Abstract
Reactive oxygen species (ROS) are known to modulate activities of a host of kinases, phosphatases and transcription factors. Rutin and chlorogenic acid (CGA) are the major polyphenolic antioxidants present in the small molecular fraction of smokeless tobacco leaf extracts, as ascertained by reverse-phase high-pressure liquid chromatography (HPLC) and mass spectrometry. Levels of intracellular ROS in resting versus antigen–immunoglobulin E (IgE)-challenged murine mast cells were measured at 510 nm by fluorescence-activated cell sorting (FACS) using carboxy-dichlorofluorescein (DCFH-DA). Enhanced ROS production was observed in IgE-sensitized mast cells following antigenic challenge. Rutin and CGA reduced ROS levels in antigen–IgE-activated mast cells. Concomitantly, they also profoundly inhibited histamine release by these activated mast cells. In contrast, rutin and CGA augmented the inducible cytokine messages, i.e. interleukin (IL)-10, IL-13, interferon-γ (IFN-γ), IL-6 and tumour necrosis factor-α (TNF-α) in IgE-sensitized mast cells following antigen challenge. This study indicates that tobacco polyphenolic antioxidants that quench intracellular ROS, differentially affect two effector functions of antigen–IgE-activated mast cells. This model system may be employed to determine the molecular target of polyphenols. The potential role of these polyphenolic antioxidants on IgE-mediated allergy in vivo depends on a balance of their differential effects on mast cell activation.
Introduction
Reactive oxygen species (ROS) are mainly derived via mitochondria's cytochrome c during aerobic respiration, via reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase cytochrome b558 during phagocytic defence, and via reactive metabolites of xenobiotics by cytochrome P450. 1–5 In contrast, antioxidants obtained from food and nutrient supplements scavenge reactive oxygen species. Thus, redox homeostasis is controlled by maintaining certain thresholds of ROS because of to steady-state generation and extinction of ROS by various cytochrome systems and antioxidants. Lymphocytes and effector leucocytes undergoing differentiation and activation in the milieu of radicals may be subjected to either normal or undue levels of oxidative stress. Multiple ROS such as hydrogen peroxide (H2O2), superoxide anions, and hydroxyl radicals are generated during normal physiological processes. There has been increasing evidence that H2O2 plays a crucial role in signal transduction of T and B cells, and other cell types. 6–11 Thus, it was proposed that ROS might serve as a second messenger for a myriad of signal transduction events. 12–15
Mast cells modulate immunoglobulin E (IgE)-mediated inflammatory responses by two well-known effector functions: degranulation of prestored inflammatory mediators versus cytokine gene expression. Epidemiological studies indicate that elevated IgE production and lesions of type I immediate hypersensitivity are associated with cigarette smokers.16,17 Tobacco polyphenols are implicated in potentiating IgE production. 18 However, it is not known whether these polyphenols also exert a direct effect on IgE-mediated mast cell activation. Because intact polyphenols may be more abundantly present in non-pyrolysed smokeless tobacco, we therefore ascertained the existence of such entities in smokeless tobacco leaves. Next, we proceeded to determine whether antioxidants concomitantly modulate degranulation as well as cytokine gene expression of antigen–IgE-activated mast cells.
In this study, two natural occurring polyphenolic anti-oxidants in non-pyrolysed, smokeless tobacco products were identified. Rutin and chlorogenic acid (CGA) effectively scavenged ROS, and profoundly inhibited histamine release by antigen–IgE-activated mast cells, while concomitantly upregulating mRNA of a host of cytokines. This model system may be employed to elucidate molecular targets of polyphenols during mast cell activation. The possible effect of tobacco polyphenols on IgE-mediated allergy in vivo may depend on a balance of modulating two distinct effector functions of mast cells.
Materials and methods
Reagents and chemicals
Rutin (quercetin-3-rutinoside; 3,3′,4,5,7-pentahydroxyflavone-3-rutinoside),3-[[6-O-(6-deoxy-a- l-mannopyranosyl)-b- d-glucopyranosyl]oxy]-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-1-benzopyran-4-one, MW 610·5), and chlorogenic acid (CGA, 3-caffeoylquinic acid), M.W. of 354, were obtained from Sigma, St Louis, MO. N-acetylcysteine (NAC) was obtained from Sigma. Dinitrophenol (DNP)-specific IgE and DNP–bovine serum albumin (BSA) was prepared as described. 19 Antigen–IgE complexes were prepared by incubating 100 µg DNP–BSA and 100 µg IgE per ml phosphate-buffered saline (PBS) at room temperature (r.t.) for 30 min. TRI ReagentR for total RNA extraction was obtained from Molecular Research Center Inc. (Cincinnati, OH).
Cell lines
Mast cell subline-9 (MC/9), obtained from ATCC (Rockville, MD), is derived from the fetal liver of (B6×A/J) F1, resembling mucosal mast cells. 20 Conditioned medium was prepared as followed. BALB/c spleen cells were stimulated with 2·5 µg/ml concanavalin A (Con A) at 5 × 105 cells/ml in Dulbecco's modified Eagle's minimal essential medium (DMEM), supplemented with 5 × 10−5 m 2-mercaptoethanol, an additional 2 mm l-glutamine, 10% heat-inactivated fetal calf serum (FCS) at 37° for 48 hr in a 10% CO2 incubator. Supernatants were harvested by centrifugation and sterilized by 0·22 µm filtration. Residual Con A was removed by passing conditioned medium through a G-50 column.
Purification and characterization of tobacco leaf extract
Research loose-leaf chewing tobacco, 1S1 (nicotine at 0·76% dry weight), was obtained from the University of Kentucky Tobacco and Health Research Institute. Crude smokeless tobacco extract (STE) was prepared as follows: Leaves were ground in water at 45% (w/v), supernatant was collected following centrifugation at 11 951 g, and filtered through 0·22 µm. The protein was determined at 1·4 mg/ml of STE by Micro BCA. STE was dialysed against membrane of MW cut-off of 1000. The volume of the dialysable small molecular fraction of STE (SM-STE, MW < 1000) was then lyophilized and re-adjusted to the volume of the initial extract. This material was further subjected to analytical reverse-phase high pressure liquid chromatography (RP-HPLC) in 0·1% trifluoroacetic acid (TFA) and acetonitrile (ACN) gradient from 20% to 70% within 45 min on a C18 column by a Dynamax Rainin's HPLC Method Manager System. Molecular weights of these naturally occurring small molecules were determined by a SCIEX-API 100 mass spectrometer (Perkin Elmer, San Francisco, CA) based on ionic spray and subsequent time-of-flight (TOF) mass determination of the ionized molecular species. Identification of MW of the natural products was aided by the software program: Dictionary of Natural Products CD-ROM Ver. 7.2 (Chapman & Hall/CRC, Boca Raton, FL). Single species of natural compounds exhibiting unique mass for polyphenols were ascertained in the database. Identification of CGA was further substantiated by chromatography. Parallel runs of polyphenol standards exhibited identical spectrum.
Measurement of ROS
Carboxydichlorofluorescein diacetate (DCFH-DA) for measuring intracellular redox was obtained from Molecular Probes (Eugene, OR). DCFH-DA is a nonpolar compound that is converted into a membrane-impermeable non-fluorescent polar derivative (DCFH) by cellular esterase after incorporation into cells. The trapped DCFH is rapidly oxidized to fluorescent 2′,7′ dichlorofluorescein (DCF) by intracellular hydrogen peroxide. 21 Cells at 1 × 106/ml were incubated overnight with IgE and polyphenols at 37° in a 10% CO2 incubator and washed. Equal number of cells were then distributed in aliquots in fresh medium, and challenged with 100 ng/ml DNP–BSA from 1 to 24 hr. Antigenic challenge was stopped by washing in fresh medium. Cells were counted and 1 × 106 cells were then resuspended in 1 ml 1× PBS with DCFH-DA at a final concentration of 10 µ m. Cells were incubated for 15 min at 37°, washed twice, and resuspended in 1 ml 1× PBS for flow cytometry analysis. Emission of the trapped, oxidized DCF in these mast cells pretreated with or without polyphenols was then analysed at 510 nm on a FACScan (Becton Dickinson, Mountain View, CA).
Histamine release
Mast cells (1 × 106) were sensitized with IgE at 2 µg/ml in the presence of different concentrations of rutin, CGA or NAC overnight. Cells were washed, resuspended in Tyrode's buffer (135 mm NaCl, 5 mm KCl, 1 mm MgCl2, 1·8 mm CaCl2, 5·6 mm glucose, and 20 mm HEPES, pH 7·4), and challenged with 100 ng/ml DNP–BSA for 30 min at 37°. Two hundred microlitre supernatants were then taken from the duplicate samples and levels of histamine were determined against the standard curve according to a quantitative histamine radioimmunoassay kit obtained from Biomerica Inc. (Newport Beach, CA).
RNase protection assay for cytokines
Levels of cytokine messages of polyphenol-treated mast cells were determined by RNase protection assay (RPA) with RiboQuant™, mCK-1 kit (for interleukin (IL)-4, IL-5, IL-10, IL-13, IL-15, IL-9, IL-2, IL-6, interferon-γ (IFN-γ)), and mCK-3 kit (for tumour necrosis factor-β (TNF-β), leukotriene-β (LTβ), TNF-α, IL-6, IFN-γ, transforming growth factor-β1 (TGF-β1) and TGF-β2) from PharMingen (San Diego, CA). Mast cells were cultured overnight in T-75 flask at 1 × 106/ml conditioned medium in the presence of IgE at 2 µg/ml with or without rutin or CGA. Cells were then harvested and challenged with DNP–BSA at 100 ng/ml for 15 min, 1 hr, and 4 hr at 37°. Cells were pelleted, total RNA extracted with TRI ReagentR. Cytokine messages were calibrated with housekeeping genes as standard by a PhosphoImager (Molecular Dynamics, Sunnyvale, CA). Units of expression were computed by dividing the intensity of the individual band over that of L32 gene in the respective lane. This minimized variations due to different amount of RNA loading from each RNA preparation.
Results
Ascertainment of free tobacco polyphenols in smokeless tobacco extract and their capacity to scavenge ROS
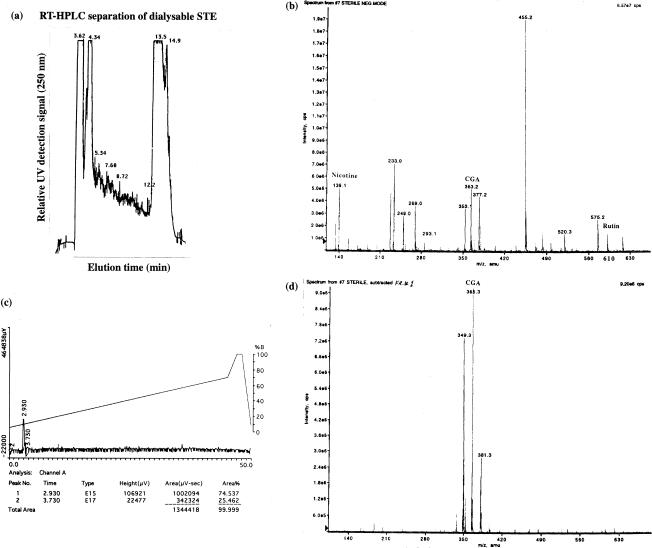
It is not known whether polyphenols exist in free form in non-pyrolysed smokeless tobacco extract. 22 Thus, we first analysed the spectrum of small molecules present in tobacco leaf extract. Supernatants were obtained after spinning at 11 951 g of a water extract of tobacco leaves, and further dialysed at the membrane MW cut-off of 1000. Figure 1(a) shows a complex elution profile of crude SM-STE, equilibrated in 0·1% TFA and eluted with a gradient of acetonitrile from 20% to 70% in 45 min by analytical RP-HPLC on a C18 column. As shown in Fig. 1(b), the crude SM-STE displayed multiple small molecules ranging from MW of 139–630, and the molecular species of 353 and 610 correspond to CGA and rutin, respectively. Next, hydrophilic materials eluted around 3 min (Fig. 1a) from the first analytical HPLC purification were collected, and were further subject to another round of analytical RP-HPLC. As shown in Fig. 1(c), the purified material was resolved into a single peak around 3 min. As shown in Fig. 1(d), this purified material was again determined to be CGA by mass spectrometry.
Figure 1.
Purification and characterization of non-pyrolysed, naturally occurring polyphenolic antioxidants in tobacco leaf extract. (a) Profile of SM-STE by RP-HPLC. SM-STE with MW of 1000 cut-off were prepared as described in Materials and Methods. One hundred µl of SM-STE were subjected to RP-HPLC (0·1% TFA; ACN 20% to 70% in 45 min) on a C18 column by a Dynamax Rainin's HPLC Method Manager System. (b) Ionogram of SM-STE. M.W. of SM-STE was determined by a SCIEX-API 100 mass spectrometer (Perkin-Elmer) based on ionic spray and subsequent TOF mass determination of the ionized molecular species. (c) Second RP-HPLC of hydrophilic substance obtained from initial purification from (a). (d) Mass determination of CGA in the purified peak of the second round of RP-HPLC from (c).
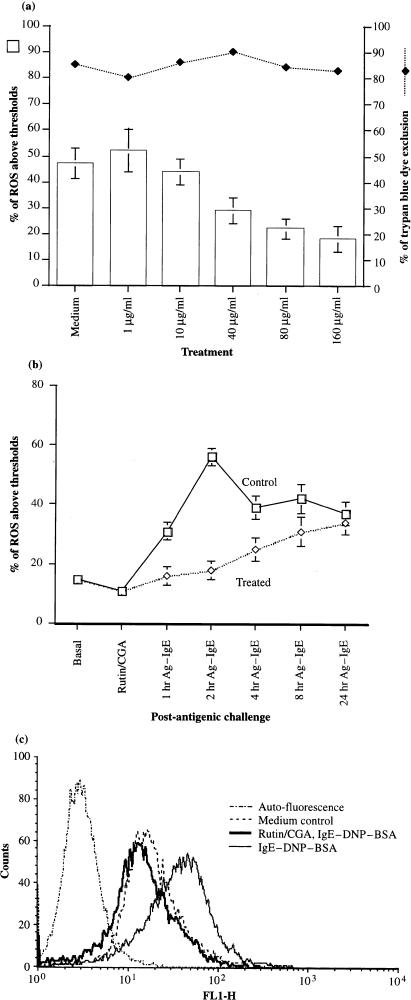
We proceeded to determine whether pure tobacco polyphenols commercially obtained could modulate endogenous ROS levels of mast cells. Constitutive superoxide anion production was detected in mast cells.23 ROS measurement in mast cells was performed by uptake of reduced fluorescent indicator, DCFH-DA, which is known to be oxidized by H2O2. 21 The resultant oxidized DCF was then determined at 510 nm. Tobacco polyphenols can scavenge inducible levels of ROS in antigen-IgE activated mast cells. As shown in Fig. 2(a), dose-dependent inhibition of ROS levels in antigen-IgE activated mast cells was observed. Levels of ROS were significantly dampened by rutin/CGA at 80–160 µg/ml. No cytotoxicity was observed in activated mast cells treated with antioxidants at different dosages. Figure 2(b) shows that ROS levels rose in IgE-sensitized mast cells 1 hr post antigenic stimulation, peaked around 2 hr and were maintained even after 24 hr post-stimulation. Treatment of antioxidants dampened ROS production by mast cells from 1 to 4 hr after antigenic challenge, and ROS levels in antioxidant-treated mast cells were recovered 8 hr post antigen challenge. Figure 2(c) shows, in detail, the changes of ROS levels at 2 hr post-antigenic stimulation. Resting mast cells exhibited constitutive basal levels of ROS. Levels of ROS in mast cells were augmented approximately 1 log of magnitude at 2 hr post-stimulation. Enhanced levels of ROS as a result of antigen-IgE activation were abrogated by pretreatment of tobacco polyphenols.
Figure 2.
Inhibition of ROS production in antigen–IgE-activated mast cells by polyphenolic antioxidants. MC/9 mast cells (1 × 106) were incubated with 2 µg/ml IgE along with rutin and CGA at 1–160 µg/ml overnight (a, dose responses). Cells were washed, and challenged with 100 ng/ml DNP-BSA from 1 to 24 hr post-antigenic challenge (b, kinetics). Cells were washed, viability determined by trypan blue and 1 × 106 cells were then incubated with DCFH-DA at 10 µ m final, and emission at 510 nm determined. Histograms of oxidized DCF for the rutin/CGA-treated group versus control at 2 hr post-antigenic challenge, including the medium and baseline controls were depicted (c, FACS diagram).
Effect of rutin and CGA on histamine release by antigen–IgE-activated mast cells
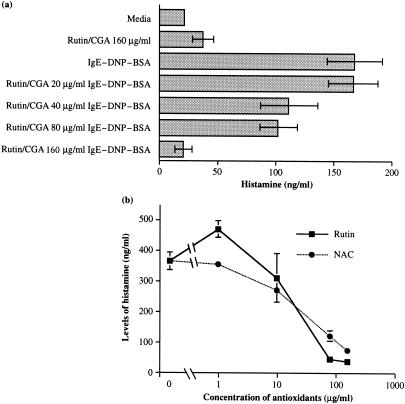
Because mast cell degranulation is a central event of antigen–IgE-activated mast cells, we determined whether treatment of tobacco polyphenols affected this process. Mast cells were sensitized overnight with IgE at 2 µg/ml in the presence of different concentrations of rutin and CGA, and then challenged with 100 ng/ml DNP-BSA. As shown in Fig. 3(a), histamine release by antigen-challenged mast cells was significantly inhibited when mast cells were preincubated overnight with 40 µg/ml rutin and CGA, and more than 90% of histamine release was inhibited by rutin/CGA at 160 µg/ml. Because NAC, a sulph-hydryl-reducing agent, acts as an antioxidant by directly replenishing endogenous glutathione, 24 we compared the dosage responses of rutin versus NAC. As shown in Fig. 3(b), about 67% and 90% of histamine release by antigen–IgE-activated mast cells were inhibited by NAC and rutin at 80 µg/ml, respectively. Approximately, 80 and 90% of histamine release were inhibited by NAC and rutin at 160 µg/ml.
Figure 3.
Effect of polyphenolic antioxidants on histamine release by antigen–IgE-activated mast cells. Mast cells were sensitized with 2 µg/ml IgE in the presence of different concentrations of rutin/CGA for 24 hr in duplicate cultures. Cells were washed, resuspended in Tyrode's buffer (135 mm KCl, 1 mm MgCl2, 1·8 mm CaCl2, 5·6 mm glucose, and 20 mm HEPES, pH 7·4), and incubated with 100 ng/ml DNP-BSA for 1 hr at 37°. Two hundred µl supernatant was then taken from the duplicate cultures. Levels of histamine were determined against the standard curve according to a quantitative histamine RIA kit obtained from Biomerica Inc. (Newport Beach, CA). Approximately 30% histamine was released in supernatants (350 ± 70 ng/106 cells) of antigen-IgE activated control mast cells. Four such experiments were performed for different doses of antioxidants.
Early cytokine gene expression in antigen-IgE activated mast cells is upregulated by rutin and CGA treatment
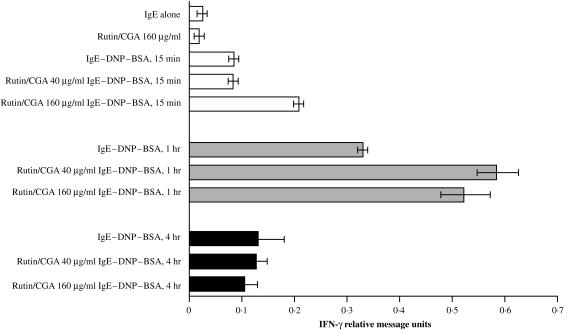
It is well documented that multiple cytokine genes are expressed in mast cells upon antigen–IgE challenge. 25 Therefore, we evaluated whether polyphenols modulate cytokine gene expression in antigen-IgE activated mast cells. First, the effect of polyphenols on kinetics of cytokine gene expression was examined. As shown in Fig. 4, IFN-γ mRNA was detected in mast cells 15 min after antigen challenge, and its level peaked at 1 hr, and declined at 4 hr post-stimulation. Polyphenols did not affect basal IFN-γ gene expression; moreover, treatment of rutin/CGA augmented IFN-γ mRNA twofold at during early and 1 hr optimal induction following antigen challenge.
Figure 4.
Time-course of IFN-γ gene expression in polyphenolic antioxidant-treated, antigen-IgE activated mast cells. Levels of cytokine messages of antioxidant-treated mast cells were determined by RNase protection assay with RiboQuant™, mCK-1 kit from PharMingen (for IL-4, IL-5, IL-10, IL-13, IL-15, IL-9, IL-2, IL-6, IFN-γ). Similar kinetics for various cytokine messages was noted, and IFN-γ was shown. Mast cells were cultured in T-75 flask at 1 × 106/ml with 2 µg/ml IgE overnight in the presence of different concentrations of rutin/CGA overnight. Cells were washed twice with Tyrode's buffer, resuspended in conditioned medium, and challenged at 37° with DNP-BSA at 100 ng/ml at different time-points. Cells were then pelleted, total RNA extracted and cytokine messages calibrated with housekeeping gene as standard by a PhosphoImager (Molecular Dynamics). Units of expression were computed by dividing the intensity of the individual band over that of L32 gene in each respective lane. Mean and SEM of densitometric readings of two similarly performed experiments are presented.
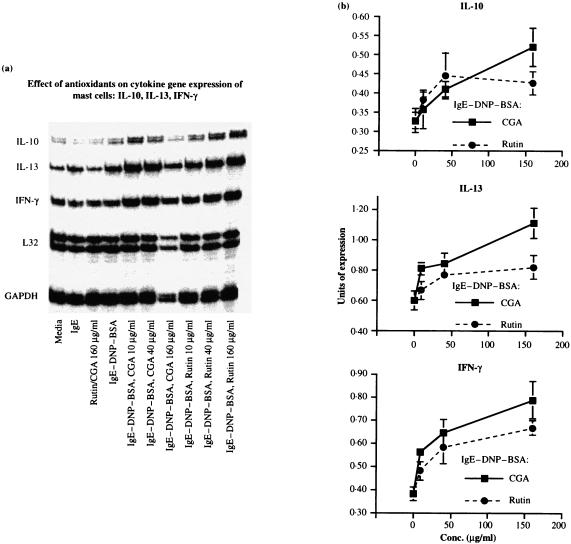
Next, the effect of different dosages of rutin and CGA on cytokine gene expression was assessed in mast cells at 1 hr following antigen challenge. As shown in Fig. 5(a), the basal levels of cytokine mRNA in mast cells treated with rutin and CGA alone were comparable to those treated with IgE or medium control. In contrast, IL-10, IL-13 and IFN-γ were upregulated in antigen-challenged mast cells. Furthermore a dose-dependent increase of cytokine mRNA for IL-10, IL-13, IFN-γ was observed in antigen-challenged mast cells preincubated with rutin or CGA from 10 to 160 µg/ml. Densitometric measurement (Fig. 5b) revealed about 200–300% increase for IL-10 mRNA at the optimal doses of polyphenols, a 50–250% increase for IL-13, and a 250–300% increase for IFN-γ. Noticeably, there was less RNA loading in Lane 7 (the group treated with 160 µg/ml CGA) of Fig. 5(b), but upon standardization with the housekeeping gene as an internal control, units of cytokine gene expression remained the highest as shown in the centre panel of Fig. 5(b).
Figure 5.
Dosage responses of IL-10, IL-13, and IFN-γ in antigen–IgE-activated mast cells treated with polyphenolic antioxidants. The procedure was similar to that described in the legend to Fig. 4. RiboQuant™, mCK-1 kit from PharMingen was employed and cytokine messages determined at 1 hr post-antigen challenge. (a) A representative autoradiogram. (b) Mean and SEM of densitometric reading of three similarly performed experiments.
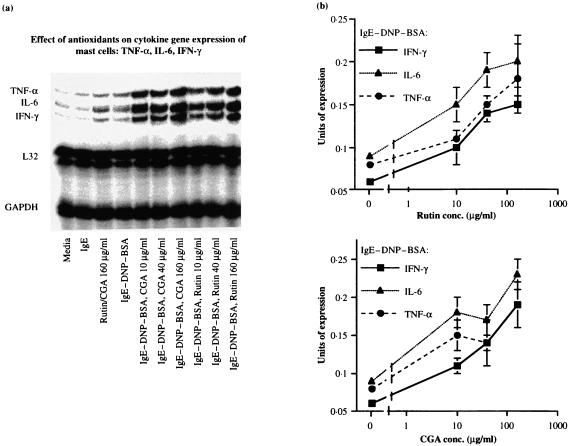
Likewise, as shown in Fig. 6(a), cytokine mRNA for TNF-α, IL-6, and IFN-γ were similar in cultures incubated with medium alone, or treated with IgE, or rutin/CGA. Induced cytokine messages in antigen-challenged mast cells were further augmented by pretreatment of rutin and CGA. Desitometric measurement (Fig. 6b) revealed about 200% increase of mRNA for TNF-α at the optimal doses of polyphenols, a 200–300% increase for IL-6, and a 200–250% increase for IFN-γ.
Figure 6.
Dosage responses of TNF-α, IL-6 in antigen-IgE activated mast cells treated with polyphenolic antioxidants. The procedure was similar to that described in the legend to Fig. 4. RiboQuant™, mCK-3 kit from PharMingen (LTβ, TNF-α, Il-6, IFN-γ) was used and cytokine messages determined at 1 hr post-antigen challenge. (a) A representative autoradiogram. (b) Mean and SEM of densitometric readings of three similarly performed experiments.
Discussion
The salient observations of this study are: (i) tobacco polyphenols, rutin and CGA were detected in non-pyrolysed, small MW fraction of SM-STE; (ii) increase of intracellular ROS was observed in mast cell line following antigen challenge of IgE-sensitized mast cells and polyphenolic antioxidants dampened levels of ROS in antigen–IgE-activated mast cells; (iii) tobacco polyphenols inhibited histamine release, but upregulated mRNA for multiple cytokines including, interleukin (IL)-10, IL-13, IFN-γ, IL-6 and TNF-α in antigen-IgE activated mast cells. Thus, this study showed that polyphenolic antioxidants differentially modulate two important effector functions, histamine release and cytokine expression of antigen-IgE activated mast cells.
Naturally occurring polyphenols, i.e. CGA and rutin (quercetin-3-rutinoside), present abundantly in fruits, tea and red wine,26,27 are also present in tobacco leaves as shown in this study. Polyphenols are known to exert three major antioxidant activities: scavenging ROS, chelation of transitional metal and induction of phase II detoxifying enzymes. 28 Thus, they are strongly implicated to play a role in chemoprotection for inflammatory diseases and cancers. 29–31 CGA, an ester of caffeic acid and quinic acid, exerts anti-inflammatory effect, reduces the mutagenic and genotoxic effects of cigarette tar and protects against DNA damage in mice.32,33 Rutin, present in tea, fruit juices, and leaves of Ginkgo biloba, is known to break the oxidative chain reaction initiated by oxidized low-density lipoproteins (LDL), and is chemoprotective for coronary heart diseases. 34 These two components were previously shown conjugated to a variety of degraded or denatured proteins in cigarette smoke condensates resulting from pyrolysis. 22 Herein, we ascertained that CGA and rutin also naturally exist in a free form in non-pyrolysed smokeless tobacco extract, and which act as antioxidants for directly scavenging ROS generated by antigen–IgE-activated mast cells (Figs 1 and 2). Their quinonic intermediate metabolites may also activate antioxidant genes; i.e. the genes for DT-diaphorase, and glutathione peroxidase that further ensure the reduced intracellular redox.24,35
ROS are small diffusible molecules generated by normal cells as side products of electron transfer reactions or environmental stress. Oxidized, ester-free fluorescein, DCF, from DCFH-DA, detected at 510 nm, mainly reflected endogenous levels of H2O2 generated caused by oxidative stress in mast cells (Fig. 2). However, DCFH-DA does not directly measure levels of superoxides and hydroxyl radicals. 21 Although H2O2 is more stable, compared to other oxygen metabolites, it is possible that superoxides required for producing H2O2, or the hydroxyl radicals whose formation depends on pre-existing H2O2 via Fenton reactions, 36 may also be pertinent species for signal transduction. The role of the respective ROS or reactive nitrogen species (RNS) in this model may be further tested with a scavenger for the respective ROS/RNS.
Redox homeostasis may play a role in modulating physiological/pathological states of many cell types.6–9,12,13 Oxidative stress is known to activate Src kinases in lymphocytes and neutrophils.8–10,37 P21Ras and protein kinase C (PKC) that contain vicinal sulph-hydryl groups can be activated directly by ROS,11,38,39 while phosphotyrosine phosphatases are inactivated by oxidative stress.8,40,41 Our observations support a role of ROS in modulating effector functions of mast cells. We propose that release of prestored mediator and de novo cytokine gene expression are mediated by PKC and mitogen-activated protein kinase (MAPK) pathways, respectively, 42–44 and these two pathways are differentially influenced by polyphenol treatments.
PKC activation plays an important role in mast cell degranulation.42,44,45 We propose that polyphenols inhibit PKC activation and henceforth histamine release. Constitutive superoxide production in cryostats of mast cell granules was detected via deposits of 3,3′-diaminobenzidine (DAB)-Mn2+/Co2+, 23 whose formation depended in turn on the presence of oxygen and intact oxidases. Thus, it appears that granules may serve as the reaction centre for generating ROS, which then permeate or diffuse to the adjacent cytosol and other subcellular compartments. Different PKC isoforms were known to be directly activated in response to H2O2 stimuli. 39 H2O2 detected by DCFH-DA, trapped in the cytosol of antigen-IgE activated mast cells, may therefore activate cytosolic PKC directly. Consequently, scavenging ROS by polyphenolic antioxidants inhibit mast cell degranulation (Figs 2 and 3). This observation is in line with others: (i) ROS generated by mercuric chloride treatment enhanced histamine release by antigen-IgE activated mast cells, and desferrioxamine or catalase reversed enhanced histamine release; 45 (ii) semiquinone oxidants enhanced mast cell degranulation. 46
In contrast, MAPK pathway and its downstream activator protein 1 (AP-1) transcription factor are considered important for cytokine gene expression in mast cells.43,44, 47,48 The observations shown in Figs 4 and 6 prompt the hypothesis that polyphenol treatment of antigen-IgE activated mast cells leads to enhanced MAPK and AP-1 activation. Elevated intracellular glutathione may offer more protection to the active vicinal sulphur catalytic centres of stimulatory CD45 than that of inhibitory phosphatases.8,41, 49,50 Cumulatively, the outcome prolongs half-lives of phosphorylated Lyn and Syk and downstream MAPK activation. Effector MAPK then leads to activation of Elk-1 transcription factor that is required for c-fos expression and AP-1 activation.51,52 Thus far, there is one study showing that overexpressed PKCβ1 but not other isoforms plays a role in augmenting IL-2 messages in antigen–IgE-activated RBL-2H3. 53 Herein, we showed that polyphenols potentiated expression of a wider variety of cytokines (Figs 4–6). It is possible that downregulation of PKCβ1 by antioxidants may be readily compensated by sustained activation of the MAPK and/or downstream transcription factors caused by antioxidants. For example, antioxidants may further ensure the supply of reduced form of redox factor-1 (Ref-1) that plays a critical role in maintaining the binding activities of AP-1.54,55 Because the active form of AP-1 is an integral constituent of nuclear factor of activated T cells (NF-AT) complexes, as demonstrated in mast cells, 56 interactions of NF-AT and AP-1 were documented as crucial for cytokine gene expression in mast cells and other cell types.47,48,52
Mast cells have been well documented as expressing a spectrum of cytokines, which mediate both inflammation and defence. 25 T helper 2 (Th2) cytokines IL-10, IL-13, Th1 cytokine IFN-γ, and inflammatory cytokines, TNF-α, IL-6 were inducible upon immunological challenge, and were further augmented by tobacco polyphenols (Figs 5 and 6). IL-4 expression in our measurement was weak and variable despite immunological challenge concomitant with polyphenol stimulation (data not shown). The consequence of cytokine production by a polyphenol-modulated mast cell in the inflammatory lesion depends in turn on its interactions with other cell types, and the outcome is likely complex. Thus, IFN-γ and IL-13 produced by mast cells can positively or negatively influence mast cell functions via other cell types. Nitric oxide (NO) produced by IFN-γ-activated macrophages in turn can inhibit IgE-mediated mast cell degranulation. 57 IFN-γ can indirectly also inhibit mast cell activation by downregulating IgE production. In contrast, IL-13 can enhance IgE production and IgE-mediated mast cell activation. 58
Development of the immune competence is constantly affected by antigenic selection, costimulatory signals as well as the metabolic microenvironment determined by status of nutrition and habitual uses of substances whereby selection and costimulation of lymphocytes take place. Evidence from both basic and clinical studies suggests an important role of pyrolysed tobacco polyphenols in IgE-mediated immediate hypersensitive diseases in cigarette smokers.16,17 On the other hand, intact polyphenols from smokeless tobacco may be chemoprotective against mast cell degranulation, while upregulating cytokine genes expression. Herein, we provide a model to examine the role of natural occurring polyphenolic antioxidants, readily available through food or consumption of smokeless tobacco, on antigen–IgE-activated mast cells. Thus, polyphenols obtained from various sources may act similarly to modulate two important effector functions of mast cells, i.e. degranulation versus cytokine gene expression. The potential outcome of intake of polyphenols through different sources on IgE-mediated allergy depends on a balance of these two effector functions, and the molecular targets of polyphenols in the respective pathway need to be ascertained.59,60
Acknowledgments
The authors gratefully acknowledge Dr Amnon Altman at LIAI for critical review of the manuscript, and Dr Howard Constant at Ancile Pharmaceutical for advice on mass spectrometry. The work is in part supported by a grant from the Smokeless Tobacco Research Council (STRC 0654-01-03).
Glossary
Abbreviations
- CGA
chlorogenic acid
- ROS
reactive oxygen radicals
- STE
crude smokeless tobacco extract
- DCFH-DA
carboxy-dichlorofluorescein diacetate
- Fce RI
Type I IgE Fc receptor
- H2O2
hydrogen peroxide
- MC/9
mast cell subline-9
- NAC
N-acetyl cysteine
- RPA
RNase protection assay
- SM-STE
small molecular fraction of smokeless tobacco extract
- TOF
time-of-flight
References
- 1.Gram TE. Chemically reactive intermediates and pulmonary xenobiotic toxicit. Am Soc Pharmacol Exp Ther. 1997;49:297. [PubMed] [Google Scholar]
- 2.Henderson LM, Chappell JB. NADPH. oxidase of neutrophils. Biochem Biophys Acta. 1996;1273:87. doi: 10.1016/0005-2728(95)00140-9. [DOI] [PubMed] [Google Scholar]
- 3.Pryor WA, Hales BJ, Premovic PI, Church DF. The radicals in cigarette tar. their nature and suggested physiological implications. Science. 1983;220:425. doi: 10.1126/science.6301009. [DOI] [PubMed] [Google Scholar]
- 4.Daniel V. Glutathione S-transferases: gene structure and regulation of expression. Crit Rev Biochem Mol Biol. 1993;28:173. doi: 10.3109/10409239309086794. [DOI] [PubMed] [Google Scholar]
- 5.Yu BP. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994;74:139. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura H, Nakamura K, Yodoi J. Redox regulation of cellular activation. Annu Rev Immunol. 1997;15:351. doi: 10.1146/annurev.immunol.15.1.351. [DOI] [PubMed] [Google Scholar]
- 7.Los M, Droge W, Stricker K, Baeuerle PA, Schulze Osthoff K. Hydrogen peroxide as a potent activator of T lymphocyte functions. Eur J Immunol. 1995;25:159. doi: 10.1002/eji.1830250127. [DOI] [PubMed] [Google Scholar]
- 8.Schieven GL, Mittler RS, Nadler SG, et al. ZAP-70 tyrosine kinase, CD45, and T cell receptor involvement in UV- and H2O2-induced T cell signal transduction. J Biol Chem. 1994;269:20718. [PubMed] [Google Scholar]
- 9.Fialkow L, Chan CK, Grinstein S, Downey GP. Regulation of tyrosine phosphorylation in neutrophils by the NADPH oxidase. Role of reactive oxygen intermediates. J Biol Chem. 1993;268:17131. [PubMed] [Google Scholar]
- 10.Schieven GL, Kirihara JM, Burg DL, Geahlen RL, Ledbetter JA. p72syk tyrosine kinase is activated by oxidizing conditions that induce lymphocyte tyrosine phosphorylation and Ca2+ signals. J Biol Chem. 1993;268:16688. [PubMed] [Google Scholar]
- 11.Lander HM, Ogiste JS, Teng KK, Novogrodsky A. p21ras as a common signaling target of reactive free radicals and cellular redox stress. J Biol Chem. 1995;270:21195. doi: 10.1074/jbc.270.36.21195. [DOI] [PubMed] [Google Scholar]
- 12.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediate as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roederer M, Staal FJ, Raju PA, Ela SW, Herzenberg LA. Cytokine-stimulated human immunodeficiency virus replication is inhibited by N-acetyl- l-cysteine. Proc Natl Acad Sci USA. 1990;87:4884. doi: 10.1073/pnas.87.12.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fialkow L, Downey GP. Reactive oxygen intermediates as signaling molecules regulating leukocyte activation. In: Forman E, Cadenas HJ, editors. Oxidative Stress and Signal Transduction. New York: Chapman & Hall; 1997. p. 200. [Google Scholar]
- 15.Satriano JA, Shuldiner M, Hora K, Xing Y, Shan Z, Schlondorff D. Oxygen radicals as second messengers for expression of the monocyte chemoattractant protein, JE/MCP-1, and the monocyte colony-stimulating factor, CSF-1, in response to tumor necrosis factor-alpha and immunoglobulin G. Evidence for involvement of reduced nicotinamide adenine dinucleotide phosphate (NADPH) -dependent oxidase. J Clin Invest. 1995;92:1564. doi: 10.1172/JCI116737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ericsson CH, Svartengren M, Mossberg B, Camner P. Bronchial reactivity, lung function, and serum immunoglobulin E in smoking-discordant monozygotic twins. Am Rev Respir Dis. 1993;147:296. doi: 10.1164/ajrccm/147.2.296. [DOI] [PubMed] [Google Scholar]
- 17.Venables KM, Topping MD, Howe W, Luczynska CM, Hawkins R, Taylor AJ. Interaction of smoking and atopy in producing specific IgE antibody against a hapten protein conjugate. Br Med J Clin Res Ed. 1985;290:201. doi: 10.1136/bmj.290.6463.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Francus T, Siskind GW, Becker CG. Role of antigen structure in the regulation of IgE isotype expression. Proc Natl Acad Sci USA. 1983;80:3430. doi: 10.1073/pnas.80.11.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen SS. Enumeration of antigen-specific IgE responses at the single-cell level by an ELISA plaque assay. J Immunol Methods. 1990;135:129. doi: 10.1016/0022-1759(90)90265-w. [DOI] [PubMed] [Google Scholar]
- 20.Galli SJ, Dvorak AM, Marcum JA, et al. Mast cell clones: a model for the analysis of cellular maturation. J Cell Biol. 1982;95:435. doi: 10.1083/jcb.95.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bass DA, Parce JW, Dechatelet LR, Szejda P, Seeds MC, Thomas M. Flow cytometric studies of oxidative product formation by neutrophils. A graded response to membrane stimulation. J Immunol. 1983;130:1910. [PubMed] [Google Scholar]
- 22.Koethe SM, Nelson KE, Becker CG. Activation of the classical pathway of complement by tobacco glycoprotein (TGP) J Immunol. 1995;155:826. [PubMed] [Google Scholar]
- 23.Frederiks WM, Bosch KS, Vreeling Sindelarova HA. In. situ detection of constitutive superoxide anion production in granules of mast cells. Histochem J. 1997;29:287. doi: 10.1023/a:1026470430151. [DOI] [PubMed] [Google Scholar]
- 24.Dalton TP, Shertzer HG, Puga A. Regulation of gene expression by reactive oxygen. Ann Rev Pharmacol Toxicol. 1999;39:37. doi: 10.1146/annurev.pharmtox.39.1.67. [DOI] [PubMed] [Google Scholar]
- 25.Plaut M, Pierce JH, Watson CJ, et al. Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature. 1989;339:64. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- 26.Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acid. Free Rad Biol Med. 1996;20:933. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 27.Hollman PC, Katan MB. Bioavailability and health effects of dietary flavonols in man. Arch Toxicol Suppl. 1998;20:237. doi: 10.1007/978-3-642-46856-8_21. [DOI] [PubMed] [Google Scholar]
- 28.Prochaska HJ, Long MJ, Talalay P. On. the mechanisms of induction of cancer-protective enzymes: a unifying proposal. Proc Natl Acad Sci USA. 1985;82:8232. doi: 10.1073/pnas.82.23.8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castonguay A. Pulmonary carcinogenesis and its prevention by dietary polyphenolic compounds. Ann N Y Acad Sci. 1993;686:177. doi: 10.1111/j.1749-6632.1993.tb39172.x. [DOI] [PubMed] [Google Scholar]
- 30.Wang ZY, Huang MT, Lou YR, et al. Inhibitory effects of black tea, green tea, decaffeinated black tea, and decaffeinated green tea on ultraviolet B light-induced skin carcinogenesis in 7,12-dimethylbenz[a]anthracene-initiated SKH-1 mice. Cancer Res. 1994;54:3428. [PubMed] [Google Scholar]
- 31.Afanas'Ev IB, Dorozhko AI, Brodskii A, Kostyuk VA, Potapovitch I. Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem Pharmacol. 1989;38:1763. doi: 10.1016/0006-2952(89)90410-3. [DOI] [PubMed] [Google Scholar]
- 32.Abraham SK, Sarma L, Kesavan PC. Protective effects of chlorogenic acid, curcumin and beta-carotene against gamma-radiation-induced in vivo chromosomal damage. Mutat Res. 1993;303:109. doi: 10.1016/0165-7992(93)90022-n. [DOI] [PubMed] [Google Scholar]
- 33.Aeschbacher HU, Jaccaud E. Inhibition by coffee of nitrosourea-mediated DNA damage in mice. Food Chem Toxicol. 1990;28:633. doi: 10.1016/0278-6915(90)90171-i. [DOI] [PubMed] [Google Scholar]
- 34.Das DK, Maulik N. Antioxidant effectiveness in ischemia reperfusion tissue injury. Meth Enzymol. 1994;233:601. doi: 10.1016/s0076-6879(94)33063-8. [DOI] [PubMed] [Google Scholar]
- 35.Cadenas E. Antioxidant and prooxidant functions of DT-diaphorase in quinone metabolism. Biochem Pharmacol. 1995;49:127. doi: 10.1016/s0006-2952(94)00333-5. [DOI] [PubMed] [Google Scholar]
- 36.Halliwell B, Gutteridge JM.C. Role of free radicals and catalytic metal ions in human disease: an overview. Free Rad Biol Med. 1990;186:1. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 37.Hardwick JS, Sefton BM. Activation of the Lck tyrosine protein kinase by hydrogen peroxide requires the phosphorylation of Tyr-394. Proc Natl Acad Sci USA. 1995;92:4527. doi: 10.1073/pnas.92.10.4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kass GE, Duddy SK, Orrenius S. Activation of hepatocyte protein kinase C by redox-cycling quinones. Biochem J. 1989;260:499. doi: 10.1042/bj2600499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Konishi H, Tanaka M, Takemura Y, et al. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc Natl Acad Sci USA. 1997;94:11233. doi: 10.1073/pnas.94.21.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guy GR, Cairns J, Ng SB, Tan YH. Inactivation of a redox-sensitive protein phosphatase during the early events of tumor necrosis factor/interleukin-1 signal transduction. J Biol Chem. 1993;268:2141. [PubMed] [Google Scholar]
- 41.Fialkow L, Chan CK, Downey GP. Inhibition of CD45 during neutrophil activation. J Immunol. 1997;158:5409. [PubMed] [Google Scholar]
- 42.Beaven MA, Ozawa K. Role of calcium, protein kinase C and MAP kinase in the activation of mast cells. Allergol Int. 1996;45:73. [Google Scholar]
- 43.Ishizuka T, Oshiba A, Sakata N, Terada N, Johnson GL, Gelfand EW. Aggregation of the FcepsilonRI on mast cells stimulates c-Jun amino-terminal kinase activity. A response inhibited by wortmannin. J Biol Chem. 1996;271:12762. doi: 10.1074/jbc.271.22.12762. [DOI] [PubMed] [Google Scholar]
- 44.Kinet JP. The high-affinity IgE receptor (Fc epsilon RI): from physiology to pathology. Annu Rev Immunol. 1999;17:931. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- 45.Wolfreys K, Oliveira DB. Alterations in intracellular reactive oxygen species generation and redox potential modulate mast cell function. Eur J Immunol. 1997;27:297. doi: 10.1002/eji.1830270143. [DOI] [PubMed] [Google Scholar]
- 46.Akasaka R, Teshima R, Kitajima S, et al. Effects of hydroquinone-type and phenolic antioxidants on calcium signals and degranulation of RBL-2H3 cells. Biochem Pharmacol. 1996;51:1513. doi: 10.1016/0006-2952(96)00092-5. [DOI] [PubMed] [Google Scholar]
- 47.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 48.Prieschl EE, Gouilleux Gruart V, Walker C, Harrer NE, Baumruker T. A nuclear factor of activated T cell-like transcription factor in mast cells is involved in IL-5 gene regulation after IgE plus antigen stimulation. J Immunol. 1995;154:6112. [PubMed] [Google Scholar]
- 49.Barrett WC, Degnore JP, Keng Y-F, Zhang Z-Y, Yim MB, Chock PB. Roles of superoxide radical anion in signal transduction mediated by reversible regulation of protein-tyrosine phosphatase 1B. J Biol Chem. 1999;274:34543. doi: 10.1074/jbc.274.49.34543. [DOI] [PubMed] [Google Scholar]
- 50.Caselli A, Marzocchini R, Camici G, et al. The inactivation mechanism of low molecular weight phosphotyrosine-protein phosphatase by H2O2. J Biol Chem. 1998;273:32554. doi: 10.1074/jbc.273.49.32554. [DOI] [PubMed] [Google Scholar]
- 51.Yang SH, Whitmarsh AJ, Davis RJ, Shanrock AD. Differential targeting of MAP kinases to the ETS-domain transcription factor Elk-1. EMBO J. 1998;17:1740. doi: 10.1093/emboj/17.6.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karin M, Liu ZG, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 53.Chang EY, Szallasi Z, Acs P, et al. Functional effects of overexpression of protein kinase C-alpha,-beta,-delta,-epsilon, and eta in the mast cell line RBL-2H3. J Immunol. 1997;159:2624. [PubMed] [Google Scholar]
- 54.Abate C, Patel L, Rauscher FJ, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990;249:1157. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- 55.Xanthoudakis S, Curran T. Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity. EMBO J. 1992;11:653. doi: 10.1002/j.1460-2075.1992.tb05097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hutchinson LE, McCloskey MA. Fc epsilon RI-mediated induction of nuclear factor of activated T-cells. J Biol Chem. 1995;270:16333. doi: 10.1074/jbc.270.27.16333. [DOI] [PubMed] [Google Scholar]
- 57.Eastmond NC, Banks EM, Coleman JW. Nitric oxide inhibits IgE-mediated degranulation of mast cells and is the principal intermediate in IFN-gamma-induced suppression of exocytosis. J Immunol. 1997;159:1444. [PubMed] [Google Scholar]
- 58.Coffman RL, Lebman DA, Rothman P. Mechanism and regulation of immunoglobulin isotype switching. Adv Immunol. 1993;54:229. doi: 10.1016/s0065-2776(08)60536-2. [DOI] [PubMed] [Google Scholar]
- 59.Pinkus R, Weiner LM, Daniel V. Role of oxidants and antioxidants in the induction of AP-1, NF-kappaB, and glutathione S-transferase gene expression. J Biol Chem. 1996;271:13422. doi: 10.1074/jbc.271.23.13422. [DOI] [PubMed] [Google Scholar]
- 60.Lin Y-I, Lin J-K. (–)-Epigallocatechin-3-gallate blocks the induction of nitric oxide synthase by down-regulating lipopolysaccharide-induced activity of transcription factor nuclear factor-κB. Am Soc Pharmacol Exp Ther. 1997;52:465. [PubMed] [Google Scholar]