Introduction
The unrelenting bombardment by microbial pathogens from the environment and their increasing resistance to medical treatments are a constant threat to the survival of all organisms on earth. Microbes are covered by molecular patterns that are common among a broad range of pathogens. These include the lipopolysaccharides (LPS) of Gram-negative bacteria, lipoteichoic acids of Gram-positive bacteria, lipoproteins of bacteria and parasites, glycolipids of mycobacteria, mannans of yeast and double-stranded RNAs of viruses.1,2 Recognition of and responses to these molecules are controlled by a wide variety of cellular receptors. The best characterized receptors are the T-cell receptor and B-cell antibody receptor of the adaptive immune response, the specificity of which is randomly generated and clonally selected during the development of T and B lymphocytes.3 Unlike the receptors of the adaptive immune system, the pattern recognition receptors of the innate immune system have predetermined specificity generated early on in evolution and play an essential role in the determination of self versus non-self during the initial rapid responses to infection.1,2 As described in this review, a family of cell surface receptors, termed Toll-like receptors, are emerging as key regulators of host responses to infection.
TOLL and THE DROSOPHILA HOST DEFENCE
The origins of the mammalian innate immune response are clearly seen with the involvement of the Toll protein in Drosophila development and host defence. Prior to the discovery of its involvement in Drosophila host defence, Toll was identified by a genetic screen for a defect in the establishment of dorsal–ventral polarity. This toll mutant was a dominant-ventralizing mutation in contrast to the recessive-dorsalizing mutants dorsal and gastrulation defective. Further mutant screens and double-mutant strategies characterized the order of other genes involved in this developmental pathway.4 The best characterized being the Rel signalling pathway involving the activation of family members dorsal, dif and relish.5–8
The factors involved in the embryonic Toll signalling pathway have been identified and characterized.9 Activation of Toll requires the endogenous ligand Spaetzle, which is proteolytically cleaved to its active form by a series of serine proteases genetically upstream of spaetzle and toll. Stimulation of Toll leads to the activation of Rel proteins, such as Dorsal, through signalling factors Tube and Pelle, resulting in the degradation of Cactus and release of Dorsal. Cactus is the Drosophila homologue of the mammalian I-κB family that sequesters and inhibits Rel family members in the cytoplasm prior to activation. Once released, Rel proteins travel to the nucleus where they positively or negatively regulate target genes involved in many cellular events. In fact, recent studies have placed Toll signalling pathways at the centre of mediating the up-regulation of Drosophila host responses to infection.4,10,11
The Drosophila immune response relies on the production of antimicrobial peptides, and each peptide has inherent specificity for its particular class of pathogen.12 In an analysis of the regulation of these peptides it was found that they were all highly regulated by κB sites in their promoters. In fact, the Rel family members Dorsal, Dif and Relish are all activated in response to infection.5–8 Genetic analysis identified the Spaetzle–Toll–Pelle–Tube–Cactus signalling cascade as an important regulator not only of Rel protein activation, but also in the induction of antimicrobial peptides. As a result, differential responses to yeast and bacterial infection have been observed between Toll family members. Toll and Spaetzle mutant flies suffer severe fungal infection and are unable to induce the expression of the antifungal peptide, Drosomycin. Although many of the same factors involved in the embryonic pathway are utilized, the Toll–antifungal pathway leads to the activation of Dif, but not Dorsal, in the regulation of the drosomycin gene.10 In addition, there are mechanisms in place to respond to bacterial infection. The Toll family member, 18-wheeler (18w), has been linked to the production of Attacin, an antibacterial peptide.13,14 Mutants for ird and imd have also been linked to antibacterial responses.6 In contrast to mutants biased to specific pathogens (toll, spaetzle, 18w, ird and imd), Cactus is required for both antifungal and antibacterial responses. This suggests that Cactus is a common player in both kinds of responses and may therefore involve separate Cactus/Rel complexes activated by independent upstream pathways.8 This is a potential mechanism for the differential regulation of Rel family target genes. Strikingly, similar receptors and signalling pathways have been observed recently in other species, ranging from plants to mammals, a fact which emphasizes the conservation of molecules throughout evolution.
Toll-Like Receptors
The link between Drosophila Toll (dToll) and mammalian Toll-like receptors exemplifies the evolutionary connection between Drosophila host defence and the mammalian innate immune response, which has been considered for many years to be the ancient arm of the mammalian immune system. Mammalian Toll homologues have been termed Toll-like receptors. Nine have been published.15 The first functional analysis of mammalian Toll-like receptor was the study of hToll4 by Medzhitov et al.16 The hToll4 mRNA was isolated from dendritic cells, γδ T cells, T helper type 1 (Th1) and 2 (Th2) αβ T cells and B cells. A dominant active form of the receptor induced cytokine expression and NF-κB activation when expressed in monocyte cell lines.16 These findings and the homology with dToll suggest that Toll-like receptors may play an important role in mammalian host responses to infection.
Toll-like receptors have moderate homology with dToll, but more importantly share a common structure. Toll-like receptor structure includes leucine-rich repeats in the extracellular domain15,17,18 and a cytoplasmic domain which shares significant homology with the interleukin-1 receptor (IL-1R) signalling domain, termed the Toll/IL-1 receptor homologous region.19 The leucine-rich repeats of the extracellular domain mediate the response to conserved pathogen-associated molecular patterns commonly found among microbial organisms. A number of pattern recognition receptors recognize these molecules, including mannose and scavenger receptors, Toll-like receptors and CD14.1,2 There is considerably more diversity found in the extracellular domains of Toll-like receptors, when compared to the intracellular signalling domain.20 This has also been observed in the Toll-like receptors of plant species.21 Analysis of the leucine-rich repeats of plants suggests that the extracellular domain plays an important role in the specificity of host responses to infection.22 Although leucine-rich repeats play a role in the recognition of microbial ligands, their involvement in Toll-like receptor function has yet to be fully addressed.
TOLL-LIKE RECEPTOR GENETICS and THE ‘LPS RECEPTOR’
Responses to LPS can cause septic shock in extreme Gram-negative bacterial infections, causing a severe health risk to the population every year. The search for the ‘LPS receptor’ has a long history. Three strains of mice, C3H/HeJ, C57BL/10ScCr and C57BL/10ScN, are naturally hyporesponsive to high doses of LPS. These mutations are referred to as the lpsd gene.23,24 These strains of mice showed inherent specificity by their increased susceptibility to Gram-negative infection, and normal responses to Gram-positive bacteria. Furthermore, LPS hyporesponsive macrophages from lpsd mutant mouse strains respond normally to microbial lipoproteins.23–25 This suggested that different receptors were involved in responding to different pathogens. Following the identification of the mouse tlr4 gene and considerable mapping of the lpsd gene locus, lpsd was identified as the lps gene.26–28 In a 1999 review, Samuel D. Wright described Toll-like receptors as, ‘the missing piece of the puzzle’.29 In fact, the mutations that occurred in all three mouse strains occurred in the tlr4 gene. The C3H/HeJ hyporesponsiveness is caused by a dominant negative mutation30 owing to a proline to histidine substitution mutation at amino acid 712. C57BL/10ScCr and C57BL/10ScN strains carry a recessive tlr4 null mutation.26–28
Another Toll-like receptor, TLR2, was also implicated in responses to LPS. TLR2 conferred LPS responsiveness to a normally non-responsive cell line, HEK293. TLR2 activated NF-κB in response to LPS and an anti-TLR2 antibody inhibited the production of tumour necrosis factor-α (TNF-α) and IL-12 p40 from monocytes stimulated with LPS.31–33 (Please see NOTE ADDED IN PROOF at end of Reference list.) Hamster macrophages and the Chinese hamster ovary CHO cell line carry a TLR2 null mutation, but are still responsive to LPS.34 While TLR2 may be LPS responsive in stably transfected cell lines, genetic evidence supports TLR4 as the LPS receptor. Toll-like receptor-deficient (TLR–/–) mice support this model. TLR4–/– mice are hyporesponsive to LPS, while TLR2–/– mice still respond normally. The results from the Toll-like receptor knockouts also suggest that there is clear specificity for microbial ligands between Toll-like receptor family members.35,36 Although the evidence supports TLR4 as a key component of the LPS receptor, TLR4 mutant mice have been observed to be responsive to LPS under some conditions. Responsiveness has been observed with LPS from different species of Gram-negative bacteria37 as well as with extremely high doses of LPS.38 In addition, activation can be restored to normal levels by treatment with interferon-γ (IFN-γ).39 Although there appear to be clear differences in specificity between TLR4 and TLR2 in mouse, the ability of human TLR2 to be activated by LPS and the inhibition of LPS-induced cytokine activation by an anti-human TLR2 blocking antibody suggest that there may either be some factors in common between TLR4 and TLR2 specificities or species differences between human and mouse Toll-like receptors.31–33 Clearly, the mechanisms underlying responses to microbial ligands are very complex and may involve multiple factors that determine the specificity of Toll-like receptor responsiveness. Much like the specificity of Drosophila Toll family members, mammalian Toll-like receptor family members are emerging as having their own specificity for different classes of microbial molecules.
MICROBIAL LIGANDS (PATHOGEN-ASSOCIATED MOLECULAR PATTERNS) and TOLL-LIKE RECEPTOR SPECIFICITY
Microbial molecules which elicit strong responses from the innate immune system are quite common among a broad range of pathogens. These pathogen-associated molecular patterns are generally repetitive in nature and are distinct from the molecules found in the host. In addition, pathogen-associated molecular patterns are not subject to antigenic variation, but are essential for the survival and pathogenicity of the organism.1,2 Microbial carbohydrates are a good example of this, which are a common structure that decorate the cell walls of many species of bacteria.40–42 Lipid modifications are also common among microbial molecules.43–48 It is thought that pathogen-associated molecular patterns are the target of pattern recognition receptors, such as Toll-like receptors; however, the exact mechanism of this interaction is under much debate.
While much of the focus of Toll-like receptor biology has centred on its role in mediating LPS responsiveness, there has been much interest in identifying other pathogens and molecules that activate TLRs. Although a growing number of Toll-like receptors have been identified, TLR2 and TLR4 are the only Toll-like receptors that have been shown to be responsive to microbial ligands. TLR4 is clearly the main LPS receptor, but what role do TLR4 and TLR2 play in the responses to other antigens? Microbial lipoproteins are very common among bacteria including mycobacteria, Gram-positive and Gram-negative bacteria, such as the spirochaetes Borrelia burgdorferi and Treponema pallidum. These microbial lipoproteins share a common triacyl motif at their N-termini that is responsible for their stimulatory properties.43–47 Much like many of these bacterial ligands, microbial lipoproteins are strong stimulators of macrophage activation, including the production of cytokines and the up-regulation of inflammatory events.48–52 TLR2 was found to mediate responsiveness to lipoproteins from mycobacteria,33 mycoplasma53 and spirochaete species.54 TLR2 was found to be the primary mediator of lipoprotein activation, while TLR4/MD2 were found not to be involved.33,53 An anti-TLR2 antibody inhibited lipoprotein stimulation of cytokine production from macrophages stimulated with lipoproteins.33 Transfection experiments, as well as the Toll-like receptor-deficient mice, demonstrate that TLR2 is the primary receptor for microbial lipoproteins.33,53,54 While TLR4 has been shown to also mediate responses to some forms lipoteichoic acids and LPS, TLR2 responds to a broad range of molecules from a wide variety of pathogens. TLR2 has been shown to be involved in the phagocytosis of yeast,55 as well as in responses to mycobacteria,33,49,55 specifically to the 19 000 MW lipoprotein33 and lipoarabinomannan from Mycobacterium tuberculosis.49 TLR2 appears to be the primary regulator of Gram-positive responses. Interestingly, lpsd mice respond normally to Gram-positive infection while being more susceptible to Gram-negative infection.26 Gram-positive bacterial ligands that stimulate through TLR2 include Listeria monocytogenes,56 Staphylococcus aureus cell walls, peptidoglycan, and lipoteichoic acids.36,49,53,57–59 Differential responsiveness to pathogens by multiple receptors is reminiscent of Drosophila host defence. Drosophila Toll receptors mediate responses to a specific class of pathogen resulting in the production of target genes that specifically fight that infection. It appears that this has been conserved at some level with mammalian Toll-like receptors which have an ever growing array of ligands. This could lead to the activation of Toll-like receptor- specific target genes. With the vast range of microbial ligands, these innate receptors provide a mechanism by which the immune system can respond specifically to distinct classes of molecules. Further studies with Toll-like receptor-deficient mice will be essential to verify TLR2 transfection experiments in addition to understanding the specificity of responses and the susceptibility to infection.
Mechanism of toll-like receptor activation
The parallels between Drosophila host defence and mammalian innate immunity are striking. Although the data suggest that TLR2 and TLR4 have clear specificity for different microbial ligands, the actual mechanism of TLR activation is still unclear. Is there a direct interaction between Toll-like receptors and their putative ligands, or are there endogenous ligands, similar to the Drosophila Spaetzle, that activate the receptors in response to infection? Furthermore, what role do Toll-like receptor co-receptors, such as CD14 and MD2, play in this process?
One protein which may play a critical role in Toll-like receptor function is CD14. CD14 was the first protein to be identified as an LPS receptor. CD14 binds many lipid-containing molecules, including LPS, microbial lipoproteins, Streptococcal cell walls and M. tuberculosis lipoarabinomannan.50,60–68 Once shed from the surface of the bacterium, LPS is brought to membrane-bound or soluble forms of CD14 by serum protein LPS-binding protein (LBP). The presence of a LPS/CD14/LBP complex at the membrane results in cellular activation of LPS-responsive cell types.69 CD14 expression correlates with increased sensitivity of many cell types to LPS and other microbial molecules in their ability to activate downstream signalling events and cytokine production.69–77 CD14 is attached to the outer leaflet of the membrane by a glycosylphosphatidyl inositol (GPI) anchor. Since CD14 does not traverse the membrane into the cytoplasm, it cannot mediate LPS signalling events alone, but may require a co-receptor to activate intracellular signalling pathways.78 Despite this inability to signal, CD14-deficient mice are hyporesponsive to LPS, suggesting that CD14 plays a critical role in this process.79 TLRs seem to fit this requirement for a co-receptor. CD14 expression alone does not confer LPS responsiveness to cell lines. However, when CD14 is co-expressed with TLR2 or TLR4, activation of NF-κB by LPS, microbial lipoproteins, or lipoarabinomannan is enhanced.31–33,54,58 These observations are consistent with the role of CD14 in cellular activation and cytokine production.
Other co-receptors may be involved in regulating TLR responsiveness. Initial studies of TLR4 suggested that it was not responsive to LPS activation when stably expressed in HEK293 cells. However, subsequent studies identified a novel secreted protein, MD2, which was shown to associate with TLR4 and enhance its responsiveness to LPS. Furthermore, MD2 may be required for TLR4 function.80 Therefore, the question remains, do other Toll-like receptors require similar co-factors to function? To date, other Toll-like receptor family members have not been shown to be responsive to microbial ligands.32,54,59,81 Therefore, they may require MD2-like co-factors or heterodimerization with other Toll-like receptor family members to function.
Is there direct interaction between Toll-like receptors and their putative microbial ligands? The specificity demonstrated in Toll-like receptor-transfected cell lines, as well as the knockout mice, suggests that direct interaction may be involved. However, the biochemistry of this interaction is lagging behind the molecular biology. Unlike the strong association of CD14 with ligands LPS and microbial lipoproteins, the binding affinity of TLRs with LPS is much lower than would be expected for this kind of interaction.31 However, other proteins, such as CD14 and MD2, may enhance this interaction.20 Recent studies suggest that there is a direct TLR4–LPS interaction in a comparison of species-specific responsiveness to different forms of LPS–lipid A by genetic complementation. Human and mouse TLR4 were able to discriminate between different forms of LPS–lipid A when overexpressed in C3H/HeJ mice.82 However, there have yet to be studies published demonstrating a strong biochemical association between Toll-like receptors and their putative ligands.
The activation of Drosophila Toll during infection requires the endogenous ligand Spaetzle. Much like the processing of Spaetzle in the developmental Toll pathway, Pro-Spaeztle is proteolytically cleaved to its active form, Spaetzle, following stimulation by fungal spores.10 The serine proteases Snake and Easter involved in the developmental pathway, however, are not involved in the response to fungal infection.10 Cleavage of Spaetzle is required for induced expression of antimicrobial peptides via Toll in Drosophila, but the protease(s) involved in the immune response has yet to be identified.9,12,83 Although, Spaetzle and Toll are required for antifungal responses in Drosophila, no endogenous ligand has been identified for antimicrobial responses upstream of the Toll family member, 18-wheeler.10
Similar protease cascades have been observed in other species and are thought to play a role in mammalian immune response, as well. For example, serine proteases have been observed in the horseshoe crab clotting cascade. These proteases share sequence similarities with the Drosophila Easter serine protease.84,85 The limulus proteases factor B and proclotting enzyme, which cleave pro-coagulogen, are homologues of Snake and Easter, respectively. Interestingly, coagulogen contains cysteine knots similar to those found in Spaetzle.86 The activation of the mammalian complement cascade involves a pathway of proteases activated during infection.87 These parallel mechanisms clearly demonstrate factors in common between widely diverse organisms.
Is there a protease cascade and endogenous ligand upstream of Toll-like receptor activation? Many have hypothesized that Toll-like receptors are activated by an endogenous ligand. However, studies of endogenous ligands for Toll-like receptors have only recently started to emerge. A study by Ohashi et al. implicated the heat-shock protein (hsp 60) chaperone as an endogenous TLR4 ligand, suggesting that it acts as a ‘danger antigen to the innate immune response’.88 In fact, hsp 60 is normally sequestered in the cell interior, but is rapidly translocated to the membrane in response to cell stress. Responses to LPS and hsp 60 are very similar. Interestingly, C3H/HeJ mice are not responsive to hsp 60, suggesting that like LPS, TLR4 mediates stimulatory effects of hsp 60. Although this is the first example of an endogenous protein activating Toll-like receptor signalling pathways, a direct interaction of TLR4 with hsp 60 has yet to be demonstrated. A role for protease cascades in the activation of responses to LPS is not unprecedented. Jin et al. identified ‘epithelial cell-derived inhibitor of leucocyte serine proteases’ (SLPI) from LPS-stimulated epithelial cells.89 Overexpression of SLPI suppressed LPS-induced activation of NF-κB, and the production of nitric oxide and TNF-α. SLPI renders macrophage cell lines hyporesponsiveness to LPS. Again, there are interesting parallels between organisms. Drosophila cleavage of pro-Spaetzle is regulated by the serine protease inhibitor, Spn43Ac, suggesting that there may be multiple levels of regulation in LPS response mechanisms.90 Interestingly, SLPI expression is inhibited by IFN-γ and thus enhances LPS responsiveness. This responsiveness is consistent with IFN-γ enhancement of LPS stimulation, and may serve to prolong activation by LPS. Further exploration of an endogenous Toll-like receptor ligand will require more thorough biochemistry and genetic screening to identify molecules involved in Toll-like receptor activation.
Toll-like receptor signalling
The immune responses of Drosophila and mammals not only share common receptors, but also share evolutionarily conserved downstream signalling pathways involved in the activation of host defences. The Toll-like receptors share significant homology with the Toll/IL-1R intracellular signalling domain.19 In fact, they share common downstream signalling molecules of the Rel/NF-κB pathway.16,91 Activation of NF-κB occurs downstream of a number of receptors including TNF receptors, CD40, the IL-1 receptor, dToll and Toll-like receptors.92 Activation of the Toll/IL-1R receptor domain results in sequential recruitment of the adapter molecule MyD88, through its own Toll/IL-1R receptor domain.93 Furthermore, MyD88-deficient mice do not respond to either IL-1 or LPS, thus demonstrating its bifunctional role in these signalling pathways.94,95 MyD88 recruits IRAK (IL-1 receptor accessory protein kinase) through death domain interactions of both proteins resulting in the autophosphorylation of IRAK.93,95–99 TRAF6 (TNF-receptor-associated factor) is then recruited leading to the activation of MAP kinase kinase kinases (MAPKKK), such as NF-κB-inducing kinase (NIK).100 The NF-κB-inducing kinase activates I-κB-alpha and -beta kinases which are involved in the phosphorylation of members of the NF-κB inhibitory family, I-κB.101–103 I-κB family members are ubiquitinated following phosphorylation, resulting in I-κB degradation. NF-κB then translocates to the nucleus where the active dimer can transactivate gene expression of such cytokines as IL-1β, IL-6, IL-8, IL-12 p40, and co-stimulatory molecules CD80 and CD86.104–107
A number of signalling pathways have been implicated downstream of microbial ligands, such as LPS and lipoproteins. In fact, the regulation of many immunomodulatory genes requires more than one signalling pathway. For example, the IL-12 p40 gene is regulated by NF-κB and CCAAT/enhancer binding protein (C/EBP) family members.108,109 In addition to the NF-κB signalling pathway, other signalling pathways have been linked to Toll-like receptor signalling pathways. Overexpression of the dominant active form of TLR4 activates not only NF-κB but also Ap1 and Jun N-terminal kinase (JNK).16,97,98 In fact NF-κB, C/EBP and Ap1 are prominently involved in the regulation of many pro-inflammatory and immunomodulatory genes.109,110 The mitogen-activated protein (MAP) kinase pathway is also activated in response to microbial ligands, possibly downstream of Toll-like receptor activation as well. The MAPKKK, TAK1, appears to be involved in LPS-induced NF-κB activation downstream of TLR2 and TLR4 in transfected cell lines and in the murine macrophage cell line RAW264.7.111 A TAK1-dominant negative mutant blocked NF-κB activation in both of these cell types, and TAK1 is phosphorylated in response to LPS. The specific interactions leading to the activation of MAP kinases downstream of Toll-like receptors are unclear; however, the MyD88 knockout suggests that while MyD88 is essential for responses to LPS, MyD88 alone is not required for the activation of NF-κB or MAP kinase pathways.94 The B-cell-specific Toll-like receptor, RP105, activates Src-family tyrosine kinase Lyn, protein kinase C beta and MAP kinase pathways.112 There is growing evidence that Toll-like receptors lead to the activation of a number of different signalling pathways that contribute to the specificity of target gene activation and cellular responses.
TOLL-LIKE RECEPTOR and HOST RESPONSES TO INFECTION
Toll-like receptors appear to be involved in the activation of a growing list of mammalian host responses to infection. The signalling pathways and target genes downstream of Toll-like receptors have drastic effects on how the host responses are activated. The innate immune response is rapid, allowing for the early detection of microbial pathogens and the control of infection. In contrast, the adaptive immune response is delayed and involves immunological memory. Not only do innate and adaptive immune responses complement each other, they are interactive. The innate immune response influences the type of adaptive immune response elicited through the production of immunomodulatory genes, which are regulated in large part by Toll-like receptor activation.1,2,16,31,33
Activation of lymphocytes is not preprogrammed, but often depends on activation signals generated by cells of the innate immune response.1,2,113,114 Cells of the innate immune system, antigen-presenting cells (APC) that have been in contact with the pathogen can polarize lymphocyte responses through the production of cytokines and co-stimulatory molecules.115,116 Toll-like receptors are clearly involved in the activation of cytokines by microbial ligands. Overexpression of a dominant active TLR4 in macrophage cell lines induces cytokine production and the expression of co-stimulatory molecules.16 IL-12 is a critical regulator of cell-mediated immune responses influencing the induction of Th1-type responses from T and B lymphocytes.117 Microbial lipoproteins activate the IL-12 p40 promoter through a TLR2-dependent mechanism. A TLR2-dominant negative mutant inhibits the induction of the IL-12 p40 and inducible nitric oxide synthase (iNOS) promoter following stimulation by microbial lipoproteins. Furthermore, an anti-TLR2 inhibitory antibody blocks the production of IL-12 and other pro-inflammatory cytokines, such as TNF-α, from primary monocytes.33 Activation of Toll-like receptors may also lead to the induction of down-regulatory cytokines, such as IL-10. As a result, the balance of cell-mediated (Th1) and humoral (Th2) immune responses can be directly influenced by Toll-like receptor target genes. Previous studies have suggested that pro- and anti-inflam- matory cytokines are differentially regulated in response to the same microbial ligands.110,118–123 In fact, the differential expression of IL-12 versus IL-10 has been observed in disease models, such as leprosy, where the dysregulation of these two cytokines can have drastic effects on the state of disease.124,125 The involvement of TLRs in this differential regulation of immunomodulatory genes will be an intriguing avenue to pursue in future studies.
Toll-like receptors are clearly activated by mycobacterial components.33,49,53,57 Mycobacteria are strong stimulators of IL-12 production and cell-mediated immune responses.33,120,126–128 The M. tuberculosis 19 000 MW lipoprotein can activate the iNOS promoter and nitric oxide production from murine macrophages in a TLR2-dependent manner.33 Inducible NOS is critical for the production of nitric oxide from macrophages. Nitric oxide represents a powerful antimicrobial pathway. In particular, nitric oxide generation is required for efficient host defence against M. tuberculosis, since iNOS-deficient mice are highly susceptible to infection.129 Therefore, activation of mammalian Toll-like receptors, by induction of nitric oxide, can directly contribute to an antimicrobial response. Since a main function of Drosophila Toll is the induction of antimicrobial peptides, the function of Toll in host defence has been conserved throughout evolution.127,128
In addition to macrophage activation and pro-inflammatory responses attributed to Toll-like receptor activation, the activation of down-modulatory mechanisms has also been observed. Aliprantis et al. demonstrated that microbial lipoproteins induced features of apoptosis in human monocytes, including cell shrinkage and membrane blebbing, by a TLR2-dependent mechanism.130 Induction of apoptosis was confirmed by TdT-mediated dUTP nick-end labelling (TUNEL) assay and cell lysis was demonstrated according to lactate dehydrogenase release. Thus, microbial lipoproteins have the ability to induce both TLR-dependent activation of host defence and tissue pathology. This dual signalling pathway is similar to TNF receptor and CD40 signalling, which can induce both NF-κB activation and apoptosis.131 In this manner, it is possible for the immune system to activate host defence mechanisms, then by apoptosis down-regulate the response from causing tissue injury, such as in sepsis. Alternatively, by leading to apoptosis, activation of TLRs may also contribute to tissue damage during the course of inflammation.
Conclusion
In a recent review, K. Anderson suggested that the Drosophila immune response is ‘genetically hardwired’ to respond specifically to pathogenic infection.20 Drosophila Toll receptors have clear specificity for the class of antimicrobial peptides that they activate. Toll induces the antifungal peptide gene, drosomycin, and 18-wheeler induces the antibacterial peptide, attacin. Interestingly, Rel family members have been found to influence the specificity of target gene activation as well; for example, dif mutants exhibit reduced production of drosomycin and defensin.11,132 Similarly, relish mutants fail to produce cecropin A and diptericin, in addition to having reduced production of other antimicrobial peptides.133
Is there a similar hardwired genetic mechanism in the mammalian immune response? The complexity of the mammalian Toll-like receptor responses involves multiple levels of regulation. Although individual target genes for each Toll-like receptor have not been identified yet, the Toll-like receptors clearly have individual specificity for certain classes of microbial ligands (Figure 1). Toll-like receptors may be able to heterodimerize, thus increasing the combinatorial diversity of ligands that these receptors could respond to, as well as, the diversity of target gene activation downstream. NF-κB-deficient mice have been generated and are currently being characterized for NF-κB family member involvement in the activation of specific target genes. Altered expression of NF-κB family members has critical effects on the production of cytokines and immune responses to infection.134–136 Therefore, as more studies emerge, the effects of NF-κB family members on host responses will become more evident. A wide variety of cell types are responsive to microbial ligands and express a battery of Toll-like receptors. The combination of these receptors may be critical to a number of cell-type-specific host responses ranging from the production of macrophage cytokines to B-cell proliferation and antibody production. The future of the Toll-like receptor field appears to be rich with many avenues to traverse.
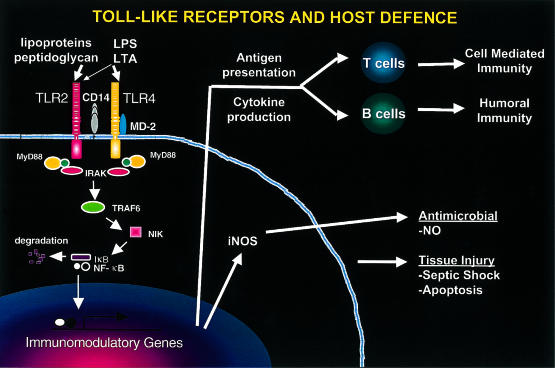
Figure 1.
Toll-like receptors and host defence: LPS, lipopolysaccharide; LTA, lipoteichoic acid; TLR, Toll-like receptors; IRAK, interleukin-1 receptor accessory protein kinase; TRAF6, tumour necrosis factor-receptor-associated factor; NIK, NF-κB-inducing kinase: iNOS, inducible nitric oxide synthase; NO, nitric oxide.
The presence of Toll in Drosophila indicates that Toll proteins represent a host defence mechanism that has been conserved over hundreds of millions of years of evolution. In mammals, Toll-like receptors provide the innate immune system with the ability to recognize and react to a wide spectrum of microbial pathogens expressing lipoproteins and lipopolysaccharides. Toll-like receptors can activate innate immune responses including iNOS, a direct microbicidal mechanism, and IL-12, which functions as a biological adjuvant amplifying acquired T-cell responses to pathogens. Thus microbial ligand activation of Toll-like receptors is probably an early signal for the induction of IL-12 and hence the generation of Th1 cytokine responses. It will be important to determine whether by induction of nitric oxide or other mechanisms, activation of TLRs leads to a direct anti-microbial effector pathway, as is evident in Drosophila. Under certain conditions, the Toll-like receptor signalling pathway can lead to the activation of down-modulatory responses, including apoptosis of the target cells resulting in down-regulation of the immune response or pathology to the host130(Fig. 1). It should be possible, however, to develop strategies to stimulate Toll-like receptors in order to activate host defence mechanisms and to enhance immune responses to a wide variety of antigens in vaccines.
NOTE ADDED IN PROOF
A recent study suggests that LPS preparations contain a mix of microbial ligands with distinct TLR specificities.
Hirschfield M, Ma Y, Weis JH, Vogel SN, Weis JJ. Repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol 2000; 165:618–22.
References
- 1.Medzhitov R, Janeway Ca., Jr Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R, Janeway Ca., Jr Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–8. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 3.Thompson CB. New insights into V(D)J recombination and its role in the evolution of the immune system. Immunity. 1995;3:531–9. doi: 10.1016/1074-7613(95)90124-8. [DOI] [PubMed] [Google Scholar]
- 4.Belvin MP, Anderson KV. A conserved signaling pathway: the Drosophila Toll-Dorsal Pathway. Annu Rev Cell Dev Biol. 1996;12:3343–416. doi: 10.1146/annurev.cellbio.12.1.393. [DOI] [PubMed] [Google Scholar]
- 5.Ip YT, Reach M, Engstrom Y, Kadalayil L, Cai H, Gonzalez-Crespo S, Tatei K, Levine M. Dif, a dorsal-related gene that mediates an immune response in Drosophila. Cell. 1993;75:753–63. doi: 10.1016/0092-8674(93)90495-c. [DOI] [PubMed] [Google Scholar]
- 6.Lemaitre B, Meister M, Govind S, Georgel P, Steward R, Reichhart JM, Hoffmann JA. Functional analysis and regulation of nuclear import of Dorsal during the immune response in Drosophila. EMBO J. 1995;14:536–45. doi: 10.1002/j.1460-2075.1995.tb07029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dushay MS, Asling B, Hultmark D. Origins of immunity: Relish, a compound Rel-like gene in the antibacterial defense of Drosophila. Proc Natl Acad Sci USA. 1996;93:10343–7. doi: 10.1073/pnas.93.19.10343. 10.1073/pnas.93.19.10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu LP, Anderson KV. Regulated nuclear import of Rel proteins in the Drosophila immune response. Nature. 1998;392:93–7. doi: 10.1038/32195. [DOI] [PubMed] [Google Scholar]
- 9.Anderson KV. Pinning down positional information: dorsal–ventral polarity in the Drosophila embryo. Cell. 1998;95:439–42. doi: 10.1016/s0092-8674(00)81610-4. [DOI] [PubMed] [Google Scholar]
- 10.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spaetzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–83. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 11.Meng X, Khanuja BS, Ip YT. Toll receptor-mediated Drosophila immune response requires Dif, an NF-κB factor. Genes Dev. 1999;13:792–7. doi: 10.1101/gad.13.7.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemaitre B, Reichhart JM, Hoffmann JA. Drosophila host defense: differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc Natl Acad Sci USA. 1997;94:14614–9. doi: 10.1073/pnas.94.26.14614. 10.1073/pnas.94.26.14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eldon E, Kooyer S, D'evelyn D, Duman M, Lawinger P, Botas J, Bellen H. The Drosophila 18 wheeler is required for morphogenesis and has striking similarities to Toll. Development. 1994;120:885–99. doi: 10.1242/dev.120.4.885. [DOI] [PubMed] [Google Scholar]
- 14.Williams MJ, Rodriguez A, Kimbrell DA, Eldon ED. The 18-wheeler mutation reveals complex antibacterial gene regulation in Drosophila host defense. EMBO J. 1997;16:6120–30. doi: 10.1093/emboj/16.20.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci USA. 1998;95:588–93. doi: 10.1073/pnas.95.2.588. 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 17.Anderson KV, Jurgens G, Nusslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene product. Cell. 1985;42:779–89. doi: 10.1016/0092-8674(85)90274-0. [DOI] [PubMed] [Google Scholar]
- 18.Wasserman SA, Sanghera JS, Lemke K, DeFranco AL, Pelech SL. A conserved signal transduction pathway regulating the activity of the rel-like proteins dorsal and NF-kappa B. Mol Biol Cell. 1993;4:767–71. doi: 10.1091/mbc.4.8.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kopp EB, Medzhitov R. The Toll-receptor family and control of innate immunity. Curr Opin Immunol. 1999;11:13–18. doi: 10.1016/s0952-7915(99)80003-x. 10.1016/s0952-7915(99)80003-x. [DOI] [PubMed] [Google Scholar]
- 20.Anderson KV. Toll signaling pathways in the innate immune response. Curr Opin Immunol. 2000;12:13–19. doi: 10.1016/s0952-7915(99)00045-x. [DOI] [PubMed] [Google Scholar]
- 21.van der Biezen EA, Jones JD. Plant disease-resistance proteins and the gene- or-gene concept. Trends Biochem Sci. 1998;12:454–6. doi: 10.1016/s0968-0004(98)01311-5. [DOI] [PubMed] [Google Scholar]
- 22.McDowell JM, Dhandaydham M, Long TA, Aarts MG, Goff S, Holub EB, Dangl JL. Intragenic recombination and diversifying selection contribute to the evolution of downy mildew resistance at the RPP8 locus of Arabidopsis. Plant Cell. 1998;11:1861–74. doi: 10.1105/tpc.10.11.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogel SN. The Lps gene. Insights into the genetic and molecular basis of LPS responsiveness and macrophage differentiation. In: Beutler B, editor. Tumor Necrosis Factors: the Molecules and Their Emerging Role in Medicine. New York: Raven Press; 1992. pp. 485–513. [Google Scholar]
- 24.Qureshi ST, Gros P, Malo D. Host resistance to infection: genetic control of lipopolysaccharide responsiveness by Toll-like receptor genes. Trends Genet. 1999;8:291–4. doi: 10.1016/s0168-9525(99)01782-5. [DOI] [PubMed] [Google Scholar]
- 25.Ma Y, Weis JJ. Borrelia burgdorferi outer surface lipoproteins OspA and OspB possess B-cell mitogenic and cytokine-stimulatory properties. Infect Immun. 1993;61:3843–53. doi: 10.1128/iai.61.9.3843-3853.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 27.Poltorak A, Smirnova I, He X, et al. Genetic and physical mapping of the Lps locus: identification of the toll-4 receptor as a candidate gene in the critical region. Blood Cells Mol Dis. 1998;24:340–55. doi: 10.1006/bcmd.1998.0201. 10.1006/bcmd.1998.0201. [DOI] [PubMed] [Google Scholar]
- 28.Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4. J Exp Med. 1999;189:615–25. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright SD. Toll, a new piece of the puzzle of innate immunity. J Exp Med. 1999;189:605–9. doi: 10.1084/jem.189.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vogel SN, Johnson D, Perera PY, Medvedev A, Lariviere L, Quershi ST, Malo D. Functional characterization of the effect of the C3H/HeJ defect in mice that lack an lpsn gene: in vivo evidence for a dominant negative mutation. J Immunol. 1999;162:5666–70. [PubMed] [Google Scholar]
- 31.Yang BR, Mark MR, Gray A, et al. Toll-like receptor-2 mediated lipopolysaccharide-induced cellular signaling. Nature. 1998;395:284–8. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- 32.Kirshning CJ, Wesche H, Ayres TM, Roth M. Human Toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med. 1999;188:2091–7. doi: 10.1084/jem.188.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brightbill HD, Libraty DH, Krutzik SR, et al. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science. 1999;285:732–6. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 34.Heine H, Kirschning CJ, Lien E, Monks BG, Rothe M, Golenbock DT. Cells that carry a null allele for toll-like receptor 2 are capable of responding to endotoxin. J Immunol. 1999;162:6971–5. [PubMed] [Google Scholar]
- 35.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Toll-like receptor 4 (TLR4) -deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the lps gene product. J Immunol. 1999;162:3749–52. [PubMed] [Google Scholar]
- 36.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–51. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 37.Tanamoto K, Azumi S, Haishima Y, Kumada H, Umemoto T. The lipid A moiety of Porphyromonas gingivalis lipopolysaccharide specifically mediates the activation of C3H/HeJ mice. J Immunol. 1997;158:4430–6. [PubMed] [Google Scholar]
- 38.Rosenstreich DL. Genetic control of endotoxin response: C3H/HeJ mice. In: Berry LJ, editor. Handbook of Endotoxin, Cellular Biology of Endotoxin. Vol. 3. New York: Elsevier Science Publishers; 1985. pp. 82–122. [Google Scholar]
- 39.Beutler B, Tkacenko V, Milsark I, Krochin N, Cerami A. Effect of gamma interferon on cachectin expression by mononuclear phagocytes. Reversal of the lpsd (endotoxin resistance) phenotype. J Exp Med. 1986;164:1791–6. doi: 10.1084/jem.164.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weis WI, Drickamer K, Hendrickson WA. Structure of a C-type mannose-binding protein complexed with an oligosaccharide. Nature. 1992;360:127–34. doi: 10.1038/360127a0. [DOI] [PubMed] [Google Scholar]
- 41.Weis WI, Drickamer K. Structural basis of lectin-carbohydrate recognition. Annu Rev Biochem. 1996;65:441–73. doi: 10.1146/annurev.bi.65.070196.002301. [DOI] [PubMed] [Google Scholar]
- 42.Weis WI, Taylor ME, Drickamer K. The C-type lectin superfamily in the immune system. Immunol Rev. 1998;163:19–34. doi: 10.1111/j.1600-065x.1998.tb01185.x. [DOI] [PubMed] [Google Scholar]
- 43.Brandt ME, Riley BS, Radolf JD, Norgard MV. Immunogenic integral membrane proteins of Borrelia burgdorferi are lipo-proteins. Infect Immun. 1990;58:983–91. doi: 10.1128/iai.58.4.983-991.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bergstrom S, Bundoc VG, Barbour AG. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol Microbiol. 1989;3:479–86. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 45.Belisle JT, Brandt ME, Radolf JD, Norgard MV. Fatty acids of Treponema pallidum and Borrelia burgdorferi lipoproteins. J Bacteriol. 1994;176:2151–7. doi: 10.1128/jb.176.8.2151-2157.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bessler WG, Cox M, Lex A, Suhr B, Wiesmuller KH, Jung G. Synthetic lipopeptide analogs of bacterial lipoprotein are potent polyclonal activators for murine B lymphocytes. J Immunol. 1985;135:1900–5. [PubMed] [Google Scholar]
- 47.Radolf JD, Norgard MV, Brandt ME, Isaacs RD, Thompson PA, Beutler B. Lipoproteins of Borrelia burgdorferi and Treponema pallidum activate cachectin/tumor necrosis factor synthesis. Analysis using a CAT reporter construct. J Immunol. 1991;147:1968–74. [PubMed] [Google Scholar]
- 48.Marie C, Roman-Roman S, Rawadi G. Involvement of mitogen-activated protein kinase pathways in interleukin-8 production by human monocytes and polymorphonuclear cells stimulated with lipopolysaccharide or Mycoplasma fermentans membrane lipoproteins. Infect Immun. 1999;67:688–93. doi: 10.1128/iai.67.2.688-693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Means TK, Wang S, Lien E, Yoshimura A, Golenbock DT, Fenton MJ. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol. 1999;163:3920–7. [PubMed] [Google Scholar]
- 50.Wooten RM, Modur VR, McIntyre TM, Weis JJ. Borrelia burgdorferi outer membrane protein A induces nuclear translocation of nuclear factor-kappa B and inflammatory activation in human endothelial cells. J Immunol. 1996;157:4584–90. [PubMed] [Google Scholar]
- 51.Sellati TJ, Bouis DA, Kitchens RL, et al. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytic cells via a CD14-dependent pathway distinct from that used by lipopolysaccharide. J Immunol. 1998;160:5455–64. [PubMed] [Google Scholar]
- 52.Ebnet K, Brown KD, Siebenlist UK, Simon MM, Shaw S. Borrelia burgdorferi activates nuclear factor-kappa B and is a potent inducer of chemokine and adhesion molecule gene expression in endothelial cells and fibroblasts. J Immunol. 1997;158:3285–92. [PubMed] [Google Scholar]
- 53.Takeuchi O, Kaufmann A, Grote K, Kawai T, Hoshino K, Morr M, Muhlradt PF, Akira S. Preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a toll-like receptor 2- and MyD88-dependent signaling pathway. J Immunol. 2000;164:554–7. doi: 10.4049/jimmunol.164.2.554. [DOI] [PubMed] [Google Scholar]
- 54.Hirschfeld M, Kirschning CJ, Schwandner R, Wesche H, Weis JH, Wooten RM, Weis JJ. Inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J Immunol. 1999;163:2382–6. [PubMed] [Google Scholar]
- 55.Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–5. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 56.Flo TH, Halaas O, Lien E, Ryan L, Teti G, Golenbock DT, Sundan A, Espevik T. Human toll-like receptor 2 mediates monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. J Immunol. 2000;164:2064–9. doi: 10.4049/jimmunol.164.4.2064. [DOI] [PubMed] [Google Scholar]
- 57.Means TK, Lien E, Yoshimura A, Wang S, Golenbock DR, Fenton MJ. The CD14 ligands lipoarabinomannan and lipopolysaccharide differ in their requirement for Toll-like receptors. J Immunol. 1999;163:6748–55. [PubMed] [Google Scholar]
- 58.Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. Recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]
- 59.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999;274:17406–9. doi: 10.1074/jbc.274.25.17406. 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 60.Pugin J, Heumann ID, Tomasz A, et al. CD14 is a pattern recognition receptor. Immunity. 1994;1:509–16. doi: 10.1016/1074-7613(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 61.Kusunoki T, Hailman E, Juan TS, Lichenstein HS, Wright SD. Molecules from Staphylococcus aureus that bind CD14 and stimulate innate immune responses. J Exp Med. 1995;182:1673–82. doi: 10.1084/jem.182.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kusunoki T, Wright SD. Chemical characterisitics of Staph aureus molecules that have CD14-dependent cell-stimulating activity. J Immunol. 1996;157:5112–17. [PubMed] [Google Scholar]
- 63.Espevik T, Otterlei M, Skjak-Braek G, Ryan L, Wright SD, Sundan A. The involvement of CD14 in stimulation of cytokine production by uronic acid polymers. Eur J Immunol. 1993;23:255–61. doi: 10.1002/eji.1830230140. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y, Doerfler M, Lee TC, Guillemin B, Rom WN. Mechanisms of stimulation of interleukin-1 beta and tumor necrosis factor-alpha by Mycobacterium tuberculosis components. J Clin Invest. 1993;91:2076–83. doi: 10.1172/JCI116430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jahr TG, Ryan L, Sundan A, Lichenstein HS, Skjak-Braek G, Espevik T. Induction of tumor necrosis factor production from monocytes stimulated with mannuronic acid polymers and involvement of lipopolysaccharide-binding protein, CD14, and bactericidal/permeability-increasing factor. Infect Immun. 1997;65:89–94. doi: 10.1128/iai.65.1.89-94.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cauwels A, Wan E, Leismann M, Tuomanen E. Coexistence of CD14-dependent and independent pathways for stimulation of human monocytes by gram-positive bacteria. Infect Immun. 1997;65:3255–60. doi: 10.1128/iai.65.8.3255-3260.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Savedra R, Jr, Delude RL, Ingalls RR, Fenton MJ, Golenbock DT. Mycobacterial lipoarabinomannan recognition requires a receptor that shares components of the endotoxin signaling system. J Immunol. 1996;157:2549–54. [PubMed] [Google Scholar]
- 68.Cleveland MG, Gorham JD, Murphy TL, Tuomanen E, Murphy KM. Lipoteichoic acid preparations of gram-positive bacteria induce interleukin-12 through a CD14-dependent pathway. Infect Immun. 1996;64:1906–12. doi: 10.1128/iai.64.6.1906-1912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wright SD. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–3. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 70.Wright SD. CD14 and innate recognition of bacteria. J Immunol. 1995;155:6–8. [PubMed] [Google Scholar]
- 71.Ulevitch RJ, Tobias PS. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–57. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 72.Weinstein SL. Bacterial lipopolysaccharide induces tyrosine phosphorylation and activation of mitogen-activated protein kinases in macrophages. J Biol Chem. 1991;267:14955–62. [PubMed] [Google Scholar]
- 73.Dong Z, Qi X, Xie K, Fidler IJ. Protein tyrosine inhibitors decrease induction of nitric oxide synthase activity in lipopolysaccharide-responsive and lipopolysaccharide-nonresponsice murine macrophages. J Immunol. 1993;151:2717–24. [PubMed] [Google Scholar]
- 74.Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–11. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 75.Shinji H, Akagawa KS, Yoshida T. LPS induces selective translocation of protein kinase C-beta in LPS-responsive mouse macrophages, but not in LPS-nonresponsive mouse macrophages. J Immunol. 1994;153:5760–71. [PubMed] [Google Scholar]
- 76.Hambleton J, Weinstein SL, Lem L, DeFranco AL. Activation of c-Jun N-terminal kinase in bacterial lipopolysaccharide-stimulated macrophages. Proc Natl Acad Sci USA. 1996;93:2774–8. doi: 10.1073/pnas.93.7.2774. 10.1073/pnas.93.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sanghera JS, Weinstein SL, Aluwalia M. Activation of multiple proline-directed kinases by bacterial lipopolysaccharide in murine macrophages. J Immunol. 1996;156:4457–65. [PubMed] [Google Scholar]
- 78.Perera PY, Vogel SN, Detore GR, Haziot A, Goyert SM. CD14-dependent and CD14-independent signaling pathways in murine macrophages from normal and CD14 knockout mice stimulated with lipopolysaccharide or taxol. J Immunol. 1997;158:4422–9. [PubMed] [Google Scholar]
- 79.Haziot A, Ferrero E, Kontgen F, Hijiya N, Yamamoto S, Silver J, Stewart CL, Goyert SM. Resistance to endotoxin shock and reduced dissemination of gram-negative bacteria in CD14-deficient mice. Immunity. 1996;4:407–14. doi: 10.1016/s1074-7613(00)80254-x. [DOI] [PubMed] [Google Scholar]
- 80.Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kinoto M. MD-2, a molecule that confers lipopoylsaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–82. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takeuchi O, Kawai T, Sanjo H, Copeland NG, Gilbert DJ, Jenkins NA, Takeda K, Akira S. TLR6: A novel member of an expanding toll-like receptor family. Gene. 1999;231:59–65. doi: 10.1016/s0378-1119(99)00098-0. [DOI] [PubMed] [Google Scholar]
- 82.Poltorak A, Ricciardi-Castagnoli P, Citterio S, Beutler B. Physical contact between lipopolysaccharide and toll-like receptor 4 revealed by genetic complementation. Proc Natl Acad Sci USA. 2000;97:2163–7. doi: 10.1073/pnas.040565397. 10.1073/pnas.040565397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morisato D, Anderson KV. The Spaetzle gene encodes a component of the extracellular signaling pathway establishing the dorsal–ventral pattern of the Drosophila embryo. Cell. 1994;76:677–88. doi: 10.1016/0092-8674(94)90507-x. [DOI] [PubMed] [Google Scholar]
- 84.Hecht PM, Anderson KV. Extracellular proteases and embryonic pattern formation. Trends Cell Biol. 1992;2:197–202. doi: 10.1016/0962-8924(92)90246-j. [DOI] [PubMed] [Google Scholar]
- 85.Iwagana S. The limulus clotting reaction. Curr Opin Immunol. 1993;5:74–82. doi: 10.1016/0952-7915(93)90084-6. [DOI] [PubMed] [Google Scholar]
- 86.Bergner A, Oganessyan V, Muta T, Iwanaga S, Tyoke D, Huber R, Bode W. Crystal structure of coagulogen, the clotting protein from horseshoe crab: a structural homologue of nerve growth factor. EMBO J. 1996;15:6789–97. [PMC free article] [PubMed] [Google Scholar]
- 87.Hoffmann JA, Kafatos FC, Janeway CA, Ezkowitz RA. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–18. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 88.Ohashi K, Burkhart V, Flohe S, Kolb H. Heat shock protein 60 is a putative endogenous ligand of the Toll-like receptor-4 complex. J Immunol. 2000;164:558–61. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 89.Jin FY, Nathan C, Radzioch D, Ding A. Secretory leukocyte protease inhibitor: a macrophage product induced by and antagonistic to bacterial lipopolysaccharide. Cell. 1997;88:417–26. doi: 10.1016/s0092-8674(00)81880-2. [DOI] [PubMed] [Google Scholar]
- 90.Levashina EA, Langley E, Green C, Gubb D, Ashburner M, Hoffmann JA, Reichhart JM. Constitutive activation of toll-mediated antifungal defense in serpin-deficient Drosophila. Science. 1999;285:1917–9. doi: 10.1126/science.285.5435.1917. [DOI] [PubMed] [Google Scholar]
- 91.O'neill LA, Greene C. Signal transduction pathways activated by the IL-1 receptor family: ancient signaling machinery in mammals, insects, and plants. J Leukoc Biol. 1998;63:650–7. [PubMed] [Google Scholar]
- 92.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–60. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 93.Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway Ca., Jr MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2:253–8. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 94.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–22. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 95.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–50. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 96.Muzio M, Ni J, Feng P, Dixit VM. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science. 1997;278:1612–15. doi: 10.1126/science.278.5343.1612. [DOI] [PubMed] [Google Scholar]
- 97.Muzio M, Natoli G, Saccani S, Levrero M, Mantovani A. The human toll signaling pathway: divergence of nuclear factor kappaB and JNK/SAPK activation upstream of tumor necrosis factor receptor-associated factor 6 (TRAF6) J Exp Med. 1998;187:2097–101. doi: 10.1084/jem.187.12.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wesche H, Henzel WJ, Shillinglaw W, Li S, Cao Z. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 1997;7:837–47. doi: 10.1016/s1074-7613(00)80402-1. [DOI] [PubMed] [Google Scholar]
- 99.Yang RB, Mark MR, Gurney AL, Godowski PJ. Signaling events induced by lipopolysaccharide-activated toll-like receptor 2. J Immunol. 1999;163:639–43. [PubMed] [Google Scholar]
- 100.Song HY, Regnier CH, Kirschning CJ, Goeddel DV, Rothe M. Tumor necrosis factor (TNF) -mediated kinase cascades: bifurcation of nuclear factor-kappaB and c-jun N-terminal kinase (JNK/SAPK) pathways at TNF receptor-associated factor 2. Proc Natl Acad Sci USA. 1997;94:9792–6. doi: 10.1073/pnas.94.18.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature. 1997;388:548–54. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 102.Stancovski I, Baltimore D. NF-kappaB activation: the I kappaB kinase revealed? Cell. 1997;91:299–302. doi: 10.1016/s0092-8674(00)80413-4. [DOI] [PubMed] [Google Scholar]
- 103.Scheidereit C. Signal transduction. Docking IkappaB kinases. Nature. 1998;395:225–6. doi: 10.1038/26121. [DOI] [PubMed] [Google Scholar]
- 104.Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel DV. TRAF6 is a signal transducer for interleukin-1. Nature. 1996;383:443–6. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 105.Malinin NL, Boldin MP, Kovalenko AV, Wallach D. MAP3K-related kinase involved in NF-kappaB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–4. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 106.Baeuerle PA, Baltimore D. NF-kappa B ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 107.Baeuerle PA. Pro-inflammatory signaling: last pieces in the NF-kappaB puzzle? Curr Biol. 1998;8:R19–22. doi: 10.1016/s0960-9822(98)70010-7. [DOI] [PubMed] [Google Scholar]
- 108.Murphy TL, Cleveland MG, Kulesza P, Magram J, Murphy KM. Regulation of interleukin 12 p40 expression through an NF-kappa B half-site. Mol Cell Biol. 1995;15:5258–67. doi: 10.1128/mcb.15.10.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Plevy SE, Gemberling JH, Hsu S, Dorner AJ, Smale ST. Multiple control elements mediate activation of the murine and human interleukin 12 p40 promoters: evidence of functional synergy between C/EBP and Rel proteins. Mol Cell Biol. 1997;17:4572–88. doi: 10.1128/mcb.17.8.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brightbill HD, Plevy SE, Modlin RL, Smale ST. A prominent role for Sp1 during LPS-mediated induction of the IL-10 promoter in macrophages. J Immunol. 2000;164:1940–51. doi: 10.4049/jimmunol.164.4.1940. [DOI] [PubMed] [Google Scholar]
- 111.Irie T, Muta T, Takeshige K. TAK1 mediates an activation signal from toll-like receptor(s) to nuclear factor-kappaB in lipopolysaccharide-stimulated macrophages. FEBS Lett. 2000;467:160–4. doi: 10.1016/s0014-5793(00)01146-7. [DOI] [PubMed] [Google Scholar]
- 112.Chan VW, Mecklenbrauker I, Su I, et al. The molecular mechanism of B cell activation by toll-like receptor RP-105. J Exp Med. 1998;188:93–101. doi: 10.1084/jem.188.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–73. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 114.Romagnani S. Induction of TH1 and TH2 responses: a key role for the ‘natural’ immune response? Immunol Today. 1992;13:379–81. doi: 10.1016/0167-5699(92)90083-J. [DOI] [PubMed] [Google Scholar]
- 115.Liu Y, Janeway Ca., Jr Cells that present both specific ligand and costimulatory activity are the most efficient inducers of clonal expansion of normal CD4 T cells. Proc Natl Acad Sci USA. 1992;89:3845–9. doi: 10.1073/pnas.89.9.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–58. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 117.Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–3. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 118.De Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin-10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.De Waal Malefyt R, Haanen J, Spits H, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via down-regulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–24. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Libraty DH, Airan LE, Uyemura K, Jullien D, Spellberg B, Rea TH, Modlin RL. Interferon differentially regulates interleukin-12 and interleukin-10 production in leprosy. J Clin Invest. 1997;99:336–41. doi: 10.1172/JCI119162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bondeson J, Brow wne KA, Brennan FM, Foxwell BM, Feldmann M. Selective regulation of cytokine induction by adenoviral gene transfer of I-κB into human macrophages: lipopolysaccharide-induced, but not zymosan-induced, proinflammatory cytokines are inhibited, but IL-10 is nuclear factor-κB independent. J Immunol. 1999;162:2939–45. [PubMed] [Google Scholar]
- 122.Nemeth ZH, Hasko G, Vizi ES. Pyrrolidine dithiocarbamate augments IL-10, inhibits TNF-α, MIP-1, IL-12 and nitric oxide production and protects from the lethal effects of endotoxin. Shock. 1998;10:49–53. doi: 10.1097/00024382-199807000-00009. [DOI] [PubMed] [Google Scholar]
- 123.Yssel H, De Waal Malefyt R, Roncarolo MG, Abrams JS, Lahesmaa R, Spits H, de Vries JE. IL-10 is produced by subsets of human CD4+ T cell clones and peripheral blood T cells. J Immunol. 1992;149:2378–84. [PubMed] [Google Scholar]
- 124.Ghalib HW, Whittle JA, Kubin M, Hashim FA, el-Hassan AM, Grabstein KH, Trinchieri G, Reed SG. IL-12 enhances Th1-type responses in human Leishmania donovani infections. J Immunol. 1995;154:4623–9. [PubMed] [Google Scholar]
- 125.Modlin RL. Th1-Th2 paradigm: insights from leprosy. J Invest Dermatol. 1994;102:828–32. doi: 10.1111/1523-1747.ep12381958. [DOI] [PubMed] [Google Scholar]
- 126.Wang J, Wakeham J, Harkness R, Xing Z. Macrophages are a significant source of type 1 cytokines during mycobacterial infection. J Clin Invest. 1999;103:1023–9. doi: 10.1172/JCI6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.MacMicking JD, North RJ, Mudgett JS, Shah SK, Nathan CF. Identification of nitric oxide synthase as a protective locus against tuberculosis. Pro Natl Acad Sci USA. 1997;94:5243–8. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chan J, Tanaka K, Carroll D, Flynn J, Bloom BR. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:736–40. doi: 10.1128/iai.63.2.736-740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chan J, Xing Y, Magliozzo RS, Bloom BR. Killing of virulent M. tuberculosis by the nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175:1111–22. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Aliprantis AO, Yang RB, Mark MR, et al. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science. 1999;285:736–9. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 131.Ware CF, VanArsdale S, VanArsdale TL. Apoptosis mediated by the TNF-related cytokine and receptor families. J Cell Biochem. 1996;60:47–55. doi: 10.1002/(SICI)1097-4644(19960101)60:1%3C47::AID-JCB8%3E3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 132.Manfruelli P, Reichhart JM, Steward R, Hoffmann JA, Lemaitre B. A mosaic analysis in Drosophila fat body cells of the control of antimicrobial peptide genes by Rel proteins Dorsal and Dif. EMBO J. 1999;18:3380–91. doi: 10.1093/emboj/18.12.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hedengren M, Asling B, Dushay MS, Ando I, Ekengren S, Wihlborg M, Hultmark D. Relish, a central factor in the control of humoral, but not cellular immunity in Drosophila. Mol Cell. 1999;4:827–37. doi: 10.1016/s1097-2765(00)80392-5. [DOI] [PubMed] [Google Scholar]
- 134.Kèontgen F, Grumont RJ, StrasSeries A, Metcalf D, Li R, Tarlinton D, Gerondakis S. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes Dev. 1995;9:1965–77. doi: 10.1101/gad.9.16.1965. [DOI] [PubMed] [Google Scholar]
- 135.Sha WC, Liou HC, Tuomanen EI, Baltimore D. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell. 1995;80:321–30. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- 136.Grigoriadis G, Zhan Y, Grumont RJ, Metcalf D, Handman E, Cheers C, Gerondakis S. The Rel subunit of NF-kappaB-like transcription factors is a positive and negative regulator of macrophage gene expression: distinct roles for Rel in different macrophage populations. Embo J. 1996;15:7099–107. [PMC free article] [PubMed] [Google Scholar]