Abstract
Pertussis toxin (PTX) has been shown previously to promote myelomonocytic cell adhesion in serum. The aim of the present study was to identify, using transforming growth factor-β1 and 1,25-(OH)2 vitamin D3 (TGF-β1/D3)-primed U937 cells, the PTX-binding site(s) and the adhesion molecule(s) responsible for PTX-induced myelomonocytic cell adhesion. Monoclonal antibodies (mAbs) directed against CD14, CD11b, CD18 or urokinase receptor (uPAR) significantly inhibited PTX-induced primed U937 cell adhesion in serum in a concentration-dependent manner. However, only anti-CD14 and anti-CD18 mAbs were able to prevent the myeloid cells from binding to PTX-coated plates and significantly inhibited a PTX-induced rise of [Ca2+]i in primed U937 cells. A receptor-isolation study showed that biotinylated PTX recognized a 48 000-molecular weight protein in primed U937 cell lysates, which could be specifically blocked by excess unlabelled PTX or by anti-CD14 mAb. On the other hand, mAb directed against uPAR significantly blocked PTX-induced myeloid cell adhesion to serum and to immobilized vitronectin, a major extracellular matrix protein in serum. Taken together, our data suggest that PTX may bind to cell-surface CD14 to induce myelomonocytic cell adhesion to vitronectin in serum via uPAR activation, which may represent a pathogenetic mechanism for the respiratory tract infection induced by Bordetella pertussis.
Introduction
Pertussis toxin (PTX) is a major virulence factor mediating the pathogenesis of respiratory tract infection induced by Bordetella pertussis1 and is the only virulence component present in all types of acellular pertussis vaccine preparations.2 PTX is a heterohexamer that is functionally divided into A and B subunits, analogous to cholera toxin. The A-protomer consists of a single polypeptide (S1) that possesses ADP-ribosyltransferase activity, and the B-oligomer confers binding specificity to the target cell membrane.3,4
Although B. pertussis infection is a well-recognized disease, the pathogenesis of the disease process is still poorly understood. Upon prolonged incubation (at least 1–2 hr) with PTX, the A-protomer will be internalized by certain cells and ADP ribosylates the α-subunit of the membrane-bound Gi-like protein, leading to blockade of certain transmembrane signalling process and eventually cellular intoxication.5 In addition to its delayed inhibitory effect on the Gi protein, PTX has also been shown to elicit rapid responses (in minutes) in a variety of cell types,6 which may have profound pathological effects as important as its ADP-ribosylation activity. All of these rapid cellular responses can be reproduced by the purified PTX B-oligomer alone, suggesting that occupation of the PTX-binding site(s) mediates these early cellular events.
Progress has been made regarding the binding properties of PTX. It has been shown that the S2 and S3 subunits of the B-oligomer possess a carbohydrate-recognition domain that could selectively bind to Lewis a (Lea) and Lewis x (Lex) determinants.7 In separate receptor-binding studies, PTX was found to bind to a 165 000-molecular weight (MW) sialylated glycoconjugate on Chinese hamster ovary (CHO) cells,8 to 43 000-MW and 70 000-MW cell-surface proteins on a Jurket cell line,9–11 and to 164 000-MW sialoglycoprotein Ib (GPIb), known to be activated by von Willebrand factor, on the platelet membrane.12
More recently, PTX holotoxin, as well as its binding subunit, B-oligomer, have been shown to block entry of monotropic (R5) strains of human immunodeficiency virus-1 (HIV-1) in primary T lymphocytes. In addition, the PTX B-oligomer inhibited virus production in peripheral blood mononuclear cells infected with either R5 or X4 strains of HIV-1.13 These findings suggested that the PTX B-oligomer deactivated a chemokine receptor (CCR5, a co-receptor for HIV-1) via binding to a yet-unknown PTX receptor on T cells and initiation of a series of cellular signalling events.13,14 There is an unequivocal need to identify the cellular binding site(s) for PTX and to ascertain the B-oligomer-mediated cellular signalling events.
As monocytes/macrophages are the major host defence against B. pertussis infection and a major target for HIV-1, it becomes very important to identify the binding site(s) for PTX on myelomonocytic cells and to understand the functional consequences upon receptor occupation. Our recent study showed that PTX holotoxin, as well as PTX B-oligomer, induced a rapid adherent response of myelomonocytic cells to serum via urokinase receptor (uPAR), a high-affinity receptor for vitronectin.15,16 The present study was undertaken to explore the interaction between PTX and myelomonocytic cells at the receptor- and adherent-response levels using transforming growth factor-β1/1,25-(OH)2 vitamin D3 (TGF-β1/D3)-primed U937 cells. Results obtained from the receptor-isolation and cell-adhesion studies indicate that CD14 is probably a binding site for PTX on myelomonocytic cells. In addition, using monoclonal antibodies (mAbs) against the binding domain of uPAR, our data confirmed that PTX induced myeloid cell adhesion to vitronectin via activation of uPAR.
Materials and methods
Materials
The human monoblastic leukaemic U937 cell line was obtained from the American Type Culture Collection (Rockville, MD). TGF-β1 was purchased from Upstate Biotechnology, Inc. (Lake Placid, NY), and D3 was a gift of Dr M. Manganel and Dr E. M. Gutkneckt (Hoffman-LaRoche Ltd., Basel, Switzerland). RPMI-1640, methionine-free RPMI-1640, fetal bovine serum (FBS), HEPES, penicillin G/streptomycin, Geimsa stain and mouse mAb against αv integrin (clone VNR147) were from Life Technologies, Inc. (Gaithersburg, MD). Purified PTX was a gift of Dr P. Licari and Dr C. Nesman (Massachusetts Public Health Biologic Laboratories, Boston, MA). Purified PTX B-oligomer was obtained from Research Biochemicals Inter-national (Natick, MA). Mouse mAbs against CD14 (clone TÜK4), CD11b (clone 2LPM19c), CD11c (clone KB90), CD18 (clone MHM23) and fluorescein isothiocyanate (FITC)-conjugated rabbit anti-mouse F(ab′)2 fragments were obtained from DAKO (Glostrup, Denmark). Paraformaldehyde was obtained from Merck KGaA (Darmstadt, Germany). Mouse immunoglobulin G (IgG) and Fluo-3/AM were purchased from Calbiochem-Novabiochem (La Jolla, CA). Mouse mAb against domain II of uPAR was purchased from American Diagnostica, Inc. (Greenwich, CN). Vitronectin was obtained from Becton-Dickinson Labware (Bedford, MA). Bovine serum albumin (BSA), probenecid, manganese chloride (MnCl2) and ethylene glycol-bis(β-aminoethylether)-N,N,N′, N′-tetraacetic acid (EGTA) were from Sigma Chemical Co. (St. Louis, MO). Sulpho-NHS-LC-biotin and streptavidin-agarose were purchased from Pierce Chemical Co. (Rockford, IL). [35S]Methionine was purchased from Amersham Pharmacia Biotech UK Ltd. (Little Chalfont, Bucks., UK).
Cell culture
The human monoblastic leukaemic U937 cell line was maintained in RPMI-1640 supplemented to 10% (v/v) with FBS, 20 mm HEPES (pH 7·4), 100 U/ml of penicillin G and 100 µg/ml of streptomycin, at 37° in a humidified incubator in an atmosphere of 5% CO2. Before any experimentation, cells were seeded at 106/ml and differentiated to a monocyte/macrophage phenotype with 1 ng/ml of TGF-β1 and 50 nm D3 (TGF-β1/D3) for 48 hr. Differentiated U937 cells were determined by morphological changes observed using microscopy and by cell-surface expression of the monocytic differentiation antigen, CD14, and other macrophage-related integrins such as CD11b, CD11c and CD18. Cells without TGF-β1/D3 pretreatment were used as controls.
Flow cytometry
Cells (5 × 105) were suspended in 200 µl of RPMI-1640 containing 2·5% FBS, and the Fc receptors were blocked by incubation with human serum, at a final concentration of 1%, for 15 min at 4°. Mouse mAbs directed against CD14, CD11b, CD11c and CD18 were then incubated with the cell suspensions for 30 min on ice. The cells were washed three times in RPMI-1640 containing 2·5% FBS and then resuspended in 200 µl of the same medium containing FITC-conjugated rabbit anti-mouse F(ab′)2 fragments and incubated for a further 30 min on ice. Cells were washed three times in phosphate-buffered saline (PBS) containing 0·5% BSA and then fixed in 200 µl of prechilled 0·5% paraformaldehyde in PBS for 15 min. The paraformaldehyde was washed off and the cells were resuspended in PBS at a concentration of 106 cells/ml. The mean fluorescence value of the cell population was determined by measuring the fluorescence intensity of 10 000 cells using a Coulter Epics Elite Esp Flow Cytometry System (Coulter, Fullerton, CA). Excitation wavelength was set at 488 nm, and emission wavelength was at 525 nm. Non-specific fluorescence was determined on cells incubated with mouse IgG, i.e. irrelevant antigen specificity. Background was determined on cells probed with only the secondary antibody.
Adhesion assays
TGF-β1/D3-primed U937 cells were suspended at a concentration of 106 cells/ml in RPMI-1640 containing 10% FBS. Cells were treated with PTX or purified PTX B-oligomer in the presence or absence of various mAbs. Aliquots (100 µl) of treated cells were then placed in 96-well microtitre plates in duplicate. PTX-induced cell adhesion in serum was allowed to take place for 1 hr at 37°. Non-adherent cells were flicked out and the adherent cells were fixed in methanol for 15 min and then stained with Geimsa stain for 1 hr. Absorbance at 550 nm was measured using a microplate reader (Tecan, Grödig, Austria). Data obtained from PTX-induced cell adhesion in the absence of mAb treatment were considered as the 100% control adherent response, and the effects of mAbs were expressed as a percentage of the control adherent response.
To determine the interaction between PTX and the cell-surface binding site(s) on primed U937 cells, we examined the direct binding of primed U937 cells to immobilized PTX. One-hundred microlitre aliquots of PTX (10 µg/ml) in PBS were added to 96-well microtitre plate and were allowed to coat the wells overnight at 4°. After blocking the coated wells with 1% BSA for 2 hr at 37°, the experiments were then performed, as described above, in the absence of FBS and added PTX.
To confirm that PTX-induced myeloid cell adhesion to vitronectin in serum is mediated by uPAR, we examined the adherent response in the presence and absence of anti-uPAR mAb and anti-αv mAb. One-hundred microlitre aliquots of vitronectin (5 µg/ml) in PBS were added to 96-well microtitre plates and were allowed to coat the wells overnight at 4°. After blocking the coated wells with 1% BSA for 2 hr at 37°, the experiments were performed (as described above) in the absence of FBS.
Intracellular Ca2+ measurement
Cells (106/ml) were loaded with the intracellular calcium concentration ([Ca2+]i) indicator dye Fluo-3/AM (10 µm) for 1 hr at 37° in RPMI-1640 and then washed three times with a physiological buffer (140 mm NaCl, 4 mm KCl, 1 mm KH2PO4, 1·8 mm CaCl2, 0·8 mm MgCl2, 10 mm glucose and 0·1% BSA). Cells were then resuspended in this buffer at 107 cells/ml. To prevent efflux of the organic anion, Fluo-3, from the cells, probenecid at a final concentration of 10 µm was added to the cell suspension. After incubation with different mAbs for 30 min, a 40-µl aliquot of cell suspension was transferred to a cuvette containing 2 ml of physiological buffer and then challenged with PTX (10 µg/ml). (All procedures in the cuvette were performed with continuous stirring and at a constant temperature of 37°.) Ca2+-specific fluorescence was measured using a spectrofluorometer (Shimadzu, Tokyo, Japan), with excitation wavelength set at 506 nm and emission wavelength set at 526 nm. Maximum fluorescence value was achieved by the addition of 200 mm KCl and 0·5% Triton-X-100, and minimum fluorescence value was attained by the addition of 20 mm EGTA and 25 mm MnCl2. A Kd of 320 nm for Fluo-3 was used to calculate [Ca2+]i, as described previously.17
Biotinylation of PTX
Biotinylation of PTX was performed according to the manufacturer's instructions (Pierce Chemical Co., Rockford, IL). PTX was dialysed against 50 mm NaHCO3 (pH 8·0) at 4° overnight and concentrated to ≈ 1 mg/ml using microcentrifuge filters (Millipore Co., Bedford, MA). The concentrated PTX was then incubated (at room temperature for 40 min with gentle rotation) with 1 mg/ml of sulpho-NHS-LC-biotin, at a molar ratio of 1 : 50 (PTX : biotin). The biotinylation reaction was stopped by dialysing the biotinylated PTX overnight at 4° against 25 mm Tris (pH 8·0), 25 mm glycine and 0·5 m NaCl. The biotinylated PTX was stored at −20° until use.
[35S]Methionine labelling and receptor isolation
U937 cells (106/ml) were first primed with TGF-β1/D3 in RPMI-1640, containing 10% FBS, for 24 hr and then incubated in methionine-free RPMI-1640 containing 10% FBS and 100 µCi/ml of [35S]methionine (specific activity > 1000 Ci/mmol) for a further 24 hr. The radiolabelled cells were spun down at 800 g and washed four times with PBS. The cell aggregates were then incubated in lysis buffer [50 mm Tris-HCl, pH 7·5, 140 mm NaCl, 1% Triton-X-100, 2 mm phenylmethylsulphonyl fluoride (PMSF), 10 µg/ml of aprotinin, 50 µg/ml of benzamidine, and 5 µg/ml of pepstatin, leupeptin and antipain] for 1 hr at 4° with gentle shaking. The lysate was centrifuged for 20 min at 24 000 g and the supernatant was precleared by incubation with streptavidin-agarose (1 µl/100 µl of lysate) for 30 min at 4° with gentle rotation. After centrifugation, the supernatant was incubated with biotinylated PTX (10 µg/106 cells) in the presence or absence of excess unlabelled PTX (40 µg/106 cells) or different mAbs for 1 hr at room temperature. The biotinylated PTX : receptor complex was then precipitated by streptavidin-agarose, at a ratio of 1 µl/µg of biotinylated PTX,18 for 1·5 hr at 4°. After centrifugation at 800 g for 1 min at 4°, the pellet was washed four times with PBS containing 0·1% Nonidet P-40 (NP-40) and 100 µg/ml of PMSF. The bound receptors for PTX were then eluted with 0·1 m glycine-HCl (pH 2·8) for 30 min at 4°, and the collected supernatant was titrated to neutral pH with 0·25 m NaOH, mixed with 0·25 vol. of 5 × electrophoresis sample buffer, boiled for 5 min, and subjected to 4–20% gradient sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE). The gel was dried and developed by autoradiography.
Data analysis
All data are presented as mean ± SEM. Statistical differences in adherent responses between the controls and the treatment groups were analysed using analysis of variance (anova) followed by the Student Newman–Keuls test. The critical level for significance was set at P < 0·05.
Results
TGF-β1/D3-induced U937 cell differentiation
Human monoblastic leukaemic U937 cells differentiated to a monocyte/macrophage phenotype after TGF-β1/D3 treatment for 48 hr. The expression of monocyte differentiation antigen, CD14, and other differentiation-related adhesion proteins, including CD11c, CD11b and CD18, was monitored (using flow cytometry) before and after TGF-β1/D3 priming. In control U937 cells, the levels of CD14 and CD11c were as low as the background value whereas the expression of CD11b and CD18 was substantially higher. The rank order for adhesion protein expression in control U937 cells was CD18 >> CD11b > CD11c > CD14. After priming with TGF-β1/D3 for 48 hr, the mean fluorescence intensities for cell-surface expression of CD18, CD11b, CD11c and CD14 were significantly increased by 2·6-, 7·5-, 1·2- and 5·1-fold, respectively. The level of CD18 expressed on differentiated U937 cells was substantially higher than that of the other three antigens (Table 1).
Table 1.
Expression of monocyte-differentiation antigens on U937 cells before and after treatment with transforming growth factor-β1 and 1,25-(OH)2 vitamin D3 (TGF-β1/D3)
| Mean fluorescence intensities | ||
|---|---|---|
| Control | TGF-β1/D3 | |
| Background (FITC) | 3·0 ± 0·4 | 3·2 ± 0·8 |
| IgG | 2·7 ± 0·2 | 2·9 ± 0·3 |
| CD11c | 5·3 ± 0·2 | 6·6 ± 0·2* |
| CD14 | 3·0 ± 0·2 | 15·5 ± 2·0* |
| CD11b | 13·5 ± 0·8 | 101·3 ± 12·4* |
| CD18 | 115·9 ± 3·3 | 296·7 ± 11·4* |
P < 0·05 compared with control U937 cells.
Each value represents mean ± SEM of three to four experiments. The mean fluorescence value of the cell population was determined by measuring the fluorescence intensity of 10 000 cells.
FITC, fluorescein isothiocyanate.
PTX-induced myelomonocytic cell adhesion
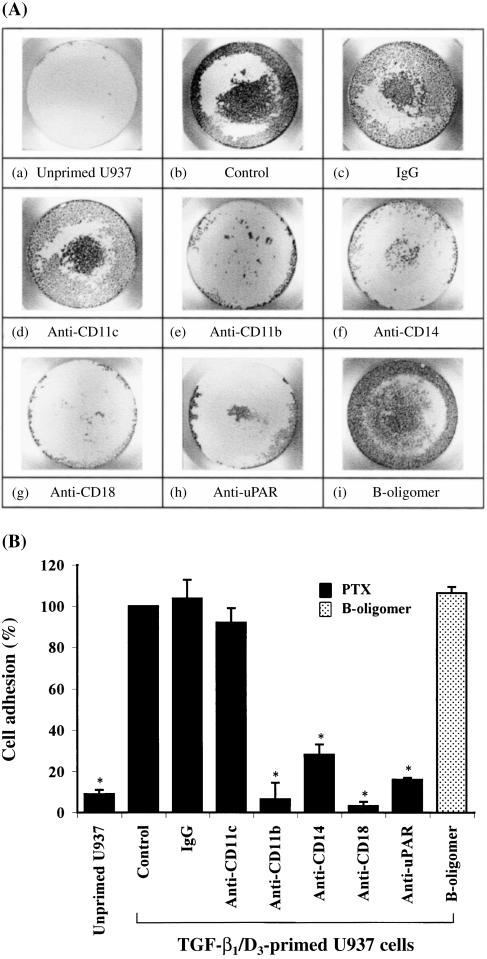
Figure 1(A) shows that only TGF-β1/D3-primed (panel b), but not unprimed (panel a), U937 cells adhered to serum in response to PTX. Both PTX holotoxin and PTX B-oligomer were able to promote primed U937 cell adhesion to the same extent. Mouse mAbs directed against CD14, CD11b and CD18, but not against CD11c, significantly inhibited PTX-induced primed U937 cell adhesion by 72%, 94% and 97%, respectively (Fig. 1B). In addition, mAb against domain II of uPAR (CD87), the binding domain for vitronectin,16 substantially reduced the cell adhesion by 84%. These results indicate that CD14, CD11b, CD18 and uPAR are involved in PTX-induced myeloid cell adhesion in serum.
Figure 1.
Pertussis toxin (PTX)-induced myeloid cell adhesion in serum. (A) Representative photographs showing PTX-induced myeloid cell adhesion in a 96-well plate. Panel (a) represents unprimed U937 cells and panels (b) to (i) represent transforming growth factor-β1/1,25-(OH)2 vitamin D3 (TGF-β1/D3)-primed U937 cells. Panels (a) to (h) display the adherent responses of myeloid cells to PTX holotoxin (10 µg/ml) in the presence and absence of mouse monoclonal antibodies (mAbs) (2 µg/ml) directed against CD11c, CD11b, CD14, CD18 or urokinase receptor (uPAR). Panel (i) shows the adherent response of myeloid cells to PTX B-oligomer (3 µg/ml) alone. (B) Cumulative results showing the effects of different mAbs on PTX-induced myeloid cell adhesion. Each value represents the mean ± SEM of four to six experiments performed in duplicate and is expressed as the percentage of control adherent responses to PTX. *P < 0·05 compared with primed U937 cell controls. IgG, immunoglobulin G.
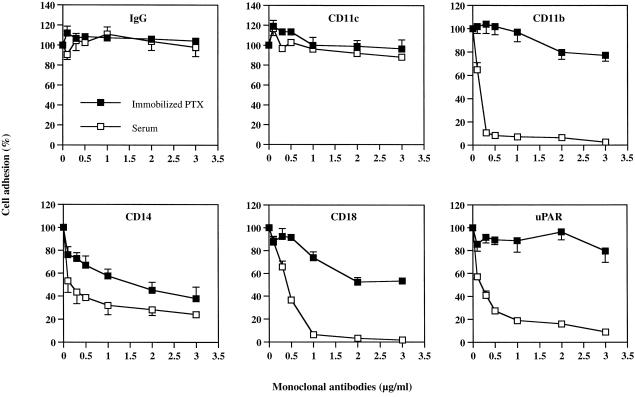
To further delineate which cell-surface proteins are the binding site(s) for PTX and which are responsible for the focal adhesion to serum, we examined the effects of increasing concentrations of mAbs on PTX-induced myelomonocytic cell adhesion in serum (for focal adhesion) and on cell binding to immobilized PTX (for PTX-binding sites). As shown in Fig. 2, anti-CD11c failed to block either PTX-induced cell adhesion to serum or cell binding to immobilized PTX at a concentration up to 3 µg/ml. However, anti-CD14 inhibited, in a concentration-dependent manner, both the PTX-induced cell adhesion to serum (by up to 76%) and the cell binding to immobilized PTX (by up to 62%) at 3 µg/ml, in a highly correlated manner (r = 0·93). In contrast, while the mAbs against CD11b, CD18 and uPAR dramatically abolished the PTX-induced cell adhesion to serum (focal adhesion) by 92%, 93% and 81%, respectively, at 0·5–1 µg/ml, they failed to prevent primed U937 cells from binding to immobilized PTX (binding sites) at these same concentrations. At a higher concentration (2 µg/ml), anti-CD18 mAb afforded a 48% inhibition on cell binding to PTX-coated plates.
Figure 2.
Concentration–response studies of the effects of different monoclonal antibodies (mAbs) on pertussis toxin (PTX) (10 µg/ml)-induced primed U937 cell adhesion in serum and on direct cell binding to immobilized PTX (10 µg/ml). Each value represents the mean ± SEM of four to six experiments performed in duplicate and is expressed as the percentage of control adherent responses to PTX. IgG, immunoglobulin G; uPAR, urokinase receptor.
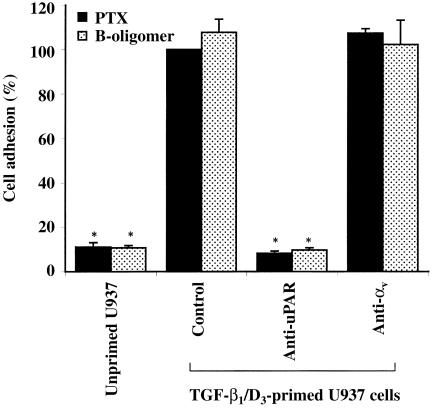
uPAR has recently been shown to be a specific receptor for vitronectin, a major extracellular matrix protein present in serum.16,19 We examined PTX-induced myelomonocytic cell adhesion to vitronectin-coated plates in the presence and absence of mAbs against uPAR. Figure 3 shows that PTX promoted primed, but not unprimed, U937 cell adhesion to vitronectin-coated plates. Mouse mAb directed against domain II of uPAR blocked PTX holotoxin- or PTX B-oligomer-induced cell adhesion to vitronectin by more than 90%. Anti-αv mAb directed against other vitronectin receptors, such as αvβ3 and αvβ5,20 did not inhibit the PTX-induced cell adhesion to vitronectin-coated plates.
Figure 3.
Pertussis toxin (PTX)-induced primed U937 cell adhesion to vitronectin (5 µg/ml)-coated plates. U937 cells were treated with PTX holotoxin (10 µg/ml) or PTX B-oligomer (3 µg/ml) in the presence or absence of monoclonal antibodies (mAbs) directed against domain II of the urokinase receptor (uPAR) (2 µg/ml) or αv integrin (1 : 100 dilution). Each value represents the mean ± SEM of four to six experiments performed in duplicate and is expressed as the percentage of control adherent responses to PTX. *P < 0·05 compared with primed U937 cell controls. D3, 1,25-(OH)2 vitamin D3; TGF-β1, transforming growth factor-β1.
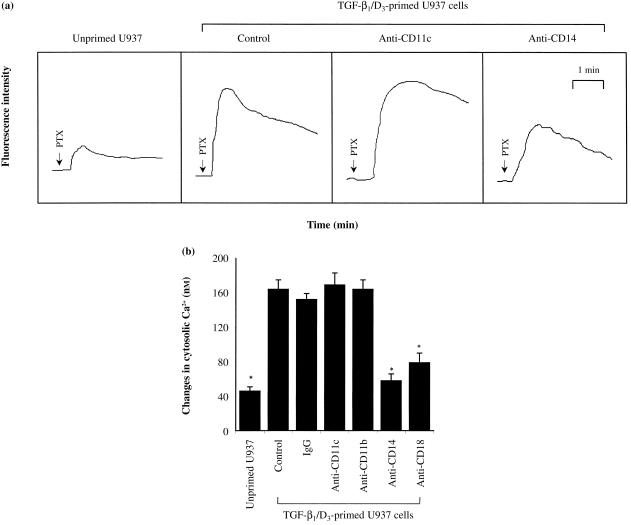
PTX-induced rise in [Ca2+]i in myelomonocytic cells
To functionally confirm the binding site(s) for PTX on myelomonocytic cells, we studied the PTX-induced rise of [Ca2+]i in the presence and absence of various mAbs. Figure 4(A) displays representative tracings of the changes in Ca2+ fluorescence intensity in myeloid cells treated with PTX. As shown in Fig. 4(B), PTX induced a substantially greater increase of [Ca2+]i in the TGF-β1/D3-primed U937 cells than in the unprimed U937 cells. The PTX-induced rise of [Ca2+]i was significantly inhibited by anti-CD14 mAb and, to a lesser extent, by anti-CD18 mAb. Whereas mAbs against CD11c and CD11b failed to block the increase of [Ca2+]i induced by PTX. These observations indicate that CD14, and probably CD18, are the potential binding sites for PTX on myelomonocytic cells.
Figure 4.
Pertussis toxin (PTX)-induced increase of intracellular calcium concentration ([Ca2+]i) in primed U937 cells in the presence and absence of mouse monoclonal antibodies (mAbs) (2 µg/ml) directed against CD11c, CD11b, CD14 or CD18. Cells (106/ml) were loaded with the [Ca2+]i indicator dye, Fluo-3/AM (10 µm), for 1 hr at 37° in RPMI-1640. After incubation with different mAbs for 30 min, aliquots of cell suspension were transferred to cuvettes and then challenged with PTX (10 µg/ml). Changes in Ca2+-specific fluorescence were measured using a spectrofluorometer. (a) Representative tracings of changes in cytosolic Ca2+ in myeloid cells, induced by PTX, as reflected by the changes in fluorescence intensity. (b) Cumulative results showing the changes in cytosolic Ca2+ (nm) in myeloid cells induced by PTX. Basal Ca2+ concentration was 117 ± 6 nm (n = 7). Each value represents the mean ± SEM of four to six experiments performed in duplicate. *P < 0·05 compared with control, unprimed, U937 cells. D3, 1,25-(OH)2 vitamin D3; IgG, immunoglobulin G; TGF-β1, transforming growth factor-β1.
PTX-binding site on myelomonocytic cells
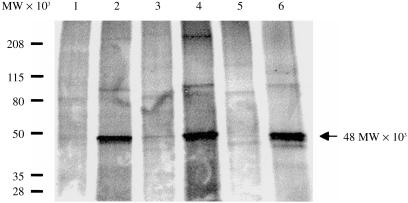
To obtain direct identification of the PTX-binding site on primed U937 cells, we conducted radiolabelled receptor-isolation experiments using biotinylated PTX. Our autoradiography results (Fig. 5) show that the biotinylated PTX isolated a protein of 48 000 MW from the primed U937 cells (lane 2), which is of the same molecular weight as human CD14 antigen isolated from HL-60 cells induced to differentiate to monocytes.21 This protein band disappeared when excess unlabelled PTX (lane 3) or anti-CD14 (lane 5), but not anti-CD11b (lane 4) or anti-CD18 (lane 6), was added to the receptor precipitation mixture. As expected, biotinylated PTX did not detect the 48 000-MW protein in unprimed U937 cells (lane 1) because the expression of CD14 in control U937 cells was very low. Taken together, our findings show that CD14 is a binding site for PTX on myelomonocytic cells.
Figure 5.
Isolation of pertussis toxin (PTX) receptor on myelomonocytic cells. U937 cells (106/ml) were first primed with transforming growth factor-β1/1,25-(OH)2 vitamin D3 (TGF-β1/D3) in RPMI-1640 for 24 hr and then in methionine-free RPMI-1640, together with 100 µCi/ml of [35S]methionine, for a further 24 hr. The radiolabelled cells were lysed, precleared with streptavidin-agarose and then incubated with biotinylated PTX (10 µg/106 cells) in the absence (lane 1, U937 cells; lane 2, primed U937 cells) and presence of a fourfold excess of unlabelled PTX (lane 3), anti-CD11b (lane 4), anti-CD14 (lane 5) or anti-CD18 (lane 6), for 1 hr at room temperature. The PTX-bound receptors were precipitated by streptavidin-agarose, eluted with 0·1 m glycine-HCl, pH 2·8, and then separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) (4–20%). The gel was dried and developed by autoradiography. The arrowhead indicates the protein band recognized by biotinylated PTX.
Discussion
The present study was undertaken to identify the binding site(s) and the adhesion molecule(s) responsible for PTX-induced myelomonocytic cell adhesion. We have presented evidence from functional studies and receptor-isolation experiments to show that CD14 is a binding site for PTX on myelomonocytic cells. Our data showed that anti-CD14 mAb inhibited PTX-induced myeloid cell adhesion to serum in a concentration-dependent manner, which correlates strongly (r = 0·93) with the prevention of binding of primed U937 cells to PTX-coated plates. In contrast to anti-CD14, mAbs directed against CD11b, CD18 and uPAR dramatically blocked PTX-induced myeloid cell adhesion in serum at a concentration that failed to prevent any substantial binding of primed U937 cells to immobilized PTX. In our receptor-isolation study, biotinylated PTX was able to cross-link a 48 000-MW protein in TGF-β1/D3-primed U937 cells, and the PTX binding to this protein was specifically blocked by a fourfold excess of non-biotinylated PTX or by anti-CD14 mAb. The mouse anti-CD14 mAb used in this study (clone TÜK4) has been characterized by a number of laboratories, confirming its reactivity with the CD14 antigen.22 This 48 000-MW protein will probably be the same human CD14 antigen lacking the glycosyl-phosphatidylinositol (GPI) linkage isolated from HL-60 cells induced to differentiate to monocytes.21 In line with the findings of van't Wout et al.,7 that PTX B-oligomer contains a carbohydrate-recognition domain which recognizes Lea and Lex determinants on human macrophages, CD14 has been identified to possess sialylated carbohydrate moieties at the N-glycosylation sites.23 PTX was shown to bind to human platelet GPIb, inducing rapid platelet aggregation and an increase of [Ca2+]i.12,24 It has been documented that both human platelet GPIb and CD14 are leucine-rich glycoproteins with a similar leucine motif, which is believed to play a role in ligand binding and adhesion.25 These findings, together with our receptor-binding results, support the role of CD14 as a binding site for PTX on myelomonocytic cells.
Although CD14 belongs to a family of GPI-anchored cell-surface proteins that lack a traditional transmembrane sequence for signal transduction,26,27 cumulative evidence has shown that CD14 is physically associated with src-related protein tyrosine kinases (e.g. p53/56lyn and p56/p59hck), the αi/αo subunit of the heterotrimeric G proteins and β2-integrin (CD18) for signal transduction.28–30 Functional studies have also shown that cross-linking of CD14 by lipopolysaccharide (LPS) induces protein tyrosine phosphorylation, MAPK activation, protein kinase C activity and an increase of [Ca2+]i in monocytes and other cell types.28,31–33 Our previous studies showed that PTX elicited protein tyrosine phosphorylation and an increase of [Ca2+]i in TGF-β1/D3-primed U937 cells.15,34 The present study extended these findings by showing that anti-CD14 and anti-CD18, but not anti-CD11b or anti-uPAR, significantly inhibited a PTX-induced rise of [Ca2+]i in primed U937 cells. These observations further support the notion that CD14 is a binding site for PTX on myelomonocytic cells. The inhibitory effect of anti-CD18 on [Ca2+]i might be the result of steric interference, as CD14 and CD18 have been shown to be closely associated.30,35
The inhibitory effects elicited by anti-CD11b, anti-CD18 and anti-uPAR on PTX-induced myelomonocytic cell adhesion in serum probably occur at the focal adhesion sites. This can be explained by the findings that at the same concentration which significantly inhibited PTX-induced myelomonocytic cell adhesion in serum, these mAbs failed to prevent the cells from binding to immobilized PTX. Substantial evidence showed that Mac-1 (CD11b/CD18) and uPAR were isolated as a receptor complex from human monocytes36 and acted as a functional unit to complement each other's activities.36–38 In addition, uPAR has recently been identified as a high-affinity adhesion molecule for vitronectin,16 a major extracellular matrix protein for cell attachment in serum.19 Our results show that PTX promoted myeloid cell adhesion to vitronectin-coated plates and that the adhesion was abolished by anti-uPAR mAb directed against domain II of the receptor that recognizes vitronectin.16 We believe that PTX-induced myelomonocytic cell adhesion in serum is specifically mediated by uPAR because the anti-αv mAb directed against the αv chain of other vitronectin receptors (e.g. αvβ3 and αvβ5)20 failed to block the adhesion to vitronectin. In addition, the uPAR-mediated adherent response is closely regulated by Mac-1 (CD11b/CD18) as mAbs against CD11b and CD18 also inhibited the PTX-induced myeloid cell adhesion in serum.
Hence, we propose that CD14 is a binding site for PTX on myelomonocytic cells, and uPAR is the adhesion molecule responsible for the PTX-induced myeloid cell adhesion in serum. The regulation of uPAR activity induced by CD14 interaction with PTX may present an early pathological step utilized by B. pertussis to down-regulate macrophage migration capacity in the airway and to facilitate bacterial internalization into the macrophages.39 Further study to investigate the in vivo protective effects of anti-CD14 mAb in PTX-induced respiratory tract infection is warranted. In addition, the present study may provide insight into a potential PTX-mediated regulation of HIV infection in myeloid cells, as has been illustrated in T lymphocytes.13,14
Acknowledgments
The authors wish to thank Dr K. Y. Leung and Dr Y. K. Ip of the Department of Biological Sciences at the National University of Singapore for their assistance in [Ca2+]i measurement; Dr M. Manganel and Dr E. M. Gutkneckt of Hoffman-LaRoche Ltd. (Basel, Switzerland) for the kind gift of 1,25-(OH)2 vitamin D3; and Dr P. Licari and Dr C. Nesman of Massachusetts Public Health Biologic Laboratories (Boston, MA) for the generous gift of purified PTX. This work was supported by grant NMRC/0098/1995 of the National Medical Research Council of Singapore (to W. S. Fred Wong).
Glossary
Abbreviations
- [Ca2+]i
intracellular calcium concentration
- D3
1,25-(OH)2 vitamin D3
- FITC
fluorescein isothiocyanate
- HIV
human immunodeficiency virus
- mAb
monoclonal antibody
- PTX
pertussis toxin
- TGF-β1
transforming growth factor-β1
- uPAR
urokinase receptor
References
- 1.Pizza MG, Covacci A, Bartoloni A, et al. Mutants of pertussis toxin suitable for vaccine development. Science. 1989;246:497. doi: 10.1126/science.2683073. [DOI] [PubMed] [Google Scholar]
- 2.Edwards KM. Acellular pertussis vaccines – a solution to the pertussis problem? J Infect Dis. 1993;168:15. doi: 10.1093/infdis/168.1.15. [DOI] [PubMed] [Google Scholar]
- 3.Tamura M, Nogimori K, Murai S, et al. Subunit structure of islet-activating protein, pertussis toxin, in conformity with the A-B model. Biochemistry. 1982;21:5516. doi: 10.1021/bi00265a021. [DOI] [PubMed] [Google Scholar]
- 4.Nicosia A, Perugini M, Finzini C, et al. Cloning and sequencing of the pertussis toxin genes: operon structure and gene duplication. Proc Natl Acad Sci USA. 1986;83:4631. doi: 10.1073/pnas.83.13.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gierschik P. ADP-ribosylation of signal-transducing guanine nucleotide-binding proteins by pertussis toxin. Curr Top Microbiol Immunol. 1992;175:69. doi: 10.1007/978-3-642-76966-5_4. [DOI] [PubMed] [Google Scholar]
- 6.Wong WSF, Rosoff PM. Pharmacology of pertussis toxin B-oligomer. Can J Physiol Pharmacol. 1996;74:559. doi: 10.1139/cjpp-74-5-559. [DOI] [PubMed] [Google Scholar]
- 7.van'T Wout J, Burnette WN, Mar VL, Rozdzinski E, Wright SD, Tuomanen EI. Role of carbohydrate recognition domains of pertussis toxin in adherence of Bordetella pertussis to human macrophages. Infect Immun. 1992;60:3303. doi: 10.1128/iai.60.8.3303-3308.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennan MJ, David JL, Kenimer JG, Manclark CR. Lectin-like binding of pertussis toxin to a 165-kilodalton Chinese hamster ovary cell glycoprotein. J Biol Chem. 1988;263:4895. [PubMed] [Google Scholar]
- 9.Rogers TS, Corey SJ, Rosoff PM. Identification of a 43-kilodalton human T lymphocyte membrane protein as a receptor for pertussis toxin. J Immunol. 1990;145:678. [PubMed] [Google Scholar]
- 10.Lei MG, Morrison DC. Evidence that lipopolysaccharide and pertussis toxin bind to different domains on the same p73 receptor on murine splenocytes. Infect Immun. 1993;61:1359. doi: 10.1128/iai.61.4.1359-1364.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armstrong GD, Clark CG, Heerze LD. The 70-kilodalton pertussis toxin-binding protein in Jurket cells. Infect Immun. 1994;62:2236. doi: 10.1128/iai.62.6.2236-2243.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sindt KA, Hewlett EL, Redpath GT, Rappuoli R, Gray LS, Vandenberg SR. Pertussis toxin activates platelets through an interaction with platelet glycoprotein Ib. Infect Immun. 1994;62:3108. doi: 10.1128/iai.62.8.3108-3114.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alfano M, Schmidtmayerova H, Amella CA, Pushkarsky T, Bukrinsky M. The B-oligomer of pertussis toxin deactivates CC chemokine receptor 5 and blocks entry of M-tropic HIV-1 strains. J Exp Med. 1999;190:597. doi: 10.1084/jem.190.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang JM, Oppenheim JJ. Interference with the signaling capacity of CC chemokine receptor 5 can compromise its role as an HIV-1 entry coreceptor in primary T lymphocytes. J Exp Med. 1999;190:591. doi: 10.1084/jem.190.5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong WSF, Simon DI, Rosoff PM, Rao NK, Chapman HA. Mechanisms of pertussis toxin-induced myelomonocytic cell adhesion: role of Mac-1 (CD11b/CD18) and urokinase receptor (CD87) Immunology. 1996;88:90. doi: 10.1046/j.1365-2567.1996.d01-646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei Y, Waltz DA, Rao N, Drummond RJ, Rosenberg S, Chapman HA. Identification of the urokinase receptor as an adhesion receptor for vitronectin. J Biol Chem. 1994;269:32380. [PubMed] [Google Scholar]
- 17.Kao JPY, Harootunian AT, Tsien RY. Photochemically generated cytosolic calcium pulses and their detection by fluo-3. J Biol Chem. 1989;264:8179. [PubMed] [Google Scholar]
- 18.Updyke TV, Nicolson GL. Immunoaffinity isolation of membrane antigens with biotinylated monoclonal antibodies and streptavidin-agarose. Methods Enzymol. 1986;121:717. doi: 10.1016/0076-6879(86)21070-8. [DOI] [PubMed] [Google Scholar]
- 19.Preissner KT, Jenne D. Vitronectin: a new molecular connection in haemostasis. Thromb Haemost. 1991;66:189. [PubMed] [Google Scholar]
- 20.Smith JW, Vestal DJ, Irwin SV, Burke TA, Cheresh DA. Purification and functional characterization of integrin αvβ5. J Biol Chem. 1990;265:11008. [PubMed] [Google Scholar]
- 21.Maliszewski CR, Ball ED, Graziano RF, Fanger MW. Isolation and characterization of My23, a myeloid cell-derived antigen reactive with the monoclonal antibody AML-2. J Immunol. 1984;135:1929. [PubMed] [Google Scholar]
- 22.Goyert SM, Tesio L, Ashman LK, et al. Report on the CD14 cluster workshop. In: Knapp W, editor. Leukocyte Typing IV, White Cell Differentiation Antigens. Oxford: Oxford University Press; 1989. p. 789. [Google Scholar]
- 23.Bazil V, Horejsi V, Baudys M, et al. Biochemical characterization of a soluble form of the 53-kDa monocyte surface antigen. Eur J Immunol. 1986;16:1583. doi: 10.1002/eji.1830161218. [DOI] [PubMed] [Google Scholar]
- 24.Banga HS, Walker RK, Winberry LK, Rittenhouse SE. Pertussis toxin can activate human platelets. J Biol Chem. 1987;262:14871. [PubMed] [Google Scholar]
- 25.Setoguchi M, Nasu N, Yoshida S, Higuchi Y, Akizuki S, Yamamoto S. Mouse and human CD14 (myeloid cell-specific leucine-rich glycoprotein) primary structure deduced from cDNA clones. Biochim Biophys Acta. 1989;1008:213. doi: 10.1016/0167-4781(80)90012-3. [DOI] [PubMed] [Google Scholar]
- 26.Simmons DL, Tan S, Tenen DG, Nicholson-Weller A, Seed B. Monocyte antigen CD14 is a phospholipid anchored membrane protein. Blood. 1989;73:284. [PubMed] [Google Scholar]
- 27.Kielian TL, Blecha F. CD14 and other recognition molecules for lipopolysaccharide: a review. Immunopharmacology. 1995;29:187. doi: 10.1016/0162-3109(95)00003-c. [DOI] [PubMed] [Google Scholar]
- 28.Stefanova I, Coreoran ML, Horak EM, Wahl LM, Bolen JB, Horak ID. Lipopolysaccharide induces activation of CD14-associated protein tyrosine kinase p53/56lyn. J Biol Chem. 1993;268:20725. [PubMed] [Google Scholar]
- 29.Solomon KR, Kurt-Jones EA, Saladino RA, et al. Heterotrimeric G proteins physically associated with the lipopolysaccharide receptor CD14 modulate both in vivo and in vitro responses to lipopolysaccharide. J Clin Invest. 1998;102:2019. doi: 10.1172/JCI4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Todd RF, Petty HR. β2(CD11/CD18) integrins can serve as signaling partners for other leukocyte receptors. J Lab Clin Med. 1997;129:492. doi: 10.1016/s0022-2143(97)90003-2. [DOI] [PubMed] [Google Scholar]
- 31.Weinstein SL, Sanghera JS, Lemke K, Defranco AL, Pelech SL. Bacterial lipopolysaccharide induces tyrosine phosphorylation and activation of mitogen-activated protein kinases in macrophages. J Biol Chem. 1992;267:14955. [PubMed] [Google Scholar]
- 32.Shapira L, Takashiba S, Champagne C, Anar S, Van Dyke TE. Involvement of protein kinase C and protein tyrosine kinase in lipopolysaccharide-induced TNF-α and IL-1β production by human monocytes. J Immunol. 1994;153:1818. [PubMed] [Google Scholar]
- 33.Ikejima K, Enomoto N, Seabra V, Ikejima A, Brenner DA, Thurman RG. Pronase destroys the lipopolysaccharide receptor CD14 on Kupffer cells. Am J Physiol. 1999;276:G591. doi: 10.1152/ajpgi.1999.276.3.G591. [DOI] [PubMed] [Google Scholar]
- 34.Wong WSF, Luk JL. Signaling mechanisms of pertussis toxin-induced myelomonocytic cell adhesion: role of tyrosine phosphorylation. Biochem Biophys Res Commun. 1997;236:479. doi: 10.1006/bbrc.1997.6986. [DOI] [PubMed] [Google Scholar]
- 35.Petty HR, Kindzelskii AL, Adachi Y, Todd RF., III Ectodomain interactions of leukocyte integrins and pro-inflammatory GPI-linked membrane proteins. J Pharm Biomed Anal. 1997;15:1405. doi: 10.1016/s0731-7085(96)02030-4. [DOI] [PubMed] [Google Scholar]
- 36.Bohuslav J, Horejsi V, Hansmann C, et al. Urokinase plasminogen activator receptor, β2-integrins, and src-kinases within a single receptor complex of human monocytes. J Exp Med. 1995;181:1381. doi: 10.1084/jem.181.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sitrin RG, Todd RF, Petty HR, et al. The urokinase receptor (CD87) facilitates CD11b/CD18-mediated adhesion of human monocytes. J Clin Invest. 1996;97:1942. doi: 10.1172/JCI118626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simon DI, Rao NK, Xu H, et al. Mac-1 (CD11b/CD18) and the urokinase receptor (CD87) form a functional unit on monocytic cells. Blood. 1996;88:3185. [PubMed] [Google Scholar]
- 39.Saukkonen K, Cabellos C, Burroughs M, Prasad S, Tuomanen E. Integrin-mediated localization of Bordetella pertussis within macrophages: role in pulmonary colonization. J Exp Med. 1991;173:1143. doi: 10.1084/jem.173.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]