Abstract
Interleukin-10 (IL-10) down-regulates T helper type 1 cell and macrophage functions. As IL-10 is induced along with tumour necrosis factor (TNF) and IL-12 in mycobacterial infection, we asked whether endogenous IL-10 plays a role in the antimycobacterial response. We demonstrate here that IL-10-deficient mice eliminate Mycobacterium bovis Calmette–Guérin bacillus faster than wild-type mice. Granulomas are significantly larger, containing more CD-11b- and CD11c-positive antigen-presenting cells and T cells, and the expression of major histocompatibility complex class II and intracellular adhesion molecule-1 is increased. Macrophages in granulomas of IL-10-deficient mice express high levels of TNF, acid phosphatase and inducible nitric oxide synthase (iNOS). Finally, an increased cutaneous delayed-type hypersensitivity reaction to mycobacterial proteins is further evidence of an augmented cell-mediated immune response. In conclusion, the cell-mediated immunity is enhanced in the absence of IL-10, resulting in a robust granuloma response, which accelerates the clearance of mycobacteria. Therefore, endogenous IL-10 attenuates mycobacterial immunity.
Introduction
Infection with Mycobacterium tuberculosis is a leading global health threat.1,2 Incomplete understanding of the nature of protective immune-inflammatory responses has hampered the development of effective vaccines and therapies. The cell-mediated immune response plays a key role in host resistance to mycobacterial infection. The cell-mediated immune response is tightly regulated by a fine balance between T helper type 1 (Th1) cytokines [interferon-γ (IFN-γ), interleukin-2 (IL-12), tumour necrosis factor (TNF), IL-18) and Th2 cytokines (IL-4, IL-10, IL-13). 3 Activation of macrophages by IFN-γ derived from T and natural killer cells is essential for the ultimate eradication of M. tuberculosis bacilli. Mice with disruption of Th1 cytokine signalling, e.g. IFN-γ and IL-12, are unable to restrict the growth of M. tuberculosis and succumb to infection. 4–8 In humans, deficiencies of the IFN-γ and IL-12 receptors9,10 are also associated with a predisposition to mycobacterial infections. TNF is induced in human and experimental tuberculosis 2 and its role in mycobacterial defence is inferred from neutralization and deletion experiments in mice.7,11,12
Besides the dominant Th1 response, mycobacterial infections also induce a Th2 component. 13 IL-4 and IL-10 expression may down-modulate macrophage function and thereby reduce their microbicidal functions. 14 Transgenic expression of IL-10 was demonstrated to antagonize macrophage activity in mycobacterial infection. 15 By contrast, neutralization of IL-10 enhanced host resistance to M. avium infection,13,16 indicating that endogenous IL-10 attenuates the host response. However, based on recent data from IL-10-deficient mice the role of endogenous IL-10 is controversial: Both no effect on M. bovis bacillus Calmette–Guérin (BCG) 17 or M. tuberculosis infection 18 and increased antimycobacterial immunity to M. bovis BCG infection have been reported. 19
We asked here whether endogenous IL-10 induced by M. bovis BCG infection plays a role in the early phase of host resistance. Using IL-10-deficient mice 20 we show that endogenous IL-10 has indeed a regulatory role and attenuates the Th1-type immune response in M. bovis infection. In the absence of IL-10, large granulomas are formed containing highly activated macrophages allowing a rapid clearance of bacilli.
Materials and methods
Animals
Adult, 8–10-week-old female mice of the wild-type strain C57BL6/129, homozygous IL-10-deficient strains, 20 all on a C57BL6/129 genetic background were kept under specific pathogen-free conditions. The genotypes of the mouse populations were confirmed by polymerase chain reaction (PCR) analysis of DNA from tail biopsies.
Antibodies
The following rat anti-mouse monoclonal antibodies were used for immunohistochemical detection: intracellular adhesion molecule-1 (ICAM-1), F4/80, major histocompatibility complex (MHC) class II antigen, TNF (MP6-XT22), IFN-γ (XMG1), IL-12 (C15·6), CD3, CD4 and CD8, CD11b and CD11c from Pharmingen. Rabbit antibody against inducible nitric oxide synthase (iNOS) was diluted 1 : 2000 (obtained from Prof. J. Pfeilschifter, Department of Pharmacology, University of Frankfurt, Germany).
Experimental design
Wild-type and IL-10-deficient mice were infected intravenously with 106 colony-forming units (CFU) BCG (Pasteur strain). Groups consisted of five mice and the experiments were repeated three times. At 2, 4 and 8 weeks after infection blood was taken by retro-orbital venous puncture, and mice were killed at each time-point. The lung, liver and spleen were weighed. Parts of the organs were either fixed in 4% buffered formalin, or frozen on dry ice and maintained at −80°. About 2-µm thick sections of the spleen, liver and lungs were cut and stained with either haematoxylin & eosin or Ziehl–Neelsen for staining the acid-fast bacilli.
Mononuclear cell clusters of more than 15 cells were considered to be granulomas. Two independent observers (NB and BR) counted granulomas and acid-fast bacilli in 25 randomly selected high-power fields (magnification 200 × and 400 ×, respectively) from five liver cross-sections per animal without knowledge of the group. Mean values ± SD (n = 5 mice) are given.
Immunohistochemistry
The frozen tissues (liver, spleen and lungs) were cut 6-µm thick on a cryostat, air-dried for storage at −80° and incubated as described. 21 Fixation in acetone (10 min at 4°) was performed just before the immunolabelling. After a rinse in phosphate-buffered saline (PBS) the sections were incubated for 16 hr at 4° with the primary antibodies diluted in PBS. The sections were then washed in PBS and incubated for 30 min at room temperature with rabbit anti-rat serum ABC system (Vector Lab, Burlington), followed by DAB substrate. After rinses in PBS the sections were mounted in Immunomount (Shandon, Pittsburgh, PA).
Colony-forming units
Bacterial loads in the lung, liver and spleen of infected mice were evaluated at 1 day and at 2, 4 and 8 weeks post-infection. Organs were weighed and defined aliquots were homogenized in saline containing 0·04% Tween. Ten-fold serial dilutions of organ homogenates were plated in duplicates onto Middlebrook 7H10 agar plates containing 10% OADC (Difco, Detroit, MI). Plates were incubated at 37° for 3 weeks and colonies were counted. Data are expressed as CFU per organ (n = 4 mice per group).
Enzyme-linked immunosorbent assays (ELISAs) for cytokines
TNF, IL-12 and IFN-γ levels in the serum of BCG infected mice at 4 weeks were measured using commercial ELISA kits (R & D Systems, Abingdon, UK).
Detection of acid phosphatase in tissues
Acid phosphatase on frozen tissue sections was performed as reported previously. 22 In order to quantify the enzymatic activity fresh livers and spleens were homogenized in 25 mm Tris-HCl (pH 7·4), 10 mm EDTA and 10 mm EGTA (250 mg of tissue/ml). Crude supernatant was obtained by centrifuging the homogenate at 10 000 g for 15 min. Acid phosphatase activity was determined by using naphtol AS-Bi phosphate as substrate. In brief, 100 µl of spleen extracts was mixed with 900 µl of reaction buffer: Michaelis buffer containing 0·04% pararosaniline and 0·04% sodium nitrate at pH5 and 0·03% naphtol SA-Bi phosphate. Enzyme activity was determined after 20 min at room temperature at OD 570.
Delayed-type hypersensitivity (DTH) response
BCG-infected mice were given a subcutaneous injection of 10 µg purified protein derivative (PPD, State Vaccine Institute, Pinelands, South Africa) in the right hind footpad and the swelling was measured at 48 hr using a Mitutoyo micrometer (Bruetsch AG, Zurich, Switzerland). The differences of swelling of the right and left (saline-injected, control) hind footpads were compared.
Statistical analysis
Statistical evaluation of differences between the experimental groups was determined by Student's t-test.
Results
Rapid clearance of BCG infection in IL-10-deficient mice
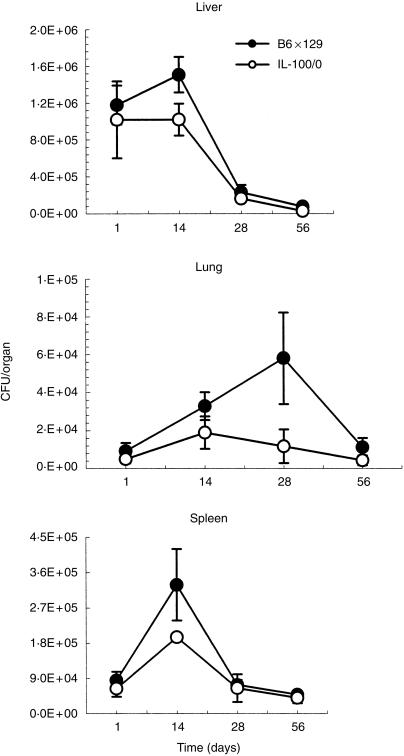
The course of infection of mice injected intravenously with 106 CFU BCG was followed over 8 weeks. Wild-type and IL-10-deficient mice were exposed to a comparable inoculum as assessed by mycobacterial colony counts (CFU) per organ at day 1. The mycobacterial organ burden did not increase over the next 2 weeks in liver and lungs, and was only slightly elevated in the spleen of IL-10-deficient mice, while the wild-type mice showed the usual increase of CFU in liver (P < 0·01), lung (P < 0·05) and spleen (P < 0·05) over 2 weeks, and at 4 weeks in lungs (P < 0·01) (Fig. 1). The elimination of the mycobacteria was significantly faster over the next 4-week period from the lungs of IL-10-deficient mice as compared to the wild-type controls. In addition, the CFU counts from the liver of IL-10-deficient mice at 56 days were still significantly lower (P < 0·05) s compared to the wild-type mice (Fig. 1). Therefore, the absence of IL-10 favoured the clearance of mycobacteria, at least during the initial phase of infection. Similar results were obtained at a high inoculum of BCG, at 107 CFU. More importantly no mortality was recorded over a 3-month observation period.
Figure 1.
Bacterial burden in organs of IL-10-deficient mice infected with M. bovis BCG.The bacterial load in liver, lung and spleen (CFU) was determined at 1, 14, 28 and 56 days after BCG infection (106 BCG intravenously) of IL-10-deficient and wild-type mice. The mean CFU values from four mice are given ± SD.
These results suggest that endogenous IL-10 might attenuate a Th1 immune response. We therefore asked whether the correlate of cell-mediated immunity, the mycobacterial granuloma formation, is enhanced in the absence of IL-10.
Enhanced granuloma formation in IL-10-deficient mice
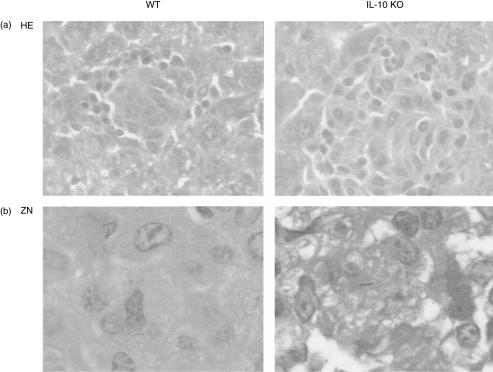
We analysed mycobacterial granulomas on microscopic sections of liver, spleen and lung from wild-type and IL-10-deficient mice 2, 4 and 8 weeks after BCG infection (106 CFU adminstered by intravenous injection). Typical granulomas were already found at 2 weeks in the liver of wild-type mice. They augmented in number and size over the next 2 weeks and consisted of activated macrophages resembling epithelioid cells and a darker lymphocyte mantle zone. In IL-10-deficient mice the granulomas had a higher cellularity and were increased in size as compared to wild-type mice (Fig. 2a). The bacterial load in tissues was further assessed by Ziehl–Neelsen staining of acid-fast bacilli in liver and spleen. Acid-fast bacilli were almost undetectable in the livers of IL-10-deficient mice at 2 weeks (Fig. 2b).
Figure 2.
Hepatic granulomas from M. bovis BCG-infected wild-type and IL-10-deficient mice. Mice were killed at 2 weeks and the sections were stained with haematoxylin & eosin (a) or with Ziehl–Neelsen (b) to detect the acid-fast bacilli. Magnification × 135.
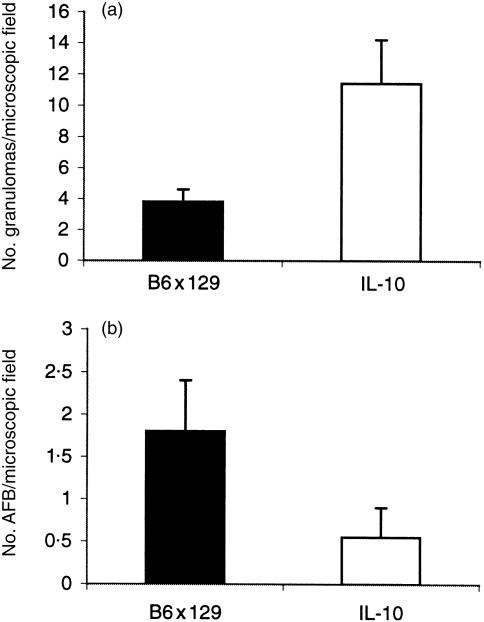
We further quantified the number of granulomas in liver sections. In the absence of IL-10 more granulomas were found per liver cross-section at 2 weeks (Fig. 3a) as compared to controls (P < 0·05). Furthermore, a semiquantitative assessment of acid-fast bacilli per granuloma in tissue sections confirmed significantly fewer mycobacteria (P < 0·05) in the liver (Fig. 3b) and lung (not shown) from IL-10-deficient mice. Therefore, a strong granulomatous response in IL-10-deficient mice correlated with a faster clearance of mycobacteria.
Figure 3.
Number of granulomas and of acid-fast bacilli in the liver of wild-type and IL-10-deficient mice 2 weeks post-infection (106 BCG intravenously). (a) Number of granulomas: mononuclear cell clusters of more than 15 cells were counted as granulomas; the results are expressed as the mean ± SD (n = 5 mice). (b) Number of acid-fast bacilli per granuloma per high-power microscopic fields, expressed as the mean ± SD (n = 5 mice).
Increased cell recruitment and activation of macrophages in granulomas
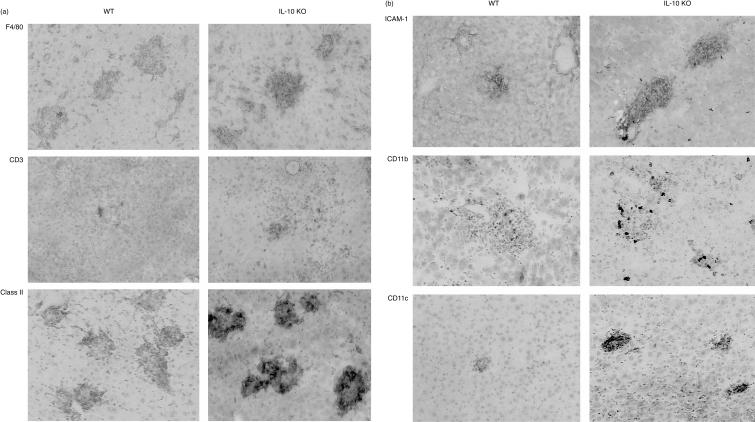
We then investigated the functionality of the granulomas and asked whether the inflammatory reaction in the liver and lungs of BCG-infected mice is enhanced in the absence of IL-10. Firstly, the recruitment of T cells (both CD4 and CD8 cells) and F4/80-positive macrophages was enhanced in the absence of IL-10 (Fig. 4a). Second, the expression of MHC class II, ICAM-1, CD11c and CD11b was distinctly higher in IL-10-deficient mice in comparison to wild-type controls (Fig. 4b). Although a few neutrophils could be found in the granuloma, neutrophils were not prominent in the granuloma and only slightly increased in the peripheral blood (not shown). Therefore, the increased size of granuloma was due to an augmented recruitment of T cells and macrophages.
Figure 4.
Cellular composition of hepatic granulomas in wild-type and IL-10-deficient mice at 4 weeks post-infection. Immunohistochemistry: expression of (a) F4/80, CD3, MHC class II and (b) ICAM-1, CD11b and CD11c are distinctly increased in the absence of IL-10. Magnification x 180.
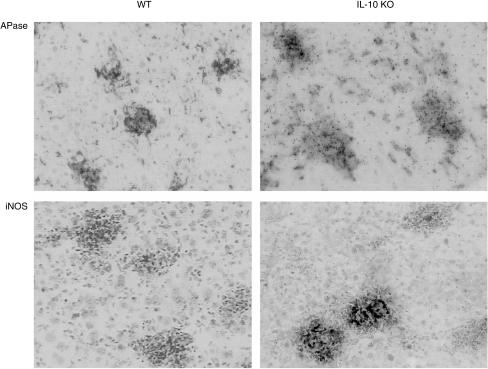
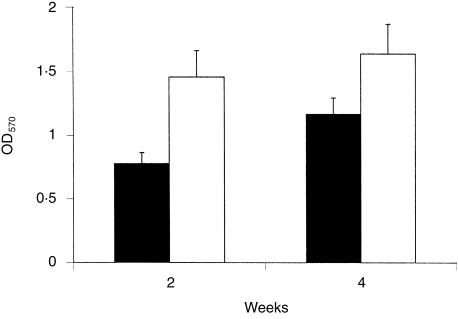
We further asked whether macrophages in granulomas showed enhanced activation and determined acid phosphatase activity and iNOS expression. The expression of iNOS by immunostaining and acid phosphatase activity of macrophages in hepatic tissue sections was heightened in the absence of IL-10 (Fig. 5). We further quantified acid phosphatase activity in tissue homogenates, which was increased in the spleen in the absence of IL-10 (Fig. 6). The differences were statistically significant (P < 0·05).
Figure 5.
Activated macrophages in hepatic granulomas from wild-type and IL-10-deficient mice at 4 weeks post-BCG infection. Distinct up-regulation of acid phosphatase activity and iNOS expression in hepatic granulomas assesssed by histochemical reaction and immunostaining, respectively, as described in the Materials and Methods. Magnification × 320.
Figure 6.
Increased acid phosphatase activity in liver and spleen of IL-10-deficient mice infected with M. bovis BCG. IL-10-deficient mice and wild-type mice were infected intravenously with 106 BCG bacilli. Fresh spleen was processed for enzymatic analysis at 2 and 4 weeks as described. Data are expressed as the mean ± SD (n = 4 mice).
These results suggest, that in the absence of endogenous IL-10 macrophages in granulomas have signs of strong activation, explaining the more rapid clearance of mycobacteria.
Enhanced cell-mediated immune response
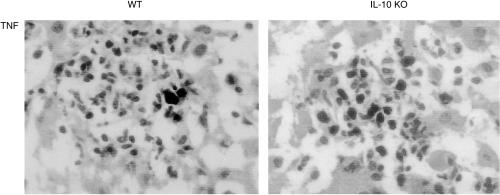
In order to confirm whether a Th1 response prevailed in IL-10-deficient mice infected with M. bovis BCG, we tested the expression of TNF, IL-12 and IFN-γ in mononuclear cells forming the granuloma by immunostaining. Macrophages reacted strongly with the TNF antibody in IL-10-deficient mice, while the immunoreactivity was very low in wild-type mice (Fig. 7). As specificity control for the anti-TNF antibody tissue from TNF-deficient mice were used which were completely negative (not shown). By contrast, no immunoreactivity was found for IL-12 and IFN-γ in the granuloma. We further measured cytokine level in serum for TNF, IFN-γ and IL-12 levels at 4 weeks after the injection of 107 CFU. Although the levels were slightly elevated, no significant differences of cytokine serum levels were found in the two experimental groups (data not shown).
Figure 7.
TNF immunoreactivity in mononuclear cells of hepatic granulomas in wild-type and IL-10-deficient mice at 4 weeks post-BCG infection. Immunostaining with TNF antibody of frozen liver sections as described in the Materials and Methods. Magnification × 320.
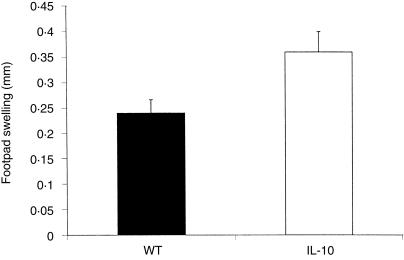
Finally, we tested the cutaneous DTH response using PPD as antigen for the challenge. The DTH response in IL-10-deficient mice was significantly (P < 0·05) increased as compared to the wild-type controls (Fig. 8). These results suggest that the cell-mediated immune response is enhanced in the absence of IL-10, which is a plausible explanation for the augmented antimycobacterial response.
Figure 8.
Cutaneous delayed-type hypersensitivity reaction. BCG-infected wild-type and IL-10-deficient mice received an intradermal injection of PPD into the right footpad and the difference of the swelling of the right and left food-pad was measured. Mean values (mm) ± SD (n = 5 mice).
Discussion
We demonstrate here a vigorous cell-mediated immune response to M. bovis BCG infection with a robust granuloma response and enhanced DTH reaction in the absence of IL-10, which favours mycobacterial clearance at least in the initial phase of infection.
Increased mycobacterial clearance from liver and spleen has been demonstrated in M. bovis BCG-infected IL-10-deficient mice. 19 In addition, we show a rapid elimination of BCG from the lung, which is usually delayed after intravenous infection. Furthermore, the accelerated mycobacterial clearance was associated with an augmented granuloma response. The enlarged granulomas contained increased numbers of T cells and activated macrophages. While no differences in circulating levels of Th1-type cytokines were found, the activated macrophages expressed large amounts of TNF. Therefore, unrestricted local TNF production may have contributed to the vigorous granuloma response and rapid mycobacterial clearance.
By contrast, Erb et al. reported that IL-10, as well IL-4 or IL-5, is not required for the control in M. bovis BCG-infected gene-deficient mice. 17In vitro antigen re-stimulation assays of lymph node lymphocytes from wild-type and IL-10-deficient M. bovis BCG-infected mice showed no differences in IFN-γ production.17,19 These data therefore suggest that T-cell-derived cytokines may not be important for the biological effect. We show here that macrophages from IL-10-deficient mice express increased tissue levels of TNF and iNOS, which most likely account for the increased antimycobacterial response. These data are corroborated by the report of increased pro-inflammatory cytokines, nitric oxide and prostaglandin formation in in vitro cultured macrophages from IL-10-deficient mice. 19
In the present study we extend previous findings by demonstrating significant increased recruitment and activation of mononuclear cells into granulomas, and an enhanced antimycobacterial activity of the granuloma macrophages in situ. The augmented granulomatous response is correlated with an increased cutaneous DTH reaction to PPD.
A regulatory role of endogenous IL-10 in mycobacterial infection was expected, since previous experiments using neutralizing antibodies in M. avium infection revealed enhanced bacterial clearance. The discrepant results from the previous investigations in IL-10-deficient mice may be related to different strains of mycobacteria, dose and time of analysis, as the enhanced clearance in a resistant mouse strain is only visible in the early phase of infection.17,18
An IL-10-regulated Th1-like protective immune response had also be shown in Chlamydia trachomatis infection. 23 In the absence of IL-10 the clearance of chlamydia was accelerated, which correlated with enhanced DTH response and increased serum IFN-γ, TNF and IL-12 levels. No systemic release of pro-inflammatory cytokines was found resulting in toxicity in IL-10-deficient mice as reported for parasitic protozoan infections. 23 Even at the high BCG inoculum of 107 CFU no mortality was recorded.
Since the absence of IL-10 increased antimycobacterial immunity, it follows that IL-10 overexpression may reduce host resistance. This hypothesis was confirmed by experiments, in which transgenic mice overexpressing IL-10 from the T-cell compartment were infected with M. bovis BCG. These mice were unable to control the infection and developed large bacterial burdens. 15 Interestingly, macrophages, but not T-cell functions, were suppressed. Another IL-10 transgenic mouse strain expressing IL-10 in antigen-presenting cells displayed a similar high susceptibility to infection with intracellular pathogens like Listeria monocytogenes and Leishmania major. 24
The comparison of the two extreme situations – absence or overexpression of IL-10 – demonstrates opposing effects on mycobacterial infection and suggests that IL-10 indeed has a regulatory role in the control of this intracellular micro-organism.
In conclusion, we demonstrate a rapid and augmented cell-mediated immune response to M. bovis BCG infection with the formation of highly mycobactericidal granulomas and reduced bacillary burden in the absence of IL-10, suggesting a regulatory role of IL-10 in mycobacterial infection.
Acknowledgments
The work was supported by Action TB from Glaxo-Wellcome, FRD Pretoria and MRC Cape Town. We are thankful for the advice and support of Dr Irene Garcia, Department of Pathology, University of Geneva.
References
- 1.Cooper AM, Flynn JL. The protective immune response to Mycobacterium tuberculosis. Curr Opin Immunol. 1995;7:512. doi: 10.1016/0952-7915(95)80096-4. [DOI] [PubMed] [Google Scholar]
- 2.Bloom BR. Tuberculosi. In: Bloom BR, editor. Tuberculosis, Pathogenesis, Protection and Control. New York: A.S.M. Press; 1994. pp. 1–43. [Google Scholar]
- 3.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 4.Cooper AM, Roberts AD, Rhoades ER, Callahan JE, Getzy DM, Orme IM. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology. 1995;84:423. [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper AM, Magram J, Ferrante J, Orme IM. Interleukin 12 is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J Exp Med. 1997;186:39. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flynn JL, Goldstein MM, Chan J, Triebold KJ, Bloom BR. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 8.Kamijo R, Le J, Shapiro D, et al. Mice that lack the interferon-gamma receptor have profoundly altered responses to infection with Bacillus Calmette-Guerin and subsequent challenge with lipopolysaccharide. J Exp Med. 1993;178:1435. doi: 10.1084/jem.178.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altare F, Durandy A, Lammas D, et al. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280:1432. doi: 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- 10.De Jong R, Altare F, Haagen IA, et al. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science. 1998;280:1435. doi: 10.1126/science.280.5368.1435. [DOI] [PubMed] [Google Scholar]
- 11.Kindler V, Sappino A-P, Grau GE, Piguet P-F, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 12.Senaldi G, Yin S, Shaklee CL, Piguet P-F, Mak TW, Ulich TR. Corynebacterium parvum- and Mycobacterium bovis bacillus Calmette-Guerin-induced granuloma formation is inhibited in TNF receptor 1 (TNF-RI) knockout mice and by treatment with soluble TNF-R1. J Immunol. 1996;157:5022. [PubMed] [Google Scholar]
- 13.Bermudez LE, Champsi J. Infection with Mycobacterium avium induces production of interleukin-10, and administration of anti IL-10 antibody is associated with enhanced resistance in mice. Infect Immun. 1993;16:3093. doi: 10.1128/iai.61.7.3093-3097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bogdan C, Nathan C. Modulation of macrophage function by transforming growth factor beta, interleukin-10. Ann N Y Acad Sci. 1993;685:713. doi: 10.1111/j.1749-6632.1993.tb35934.x. [DOI] [PubMed] [Google Scholar]
- 15.Murray PJ, Wang L, Onufryk C, Tepper RI, Young RA. T cell-derived IL-10 antagonizes macrophage function mycobacterial infecton. J Immunol. 1997;158:315. [PubMed] [Google Scholar]
- 16.Denis M, Ghadirian E. IL-10 neutralization augments mouse resistance to systemic Mycobacterium avium infections. J Immunol. 1993;151:5425. [PubMed] [Google Scholar]
- 17.Erb KJ, Kirman J, Delahunt B, Chen W, Le Gros G. IL-4, IL-5 and IL-10 are not required for the control of M. bovis-BCG infection in mice. Immunol Cell Biol. 1998;76:41. doi: 10.1046/j.1440-1711.1998.00719.x. [DOI] [PubMed] [Google Scholar]
- 18.North RJ. Mice incapable of making IL-4 or IL-10 display normal resistance to infection with Mycobacterium tuberculosis. Clin Exp Immunol. 1998;113:55. doi: 10.1046/j.1365-2249.1998.00636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray PJ, Young AY. Increased antimycobacterial immunity in interleukin-10-deficient mice. Infect Immun. 1999;67:3087. doi: 10.1128/iai.67.6.3087-3095.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 21.Ryffel B, Car BD, Gunn H, Roman D, Mihatsch MJ. Interleukin-6 exacerbates glomerulonephritis in (NZB x NZW) F1 mice. Am J Pathol. 1994;144:927. [PMC free article] [PubMed] [Google Scholar]
- 22.Lucas R, Tacchini-Cottier F, Guler R, et al. A role for lymphotoxin beta receptor in host defence against Mycobacterium bovis BCG infection. Eur J Immunol. 1999;29:4002. doi: 10.1002/(SICI)1521-4141(199912)29:12<4002::AID-IMMU4002>3.0.CO;2-S. 10.1002/(sici)1521-4141(199912)29:12<4002::aid-immu4002>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 23.Yang X, Gartner J, Zhu L, Wang S, Brunham RC. IL-10 gene knockout mice show enhanced Th1-like protective immunity and absent granulomaa formation following Chlamydia trachomatis lung infection. J Immunol. 1999;162:1010. [PubMed] [Google Scholar]
- 24.Groux H, Cottrez F, Rouleau M, Roncarolo MG, Coffman RL. A transgenic model to analyze the immunoregulatory role of IL-10 secreted by antigen-presenting cells. J Immunol. 1998;162:1723. [PubMed] [Google Scholar]