Abstract
B220 is the full-length splicing isoform of a tyrosine phosphatase CD45 and is predominantly expressed as a transmembrane protein on B cells. Other splicing isoforms of CD45 are yielded by alternative splicing of exons 4, 5 and 6. Recently, the expression of B220 on peripheral T cells during activation-induced cell death has been reported. To investigate whether B220 is implicated in apoptosis of immature T cells, we analysed (by flow cytometry using the anti-B220 monoclonal antibody, RA3-6B2) the expression of B220 on mouse thymocytes undergoing X-irradiation- and dexamethasone (DEX)-induced apoptosis. The expression of B220 on thymocytes positive for Thy-1 was induced by X-irradiation or DEX treatment and increased with length of incubation. The expression of B220 was pronounced on the apoptotic hypodiploid cells in the fraction showing lower forward scattering values. Reverse transcription–polymerase chain reaction detected mRNA containing exons 4, 5 and 6 of CD45 in normal thymocytes as well as those exposed to X-rays or DEX. Surprisingly, cytoplasmic B220 antigens were detected in a considerable fraction of normal thymocytes. Moreover, the expression level of the 220 000-MW protein in normal thymocytes was similar to that in the thymocytes undergoing apoptosis. During apoptosis, the expression level of B220 antigen was reduced in the cytoplasm but, conversely, up-regulated on the surface of thymocytes. These results suggest that B220 is constitutively expressed as a cytoplasmic form within thymocytes and possibly translocated to the cell membrane during apoptosis.
Introduction
CD45 is a major cell-membrane glycoprotein expressed on all types of haematopoietic cells except platelets and mature erythrocytes.1 CD45 molecules occupy ≈ 10% of the surface of T and B cells and play important roles in the regulation of proliferation and differentiation of these cells.2–4 These functions stem from the protein tyrosine phosphatase activity in the cytoplasmic domain of the molecule.5,6 Alternative splicing of exons 4, 5 and 6 (also referred to as exons A, B and C, respectively) yields major isoforms of CD45 with different molecular weights (MW) between 180 000 and 220 000.7 In addition, the presence of splicing variants lacking exons 7, 8 and 10 has recently been reported.8 The predominant CD45 isoform on thymocytes is CD45RO, which lacks exons 4–6.9 On the other hand, the 220 000-MW isoform of CD45 (containing all these three exons) is designated ‘B220’ because it was initially thought to be a marker of the B-cell lineage.10
Recently, however, the expression of B220 on peripheral T cells activated by staphylococcal enterotoxin B, concanavalin A or anti-CD3 monoclonal antibody (mAb), has been reported.11,12 Moreover, the expression of B220 on mature T cells precedes apoptosis following the activation and proliferation of these cells.13 Accumulation of activated T cells triggers the Fas–Fas ligand (FasL) apoptotic system, which terminates the immune response. Peripheral T cells that accumulate in gld and lpr mice with lymphadenopathy caused by a defective Fas–FasL system, are also positive for B220.14 These cells are thought to be arrested at the prestage for Fas-mediated apoptosis. Collectively, the expression of B220 on activated T cells may be a prerequisite for Fas-mediated apoptosis.
In thymus, prothymocytes migrating from bone marrow proliferate and differentiate through a complicated process, including T-cell receptor gene rearrangement. Most thymocytes are positive for Thy-1, a marker for the T-cell lineage, but negative for the B-cell marker, B220. The majority of thymocytes are quiescent and inactive to antigen stimulation, dying in situ through positive and negative selection.15 Thymocytes are highly sensitive to apoptosis induced by various types of stress, such as DNA damage induced by ionizing radiation or exposure to glucocorticoids induced by stress. Stress-induced apoptosis of thymocytes is independent of the Fas–FasL system and triggered by cytochrome c release from mitochondria.16 Thus, stress-induced apoptosis of thymocytes offers an experimental system suitable for studying the involvement of B220 in the apoptosis of immature T cells.
In the present study, we show the expression of B220 on thymocytes undergoing apoptosis induced by X-irradiation and a synthetic glucocorticoid, dexamethasone (DEX). The B220 detected on apoptotic thymocytes was not biochemically distinct from the 220 000-MW CD45 isoform expressed on B cells. Moreover, we present evidence to support the notion that the expression of B220 on apoptotic thymocytes occurs by the translocation of a constitutively expressed cytoplasmic form of B220 from the cytoplasm to the cell membrane.
Materials and methods
Animals
Six to 10-week-old female NFS mice, originally provided by Dr M. Okumoto (Research Institute for Advanced Science and Technology, Osaka Prefecture University, Japan) and maintained at the Laboratory of Veterinary Radiology, College of Agriculture, Osaka Prefecture University, were used.
Cell preparation, X-irradiation and DEX treatment
Thymocytes were suspended in phosphate-buffered saline (PBS) containing 137 mm NaCl, 2·7 mm KCl, 4·3 mm Na2HPO4, 1·4 mm KH2PO4 and 2% fetal calf serum (PBS-2% FCS). The cell suspension was exposed to 6·8 Gy of X-irradiation or incubated in the PBS-2% FCS containing 10−6m DEX (Wako Pure Chemicals, Osaka, Japan). X-irradiation was carried out at a dose rate of 0·46 Gy/min using an X-ray generator Radioflex-350 (Rigaku Corp., Osaka, Japan) operated at 250 kV and 12·5 mA with a filter of 0·3 mm Cu plus 0·5 mm Al.
Antibodies and flow cytometry
For the detection of cell-surface antigens, 1 × 106 thymocytes were incubated for 30 min on ice in the dark with fluorescein isothiocyanate (FITC)-conjugated anti-B220 mAb (RA3-6B2; Caltag Laboratories, Burlingame, CA) and/or phycoerythrin (PE)-conjugated anti-Thy-1.2 mAb (5a-8; Cedarlane Laboratories, Ontario, Canada) at appropriate dilutions. The cells were washed twice with PBS-2% FCS and fixed in cold PBS containing 1% paraformaldehyde. To assess the percentage of B220-positive (B220+) cells in the hypodiploid cell fraction, thymocytes were doubly stained with the FITC-conjugated anti-B220 mAb and 50 mg/ml of propidium iodide (PI; Sigma Chemicals, St. Louis, MO) using a modified method of Kishimoto et al.17 To detect cytoplasmic B220, thymocytes were stained with the FITC-conjugated anti-B220 mAb or FITC-conjugated anti-rat immunoglobulin G (IgG) polyclonal Ab (Becton-Dickinson, San Jose, CA), as a negative control, after permeabilization with 70% ethanol, according to the method of Bijman et al.18 Flow cytometry was performed on a flow cytometer, Cyto ACE-150, equipped with the Cyto ACE system program version 3·04 (JASCO Corp., Tokyo, Japan). For each data point, 1 × 104 cells were analysed.
Reverse transcription–polymerase chain reaction (RT–PCR) analysis
The expression of B220 mRNA was determined using RT–PCR analysis. Total RNA from splenocytes or thymocytes was extracted and first strands were synthesized using SuperScript II (Gibco, Grand Island, NY).19 PCR was performed in 50 μl of the reaction mixture (10 mm Tris-HCl, pH 9·0; 50 μm KCl; 0·1% Triton-X-100; 2·5 mm MgCl2; and 200 μm of each of dATP, dGTP, dCTP and dTTP) containing 1 μm each of the murine CD45 primer set, 5 μl of the first-strand mixture, 0·2 mg/ml of RNase A (Sigma Chemicals) and 2·5 U of Taq polymerase (Toyobo, Osaka, Japan) as follows: 94° for 1 min; 30 thermal cycles at 94° for 30 seconds, 55° for 1 min and 72° for 2 min; and a final cycle at 72° for 5 min19 The murine CD45 sense and antisense primer sequences were: 5′-CAAAGTGACCCCTTACCTGCT-3′ (exon 4) and 5′-CTGACATTGGAGGTGTGTGT-3′ (exon 6), respectively. Murine α-tubulin primer set (sense: 5′-TCCATCCTCACCACCCACAC-3′ and antisense: 5′-CGCTTGGTCTTGATGGTGGC-3′) were used as an internal standard. The PCR products were analysed using 1·5% agarose-gel electrophoresis at 12·5 V/cm for 90 min.
Western blotting analysis
X-irradiated or DEX-treated thymocytes (5 × 106) were lysed in buffer containing 20 mm Tris-HCl (pH 8·0), 137 mm NaCl, 10% glycerol, 1% Triton-X-100, 2 mm EDTA and 1 mm phenylmethylsulphonyl fluoride (PMSF), at 4° for 1 hr. Total cell lysates were separated by 5% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to nylon membrane. Detection of B220 was carried out using 10 mg/ml of anti-B220 mAb solution (RA3-6B2), horseradish peroxidase (HRP)-conjugated goat anti-rat IgG Ab (1 : 1000 dilution; Zymed Laboratories, South San Francisco, CA) and BLAST Biotin Reagent (NEN™; Life Science Products, Boston, MA), according to the instructions for the products.
Results
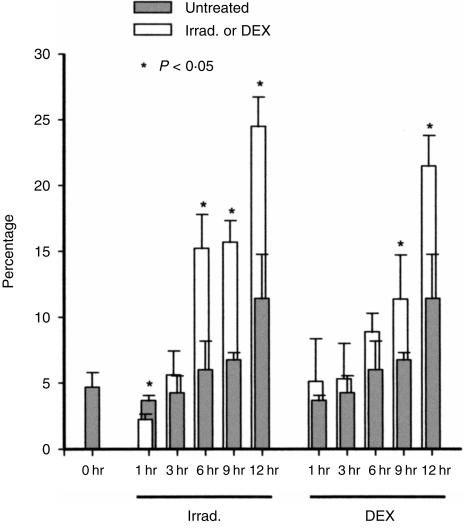
Figure 1 shows the change of B220 expression on thymocytes, relative to duration of incubation, after X-irradiation or DEX treatment. The specificity of anti-B220 mAb RA3-6B2 used in the present study has been verified by other investigators.11,12,20 In order to exclude the possibility of detecting contaminating peripheral B cells, thymocytes were doubly stained for B220 and Thy-1 and the content of B220+ cells in the Thy-1 positive (Thy-1+) fraction was analysed. In our experiments, > 95% of thymocytes were positive for Thy-1 and this percentage did not change significantly during incubation after X-irradiation or DEX treatment. Five per cent of normal thymocytes were B220+ and this proportion showed a slight increase during incubation (6·0% and 11·4% at 6 and 12 hr, respectively). In X-irradiated thymocytes, the percentage of B220+ cells significantly increased with incubation time (15·2% and 24·5% at 6 and 12 hr, respectively). Similarly, the expression of B220 was induced on Thy-1+ thymocytes exposed to DEX during incubation (8·9% and 21·5% at 6 and 12 hr, respectively). Differences amongst the data were analysed by using the t-test.
Figure 1.
Induction of B220 on Thy-1+ thymocytes by X-irradiation (Irrad.) or dexamethasone (DEX). Thymocytes were irradiated with 6·8 Gy of X-rays or cultured with 10−6m of DEX. Cells were harvested at different time-points during incubation, stained for B220 and Thy-1, and analysed by flow cytometry. The percentage of B220+ cells to Thy-1+ cells is represented as a vertical bar indicating the mean ± standard deviation (SD) of four to eight independent determinations. Values that are significantly different from each untreated control are indicated by asterisks (t-test, the level of significance, P = 0·05).
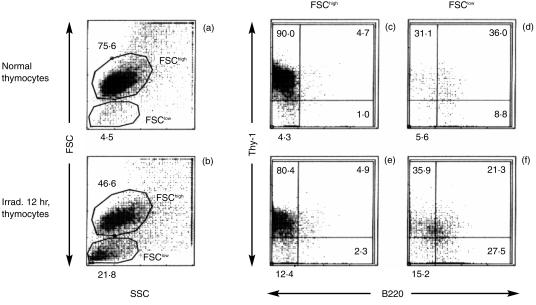
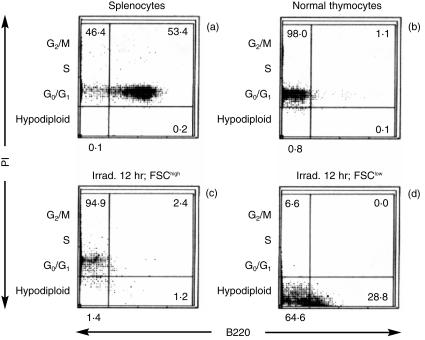
As described by Darzynkiewicz et al.,21 the cytoplasmic condensation of apoptosis can be reflected as a reduced forward light scattering in flow cytometry analysis. Furthermore, a diminished DNA content of apoptotic bodies is detectable as hypodiploid by PI staining.22 The proportion of cells with a low forward scattering value (FSClow) was increased by X-irradiation, coincident with a reduction of cell size in apoptosis (Fig. 2a, 2b). Cells doubly positive for B220 and Thy-1 were abundant in the FSClow fractions, both in normal and irradiated thymocytes, but scant in the FSChigh fractions (Fig. 2c, 2d, 2e, 2f). Most of the normal splenocytes and thymocytes were diploid (Fig. 3a, 3b). Of normal splenocytes, 53·4% were B220+, reflecting the B-cell content (Fig. 3a). In contrast, there were few B220+ cells in normal thymocytes (Fig. 3b). When irradiated thymocytes were analysed by gating in terms of FSC, most of the diploid cells were present in the FSChigh fraction; the majority of the hypodiploid cells were in the FSClow fraction (Fig. 3c, 3d). The FSClow fraction of the irradiated thymocytes comprised 28·8% B220+ cells, but very few were detected in the FSChigh fraction. A similar result was obtained in the analysis of DEX-treated thymocytes (data not shown). As shown in Fig. 3(a), 3(d), the fluorescence intensity of B220 on the FSClow thymocytes was, however, ≈ 10% of that on splenocytes.
Figure 2.
Expression of B220 on Thy-1+ thymocytes with diminished cell size. Normal and X-irradiated (Irrad.) thymocytes at 12 hr of incubation were doubly stained for B220 and Thy-1 and analysed by flow cytometry. Normal (a) and X-irradiated (b) thymocytes were gated by forward scattering (FSC); the expression of B220 on Thy-1+ thymocytes in the FSChigh (c) and (e) and FSClow (d) and (f) fractions are shown. Numbers in each panel represent the mean percentages of four to eight independent determinations.
Figure 3.
Expression of B220 on apoptotic thymocytes. Normal and 6·8 Gy-irradiated cells (Irrad.), harvested after 12 hr of incubation, were stained with propidium iodide (PI) and fluorescein isotihiocyanate (FITC)-conjugated anti-B220 monoclonal antibody (mAb) and analysed by flow cytometry. Results are shown for: normal splenocytes (a), normal thymocytes (b) and irradiated thymocytes in the forward scatter high (FSChigh) (c) and forward scatter low (FSClow) (d) fractions. The scales for the fluorescence intensity of B220 and PI are logarithmic and linear, respectively. Numbers indicated in each panel represent the mean percentages of four to eight independent determinations.
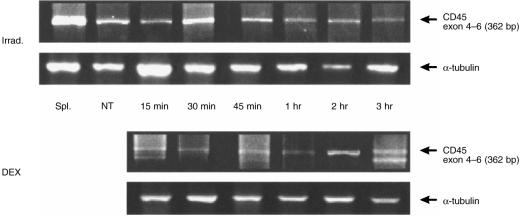
Alternative splicing of exons 4, 5 and 6 yields major CD45 isoforms, while B220 is the full-length isoform.1,3,7,10 To characterize the biochemical features of the B220 molecule detected on apoptotic thymocytes, we investigated the expression of B220 mRNA in irradiated and normal thymocytes by RT–PCR, using sense and antisense primers derived from the sequences of exons 4 and 6 of CD45, respectively. The expected size of the PCR product was 362 bp, and the identity of the product was confirmed by DNA sequencing using the DNA sequencer ABI Prism 310 (Perkin-Elmer/Cetus, Norwalk, CT). The results are shown in Fig. 4. Unexpectedly, a 362-bp sequence of DNA was detected both in normal and irradiated thymocytes from 15 min to 3 hr of irradiation. The expression levels of B220 mRNA both in normal and irradiated thymocytes were markedly lower than the expression level in splenocytes containing ≈ 50% B cells. Similar results were obtained in DEX-treated thymocytes.
Figure 4.
Reverse transcription–polymerase chain reaction (RT–PCR) analysis of B220 mRNA expression. Total RNA was extracted at the indicated time-points after 6·8 Gy of X-irradiation (Irrad.) or incubation with 10−6m dexamethasone (DEX). The cDNA synthesized from each sample was amplified with primers located in exons 4 and 6 of CD45. α-tubulin was used as a standard of mRNA content. The product was electrophoresed in a 1·5% agarose gel and visualized by ethidium bromide staining. The results shown are representative of three experiments. NT, normal thymocytes; Spl. splenocytes.
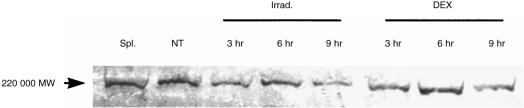
As the RT–PCR product of the same size as that in treated thymocytes was detected in normal thymocytes, the expression of a protein-bearing B220 epitope was investigated in irradiated and normal thymocytes by Western blotting. As shown in Fig. 5, the 220 000-MW B220 protein was detected both in irradiated and normal thymocytes. The expression level of B220 was not significantly changed during incubation from 3 to 9 hr. Similar results were obtained with DEX-treated thymocytes.
Figure 5.
Western blotting analysis of B220 in X-irradiated (Irrad.) and dexamethasone (DEX)-treated thymocytes. Cell lysates extracted from 5 × 106 of normal thymocytes, splenocytes, and X-irradiated or DEX-treated thymocytes at 3, 6 and 9 hr of incubation were electrophoresed in a 5% acrylamide gel. Immunoblots were sequentially incubated with anti-B220 monoclonal antibody (mAb) and horseradish peroxidase (HRP)-conjugated anti-rat immunoglobulin G (IgG) Ab, and visualized using the BLAST Biotin Reagent. NT, normal thymocytes; Spl. splenocytes.
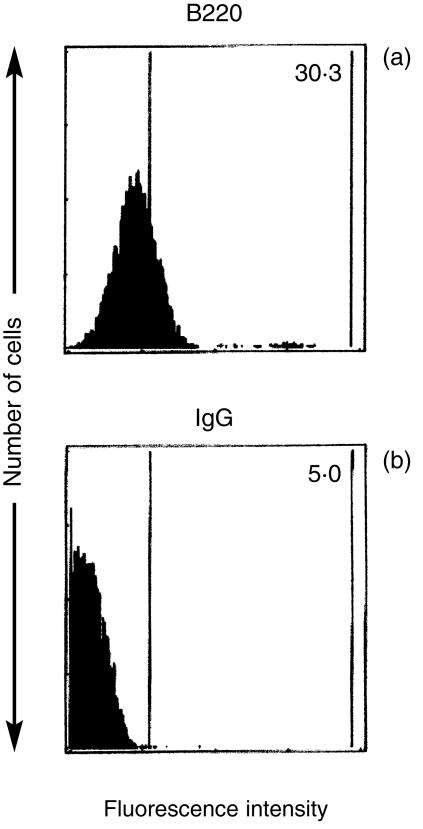
Despite the biochemical evidence indicating the presence of the B220 isoform in normal thymocytes, most of these cells were negative for B220 on their surface, as shown in Fig. 3(b). To test for cytoplasmic B220, we analysed normal thymocytes and X-irradiated or DEX-treated thymocytes by flow cytometry after staining for B220 following permeabilization with 70% ethanol. Surprisingly, a significant percentage (30·3%) of normal thymocytes was positive for B220, whereas the fluorescence intensity was relatively low (Fig. 6a). This result was not caused by non-specific staining, because a mouse fibroblast cell line, Mβ16tsA, processed in a similar manner, showed no positive signal for cytoplasmic B220 (data not shown). The expression data of surface and cytoplasmic B220 in normal, X-irradiated or DEX-treated thymocytes are summarized in Table 1. In normal thymocytes, the percentage of cells positive for surface B220 was low and slightly increased at 6 hr. The expression of surface B220 was 15·6% and 12·2% on irradiated and DEX-treated thymocytes, respectively. On the contrary, cytoplasmic B220 was reduced to less than 50% of the 0-hr control after 6 hr of incubation. The expression level of cytoplasmic B220 in irradiated and DEX-treated thymocytes was similar to that of normal thymocytes at 6 hr. The possibility of non-specific staining of cytoplasmic antigens was excluded because the percentage of the cells positive for IgG, a negative control, was similarly low in all of the samples tested.
Figure 6.
Expression of cytoplasmic B220 within normal thymocytes. Thymocytes were stained for B220 (a) or immunoglobulin G (IgG) (b) after permeabilization with 70% ethanol, and were analysed by flow cytometry. Numbers in the cytograms indicate the mean percentage calculated from three and 10 separate experiments, respectively.
Table 1.
Flow cytometric analysis of surface and cytoplasmic B220 expression in thymocytes 6 hr after X-irradiation or dexamethasone (DEX) treatment
| Untreated (%) | |||||
|---|---|---|---|---|---|
| Permeabilization with 70% ethanol* | Antibody | 0 hr | 6 hr | X-irradiation | DEX |
| – | Anti-B220 | 4·9 ± 1·0† | 6·4 ± 2·1 | 15·6 ± 2·5 | 12·2 ± 3·5 |
| + | Anti-B220 | 30·3 ± 4·7 | 13·3 ± 1·4 | 12·2 ± 1·8 | 10·1 ± 3·1 |
| + | IgG | 5·0 ± 1·0 | 3·3 ± 1·3 | 2·8 ± 0·5 | 3·1 ± 0·8 |
Antibody binds to cell surface B220 (−) or cytoplasmic B220 (+).
Mean ± SD determined from four to 10 different experiments.
IgG, immunoglobulin G.
Discussion
B220 is a full-length splicing isoform of CD45 expressed on the B-cell lineage.3,10 T-cell progenitors derived from bone marrow cells differentiate towards T cells, having a common T-cell surface marker, Thy-1, under the influence of the thymic microenvironment. Although a fraction of the marrow cells homing to thymus is positive for the B-cell marker, B220,23 this molecule is not detected on thymocytes. The majority of thymocytes are positive for Thy-1, and express the 180 000-MW splicing isoform, CD45RO, which lacks exons 4–6 (exons A, B and C).9 On the other hand, the majority of thymocytes express neither CD45RA nor CD45RC. Minor subsets of medullary thymocytes, however, express different splicing isoforms recognized by anti-CD45RA or CD45RC.24,25 The molecular weights for these minor isoforms range from 200 000 to 220 000.
In the present study, we detected the B220 epitope on thymocytes positive for Thy-1 by flow cytometry after X-irradiation or exposure to DEX. In our experiments, most of the thymocytes positive for B220 were included in the FSClow fraction, reflecting diminished cell size, which is characteristic of apoptosis. Furthermore, B220+ cells were abundant in the hypodiploid cell fraction, in association with a reduced DNA content of apoptotic cells. Hence, B220 is preferentially expressed on thymocytes undergoing X-irradiation- and DEX-induced apoptosis. A small percentage of normal thymocytes was positive for B220 in our experiments. Because these B220+ cells were included in the FSClow fraction of normal thymocytes, these cells were thought to be spontaneously dying cells. Previously, we detected the expression of B220 cells on thymocytes undergoing apoptosis after in vivo X-irradiation.26 In our in vivo study, however, a considerable percentage of B220+ cells was detected in the fractions of FSChigh, as well as FSClow. Such a difference might be attributed to the dissimilar conditions between in vitro and in vivo irradiation.
Naive T cells are positive for CD45RA.9 When naive T cells are activated by antigen stimulation or its equivalents, these cells down-regulate CD45RA and gain a high density of CD45RO and CD45RB expression.27–29 Recently, it has been reported that the B220 epitope is also expressed on T cells undergoing apoptosis following activation.11–13 According to Renno et al., the proteins carrying the B220 epitope expressed on activated T cells are 200 000 and 210 000 MW, different from the 220 000 MW of the full-length CD45 isoform.13 It is as yet unresolved whether these B220+ molecules are novel splicing variants of CD45 (containing exons 4–6 but lacking other exons) or products of other unknown genes. Contrary to the case for activation-induced death of mature T cells, we detected, in both normal and apoptotic thymocytes, a 220 000-MW protein carrying the B220 epitope. The molecule was not biochemically distinct from B220 expressed on B cells. The identity of the protein with the full-length CD45 isoform is also supported by the data from RT–PCR analysis.
Detection of B220 on normal thymocytes has not been reported. In agreement with this, most of the B220+ cells in normal thymocytes detected in our experiments were included in the FSClow fraction where apoptotic cells are prominent. On the other hand, the presence of a full-length CD45 isoform with a MW of 220 000 has been reported in normal thymocytes, while the B220 epitope was not detected in this study.30 This can be explained by our surprising results showing the presence of B220 within a considerable proportion of thymocytes. Moreover, we showed that B220 appears on the surface of dying thymocytes, although is dormant in living thymocytes. Additionally, the data of Western blotting analysis, as well as RT–PCR, indicated that B220 was not newly synthesized following stimulation for apoptosis. Taken together, B220 is constitutively expressed within thymocytes and the dormant B220 might be translocated to the surface of apoptotic cells. Previously, a similar idea was proposed by Kishimoto et al., who demonstrated that thymocytes undergoing DEX-induced apoptosis show up- and down-regulation of several surface antigens without protein synthesis.17 The idea is also supported by the report demonstrating that some B-cell surface antigens such as CD20, -21, -22, -32 and -35 are constitutively expressed within peripheral T cells as cytoplasmic forms, but appear on the cell surface of activated T cells.31 The presence of B220 within thymocytes may simply be the result of an escape from splicing regulation. Alternatively, it may be a sign of maturation towards naive T cells expressing CD45RA. Biological significance of the presence of cytoplasmic B220 within thymocytes is unknown.
In the present study, we showed expression of B220 on thymocytes undergoing stress-induced apoptosis. Considering that B220 is coincidentally expressed on apoptotic cells of the T-cell lineage, expression of B220 on these cells may play a role in the process of apoptosis. The implication of B220 expression in apoptosis remains to be elucidated.
Acknowledgments
We thank K. Horie, K. Ono, Y. Ito, M. Akiyama, M. Nakamura, K. Yonezawa, M. Fujitsuka and K. Matsukawa for their helpful research assistance.
References
- 1.Thomas ML. The leukocyte common antigen family. Annu Rev Immunol. 1989;7:339. doi: 10.1146/annurev.iy.07.040189.002011. [DOI] [PubMed] [Google Scholar]
- 2.Ong CJ, Chui D, Teh HS, Marth JD. Thymic CD45 tyrosine phosphatase regulates apoptosis and MHC-restricted negative selection. J Immunol. 1994;152:3793. [PubMed] [Google Scholar]
- 3.Shen FW, Saga Y, Litman G, et al. Cloning of Ly-5 cDNA. Proc Natl Acad Sci USA. 1985;82:7360. doi: 10.1073/pnas.82.21.7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torimoto Y, Dang NH, Streuli M, et al. Activation of T cells through a T cell-specific epitope of CD45. Cell Immunol. 1992;145:111. doi: 10.1016/0008-8749(92)90317-i. [DOI] [PubMed] [Google Scholar]
- 5.Pingel JT, Thomas ML. Evidence that the leukocyte-common antigen is required for antigen-induced T lymphocyte proliferation. Cell. 1989;58:1055. doi: 10.1016/0092-8674(89)90504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tonks NK, Charbonneau H, Diltz CD, Fischer EH, Walsh KA. Demonstration that the leukocyte common antigen CD45 is a protein tyrosine phosphatase. Biochemistry. 1988;27:8695. doi: 10.1021/bi00424a001. [DOI] [PubMed] [Google Scholar]
- 7.Streuli M, Hall LR, Saga Y, Schlossman SF, Saito H. Differential usage of three exons generates at least five different mRNAs encoding human leukocyte common antigens. J Exp Med. 1987;166:1548. doi: 10.1084/jem.166.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Virts E, Barritt D, Raschke WC. Expression of CD45 isoforms lacking exons 7, 8 and 10. Mol Immunol. 1998;35:167. doi: 10.1016/s0161-5890(98)00025-x. [DOI] [PubMed] [Google Scholar]
- 9.Merkenschlager M, Fisher AG. CD45 isoform switching precedes the activation-driven death of human thymocytes by apoptosis. Int Immunol. 1991;3:1. doi: 10.1093/intimm/3.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Coffman RL, Weissman IL. B220: a B cell-specific member of the T200 glycoprotein family. Nature. 1981;289:681. doi: 10.1038/289681a0. [DOI] [PubMed] [Google Scholar]
- 11.Renno T, Hahne M, Tschopp J, MacDonald HR. Peripheral T cells undergoing superantigen-induced apoptosis in vivo express B220 and upregulate Fas and Fas ligand. J Exp Med. 1996;183:431. doi: 10.1084/jem.183.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe Y, Akaike T. Activation signal induces the expression of B cell-specific CD45R epitope (6B2) on murine T cells. Scand J Immunol. 1994;39:419. doi: 10.1111/j.1365-3083.1994.tb03395.x. [DOI] [PubMed] [Google Scholar]
- 13.Renno T, Attinger A, Rimoldi D, Hahne M, Tschopp J, MacDonald HR. Expression of B220 on activated T cell blasts precedes apoptosis. Eur J Immunol. 1998;28:540. doi: 10.1002/(SICI)1521-4141(199802)28:02<540::AID-IMMU540>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 14.Davidson WF, Dumont FJ, Bedigian HG, Fowlkes BJ, Morse HC. Phenotypic, functional, and molecular genetic comparisons of the abnormal lymphoid cells of C3H-lpr/lpr and C3H-gld/gld mice. J Immunol. 1986;136:4075. [PubMed] [Google Scholar]
- 15.Von Boehmer H, Karjalainen K, Pelkonen J, Borgulya P, Rammensee HG. The T-cell receptor for antigen in T-cell development and repertoire selection. Immunol Rev. 1988;101:21. doi: 10.1111/j.1600-065x.1988.tb00731.x. [DOI] [PubMed] [Google Scholar]
- 16.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 17.Kishimoto H, Surh CD, Sprent J. Upregulation of surface markers on dying thymocytes. J Exp Med. 1995;181:649. doi: 10.1084/jem.181.2.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bijman JT, Wagener DJ, Wessels JM, Van Den Broek P, Ramaekers FC. Cell size, DNA, and cytokeratin analysis of human head and neck tumors by flow cytometry. Cytometry. 1986;7:76. doi: 10.1002/cyto.990070111. [DOI] [PubMed] [Google Scholar]
- 19.Matsuyama S, Kubo K, Ohashi F, Takamori Y. Partial cloning of prohibitin cDNA from canine, feline, bovine, equine, and rabbit liver mRNA by RT–PCR. J Vet Med Sci. 1997;59:201. doi: 10.1292/jvms.59.201. [DOI] [PubMed] [Google Scholar]
- 20.Johnson P, Greenbaum L, Bottomly K, Trowbridge IS. Identification of the alternatively spliced exons of murine CD45 (T200) required for reactivity with B220 and other T200-restricted antibodies. J Exp Med. 1989;169:1179. doi: 10.1084/jem.169.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darzynkiewicz Z, Bruno S, Del Bino G, et al. Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13:795. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- 22.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 23.Lepault F, Coffman RL, Weissman IL. Characteristics of thymus-homing bone marrow cells. J Immunol. 1983;131:64. [PubMed] [Google Scholar]
- 24.Lightstone EB, Marvel J. CD45RA is detected in all thymocyte subsets defined by CD4 and CD8 by using three-colour flow cytometry. Immunology. 1990;71:467. [PMC free article] [PubMed] [Google Scholar]
- 25.Pilarski LM, Gillitzer R, Zola H, Shortman K, Scollay R. Definition of the thymic generative lineage by selective expression of high molecular weight isoforms of CD45 (T200) Eur J Immunol. 1989;19:589. doi: 10.1002/eji.1830190403. [DOI] [PubMed] [Google Scholar]
- 26.Oka S, Kubo K, Matsuyama S, Takamori Y. B220 is expressed on apoptotic thymocytes induced by X-irradiation. J Vet Med Sci. 1999;61:3337. doi: 10.1292/jvms.61.337. [DOI] [PubMed] [Google Scholar]
- 27.D'Imperio Lima MR, Alvarez JM, Furtado GC, Kipnis TL, Coutinho A, Minoprio P. Ig-isotype patterns of primary and secondary B cell responses to Plasmodium chabaudi correlate with IFN-gamma and IL-4 cytokine production with CD45RB expression by CD4+ spleen cells. Scand, J Immunol. 1996;43:263. doi: 10.1046/j.1365-3083.1996.d01-35.x. [DOI] [PubMed] [Google Scholar]
- 28.Varga SM, Welsh RM. The CD45RB-associated epitope defined by monoclonal antibody CZ-1 is an activation and memory marker for mouse CD4 T cells. Cell Immunol. 1996;167:56. doi: 10.1006/cimm.1996.0007. [DOI] [PubMed] [Google Scholar]
- 29.Aversa G, Waugh JA, Hall BM. A monoclonal antibody (A6) recognizing a unique epitope restricted to CD45RO and RB isoforms of the leukocyte common antigen family identifies functional T cell subsets. Cell Immunol. 1994;158:314. doi: 10.1006/cimm.1994.1279. [DOI] [PubMed] [Google Scholar]
- 30.Zapata JM, Pulido R, Acevedo A, Sanchez-Madrid F, Landazuri MO. Human CD45RC specificity. A novel marker for T cells at different maturation and activation stages. J Immunol. 1994;152:3852. [PubMed] [Google Scholar]
- 31.Sandilands GP, Perry M, Wootton M, Hair J, More IA. B-cell antigens within normal and activated human T cells. Immunology. 1999;96:424. doi: 10.1046/j.1365-2567.1999.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]