Abstract
There is an urgent need for prophylactic and therapeutic vaccines against human immunodeficiency virus (HIV). Mucosal immunization strategies have great potential to elicit both mucosal and systemic cellular immunity required to protect against HIV-induced aquired immune deficiency syndrome (AIDS). However, mucosal immunizations with soluble protein antigens generally require adjuvants. In this study, we tested two mutants of the heat-labile enterotoxin (LT) from Escherichia coli, LTK63: with no measurable ADP-ribosyltransferase activity, and LTR72: with residual ADP-ribosyltransferase activity, as mucosal adjuvants for induction of cytotoxic T lymphocyte (CTL) responses to coadministered HIV gag p55 protein. We found that intranasal (i.n.) immunizations with HIV gag p55 protein coadministered with LTK63 or LTR72 induced systemic CTL responses comparable to that obtained following intramuscular (i.m.) immunizations with the same adjuvants. Moreover, oral coadministration of LTR72, but not LTK63, resulted in local as well as systemic p55-specific CTL responses in mesenteric lymph nodes (MLN) and spleens (SP) of the immunized mice. These data have important implications for current efforts to develop a safe vaccine against HIV.
Introduction
It is well established that during a sexually transmitted human immunodeficiency virus (HIV) infection, the HIV virus first enters through mucosal surfaces and then proceeds to spread systemically.1,2 One of the challenges in developing an effective prophylactic HIV vaccine that can protect the vaginal mucosa is to induce mucosal as well as systemic protective immunity. However, mucosal immunizations in general require adjuvants. By far the majority of studies on the use of mucosally active adjuvants have involved induction of humoral responses and relatively little data is available about the use of mucosal adjuvants to induce cell-mediated responses. It is important to note that most experimental mucosal adjuvants used in animal models are too toxic for human use.
The heat-labile enterotoxin (LT) from Escherichia coli is known to be a potent mucosal immunogen and adjuvant for elicitation of serum immunoglobulin G (IgG) and mucosal IgA against coadministered antigens.3,4 LT is toxic in its native state and produces accumulation of intestinal fluid and diarrhoea in humans.5 In order to retain the adjuvanticity of these molecules but to reduce their toxicity, several mutants have been generated by site directed mutagenesis. Of these, two mutants of the enzymatic A subunit, LTK63 and LTR72, maintain a high degree of adjuvanticity.6 LTK63 is the result of a substitution of serine 63 with a lysine in the A subunit, which renders it enzymatically inactive.5,7–9 LTR72 is derived from a substitution of alanine 72 with an arginine in the A subunit and contains about 0·6% of the enzymatic activity of wild-type LT. In addition, LTR72 is 100 000-fold less toxic than wild-type LT in Y1 cells in vitro and 25–100 times less toxic than wild-type LT in the rabbit ileal loop assay.10
This study assessed the ability of LTK63 or LTR72 adjuvants coadministered with HIV gag p55 protein through mucosal or parenteral routes to induce antigen-specific CTL responses. We found that these adjuvants mixed with HIV-1 gag p55 protein can induce strong HIV-1 gag-specific CTL responses through mucosal (oral and i.n.) immunizations comparable to that induced by parenteral (i.m.) immunizations.
Materials and methods
Mice and cell lines
Female CB6F1 mice (H-2bxd from an F1 cross between H-2b C57BL/6 and H-2d BALB/c mice) were purchased from Charles River Breeding Laboratories (Wilmington, MA) and were used between 6 and 8 weeks of age. The fibroblast cell line SvBalb (H-2d) was used as target cells. This cell line expresses class I but not class II major histocompatibility complex (MHC) molecules.
Antigens
p7g is an H-2Kd restricted HIV-1SF2p24gag CTL epitope and is a synthetic 9-mer peptide: (aa, 199-AMQMLKETI-207).11 This peptide was synthesized with free amine N termini and free acid C termini using Fmoc solid phase methods by Research Genetics (Huntsville AL).12,13 Mice were infected intraperitoneally with 1 × 107 plaque-forming units (p.f.u.) of recombinant vaccinia virus expressing HIV-1SF2 gag/pol (Vvgagpol) provided by Dr I. Ramshaw. LTK63 and LTR72 mutants were prepared as described.5,10 The endotoxin content of the LT mutant preparations was routinely tested and fell below 0·1 ng in the highest dose of LTK63 (50 µg) or LTR72 (50 µg) used in the study. Yeast-derived recombinant p55SF2 gag protein was obtained from Chiron manufacturing division. The p55 gag protein was dissolved in 50 mm sodium phosphate buffer with 0·4 m NaCl and 6 m urea at pH 6·9. The protein was subsequently dialysed into 50 mm Tris buffer with 0·5 m NaCl and 2 m urea at pH 7·5 before use.
Immunization of mice
The vaccines were administered to groups of five or 10 mice. The mice were immunized at day 0 and 21 (except for mice immunized with vaccinia virus that were immunized only once at day 0). All immunized mice were killed on day 42 and their spleens (SP) collected for analysis of the CTL responses, as described below. For i.m. and i.n. immunizations, p55 gag protein and LT mutants were resuspended in phosphate-buffered saline (PBS) with 2 m urea (pH 7·2). For i.n. immunizations, vaccines were applied to the nares of mice in a volume of 25 µl without anaesthesia. For i.n. immunization, 50 µl of the vaccines was injected into the tibialis anterior muscle of each hind leg. For oral immunizations, the immunogens were resuspended into PBS with 2 m urea and 3% bicarbonate solution in a volume of 200 µl and administered intragastrically with a feeding needle without anaesthesia. Vvgagpol was diluted into PBS to 1 × 108 p.f.u./ml and 100 µl was injected intraperitoneally (i.p.) into each mouse.
Lymphocyte cultures and cytotoxic T-cell assays
Pooled SP cells from five or 10 immunized mice per group were cultured in 24-well tissue culture dishes at 5 × 106 cells/well. A subset of the culture cells, 1 × 106 cells/well, were set aside for use as antigen-presenting cells (APC). These cells were sensitized with 10 µm of the relevant synthetic CTL epitopic peptide (p7g) for 1 hr at 37°, washed and cocultured with the remaining 4 × 106 untreated SP cells in 2 ml of medium (50% RPMI-1640 and 50% α-minimal essential medium (MEM; Gibco, Grand Island, NY)) supplemented with 10% fetal calf serum and 5% interleukin 2 (Rat T-stim, Collaborative Biomedical Products, Bedford, MA). After a stimulation period of 6–7 days, effector cells were collected and assayed for cytotoxic activity in a standard 51Cr release assay as described in detail elsewhere.14 Percentage specific release was calculated as 100 × [(release by test CTL − spontaneous release/total release − spontaneous release)] − specific release from a non-relevant target (to account for non-specific activity). The peptide used for this purpose was a 9-mer synthetic peptide displaying a non-relevant epitope. The cells were treated with this peptide in the same manner as the cells treated with p7g peptide. Non-specific release was generally 10–15% of total release. As a negative control, naïve mice were used against peptide-pulsed target cells in several experiments and the percent specific lysis for all effector : target (E : T) ratios were consistently near 0. In all experiments spleens were pooled from groups of five or 10 animals. The results shown are representative of at least two independent experiments from pools of five or 10 animals per vaccination group giving similar results as stated in the figure legends.
Statistical analysis
A standard Student's t-test in Microsoft Excel™ was used to assess the statistical significance of the differences in the mean of the CTL responses as percent lysis at the 1 : 60 E : T between the vaccination groups with adjuvants versus vaccination groups without adjuvants where the number of experiments was three (as indicated in the figure legends). In all other instances, where the number of experiments is two, the response of the adjuvant vaccination group is defined higher than the no adjuvant vaccination group if the mean of the adjuvant group is at least twofold higher than the mean of the no adjuvant group.
Results
Immunization (i.m.) with p55 gag protein coadministered with LTK63 or LTR72 elicits a vigorous cytotoxic response
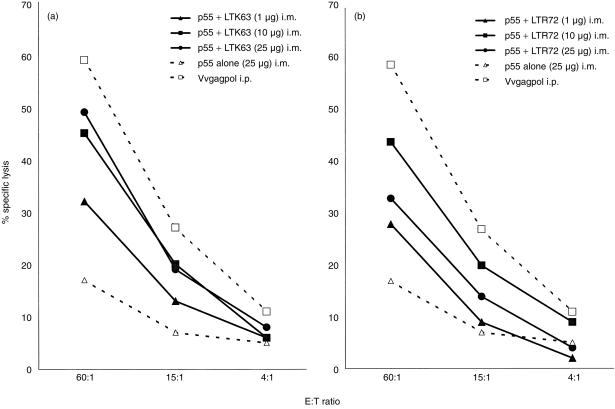
In order to determine the ability of LT mutants to act as adjuvants for induction of HIV gag p55-specific CTL responses by parenteral immunizations, CB6F1 mice were immunized by the i.m. route with LTK63 or LTR72 coadministered with HIV-1 p55 gag protein. i.m. immunization of mice with 25 µg of p55 gag protein alone did not elicit a significant cytotoxic response. However, when LTK63 was coadministered with p55 gag protein, the effectors were capable of strong cytotoxic activity (Fig. 1a). The adjuvant effect of LTK63 was evident at the 1 µg dose which clearly increased the specific cytotoxic activity over the unadjuvanted protein. Moreover, an increased dosage of LTK63 (10 µg and 25 µg) appeared to increase the specific cytotoxic activity to a potency equivalent to that of the Vvgagpol positive control.
Figure 1.
i.m. immunization with p55 gag protein coadministered with LTK63 or LTR72 elicits a vigorous cytotoxic response. CB6F1 mice were given i.m. injections on day 0, 7 and 21 and SP were harvested for assay on day 28. The immunizations included unadjuvanted protein as a negative control or a single i.p. injection with Vvgagpol on day 0 as a positive control for the assay. i.m. immunizations of p55 gag protein formulated with a range of doses of either LTK63 (a) or LTR72 (b) induced potent cytotoxic T-cell responses. Each data point is representative of two or three independent experiments with similar results: LTK63 at 25 µg induced a significantly higher response over that of protein alone (n = 3, P < 0·005). Other doses of LTK63 or all doses of LTR72 clearly induced a higher CTL response (n = 2, means at least twofold higher) compared to protein alone.
Immunizations (i.m.) with LTR72 coadministered with p55 gag protein also provided an increase in the specific cytotoxic activity over immunizations with protein alone (Fig. 1b). Similar to LTK63, the LTR72 adjuvant increased the cytotoxic activity at a dose of only 1 µg and this increase was considerably higher with doses of 10 µg and 25 µg.
These results show that LTK63 and LTR72 adjuvants coadministered with p55 gag protein by the i.m. route induce a vigorous cellular response that is not obtainable with protein alone.
Immunization (i.n.) with p55 gag protein coadministered with LTK63 or LTR72 induces specific CTL activity
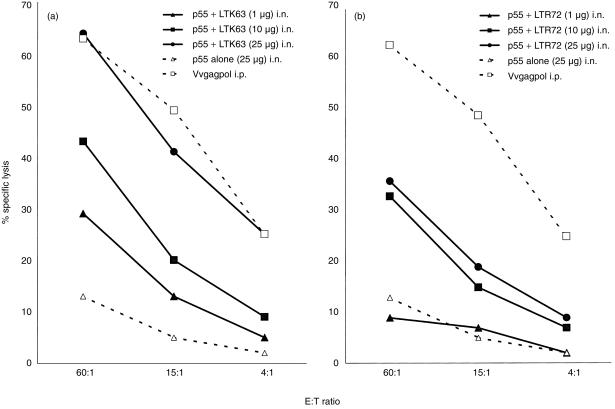
Because the mucosal delivery of safe vaccines against HIV is important, we immunized mice by the i.n. route with various concentrations of p55 gag protein mixed with LTK63 or LTR72. In Fig. 2, the potency of LT mutant adjuvants to invoke strong cytotoxic responses after mucosal immunization is demonstrated. Although both mutants are effective at increasing the specific cytotoxic responses after i.n. immunization, LTK63 appears to be more potent than LTR72.
Figure 2.
i.n. immunization with p55 gag protein coadministered with LTK63 or LTR72 boosts CTL activity. CB6F1 mice were given i.n. immunizations on day 0, 7 and 14 and SP were harvested for assay on day 28. The immunizations included unadjuvanted protein as a negative control or a single i.p. injection with Vvgagpol on day 0 as a positive control for the assay. i.n. immunizations of p55 gag protein formulated with a range of doses of either LTK63 (a) or LTR72 (b) induced potent, titratable cytotoxic T-cell responses. Each data point is representative of two or three independent experiments with similar results: LTK63 at 10 µg induced a statistically significant higher response over that of protein alone (n = 3, P < 0·005). Other doses of LTK63 or all doses of LTR72 clearly induced a higher CTL response (n = 2, means at least twofold higher) compared to protein alone.
LTK63 and p55 gag protein increased the CTL response to several-fold higher than that of the protein alone (Fig. 2a). The highest dose used, 25 µg, resulted in a potent CTL response that was comparable to that of the Vvgagpol positive control. LTR72 exhibited an adjuvant effect at a dose of 10 µg and higher (Fig. 2b). Although the lowest dose of LTR72, 1 µg, did not raise cytotoxic activity above that of protein alone, the higher doses induced a CTL response comparable to that of the 10 µg dose of LTK63 adjuvant.
These data show that i.n. immunizations with HIV gag p55 protein coadministered with LTK63 or LTR72 result in strong cytotoxic activity that is similar to that achieved by i.m. immunizations.
Local and systemic HIV-1 p55-specific CTL responses following oral immunization with p55 protein coadministered with LTR72 but not LTK63
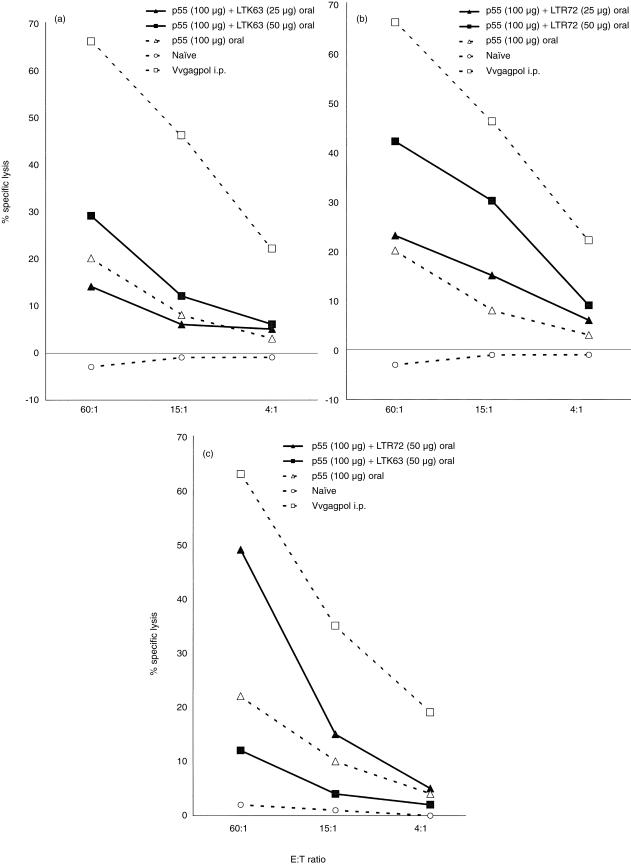
Various routes of inoculation with the simian immunodeficiency virus have been shown to rapidly decrease the numbers of CD4+ cells in the intestinal mucosa before such loss is detectable in peripheral lymphoid tissues.15,16 Therefore, it is important to explore the oral immunization route for local protection against HIV. Figure 3 demonstrates the adjuvant ability of the LT mutants to induce p55-specific CTL when administered by the oral route. Because of the acidity of the stomach and the harsh microenvironment of the intestine, the amount of p55 gag protein used for oral immunizations was higher compared to the other routes, i.e. 100 µg rather than 25 µg.
Figure 3.
Oral immunization with p55 gag protein coadministered with LTR72 boosts CTL activity. CB6F1 mice were orally immunized on days 0,7, and 14 and SP or MLN were harvested on day 28. The immunizations included protein alone as a negative control or a single i.p. injection with Vvgagpol on day 0 as a positive control for the assay. A negative control group of naïve mice was also included. Oral immunizations of p55 gag protein with LTR72 (a), but not LTK63 (c) induced potent systemic cytotoxic T-cell responses in the SP. In MLN, only the high dose of LTR72 induced a CTL response (c). Each data point is representative of two independent experiments with similar results: LTR72, but not LTK63, only at the 50 µg dose clearly induced a higher CTL response (n = 2, means at least twofold higher) compared to protein alone.
The mice immunized with protein alone had a low CTL response (Fig. 3a). The result of oral immunization with 25 µg of LTK63 was similar to that of protein alone. The 50 µg dose of LTK63 increased the CTL response above that of the protein given alone. Mice immunized with 25 µg of LTR72 (Fig. 3b) showed a higher cytotoxic response than mice immunized with protein alone, although the best results were obtained with a higher dose (50 µg) of LTR72. Importantly, mice immunized orally with 100 µg of p55 and 50 µg of LTR72 demonstrated local specific CTL responses in mesenteric lymph nodes (MLN). In contrast, mice immunized orally with even the higher dose of LTK63 failed to demonstrate local CTL responses in MLN (Fig. 3c).
These results show that LTR72 is superior to LTK63 when administered orally with p55 gag protein for induction of CTL responses.
Discussion
The principal aim of this study was to determine the ability of LT mutant adjuvants with low or no toxicity to promote local and systemic cell-mediated responses against HIV-1 gag-p55 through mucosal immunizations. As such, this is the first comparative report on the use of adjuvants with no or low toxicity for eliciting local as well as systemic anti-HIV CTL responses through mucosal immunizations. Thus, this study has important implications for development of safe mucosal vaccines against HIV.
This study also provides evidence on the importance of adjuvants with residual ADP-ribosyltransferase activity versus no ADP-ribosyltransferase activity when oral and i.n. routes of mucosal immunizations are compared. An important observation was the induction of strong local and systemic CTL responses following oral immunizations with LTR72 but not LTK63, even though LTK63 induced potent CTL responses following i.n. immunization. It appears that some ADP-ribosyltransferase activity may be required for induction of immunity through oral but not i.n. immunizations as shown here and elsewhere.17
A recent study demonstrated the capacity of various LT mutants to act as adjuvants for the induction of ovalbumin (OVA)-specific CTL responses, although the mice were immunized under anaesthesia, in effect making the immunization intratracheal.17 Compared to i.n. immunization of mice without anaesthesia, intratracheal immunization of mice under anaesthesia generally yields higher local as well as systemic immune responses.18 Because the aim of our study is to ultimately develop an HIV-related vaccine for use in humans, the intratracheal vaccine administration or the use of wild-type LT would pose undesirable health issues and would not have clinical relevance.
A noteworthy observation was that following oral immunization, LTK63 induced a low systemic CTL response in SP but no detectable local CTL responses in MLN. It is generally believed that antigens are taken up into mucosal inductive sites where antigen-specific B- and T-cell activation is induced, following which the activated lymphocytes migrate to distant mucosal and systemic lymphoid tissues.19 Therefore, it may be expected that if a systemic CTL response is detected, a local CTL response should also be detected. We do not have an explanation for this discrepancy. However, a plausible explanation may be that the CTL responses induced by oral immunization with LTK63 induced marginally poor CTL responses as measured by the 51Cr-release assay with generally low sensitivity. Whether our immunization strategies induce CTL responses in the mucosal effector sites (lamina propria) of the nasal, intestinal or vaginal mucosa following i.n. or oral immunization needs further investigation. Others have shown that cells with CTL activity reside in the lamina propria of mucosal membranes,20 and i.n. immunizations induce immune responses in the nasal as well as the vaginal mucosa.21–25
To determine whether our immunizations induced gag-specific humoral responses we performed an enzyme-linked immunosorbent assay (ELISA) on sera collected from the immunized animals. Although strong p55-specific antibody responses appeared to have been induced in mice immunized i.m. or i.n. with LTK63, the results from mucosally immunized animals were generally poor and not reproducible (data not shown). Thus, although these LT mutants induce potent antigen-specific mucosal and systemic humoral responses when administered with other protein antigens (e.g. influenza HA26), they are poor inducers of humoral responses following mucosal immunizations with HIV gag protein. Therefore, the ability of the LT mutant adjuvants to induce immunity against coadministered proteins may be antigen dependent.
Acknowledgments
Jason Neidleman would like to acknowledge Malou Valdez for her support.
Glossary
Abbreviations
- i.m.
intramuscular
- i.n.
intranasal
- i.p.
intraperitoneal
- LT
heat-labile enterotoxin
- CTL
cytotoxic T lymphocyte
- MLN
mesenteric lymph nodes
- SP
spleen
- APC
antigen-presenting cell
References
- 1.Miller CJ. Localization of simian immunodefeciency virus-infected cells in the genital tract of male and female Rhesus macaques. J Reprod Immunol. 1998;41:331. doi: 10.1016/s0165-0378(98)00069-2. 10.1016/s0165-0378(98)00069-2. [DOI] [PubMed] [Google Scholar]
- 2.Joag SV, Adany I, Li Z, et al. Animal model of mucosally transmitted human immunodefeciency virus type 1 disease; intravaginal and oral deposition of simian/human immunodefeciency virus in macaques results in systemic infection, elimination of CD4+ T cells, and AIDS. J Virol. 1997;71:4016. doi: 10.1128/jvi.71.5.4016-4023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clements JD, Dickinson BL. Use of Escherichia coli heat-labile enterotoxin as an oral adjuvant. In: Kiyono H, Ogra PL, McGhee JR, editors. Mucosal Vaccines. London, New York: Academic Press; 1996. p. 73. [Google Scholar]
- 4.Douce G, Turcotte C, Cropley I, Roberts M, Pizza M, Domenighini M, Rappuoli R, Dougan G. Mutants of Escherichia coli heat-labile toxin lacking ADP-ribosyltransferase activity act as nontoxic, mucosal adjuvants. Proc Natl Acad Sci USA. 1995;92:1644. doi: 10.1073/pnas.92.5.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pizza M, Fontana MR, Giuliani MM, et al. A genetically detoxified derivative of heat-labile Escherichia coli enterotoxin induces neutralizing antibodies against the A subunit. J Exp Med. 1994;180:2147. doi: 10.1084/jem.180.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Partidos CD, Pizza M, Rappuoli R, Steward MW. The adjuvant effect of a non-toxic mutant heat-labile enterotoxin of Escherichia coli for the induction of measles virus-specific CTL responses after intranasal co-immunization with a synthetic peptide. Immunology. 1996;89:483. doi: 10.1046/j.1365-2567.1996.d01-790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clements JD, Finkelstein RA. Isolation and characterization of homogeneous heat-labile enterotoxin with high specific activity from Escherichia coli cultures. Infect Immun. 1979;24:760. doi: 10.1128/iai.24.3.760-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clements JD, Yancy RJ, Finkelstein RA. Properties of homogeneous heat-labile enterotoxin from Escherichia coli. Infect Immun. 1980;29:91. doi: 10.1128/iai.29.1.91-97.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pizza M, Domenighini M, Hoi W, et al. Probing the structure-activity relationship of Escherichia coli LT-A by site-directed mutagenesis. Mol Microb. 1994;14:51. doi: 10.1111/j.1365-2958.1994.tb01266.x. [DOI] [PubMed] [Google Scholar]
- 10.Giuliani MM, Del Giudice G, Giannelli V, Dougan G, Douce D, Rappuoli R, Pizza M. Mucosal adjuvanticity and immunogenicity of LTR72, a novel mutant of Escherichia coli heat-labile enterotoxin with partial knockout of ADP-ribosyltransferase activity. J Exp Med. 1998;187:1. doi: 10.1084/jem.187.7.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paliard X, Doe B, Walker CM. The T cell repertoire primed by antiviral vaccination is influenced by self-tolerance. Cell Immunol. 1998;188:73. doi: 10.1006/cimm.1998.1338. 10.1006/cimm.1998.1338. [DOI] [PubMed] [Google Scholar]
- 12.Doe B, Walker CM. HIV-1 p24 gag-specific cytotoxic T-lymphocyte responses in mice. AIDS. 1996;10:793. doi: 10.1097/00002030-199606001-00015. [DOI] [PubMed] [Google Scholar]
- 13.Elvin J, Cerundolo V, Elliott T, Townsend A. A quantitative assay of peptide-dependant class I assembly. Eur J Immunol. 1991;21:2025. doi: 10.1002/eji.1830210909. [DOI] [PubMed] [Google Scholar]
- 14.Doe B, Steimer KS, Walker CM. Induction of HIV-1 envelope (gp120)-specific cytotoxic T lymphocyte responses in mice by recombinant CHO-derived gp120 is enhanced by enzymatic removal of N-linked glycans. Euro J Immunol. 1994;24:2369. doi: 10.1002/eji.1830241017. [DOI] [PubMed] [Google Scholar]
- 15.Kewenig S, Schneider T, Hohloch K, et al. Rapid mucosal CD4+ T cell depletion and enteropathy in simian immunodefeciency virus-infected rhesus macaques. Gastroenterology. 1999;116:1115. doi: 10.1016/s0016-5085(99)70014-4. [DOI] [PubMed] [Google Scholar]
- 16.Veazey RS, DeMaria M, Chalifoux LV, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 17.Simmons CP, Mastroeni P, Fowler R, Ghaem-Maghami M, Lycke N, Pizza M, Rappuoli R, Dougan G. MHC class-I restricted cytotoxic lymphocyte responses induced by enterotoxin-based mucosal adjuvants. J Immunol. 1999;163:6502. [PubMed] [Google Scholar]
- 18.Ryan EJ, McNeela E, Murphy GA, Stewart H, O'hagan D, Pizza M, Rappuoli R, Mills HG. Mutants of Escherichia coli heat-labile toxin act as effective mucosal adjuvants for nasal delivery of an acellular pertussis vaccine: differential effects of the nontoxic AB complex and enzyme activity on TH1 and TH2 cells. Infect Immun. 1999;67:6270. doi: 10.1128/iai.67.12.6270-6280.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butcher EC. Lymphocyte homing and intestinal immunity. In: Ogra PL, Metecky J, Lamm ME, Stober W, Bienenstock J, McGhee JR, editors. Mucosal Immunology. London: Academic Press; 1999. p. 507. [Google Scholar]
- 20.London SD, Rubin DH. Functional role of mucosal cytotoxic lymphocytes. In: Ogra PL, Metecky J, Lamm ME, Stober W, Bienenstock J, McGee JR, editors. Mucosal Immunology. London: Academic Press; 1999. p. 643. [Google Scholar]
- 21.Bowen JC, Alpar HO, Phillpotts R, Brown MRW. Mucosal delivery of herpes simplex vaccine. Res Virol. 1992;143:269. doi: 10.1016/s0923-2516(06)80115-9. [DOI] [PubMed] [Google Scholar]
- 22.Di Tommaso A, Saletti G, Pizza M, Rappuoli R, Dougan G, De Magistris MT. Induction of antigen-specific antibodies in vaginal secretions by using a nontoxic mutant of heat-labile enterotoxin as a mucosal adjuvant. Infect Immun. 1996;64:974. doi: 10.1128/iai.64.3.974-979.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallichan WS, Rosenthal KL. Specific secretory immune responses in the female genital tract following intranasal immunization with a recombinant adenovirus expressing glycoprotein B of herpes simplex virus. Vaccine. 1995;13:1589. doi: 10.1016/0264-410x(95)00100-f. 10.1016/0264-410x(95)00100-f. [DOI] [PubMed] [Google Scholar]
- 24.Lowell GH, Kaminski RW, VanCott TC, et al. Proteosomes, emulsomes, and cholera toxin B improve nasal immunogenicity of human immunodefeciency virus gp160 in mice: induction of serum, intestinal, vaginal, and lung IgA and IgG. J Inf Dis. 1997;175:292. doi: 10.1093/infdis/175.2.292. [DOI] [PubMed] [Google Scholar]
- 25.VanCott TC, Kaminski RW, Mascola JR, et al. HIV-1 neutralizing antibodies in the genital and respiratory tract of intranasally immunized with oligomeric gp160. J Immunol. 1998;160:2000. [PubMed] [Google Scholar]
- 26.Barackman JD, Ott G, O'hagan DT. Intranasal immunization of mice with influenza vaccine in combination with the adjuvant LT-R72 induces potent mucosal and serum immunity which is stronger than that with traditional intramuscular immunization. Infect Immun. 1999;67:4276. doi: 10.1128/iai.67.8.4276-4279.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]