complement is of paramount importance.
Introduction
Cells express on their surface several proteins which protect against complement (C) attack, namely C receptor 1 (CR1), decay-accelerating factor (DAF), membrane cofactor protein (MCP) and CD59.1 CR1, DAF and MCP regulate the activation pathways of C by either accelerating decay of the C3 and C5 convertase (CR1, DAF), or acting as cofactors for the serine protease factor I, which cleaves and irreversibly inactivates C3b (CR1, MCP). CD59 acts to inhibit the terminal pathway of C by binding to C8 during membrane attack complex (MAC) formation and preventing C9 polymerization. These membrane regulators together confer resistance against homologous C. Both CD59 and DAF are linked to the membrane by a glycosyl phosphatidylinositol (GPI) anchor. Treatment of cells with phosphatidylinositol-specific phospholipase C (PIPLC) removes GPI-anchored proteins, including DAF and CD59, and increases cell susceptibility to homologous C attack.2,3
The phenomenon of species restriction of C was first recognized in 1911 when it was demonstrated that human erythrocytes (E) were more difficult to lyse with human serum than with sera from other species.4 Examination of C-mediated lysis of E from different species using a panel of sera confirmed that lysis is least effective when the source of cell and serum are matched. Restriction is apparent, regardless of the pathway used to activate C, and is evident in the terminal pathway in addition to the activation pathways.5–7 Although it is clear that membrane C regulators are extremely important in protecting against homologous C, their capacity to regulate C from other species is less certain. For each of the membrane C regulators a role in the phenomenon of species restriction has been suggested. For example, early work suggested that CD59 was species specific in its action, interacting only with C8 or C9 from the same species.8–10 However, later work did not support these early studies and it is now clear that human CD59 and analogues from other species are not species specific in that each can inhibit a range of different sera.11,12 The role of DAF in species restriction has been studied largely by using antibodies to block DAF function and assessing alteration in cell susceptibility to lysis.2,3,13–15 In some cases, blockade of human DAF on E or nucleated cells enhanced lysis of cells by homologous C whilst having no effect on lysis by heterologous C, suggesting that DAF exhibited species selectivity. However, in several of these studies, blockade of DAF also enhanced lysis of cells by other heterologous sera, indicating that DAF was not truly species restricted. The pioneering studies of Hoffmann in 1969 also indicated that DAF was not species restricted: extracts of human E membranes containing decay-accelerating activity were shown to inhibit guinea-pig C.16
DAF analogues have recently been identified in rats and mice, although only basic functional analysis has been performed.17–19 It therefore was opportune to undertake an evaluation of the capacities of human and rodent DAF analogues to inhibit C across species barriers. We undertook a comprehensive analysis of the species specificity of the C-inhibitory activities of each of the DAF analogues. We examined the differential regulation of C in homologous and heterologous sera by human, rat and mouse DAF expressed by transfection on the surface of Chinese hamster ovary (CHO) cells. Fluid-phase recombinant forms of each DAF protein were generated and used to further analyse C regulation in homologous and heterologous sera in the classical and alternative pathways.
Materials and methods
Chemicals, reagents and buffers
Chemicals and reagents were from Sigma (Poole, Dorset, UK) or Fisher Scientific (Loughborough, Leicestershire, UK) unless otherwise stated below. All tissue culture reagents and plastics were from Life Technologies (Paisley, Strathclyde, UK). pDR2ΔEF1α was a gift from Dr I. Anegon (INSERM U437; Nantes, France)20 and Signal pIgplus was from R & D Systems (Abingdon, Oxford, UK). Sheep E in Alsever's solution were from TCS Microbiology (Claydon, Bucks., UK), guinea-pig E from Harlan Sera-Labs (Loughborough, Leicestershire, UK) and rabbit E were from the local animal facility. Zymosan A was from Sigma. Human serum was obtained by venepuncture from healthy volunteers; other sera were obtained from the local animal facility. Polyclonal rabbit anti-human CD59, rabbit anti-CHO and mouse anti-rabbit erythrocyte antibodies were raised in-house using standard techniques. Rabbit anti-sheep E (Amboceptor) was from Behring Diagnostics GmbH (Marburg, Germany), rabbit anti-rat immunoglobulin G conjugated to horseradish peroxidase (IgG-HRP) and goat anti-mouse IgG-HRP were purchased from BioRad Ltd (Hemel Hempstead, Herts., UK). Rabbit anti-rat IgG-fluorescein isothiocyanate (FITC) was from Sigma and goat anti-mouse IgG-phycoerythrin (PE) was purchased from DAKO Ltd (High Wycombe, Cambs., UK). Monoclonal anti-human DAF antibodies (BRIC110 and BRIC216) were from the International Blood Group Reference Laboratory (Bristol, Avon, UK). A mouse monoclonal antibody (mAb), C3/30, that recognizes C3b and inactive C3b (iC3b), but not native C3, was a gift from Novartis (Horsham, Surrey, UK).21 mAbs recognizing mouse DAF (3D5), rat DAF (RDIII-7) and human DAF (MBC1) were raised in this laboratory and were used for flow cytometry and Western blotting.22 Rat monoclonal anti-mouse C3 (RMC11H9) was obtained from Connex GmbH (Martinsried, Germany). Soluble, recombinant human C receptor 1 (sCR1) was a gift from T Cell Sciences Inc (Needham, MA), Prosep A was from Bioprocessing Ltd (Consett, Co. Durham, UK). Restriction enzymes were from Amersham (Little Chalfont, Bucks., UK), T4 DNA ligase from Promega (Southampton, Hants., UK), dNTPs from Bioline (London, UK) and Vent DNA polymerase from New England Biolabs Ltd (Hitchin, Herts., UK). Primers and other molecular biology reagents were from Life Technologies.
Phosphate-buffered saline (PBS) (8·1 mm Na2PO4, 1·5 mm KH2PO4, 137 mm NaCl, 2·7 mm KCl, pH 7·4) was from Oxoid Ltd (Basingstoke, Hants., UK). C-fixation diluent (CFD) (2·8 mm barbituric acid, 145·5 mm NaCl, 0·8 mm MgCl2, 0·3 mm CaCl2, 0·9 mm sodium barbital, pH 7·2) was from Oxoid Ltd. gelatin veronal buffer (GVB) was CFD containing 0·1% (w/v) gelatin. Alternative pathway buffer (APB) was 5 mm sodium barbital, pH 7·4, 150 mm NaCl, 7 mm MgCl2, 10 mm ethylene glycol-bis-(β-aminoethyl ether) N,N,N′,N′-tetraacetic acid (EGTA) and 0·1% (w/v) gelatin. Flow cytometry medium (FCM) was PBS containing 15 mm EDTA, 30 mm sodium azide and 1% (w/v) bovine serum albumin (BSA).
Transfection of CHO cells
CHO cells obtained from the European Collection of Animal Cell Cultures (ECACC; Salisbury, Wilts., UK) were transfected with the empty eukaryotic expression vector, pDR2ΔEF1α, or vector containing human, rat or mouse DAF cDNA, as described previously.22 In order to remove non-expressing cells, cultures were preincubated with 10 µg/ml of specific mAb and positive cells were selected under aseptic conditions using rabbit anti-mouse immunoglobulin-conjugated magnetic beads, according to the manufacturer's instructions (Dynabeads; Dynal, Oslo, Norway). Flow cytometric analysis indicated that non-expressing cells had been removed. In all cases only one round of magnetic selection was required to obtain uniform, high-level expression of DAF.
Analysis of DAF expression
Expression of DAF analogues was analysed by flow cytometry and Western blot. For flow cytometry, transfected CHO cells were harvested, disaggregated in PBS containing 10 mm EDTA and suspended to a final concentration of 106 cells/ml in FCM. Cells (105) were incubated with primary antibodies (10 µg/ml) for 30 min on ice, washed three times in FCM and incubated for 30 min at 4° with either PE-conjugated goat anti-mouse immunoglobulin or FITC-conjugated goat anti-rat immunoglobulin. Cells were washed three times in FCM and analysed on a fluorescence-activated cell sorter (FACScalibur; Becton-Dickinson, Oxford, UK). All studies were carried out in triplicate. For Western blot analysis, cell lysates (2 × 107 cells/ml of lysis buffer) were prepared and analysed, essentially as described previously.19 Blots were probed first with specific mAbs at 0·1 µg/ml in phosphate-buffered saline Tween (PBST)/milk for 1 hr at room temperature and then with a 1 : 1000 dilution of HRP-conjugated goat anti-mouse immunoglobulin or goat anti-rat immunoglobulin. The presence of bound HRP-conjugated secondary antibody was detected using enhanced chemiluminescence (ECL; Amersham) and X-ray film. No bands were detected on blots incubated with secondary antibody only.
Calcein lysis assay
Complement-mediated lysis of control cells or cells expressing DAF was assessed by calcein release as described previously with the following modifications.19 Cells were sensitized by incubation with serum-free Dulbecco's modified Eagle's minimal essential medium (DMEM) containing a specified dilution of heat-inactivated rabbit polyclonal antiserum raised against untransfected CHO cells. Sensitized cells were rinsed and incubated with rat or human serum (0·25 ml of a 20% dilution in CFD) for 1 hr at 37°. The per cent calcein release by serum was calculated and expressed as a percentage of total calcein loaded into the cells, as described previously.19 All conditions were assayed in triplicate.
C3-deposition assays
CHO cells expressing rat, mouse, or human GPI-anchored DAF or vector control cells were disaggregated in FCM. Cells (5 × 105 per incubation) were sensitized by incubation for 15 min at room temperature with an equal volume of different dilutions of heat-inactivated polyclonal rabbit anti-CHO antiserum, as indicated in the text. Cells were washed twice in PBS (800 g, 3 min, 4°), once in CFD and then resuspended in serum diluted in CFD. Cells were incubated with serum for 15 min at 37° (human serum) or 30 min at 37° (mouse serum or C6-deficient rat serum) and then washed three times with FCM. Cell-bound C3b/iC3b was detected using mouse monoclonal C3/30 (anti-human C3, which also detects rat C3) or rat monoclonal 11H9 (anti-mouse C3). Bound anti-C3 antibody was detected with either PE-conjugated goat anti-mouse immunoglobulin or FITC-conjugated rabbit anti-rat immunoglobulin. Samples were analysed on a Becton-Dickinson FACScalibur, as described above. Background levels were set using control cells that consisted of cells incubated with anti-C3 antibody but not exposed to C, and cells exposed to C but not incubated with primary antibody.
Generation and purification of DAF-Fc fusion proteins
Total RNA was prepared from mouse testis tissue or rat lung tissue using Ultraspec Total RNA Isolation Reagent (Biotecx Laboratories Inc., Houston, TX) and was reverse transcribed, according to standard protocols, using Superscript reverse transcriptase (Life Technologies) and oligo(dT) (CCAGTGAGCAGAGTGACGAGGACTGGAGCTCA AGCT17). In order to generate soluble, recombinant forms of mouse, rat and human DAF, DNA encoding the first four short consensus repeats (SCRs) was amplified by the polymerase chain reaction (PCR) using Vent DNA polymerase, from mouse testis cDNA, rat lung cDNA or from plasmid containing full-length human DAF cDNA.20 Primers used for amplification incorporated restriction sites enabling ligation into the expression vector, Signal pIgplus (R & D Systems), following digestion of the PCR product with Xba1 and BamHI and the vector with Nhe1 and BamHI. Primers were as follows, human DAF: 5′-GTGTCTAGAGACTGTGGCCTTCCCCAG-3′ (sense) and 5′-GGTGGATCCTTGGAAGTTAGAGATTTTC-3′ (antisense); mouse DAF: 5′GGCTCTAGAGACTGCGGCCCAC CTCCAGAC-3′ (sense) and 5′-CGCGGATCCGATTTCTCTATGCAGCGGGG-3′ (antisense); rat DAF: 5′-CGCG GATCCTGTCTCTCTATGCACTTGGG-3′, sense primer as for mouse DAF. PCR products were ligated into the expression vector Signal pIgplus (R & D Systems), according to the manufacturer's instructions, ensuring that it was in-frame with DNA encoding the hinge and Fc regions of human IgG1. Expression would result in soluble forms of DAF consisting of the four SCRs linked to the Fc domain of human IgG1 (‘DAF-immunoglobulin’, DAF-Ig). Ligation into the multiple cloning site resulted in addition of five amino acids to the N-terminus of the mature protein (Asp-Lys-Leu-Ala-Arg-). The C-terminal residues of DAF were (as in the published sequences of the mature proteins): Asn253 for rat DAF;18 Ser254 for mouse DAF;17 and Lys257 for human DAF.23 In order to obtain high levels of secretion, DNA encoding the DAF-Fc fusion protein was subcloned into the expression vector, pDR2ΔEF1α. Sequencing confirmed that no errors had been introduced by PCR. CHO cells were transfected with plasmid, as described previously,19 stable cell lines were generated and culture supernatant was harvested. DAF-Ig constructs were purified from the supernatant by protein-A affinity chromatography (Prosep A; Bioprocessing Ltd). A 0·1-m citrate buffer (pH 5·0) wash was used to remove weakly bound contaminants, and fusion proteins were eluted using 0·1 m glycine/HCl, pH 2·5. Fractions containing DAF-Ig were pooled, neutralized with 1 m Tris, concentrated by ultrafiltration and dialysed against PBS. Protein concentration was determined using Coomassie protein assay reagent (Pierce & Warriner, Chester, Ches., UK), according to the manufacturer's instructions and using BSA as a standard. Purity was assessed by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE).
Lysis of human E by heterologous C
Human E were washed and resuspended in PBS at 2% (v/v). E were sensitized by incubating 1 volume for 30 min at 37° with 1 volume of PBS containing rabbit anti-human CD59 antiserum (1 : 25 dilution), in the presence or absence of neutralizing anti-DAF mAb (BRIC110 and BRIC216) at 10 µg/ml each. Antibody-coated E (EA) were washed twice in GVB and resuspended to 1% (v/v). A portion of cells was incubated for 10 min at 37° with an equal volume of a serum dilution previously titred to give subtotal levels of lysis. Per cent lysis was calculated as described previously.19
Haemolysis assays to test function of soluble DAF
Classical pathway (CP) assays
Sheep E were sensitized by incubating 1 volume of 4% E (v/v) with 1 volume of 1 : 250 rabbit anti-sheep E for 20 min at 37°. Rabbit E were sensitized by incubating 1 volume of 4% E (v/v) with 1 volume of 1 : 250 mouse anti-rabbit E for 30 min at 37°. Sensitized cells were washed twice in GVB and resuspended to 2%. Fifty microlitres of EA (sheep EA for rat and human CP; rabbit EA for mouse CP) was incubated with 50 µl of serum and 50 µl of a dilution of DAF-Ig or control protein. All dilutions were made in GVB and serum was previously titred to yield between 50 and 60% lysis in the absence of inhibitor. Cells were incubated at 37° for 30 min (sheep EA) or 40 min (rabbit EA). Per cent lysis was calculated as described previously.19
% Inhibition = 100 × [(% Lysis of the negative control - % Lysis of the test sample)/(% Lysis of the negative control)]
Alternative pathway (AP) assays
Guinea-pig E (rat AP assay) and rabbit E (human AP assay) were washed in APB and resuspended to 1% (v/v). For the mouse AP assay, 1 volume of 2% rabbit E in APB was mixed with 1 volume of 20 mg/ml zymosan in APB. Fifty microlitres of E (or E/zymosan for mouse AP) was incubated with 50 µl of serum and 50 µl of a dilution of DAF-Ig or control protein. All dilutions were made in APB and serum was previously titred to yield ≈ 70% lysis. Cells were incubated at 37° for 30 min (human AP), 40 min (rat AP) or 60 min (mouse AP). Per cent inhibition was calculated as described above.
Results
Blockade of DAF on human E enhances lysis by heterologous serum
Human E were sensitized with rabbit polyclonal anti-human CD59 in the presence or absence of function-blocking monoclonal anti-DAF antibodies (BRIC110 and BRIC216). In addition to sensitizing the cells to C attack, use of polyclonal anti-CD59 resulted in an increased susceptibility to lysis through blockade of CD59 function. Sensitized cells were then incubated with sera from different species and haemolysis was assessed by release of haemoglobin to the supernatant. Nine different sources of C were used and, in each case, preincubation of E with blocking anti-DAF antibodies increased their susceptibility to lysis (Table 1). The mouse mAb used to block DAF function were both of isotype IgG1 and have previously been shown not to activate C.24
Table 1.
Inhibition of decay-accelerating factor (DAF) on human erythrocytes (E) enhances lysis by homologous and heterologous complement (C)
| % Lysis | |||
|---|---|---|---|
| Source of serum | Dilution | Anti-DAF – | Anti-DAF + |
| Human | 1 : 160 | 24 ± 0·6 | 87 ± 5·6 |
| Rat | 1 : 160 | 31 ± 0·6 | 76 ± 2·1 |
| Sheep | 1 : 80 | 29 ± 3·6 | 84 ± 4·0 |
| Guinea-pig | 1 : 80 | 10 ± 1·7 | 88 ± 2·5 |
| Bovine | 1 : 10 | 25 ± 2·5 | 74 ± 1·0 |
| Goat | 1 : 10 | 4 ± 0·6 | 21 ± 2·6 |
| Pig | 1 : 80 | 16 ± 1·5 | 81 ± 5·6 |
| Rabbit | 1 : 80 | 41 ± 1·5 | 88 ± 4·0 |
| Mouse | 1 : 20 | 39 ± 2·6 | 65 ± 1·5 |
Sensitized cells were incubated with sera in the absence or presence of function-blocking anti-DAF antibodies. Per cent lysis was assessed by release of haemoglobin to the supernatant. Results represent the mean value ± SD of triplicate samples.
Expression and functional analysis of DAF on CHO cells
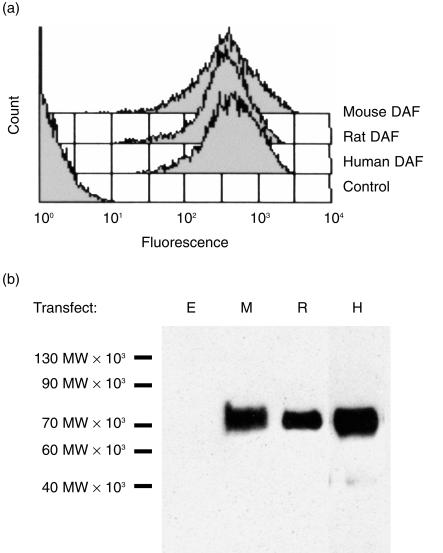
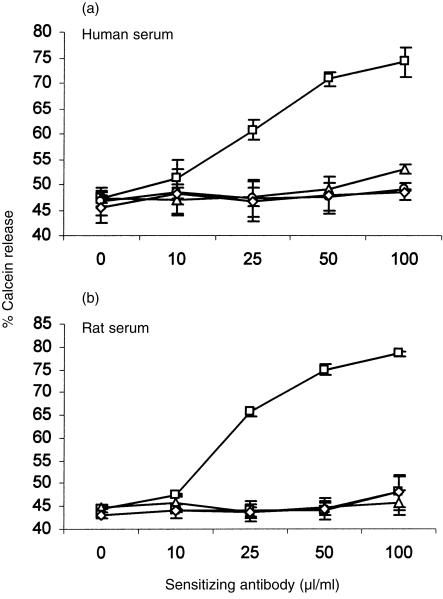
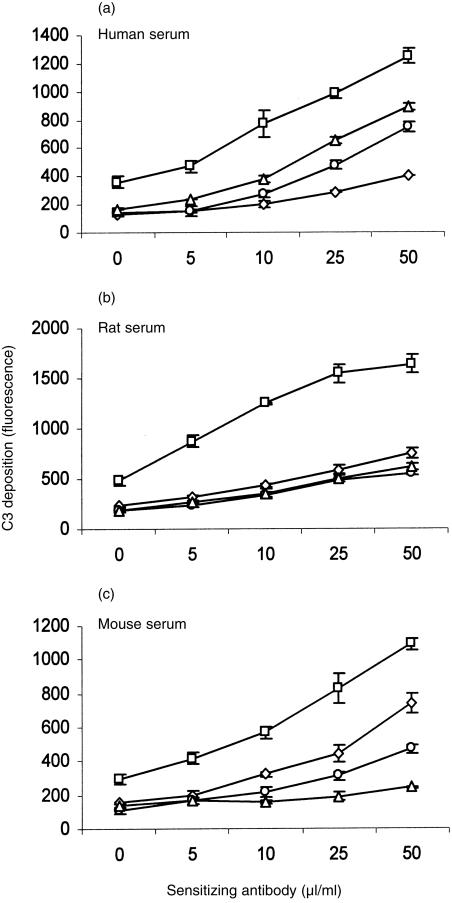
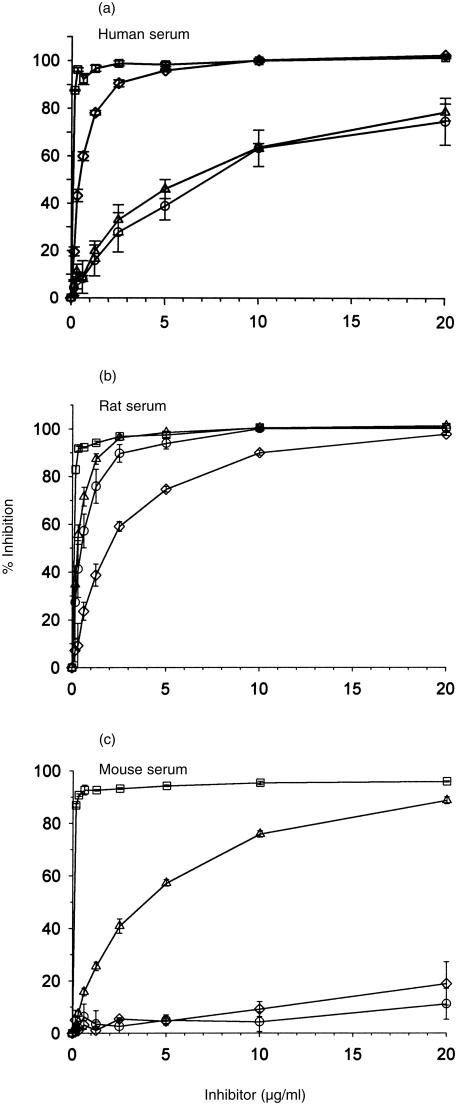
The preliminary experiment described above suggested that C inhibition by human DAF was not species specific. In order to analyse species specificity in more depth, a different approach was adopted in which CHO cells were transfected with DNA encoding DAF from different species. DNA encoding the GPI-anchored forms of human, rat and mouse DAF was cloned into the high-expression vector, pDR2ΔEF1α, and transfected into CHO cells. Stable cell lines were generated by selection. High-level expression of DAF on the cell surface was confirmed by flow cytometry using in-house-generated mAb (Fig. 1a). Western blot analysis demonstrated specific bands of the expected sizes for all species of DAF (Fig. 1b).Transfected cells expressing DAF were then analysed for protection from CP-mediated C attack. Sensitized cells were attacked with rat or human C under conditions in which lytic killing of control cells was obtained (Fig. 2). Protection from mouse C could not be determined using this assay as mouse serum was non-lytic under these experimental conditions. CHO cells expressing human, rat or mouse DAF were equally protected from attack by human or rat C, illustrating the ability of all three species of DAF to regulate heterologous C. Deposition of C3 fragments on the cell surface was used to further assess decay-accelerating activity of surface-expressed DAF. Cells were attacked under non-lytic conditions with human, mouse or rat C and deposited C3b was detected by flow cytometry using monoclonal anti-C3 antibody (Fig. 3). Protection from human C attack, as assessed by C3b deposition, was provided by all three DAF analogues in the rank order human > rat > mouse. All DAF analogues were equally effective at protection from C3b deposition from rat C, whereas C3b deposition from mouse C was inhibited in the order mouse > rat > human DAF.
Figure 1.
Expression of decay-accelerating factor (DAF) on the surface of transfected cells.(a) Cells expressing human, rat or mouse DAF were stained with specific monoclonal anti-DAF antibodies and analysed by flow cytometry. (b) Lysates from cells expressing DAF and vector control cells were subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and Western blot. Blots were probed with specific monoclonal anti-DAF antibodies and horseradish peroxidase (HRP)-linked secondary antibody. Bands were visualized using enhanced chemiluminescence (ECL). Relative molecular mass band sizes are indicated at the left of the blot. E, empty vector; M, cells expressing mouse DAF; R, cells expressing rat DAF; H, cells expressing human DAF.
Figure 2.
Protection of transfected Chinese hamster ovary (CHO) cells from lysis. Sensitized vector control cells (□) or cells expressing human decay-accelerating factor (DAF) (⋄), rat DAF (○) or mouse DAF (▵) were subjected to attack by (a) human complement (C) or (b) rat C. Lysis was assessed by release of calcein to the supernatant. Results represent the mean value ± SD of three determinations.
Figure 3.
Deposition of C3 fragments on transfected Chinese hamster ovary (CHO) cells expressing decay-accelerating factor (DAF). Sensitized vector control cells (□) or cells expressing human decay-accelerating factor (DAF) (⋄), rat DAF (○) or mouse DAF (▵) were subjected to attack by (a) human complement (C) (b) rat C, or (c) mouse C. Deposition of C3 fragments was measured using anti-C3 monoclonal antibodies (mAbs) and flow cytometry. Results represent the mean value ± SD of three determinations.
Cross-species inhibitory activity of soluble DAF
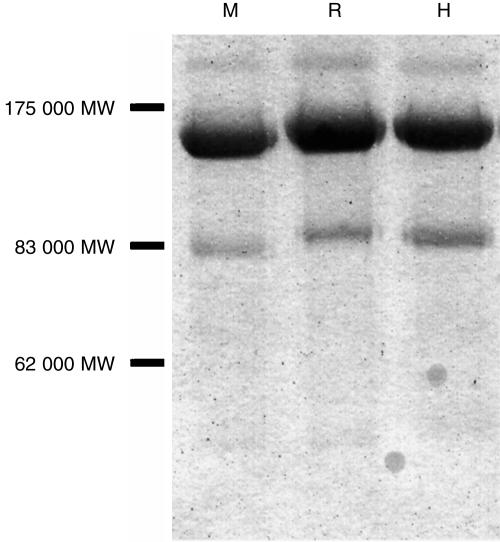
The native forms of all three DAF analogues contain four SCRs at the N-terminal end of the molecule which bind C3b/C4b and bestow the molecule with decay-accelerating activity.25,26 Soluble, recombinant forms of human, rat and mouse DAF were generated, consisting of the four SCRs linked to the Fc domain of human IgG1. These soluble forms of the regulators were ‘antibody-like’ molecules consisting of two DAF moieties linked together through the antibody hinge region, and are termed DAF-Ig. The agents were expressed in CHO cells and purified by protein-A affinity chromatography. This yielded highly purified proteins of approximate molecular mass 140 kDa (Fig. 4).
Figure 4.
Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) of purified decay-accelerating factor (DAF)-immunoglobulin fusion proteins. DAF-immunoglobulin was purified from the supernatant of transfected cells using protein-A affinity chromatography. Purified protein (4 µg) was analysed on an 8% separating polyacrylamide gel; bands were visualized using Coomassie Brilliant Blue staining. M, mouse DAF-immunoglobulin; R, rat DAF-immunoglobulin; H, human DAF-immunoglobulin. Relative molecular mass band sizes are indicated at the left of the gel.
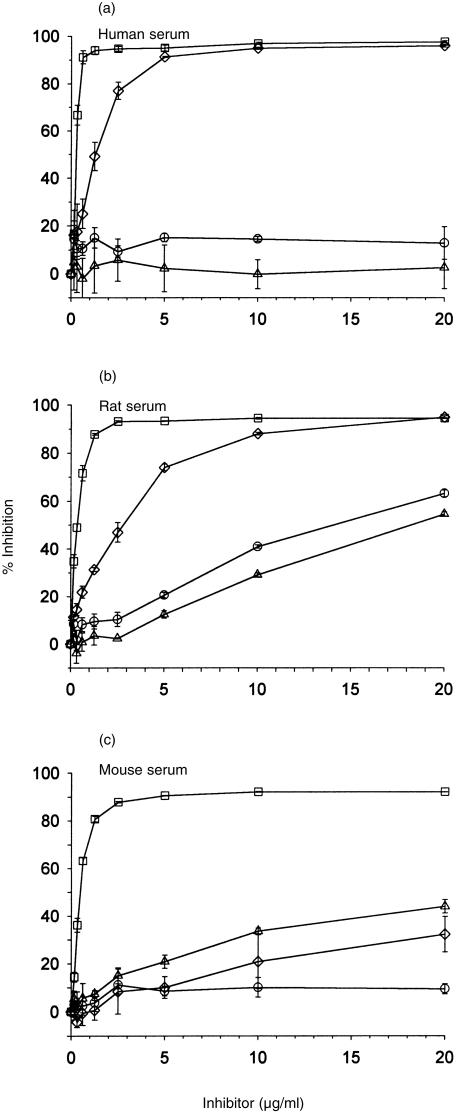
To assess inhibition of the CP, antibody-sensitized cells were incubated with different dilutions of rat, mouse or human DAF-Ig and an amount of serum previously titred to give between 50 and 60% haemolysis. Assays were performed using soluble, recombinant CR1 (sCR1), a powerful fluid-phase inhibitor of C activation, as a positive control for inhibition, and a non-regulatory SCR-containing Fc fusion protein as a negative control. Whilst some background activation of the AP may occur in these assays it is probable that complement is activated predominantly via the CP and that AP activation occurs to a very small extent, if at all. Human DAF inhibited human and rat CP, rat DAF inhibited rat and human CP, and mouse DAF inhibited CP from all three species (Fig. 5). C inhibition was best achieved by homologous DAF, except in the case of rat C where both rodent DAFs were equally effective. AP inhibition was assessed using appropriate activating surfaces and an amount of serum previously titred to give between 50 and 70% haemolysis. Soluble human DAF-immunoglobulin was the most powerful inhibitor of both human and rat AP and also inhibited mouse AP, albeit less efficiently (Fig. 6). Rat DAF inhibited only rat AP and mouse DAF inhibited both rat and mouse AP. Soluble CR1 inhibited in all these systems.
Figure 5.
Inhibition of classical pathway (CP)-mediated haemolysis by soluble, recombinant decay-accelerating factor (DAF)-Ig fusion protein. Sensitized erythrocytes (E) were incubated in gelatin veronal buffer (GVB) with different concentrations of soluble CR1 (sCR1) (□), human DAF-Ig (⋄), rat DAF-Ig (○) or mouse DAF-Ig (▵) and (a) human, (b) rat, or (c) mouse serum. Haemolysis was assessed by release of haemoglobin to the supernatant and per cent inhibition was determined. Results represent the mean value ± SD of three determinations.
Figure 6.
Inhibition of alternative pathway (AP)-mediated haemolysis by soluble, recombinant decay-accelerating factor (DAF)-Ig fusion proteins. Erythrocytes (E) were incubated in AP buffer with different concentrations of soluble CR1 (sCR1) (□), human DAF-Ig (⋄), rat DAF-Ig (○) or mouse DAF-Ig (▵) and (a) human, (b) rat, or (c) mouse serum. Haemolysis was assessed by release of haemoglobin to the supernatant and per cent inhibition was determined. Results represent the mean value ± SD of three determinations.
Discussion
E from most species are remarkably resistant to lysis by homologous C, regardless of whether attack is initiated via the CP or AP.6,7 Many factors contribute to resistance, the additive effect being protection from damage by homologous C. In humans, cell-surface characteristics, such as the density of sialic acid, have been shown to have an important role in restriction of activation on self-cells.7,27 Binding of the fluid-phase C regulator, fH, may also inhibit C activation.28 However, numerous blocking studies and evidence from deficient cell lines have shown that the membrane C-regulatory proteins DAF, MCP and CD59 together provide the principal defence against damage by homologous C in humans and other mammals.1 In contrast to this stubborn resistance to homologous C, E and nucleated cells are often vulnerable to attack by heterologous C.2,13,14,29 The molecular basis of this species-specific protection is poorly defined. The greater sensitivity to heterologous sera cannot be attributed solely to the presence of natural anti-cell antibodies, implying that mechanisms exist on cells to preferentially inhibit homologous C.6,30 The membrane C regulators CD59, DAF and MCP have been implicated as species-specific restriction factors in many studies. In several of the early studies of CD59 it was suggested that this molecule might be the elusive ‘homologous restriction factor’ responsible for protecting specifically against homologous C.8–10,15 However, it has since been demonstrated that CD59 will inhibit C from many other species, sometimes more efficiently than it regulates homologous C.11,12,31
Several studies have used blocking antibodies to address the capacity of human DAF to regulate activation of C on the cell surface.2,13–15,29 Heterologous C, in particular rabbit C, caused much greater lysis of human cells than homologous C. When DAF was blocked, lysis by human C was increased to levels similar to those obtained with rabbit C, lysis by guinea-pig C was enhanced, but lysis by rabbit C was unaffected.15 A soluble, recombinant form of human DAF was shown to inhibit human and guinea-pig C, but not mouse, rat or rabbit C.32 These confusing data sets have been interpreted as evidence that DAF is species restricted. Our first experiments replicated and extended some of these earlier studies by examining the effects of antibody blockade of DAF on lysis of human E using a range of sera. Blocking DAF enhanced lysis by every serum examined, indicating that human DAF C-regulatory activity was not species restricted (Table 1).
We subsequently proceeded to analyse whether human DAF and rat and mouse DAF analogues were species restricted in their C-inhibitory activities. CHO cells were transfected to generate CHO clones abundantly expressing each DAF, and each of the DAF analogues was tested for its ability to regulate C activation via the CP using sera from each of the three species. Measurement of C3 deposition proved to be the most sensitive indicator of C activation and demonstrated that, while C was usually best regulated by DAF from the same species, there was substantial cross-species activity, each DAF analogue causing substantial inhibition of each C (Fig. 3). In the case of rat C, all three DAF analogues were equally protective. Cross-species activities of the three DAF analogues was confirmed in lysis assays (Fig. 2). Minor discrepancies between the results obtained in the two assays may be explained by the fact that C3 deposition is a direct measure of DAF function whereas lysis is dependent upon downstream factors, including CD59.
We wished to confirm these surprising results in another system. It has previously been shown that soluble, human DAF can function to regulate C on cell surfaces or in the fluid phase.26,32 We therefore generated DAF-Ig fusion proteins for DAF from each species. These were efficiently synthesized in CHO cells and purified to homogeneity, enabling accurate quantification, essential for comparison of functional activities. The use of soluble forms of DAF for these studies eliminates any possible role of spatial orientation and length of serine/threonine/proline-rich (STP) region, previously suggested to be important for the efficient functioning of DAF on the membrane.25 Although the influence of length of STP region on DAF function in other species is uncertain,33 it is possible that the spacing requirements vary between DAF analogues and different species of C. The use of fluid-phase forms of DAF removes this confounding factor.
The three DAF-Ig fusion proteins were compared in simple haemolysis assays in which human, rat or mouse C were activated via either the CP or AP. Quantification of the inhibitor was precise, enabling comparison of functional activity using equimolar amounts of DAF, a comparison impossible to achieve using transfected cells. For each species tested, inhibition of the CP by soluble DAF was most effective with homologous DAF (Fig. 5). The human CP was also inhibited by rat and mouse DAF, and rat C by human and mouse DAF, whereas mouse C was inhibited only by mouse DAF. These findings are in broad agreement with the C3-deposition data obtained from transfected cells, except for the apparent species restriction of mouse C in the fluid-phase CP assays. We have no explanation for this inconsistency, although it is possible that cell-surface structures on the surface of E, used as the target in the haemolysis assays, may also influence regulation of C by soluble DAF. In a recently described DAF-knockout mouse, the DAF-deficient E were more susceptible to CP-mediated lysis by human and guinea-pig C, supporting the suggestion that mouse DAF is not species restricted in its mode of action.34 In the AP, human DAF was the most efficient inhibitor for both human and rat C and also inhibited the mouse AP, albeit less efficiently. Rat DAF was an AP inhibitor only for rat C, whilst mouse DAF inhibited the AP of mouse and rat C (Fig. 6). These findings indicate that species selectivity of the DAF analogues is more apparent in the AP than in the CP.
Cross-species reactivity of C regulators has implications in many fields of C research. Soluble, recombinant forms of human regulators, in particular human CR1, have been used as anti-C therapeutic reagents in animal models of disease.1 A knowledge of the capacity to regulate in each of the activation pathways across species barriers is essential for these applications. The ability of endogenous regulators to inhibit C from another species is also pertinent to the field of xenotransplantation where the target is the transplantation of pig organs into humans.35 The dogma implicating membrane C regulators as species restriction factors has driven the generation of transgenic pigs expressing human DAF and other regulators. The expectation is that organ destruction mediated by heterologous C attack will be prevented by the presence of human DAF on the endothelial cells of the transplanted organ. Our data demonstrate that neither human nor rodent DAF are species restricted in that they can regulate both homologous and heterologous C in CP and AP assays. Comparison of human and rodent DAF analogues does suggest a degree of species selectivity in some species combinations. Our preliminary studies with the porcine analogue of DAF indicate that it is a more efficient inhibitor of human than porcine C(J. M. Perez de la Lastra et al., in preparation). With improved understanding of cross-species activities of C regulators, current strategies for protection of xenografts from C will need to be revisited.
Acknowledgments
We thank Dr Neil Rushmere and Dr Stewart Hinchliffe for their advice in expression of the recombinant forms of DAF. This work was funded by the Wellcome Trust (098700/z/98/z and 043400/z/95/z). B. P. M. is a Wellcome Senior Fellow.
Glossary
Abbreviations
- AP
alternative pathway
- CHO
Chinese hamster ovary
- CP
classical pathway
- DAF
decay-accelerating factor
- EA, antibody-coated E; GPI
glycosyl phosphatidylinositol
- iC3b, inactive C3b; HRP
horseradish peroxidase
- GVB, gelatin veronal buffer; MAC
membrane attack complex
- MCP
membrane cofactor protein
- PIPLC
phosphatidylinositol-specific phospholipase C
- SCR
short consensus repeat
- STP
serine/threonine/proline-rich
References
- 1.Morgan B, Harris C. Complement Regulatory Proteins. London: Academic Press; 1999. [Google Scholar]
- 2.Quigg RJ, Nicholson-Weller A, Cybulsky AV, Badalamenti J, Salant DJ. Decay accelerating factor regulates complement activation on glomerular epithelial cells. J Immunol. 1989;142:877. [PubMed] [Google Scholar]
- 3.Brooimans RA, van Wieringen PA, van Es LA, Daha MR. Relative roles of decay-accelerating factor, membrane cofactor protein, and CD59 in the protection of human endothelial cells against complement-mediated lysis. Eur J Immunol. 1992;22:3135. doi: 10.1002/eji.1830221216. [DOI] [PubMed] [Google Scholar]
- 4.Muir R. On the relationships between the complements and immune bodies of different animals. J Pathol Bacteriol. 1911;16:523. [Google Scholar]
- 5.Hansch GM, Hammer CH, Vanguri P, Shin ML. Homologous species restriction in lysis of erythrocytes by terminal complement proteins. Proc Natl Acad Sci USA. 1981;78:5118. doi: 10.1073/pnas.78.8.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houle JJ, Hoffmann EM. Evidence for restriction of the ability of complement to lyse homologous erythrocytes. J Immunol. 1984;133:1444. [PubMed] [Google Scholar]
- 7.Ish C, Ong GL, Desai N, Mattes MJ. The specificity of alternative complement pathway-mediated lysis of erythrocytes – a survey of complement and target-cells from 25 species. Scand J Immunol. 1993;38:113. doi: 10.1111/j.1365-3083.1993.tb01701.x. [DOI] [PubMed] [Google Scholar]
- 8.Davies A, Simmons DL, Hale G, et al. CD59, an LY-6-like protein expressed in human lymphoid cells, regulates the action of the complement membrane attack complex on homologous cells. J Exp Med. 1989;170:637. doi: 10.1084/jem.170.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harada R, Okada N, Fujita T, Okada H. Purification of 1F5 antigen that prevents complement attack on homologous cell membranes. J Immunol. 1990;144:1823. [PubMed] [Google Scholar]
- 10.Rollins SA, Zhao J, Ninomiya H, Sims PJ. Inhibition of homologous complement by CD59 is mediated by a species-selective recognition conferred through binding to C8 within C5b-8 or C9 within C5b-9. J Immunol. 1991;146:2345. [PubMed] [Google Scholar]
- 11.van den Berg CW, Morgan BP. Complement-inhibiting activities of human CD59 and analogues from rat, sheep, and pig are not homologously restricted. J Immunol. 1994;152:4095. [PubMed] [Google Scholar]
- 12.Rushmere NK, Tomlinson S, Morgan BP. Expression of rat CD59: functional analysis confirms lack of species selectivity and reveals that glycosylation is not required for function. Immunology. 1997;90:640. doi: 10.1046/j.1365-2567.1997.00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto H, Blaas P, Nicholson-Weller A, Hansch GM. Homologous species restriction of the complement-mediated killing of nucleated cells. Immunology. 1990;70:422. [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong RK, Kozii R, Ball ED. Homologous restriction of complement-mediated cell lysis can be markedly enhanced by blocking decay-accelerating factor. Br J Haematol. 1995;91:269. doi: 10.1111/j.1365-2141.1995.tb05289.x. [DOI] [PubMed] [Google Scholar]
- 15.Shin ML, Hansch G, Hu VW, Nicholson-Weller A. Membrane factors responsible for homologous species restriction of complement-mediated lysis: evidence for a factor other than DAF operating at the stage of C8 and C9. J Immunol. 1986;136:1777. [PubMed] [Google Scholar]
- 16.Hoffmann EM. Inhibition of complement by a substance isolated from human erythrocytes. I. Extraction from human erythrocyte stroma. Immunochemistry. 1969;6:391. doi: 10.1016/0019-2791(69)90296-1. [DOI] [PubMed] [Google Scholar]
- 17.Spicer AP, Seldin MF, Gendler SJ. Molecular cloning and chromosomal localization of the mouse decay-accelerating factor genes. Duplicated genes encode glycosylphosphatidylinositol-anchored and transmembrane forms. J Immunol. 1995;155:3079. [PubMed] [Google Scholar]
- 18.Hinchliffe SJ, Spiller OB, Rushmere NK, Morgan BP. Molecular cloning and functional characterization of the rat analogue of human decay-accelerating factor. J Immunol. 1998;161:5695. [PubMed] [Google Scholar]
- 19.Harris CL, Rushmere NK, Morgan BP. Molecular and functional analysis of mouse decay accelerating factor (CD55) Biochem J. 1999;341:821. 10.1042/0264-6021:3410821. [PMC free article] [PubMed] [Google Scholar]
- 20.Charreau B, Cassard A, Tesson L, et al. Protection of rat endothelial cells from primate complement-mediated lysis by expression of human CD59 and/or decay-accelerating factor. Transplantation. 1994;58:1222. [PubMed] [Google Scholar]
- 21.Kemp PA, Spragg JH, Brown JC, Morgan BP, Gunn CA, Taylor PW. Immunohistochemical determination of complement activation in joint tissues of patients with rheumatoid arthritis and osteoarthritis using neoantigen-specific monoclonal antibodies. J Clin Lab Immunol. 1992;37:147. [PubMed] [Google Scholar]
- 22.Spiller OB, Harris CL, Morgan BP. Efficient generation of monoclonal antibodies against surface-expressed proteins by hyperexpression in rodent cells. J Immunol Methods. 1999;224:51. doi: 10.1016/s0022-1759(99)00008-3. [DOI] [PubMed] [Google Scholar]
- 23.Caras IW, Davitz MA, Rhee L, Weddell G, Martin DW, Nussenzweig V. Cloning of decay-accelerating factor suggests novel use of splicing to generate two proteins. Nature. 1987;325:545. doi: 10.1038/325545a0. [DOI] [PubMed] [Google Scholar]
- 24.Harris CL, Morgan BP. Characterization of a glycosyl-phosphatidylinositol anchor-deficient subline of Raji cells. An analysis of the functional importance of complement inhibitors on the Raji cell line. Immunology. 1995;86:311. [PMC free article] [PubMed] [Google Scholar]
- 25.Coyne KE, Hall SE, Thompson S, et al. Mapping of epitopes, glycosylation sites, and complement regulatory domains in human decay accelerating factor. J Immunol. 1992;149:2906. [PubMed] [Google Scholar]
- 26.Brodbeck WG, Liu D, Sperry J, Mold C, Medof ME. Localization of classical and alternative pathway regulatory activity within the decay-accelerating factor. J Immunol. 1996;156:2528. [PubMed] [Google Scholar]
- 27.Pangburn MK, Muller-Eberhard HJ. Complement C3 convertase: cell surface restriction of β1H control and generation of restriction on neuraminidase-treated cells. Proc Natl Acad Sci USA. 1978;75:2416. doi: 10.1073/pnas.75.5.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meri S, Pangburn MK. Discrimination between activators and nonactivators of the alternative pathway of complement: regulation via a sialic acid/polyanion binding site on factor H. Proc Natl Acad Sci USA. 1990;87:3982. doi: 10.1073/pnas.87.10.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brooimans RA, Van der Ark AA, Tomita M, Van Es LA, Daha MR. CD59 expressed by human endothelial cells functions as a protective molecule against complement-mediated lysis. Eur J Immunol. 1992;22:791. doi: 10.1002/eji.1830220324. [DOI] [PubMed] [Google Scholar]
- 30.Okada H, Tanaka H, Okada N. Prevention of complement activation on the homologous cell membrane of nucleated cells as well as erythrocytes. Eur J Immunol. 1983;13:340. doi: 10.1002/eji.1830130413. [DOI] [PubMed] [Google Scholar]
- 31.Hanna SM, Williams GT, Vandenberg CW, Morgan BP. Characterization in vitro and in vivo of the pig analogue of human CD59 using new monoclonal antibodies. Immunology. 1998;95:450. doi: 10.1046/j.1365-2567.1998.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moran P, Beasley H, Gorrell A, et al. Human recombinant soluble decay accelerating factor inhibits complement activation in vitro and in vivo. J Immunol. 1992;149:1736. [PubMed] [Google Scholar]
- 33.Wang G, Nonaka M, He C, Okada N, Nakashima I, Okada H. Functional differences among multiple isoforms of guinea pig decay-accelerating factor. J Immunol. 1998;160:3014. [PubMed] [Google Scholar]
- 34.Sun XJ, Funk CD, Deng CJ, Sahu A, Lambris JD, Song WC. Role of decay-accelerating factor in regulating complement activation on the erythrocyte surface as revealed by gene targeting. Proc Natl Acad Sci USA. 1999;96:628. doi: 10.1073/pnas.96.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Auchincloss H, Sachs DH. Xenogeneic transplantation. Annu Rev Immunol. 1998;16:433. doi: 10.1146/annurev.immunol.16.1.433. [DOI] [PubMed] [Google Scholar]