Abstract
Previously we showed that calcitonin gene-related peptide (CGRP), a neuropeptide, inhibited lipopolysaccharide (LPS)-induced tumour necrosis factor-α (TNF-α) production and increased interleukin (IL)-6 release at low concentrations via activation of the cAMP pathway in mouse peritoneal macrophages (Mφ). In this study we examined whether CGRP could modulate IL-12 release from mouse peritoneal Mφ, and if so, what signal transduction pathway was involved. Mφ were obtained from the peritoneal exudate of male BALB/c mice. The cells were plated on culture dishes at a density of 5 × 105 cells per well and allowed to adhere for 2 hr. After incubation for 24 hr, the Mφ were cultured with 0·1 µg/ml of LPS, alone or together with CGRP (1–1000 nm) for 24 hr. The amount of IL-12 in the cell medium was measured by enzyme-linked immunosorbent assay (ELISA). The results showed that CGRP attenuated LPS-induced IL-12 release in a concentration-dependent manner. Production of IL-12 was decreased from 95·9 ± 4·6 to 73·4 ± 5·7 pg/ml by 100 nm CGRP. The two cAMP phosphodiesterase (PDE) inhibitors, 3-isobutyl-1-methyl-xanthine (IBMX) and rolipram, significantly potentiated the CGRP response, and the level of IL-12 was further decreased by 28% and 47%, respectively. However, CGRP had no effect on IL-12 production from unstimulated Mφ. The LPS-induced IL-12 release from Mφ could also be reduced by forskolin, an activator of adenylate cyclase, and 8-Br-cAMP, an analogue of cAMP. Using the reverse transcription–polymerase chain reaction (RT–PCR), we found that CGRP also decreased the LPS-induced IL-12 p40 mRNA levels. Furthermore, pretreatment with H89 (0·1 µm or 1 µm), an inhibitor of cAMP-dependent protein kinase, diminished CGRP effects, IL-12 production and gene expression. These data suggest that LPS-induced IL-12 release and gene expression were attenuated by CGRP via an activated cAMP-protein kinase A (PKA) pathway in mouse peritoneal Mφ.
Introduction
Interleukin (IL)-12, a heterodimeric cytokine produced mainly by monocytes/macrophages (Mφ), is a central inducer of cell-mediated immunity that promotes the development, proliferation and function of T helper 1 (Th1) cells.1 Th1 cells promote the activation and function of natural killer (NK) cells, T-cytotoxic cells and monocytes/Mφ, which are the principal effectors of cellular immunity.2 In the absence of IL-12, a T helper 2 (Th2) response develops.3,4 Therefore, the modulation of IL-12 production during an immune response is crucial for the outcome of some diseases. Endogenous inhibitors of IL-12 production are IL-10, IL-4, IL-13, transforming growth factor-β (TGF-β) and the components of the complement system.5,6 Excessive production of IL-12 may be involved in the pathogenesis of autoimmunity. For example, a recent study indicated that the stimulation of IL-12 secretion by microbial products was the crucial factor for proliferation and differentiation of pathogenic autoreactive Th1 effector cells in experimental allergic encephalomyelitis.7 IL-12 also induced tissue- or organ-specific inflammatory responses in insulin-dependent diabetes mellitus of non-obese diabetic (NOD) mice8 and type II collagen-induced arthritis in DBA/1 mice.9 Conversely, IL-12 deficiency has been documented in tuberculosis and human immunodeficiency virus (HIV) infections.10
Calcitonin gene-related peptide (CGRP) is a 37-amino acid peptide widely distributed in the central and peripheral nervous systems, mainly in sensory nerves.11 As sensory nerve endings are in close contact with components of the immune system, the immune functions may be affected by CGRP released from sensory nerve endings. Specific receptors for CGRP are present in T and B lymphocytes, as well as in Mφ.12–14 CGRP has been shown previously to modulate a number of immune functions, i.e. to inhibit mitogen-stimulated T-lymphocyte proliferation15,16 and to diminish human peripheral blood mononuclear cell (PMBC) function.17 It has also been shown that CGRP inhibits the killing activity of NK cells18 and IL-2-activated lymphocytes in mice.19 Previously we have shown that CGRP can inhibit lipopolysaccharide (LPS)-induced tumour necrosis factor-α (TNF-α) production20 and increase IL-6 release, at low concentrations, via activation of the cAMP pathway, in murine peritoneal Mφ.21 LPS and granulocyte–macrophage colony-stimulating factor (GM-CSF)-induced release of IL-10 is augmented by CGRP in Mφ and the Langerhans'-like cell line, XS52, but the B7-2 expression and IL-1β production are suppressed.22 CGRP also profoundly inhibits the ability of Mφ to produce H2O2 in response to interferon-γ (IFN-γ).23 When CGRP is present during the culture of human PBMC, the level of IL-12 p40, which is one of the two unrelated and disulphide-linked subunits, is decreased by ≈ 30%.24 A suppressive effect of CGRP on the LPS-stimulated and unstimulated expression of IL-12 p40 mRNA in the Langerhans' cell-like cell line, XS52, and peritoneal Mφ has also been reported.22 However, to our knowledge there have been no reports regarding the signal transduction mechanism of CGRP on IL-12 release from Mφ. The purpose of the present study was to investigate the cellular mechanism of CGRP on IL-12 release from mouse peritoneal Mφ. Our data indicated that CGRP inhibited LPS-induced IL-12 production and gene expression, which were mediated by the cAMP–protein kinase A (PKA) pathway in mouse peritoneal Mφ.
Materials and methods
Animals
The treatment of the laboratory animals and experimental protocols of the present study adhered to the guidelines of Beijing Medical University and were approved by the Institutional Authority for Laboratory Animal Care. All experiments were carried out in healthy, male BALB/c mouse (6–8 weeks old; weight 18–22 g). Animals were obtained from the animal laboratory of Beijing Medical University and housed in wire-mesh cages at an ambient temperature of 22°, under conditions of 12-hr light/12-hr dark and fed food and water ad libitum, for 1–2 weeks prior to all experiments.
Preparation of Mφ
Mφ were obtained from the peritoneal exudate of male BALB/c mice. The cells were plated on 24-well plate culture dishes at density of 5 × 105 cells/well in 1 ml of RPMI-1640 containing 10% fetal bovine serum and antibiotics (100 µg/ml of streptomycin and 100 units/ml of penicillin G) at 37° in a humidified atmosphere of 5% CO2 and 95% air. Mφ were allowed to adhere for 2 hr and then washed three times with RPMI-1640. The cells were incubated for 24 hr with one change of RPMI-1640 before beginning the formal experiment.
Measurement of IL-12 levels by enzyme-linked immunosorbent assay
Unless otherwise indicated, the Mφ were incubated with 0·1 µg/ml of LPS alone or with CGRP (1–1000 nm) for 24 hr. In some experiments, human (h)CGRP8−37 (5 µm), an antagonist of CGRP1 receptor, was added along with CGRP (100 nm) to study its blocking effect on CGRP. In another experiment, two inhibitors of cAMP phosphodiesterase (PDE), 3-isobutyl-1-methyl-xanthine (IBMX) and rolipram, were added at 10 µm and 30 µm, respectively, 30 min before stimulation with CGRP (10 nm). In addition, H89, an inhibitor of cAMP-dependent protein kinase, was added at 0·1 µm and 1 µm 30 min before stimulation with CGRP 100 nm. The effects of 1 µm forskolin, an activator of adenylate cyclase, and 1 mm 8-Br-cAMP, an analogue of cAMP, were also tested on LPS-induced IL-12 production. The medium was harvested for measurement of IL-12 by mIL-12 ELASA kit (Biosource International Inc., Camarillo, CA), which can measure IL-12 levels in the range of 7·8–500 pg/ml. The BioSource International Cytoscreen™ Mouse Interleukin-12 (mIL-12) enzyme-linked immunosorbent assay (ELISA) recognizes both natural and recombinant murine IL-12, as well as the free p40 subunit.
RNA preparation and reverse transcription–polymerase chain reaction (RT–PCR) for IL-12 mRNA
Mouse peritoneal Mφ were cultured in 90-mm dishes for 24 hr. They were then cultured for 5 hr in medium containing 0·1 µg/ml of LPS in the presence or absence of 100 nm CGRP. RNA was extracted from the cells using a method described previously.25 Total RNA was isolated from Mφ by acid guanidium thiocyanate–phenol–chloroform extraction26 and treated with DNase to eliminate contaminating genomic DNA. The purified RNA was confirmed by visualization of the 28 S and 18 S ribosomal RNA bands after electrophoresis of RNA through a 1% agarose–formaldehyde ethidium bromide gel.
The RT reaction was conducted by heating 4·0 µg of total RNA, 0·2 µg of oligo dT, 24 U RNasin (RNase inhibitor) and 10 U AMV in a total reaction volume of 25 µl. This mixture was incubated at 42° for 1 hr. The reaction was terminated by heating at 95° for 5 min. The primers used were synthesized by Sybersyn Biology Company (Beijing, China) and are as follows: mouse IL-12 p40 (5′-GAAGTTCAACATCAAGAGCAGTAG-3′ and 5′-AGGGAGAAGTAGGAATGGGG-3′27); mouse IL-12 p35 (5′-GACTTGAAGATGTACCAGACAG-3′ and 5′-GAGATGAGATGTGATGGGAG-3′); and mouse β-actin (5′-GTGGGGCGCCCAGGCACCT-3′ and 5′-CTTCCTTAATGTCACGCACGATTG-3′). By performing the RT reaction with different numbers of cycles (10, 20, 30, 40 and 50), we demonstrated that 30 cycles represented the linear part of the RNA amplification. PCR amplification for IL-12 p40 was performed by using 30 cycles comprising 1 min of denaturation at 94°, 1 min of annealing at 55° and 1 min of extension at 72°. PCR amplification for IL-12 p35 was performed by using 30 cycles comprising 1 min of denaturation at 94°, 1 min of annealing at 55° and 2 min of extension at 72°. PCR amplification for β-actin was performed by using 30 cycles of 45 seconds of denaturation at 94°, 45 seconds of annealing at 55° and 45 seconds of extension at 72°. Samples were subjected to electrophoresis on 2% agarose gels, stained with ethidium bromide and photographed. The intensities of the IL-12 band were compared with β-actin as an internal standard.
Chemicals and drugs
Synthetic CGRP (rat sequence, CGRP type I, CGRP-α) and hCGRP8−37 were purchased from Bachem Compary (Torrance, CA). RPMI-1640 was purchased from Gibco Laboratories (Grand Island, NY), LPS (lipopolysaccharide B from Salmonella enteritidis) was purchased from Difco Laboratories (Detroit, MI). H89, forskolin and 8-Br-cAMP were purchased from RBI Co. (Natick, MA). All other chemicals and drugs were purchased from Sigma Chemical Co. (St. Louis, MO) and the Beijing Chemical Plant of China (Beijing, China).
Statistical analysis
The results were expressed as the mean value ± SEM. The data were analysed using one-way analysis of variance (anova) and further analysed using the Student-Newman-Keuls' test (for multiple comparisons) or unpaired Student t-test (for means between two groups). A P-value of < 0·05 was considered significant for differences between treatment group means.
Results
Effect of CGRP on LPS-induced IL-12 release
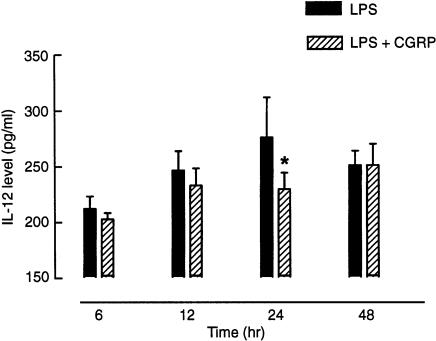
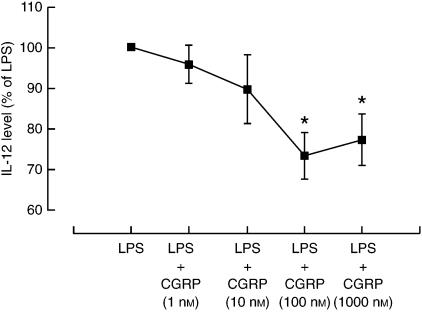
LPS (0·1–10 µg/ml for 24 hr) caused a concentration-dependent IL-12 release from mouse peritoneal Mφ (data not shown). The time-course of the effect of CGRP on LPS-induced IL-12 production from these cells was studied at 6, 12, 24 and 48 hr. The inhibitory effect of 100 nm CGRP on LPS-induced IL-12 release was observed as early as 6 hr with a maximal effect at 24 hr (Fig. 1). Therefore, the effect of LPS and CGRP was studied in the present study only after 24 hr of culture. When 0·1 µg/ml of LPS-treated Mφ was cultured in the presence of CGRP, CGRP significantly inhibited LPS-induced IL-12 release in a concentration-dependent manner. The IL-12 level was reduced from 95·9 ± 4·6 pg/ml to a low of 73·4 ± 5·7 pg/ml by 100 nm CGRP (Fig. 2). However, CGRP had no effect on IL-12 production from unstimulated Mφ.
Figure 1.
Time-course of the effect of calcitonin gene-related peptide (CGRP) on lipopolysaccharide (LPS)-induced interleukin-12 (IL-12) production in mouse peritoneal macrophages (Mφ). Mφ were stimulated with LPS (0·1 µg/ml), in the presence or absence of 100 nm CGRP, and the supernatants were harvested at different time-points. IL-12 released into the medium was assayed by using an enzyme-linked immunosorbent assay (ELISA). The inhibitory effect of CGRP on LPS-induced IL-12 release occurred as early as 6 hr after addition of CGRP, with a maximal effect at 24 hr. The data shown represent the mean ± SEM. *P < 0·05, compared with LPS alone at same time-point; n = 4 for each group.
Figure 2.
Effect of calcitonin gene-related peptide (CGRP) on lipopolysaccharide (LPS)-induced interleukin-12 (IL-12) release from mouse peritoneal macrophages (Mφ). Mφ were incubated for 24 hr with 0·1 µg/ml of LPS alone or with different concentrations (1–1000 nm) of CGRP. The IL-12 released into the medium was assayed by using an enzyme-linked immunosorbent assay (ELISA). CGRP inhibited the LPS-induced IL-12 production in a concentration-dependent manner. The data shown represent mean values ± SEM. *P < 0·05, compared with LPS alone; n = 5–7 for each group.
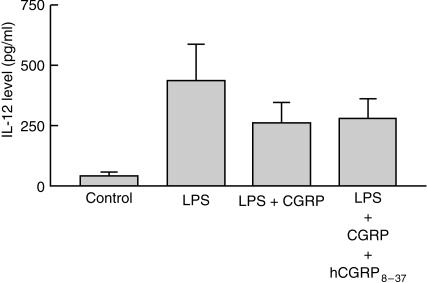
Effect of hCGRP8−37 on CGRP-diminished IL-12 production
In order investigate whether CGRP1 receptor was involved in the effect of CGRP on LPS-stimulated IL-12 production, 5 µm hCGRP8−37, an antagonist of CGRP1 receptor, was used 15 min before the addition of 100 nm CGRP to Mφ. The results showed that hCGRP8−37 did not reverse the inhibitory effect of CGRP on LPS-induced IL-12 release from the Mφ. This indicated that CGRP might act through CGRP receptors other than the CGRP1 receptor (Fig. 3).
Figure 3.
Effect of human calcitonin gene-related peptide 8–37 (hCGRP8−37) on CGRP-diminished interleukin-12 (IL-12) release from mouse peritoneal macrophages (Mφ). hCGRP8−37 (5 µm), an antagonist of the CGRP1 receptor, was added to Mφ 15 min before 100 nm CGRP, and stimulation with 0·1 µg/ml of lipopolysaccharide (LPS) was carried out for 24 hr. The IL-12 level in the medium was assayed by using an enzyme-linked immunosorbent assay (ELISA). The effect of CGRP on IL-12 release was not abrogated by hCGRP8−37. The data shown represent the mean value ± SEM, n = 6 for each group.
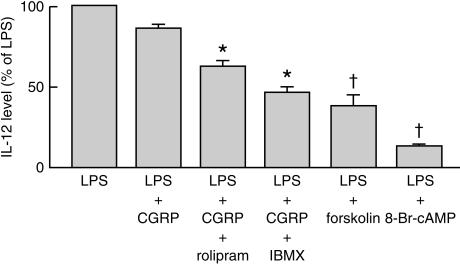
Role of cAMP and PKA in CGRP-diminished IL-12 release
To identify the second messenger involved in the inhibitory function of CGRP, we investigated the effects of two cAMP PDE inhibitors, IBMX and rolipram, on CGRP-diminished IL-12 production. Mφ were pretreated with 10 µm IBMX or 30 µm rolipram for 30 min. The inhibitory effect of CGRP 100 nm on LPS-induced IL-12 release was significantly potentiated by the two inhibitors, the level of IL-12 being further decreased by ≈ 28% and 47%, respectively. IBMX or rolipram alone at the above concentrations did not affect the LPS-induced IL-12 release from the cells. When 1 µm forskolin or 1 mm 8-Br-cAMP was added for 24 hr to the Mφ culture with 0·1 µg/ml of LPS, the LPS-induced IL-12 release was decreased from 43·1 ± 11·9 to 16·9 ± 4·5 pmol/ml and from 43·1 ± 11·9 to 6·1 ± 1·9 pmol/ml, respectively (Fig. 4).
Figure 4.
The cAMP phosphodiesterase (PDE) inhibitors potentiated the calcitonin gene-related peptide (CGRP)-inhibitory effect on lipopolysaccharide (LPS)-induced interleukin-12 (IL-12) release, and forskolin or 8-Br-cAMP inhibited LPS-induced IL-12 release from mouse peritoneal macrophages (Mφ). Mφ were pretreated with 10 µm IBMX or 30 µm rolipram for 30 min, and then 0·1 µg/ml of LPS, with 100 nm CGRP, was added for 24 hr. In order to test the effect of forskolin or 8-Br-cAMP on LPS-induced IL-12 release, Mφ were stimulated with LPS alone (0·1 µg/ml) or with LPS and either 1 µm forskolin or 1 mm 8-Br-cAMP, for 24 hr. The IL-12 level in the medium was assayed by using an enzyme-linked immunosorbent assay (ELISA). The inhibitory effect of CGRP on LPS-induced IL-12 release was significantly potentiated by the two cAMP PDE inhibitors. Both forskolin and 8-Br-cAMP markedly suppressed the LPS-induced IL-12 release. The data shown represent mean values ± SEM. *P < 0·05 compared with the LPS + CGRP group, n = 4 for each group; †P < 0·05 compared with LPS alone, n = 4–6 for each group.
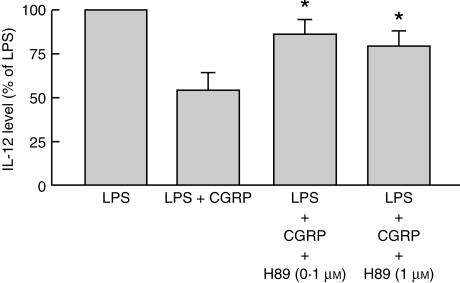
Furthermore, the effect of H89, a PKA inhibitor, was investigated on CGRP-diminished IL-12 production. When pretreated with H89 0·1 µm or 1 µm for 30 min, the inhibitory effect of CGRP 100 nm on LPS-induced IL-12 release from the Mφ was significantly reversed (Fig. 5). These data suggested that the cAMP-PKA pathway might mediate the CGRP effect on LPS-induced IL-12 production from mouse peritoneal Mφ.
Figure 5.
Protein kinase A (PKA)-mediated calcitonin gene-related peptide (CGRP) effect on lipopolysaccharide (LPS)-induced interleukin-12 (IL-12) production. Macrophages (Mφ) were pretreated with H89 (0·1 µm or 1 µm) for 30 min, and then 0·1 µg/ml of LPS, together with 100 nm CGRP, was added to the Mφ for 24 hr. The IL-12 level in the medium was assayed by using an enzyme-linked immunosorbent assay (ELISA). The inhibitory effect of CGRP on LPS-induced IL-12 release was reversed by H89. The data shown represent the mean values ± SEM. *P < 0·05, compared with the LPS + CGRP group; n = 7 for each group.
RT–PCR analysis of IL-12 mRNA expression
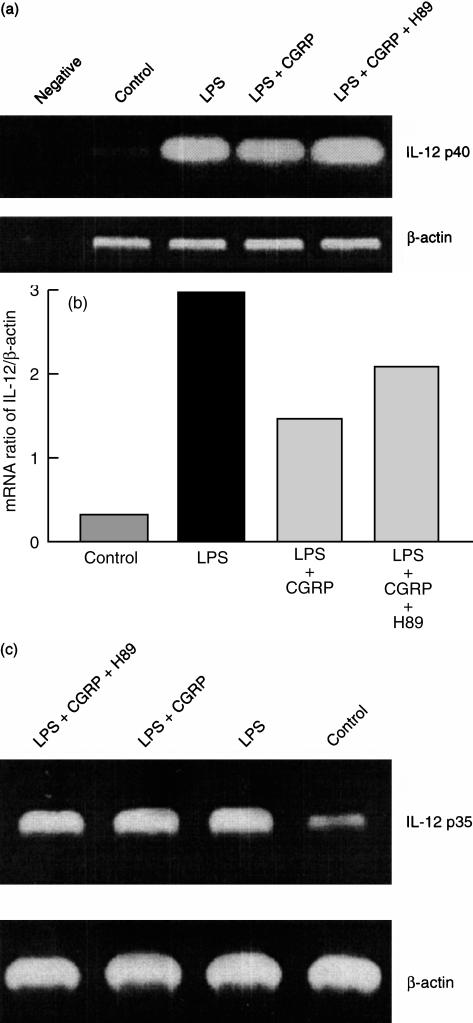
Having demonstrated that CGRP had an inhibitory effect on IL-12 production, we investigated whether this action occurred at the mRNA level. Mφ were stimulated with 0·1 µg/ml of LPS in the presence or absence of 100 nm CGRP and/or 1 µm H89 for 5 hr. CGRP significantly inhibited the level of specific IL-12 p40 mRNA, which could be reversed by 1 µm H89 (Fig. 6a,b). However, the IL-12 p35 mRNA level was not affected by CGRP (Fig. 6c). This result indicated that CGRP exerted its action at the IL-12 p40 mRNA level through the PKA pathway in the peritoneal Mφ. Bands were quantified densitometrically and expressed as mRNA ratio of IL-12 p40 to β-actin (internal standard) in the same experiment (Fig. 6b).
Figure 6.
Reverse transcription–polymerase chain reaction (RT–PCR) analysis of the calcitonin gene-related peptide (CGRP) effect on interleukin-12 (IL-12) mRNA expression. Macrophages (Mφ) were stimulated with 0·1 µg/ml of lipopolysaccharide (LPS) in the presence or absence of 100 nm CGRP and/or 1 µm H89 for 5 hr. The mRNA level of IL-12 p40 was reduced to a greater extent by CGRP in the absence of 1 µm H89 (a). However, the IL-12 p35 mRNA was not affected by CGRP on stimulated Mφ (c). Bands were quantified densitometrically and are expressed as mRNA ratio of IL-12 p40 to β-actin (internal standard) in the same experiment (b). This result was representative of three similar, independent experiments.
Discussion
These studies extend our prior observation regarding the regulatory effect of CGRP on immune function. We showed here that CGRP was potent in suppressing IL-12 production in mouse peritoneal Mφ after stimulation with LPS. Our results confirmed previous reports that CGRP inhibited IL-12 p40 production from PBMC.24 However, our data showed that the inhibitory effect of CGRP on IL-12 release occurred as early as 6 hr after addition of 100 nm CGRP, with a maximal effect at 24 hr, and that the CGRP effect was concentration dependent.
To fully understand the mechanism of CGRP action, it is important to clarify which cellular transduction pathway is involved in mediating the effect of CGRP on IL-12 production from Mφ. In the present study, the incubation of Mφ with forskolin and 8-Br-cAMP, an enhancer and an analogue of cAMP, respectively, suppressed LPS-induced IL-12 production. This suggests that both CGRP and forskolin or 8-Br-cAMP might mediate their actions through the same intracellular pathway. This also confirmed the data that the CGRP response was potentiated by the two potent cAMP PDE inhibitors, IBMX and rolipram. Furthermore, H89, a potent selective inhibitor of PKA,28 reduced the inhibitory effect of CGRP on IL-12 production and thus confirmed the role of the cAMP-PKA pathway for CGRP function.
Previously we have shown that CGRP, at low concentrations, can inhibit LPS-induced TNF-α production and increase IL-6 release via activation of the cAMP pathway in murine peritoneal Mφ.21 It has been reported that CGRP can induce cAMP accumulation in rat peritoneal Mφ29 and increase adenylate cyclase in the murine Mφ cell line, p388 D1.30 There is evidence to suggest that agents which increase cAMP level, such as prostaglandin E2, cholera toxin, β2-agonist, histamin, forskolin and db-cAMP, might cause a significant suppression of IL-12 production.31–35 In this study, we demonstrated for the first time that the inhibitory effect of CGRP on LPS-induced IL-12 production from mouse peritoneal Mφ was mediated by the cAMP-PKA pathway.
Our results showed that CGRP inhibited LPS-induced IL-12 p40 gene expression, which could be reversed by H89. However, the IL-12 p35 mRNA level was not affected by CGRP. These data were consistent with the report by Torii et al.22 that CGRP reduced LPS-induced IL-12 mRNA expression. We extended this observation, indicating that the CGRP reduction of IL-12 p40 mRNA expression was mediated through the cAMP-PKA pathway. The cAMP-responsive element-binding protein (CREB) has been shown to be activated by PKA.36 Therefore, CGRP might stimulate cAMP-PKA and the CREB system to inhibit the expression of IL-12 mRNA in mouse peritoneal Mφ.
Although CGRP inhibited the LPS-induced IL-12 release, it did not directly affect the basal IL-12 production from the mouse peritoneal Mφ. Most previous work has reported that CGRP modulated the production of cytokines, such as IL-1β, IL-10, IL-6 and TNF-α, from Mφ but required a stimulus such as LPS or GM-CSF.20–22 In the present study, CGRP together with LPS caused a significant decrease of IL-12 secretion and mRNA accumulation, and also shows that CGRP affects only the activated Mφ. Thus, the neuropeptide CGRP appears to be merely a modulator of immune functions in mouse Mφ.
In certain pathological conditions an abnormally high level of CGRP may be associated with altered immune function. The plasma level of CGRP in a rat endotoxin shock model was elevated by fourfold at 30 min and elevated further to more than 20-fold at 3 hr, the late stage of rat endotoxin shock.37 Elevated levels of CGRP in the serum of endotoxin-treated rats inhibited considerably the lymphocyte proliferation,16 suggesting that CGRP, especially during the pathogenesis of endotoxic shock, could have a profound inhibitory effect on lymphocyte proliferation and perhaps also on other immune functions.
IL-12 also plays a role in pathological situations, such as septic shock, tissue damage during inflammation and organ-specific autoimmune diseases. IL-12 is produced principally by Mφ and dendritic cells after stimulation with LPS, superantigens and parasitic antigens.38 Differentiation of CD4+ T cells into Th1 and Th2 cells, which have distinct cytokine phenotypes, is dependent on a significant level of IL-12.39 CGRP clearly inhibits the functional capacity of IL-2-activated lymphocytes to lyse tumour cells and also inhibits the growth of Candida albicans.19 CGRP also inhibits the NK cell activity of mice.12 Hence, these data suggest that CGRP might regulate the killing function of NK cells and IL-2-activated lymphocytes as well as other immune functions by acting, at least in part, on the production of IL-12. Therefore, we anticipate that CGRP may exert immunomodulatory effects by inhibiting IL-12 production from Mφ during sepsis or other inflammatory responses or in some autoimmune diseases.
In conclusion, CGRP caused a concentration-dependent inhibition of LPS-induced IL-12 production from mouse Mφ. The mode of action of CGRP seems not to be through the CGRP1 receptor. The CGRP effect on LPS-induced IL-12 production was synergized with the inhibition of cAMP PDE and in parallel with the cAMP enhancing agents. Furthermore, the inhibitory effect of CGRP on IL-12 production and mRNA expression was reversed by PKA inhibition. Therefore, our data indicate that the inhibition of CGRP on LPS-induced IL-12 production and gene expression is via the cAMP-PKA pathway in murine peritoneal Mφ.
Acknowledgments
This research project was supported by grants from the National Natural Science Foundation of China (no. 39425027) and from the China Medical Board of New York, to X. Wang.
References
- 1.Kobayashi M, Fitz L, Ryan M, et al. Identification and purificaton of natural killler cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–45. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 3.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'garra A, Murphy KM. Development of Th1, CD4+ T cells through IL−12 produced by Listeria–induced macrophages. Science. 1993;260:547–9. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 4.Scharton-Kersten T, Afonso LC, Wysocka M, Trinchieri G, Scott P. IL-12 is required for natural killer cell activation and subsequent T helper 1 cell development in experimental Leishmaniasis. J Immunol. 1995;154:5320–30. [PubMed] [Google Scholar]
- 5.D'anrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon-gamma-production by suppressing natural killer cell stimulatory factor IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–8. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'andrea A, Ma X, Aste-Amezaga M, Paganin C, Trinchieri G. Stimulatory and inhibitory effects of interleukin-4 (IL)-4 and IL-13 on the production of cytokines by human peripheral blood mononuclear cells: priming for IL-12 and tumor necrosis factor α production. J Exp Med. 1995;181:537–46. doi: 10.1084/jem.181.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Segal BM, Klinman M, Shevach EM. Microbial products induce autoimmune disease by an IL-12-dependent pathway. J Immunol. 1997;158:5087–90. [PubMed] [Google Scholar]
- 8.O'hara RM, Henderson SL, Nagelin A. Prevention of a Th1 disease by a Th1 cytokine: IL-12 and diabetes in NOD mice. Ann NY Acad Sci. 1996;795:241–9. doi: 10.1111/j.1749-6632.1996.tb52673.x. [DOI] [PubMed] [Google Scholar]
- 9.Germann T, Hess H, Szeliga J, Rude E. Characterization of the adjuvant effect of IL-12 and efficacy of IL-12 inhibitors in type II collagen-induced arthritis. Ann NY Acad Sci. 1996;795:227–40. doi: 10.1111/j.1749-6632.1996.tb52672.x. [DOI] [PubMed] [Google Scholar]
- 10.Chougnet C, Wynn TA, Clerici M, et al. Molecular analysis of decreased interleukin-12 production in persons infected with human immunodeficiency virus. J Infect Dis. 1996;174:46–53. doi: 10.1093/infdis/174.1.46. [DOI] [PubMed] [Google Scholar]
- 11.Poyner DR. Calcitonin gene-related peptide: multiple actions, multiple receptors. Pharmacol Ther. 1992;56:23–51. doi: 10.1016/0163-7258(92)90036-y. [DOI] [PubMed] [Google Scholar]
- 12.Umeda Y, Arisawa M. Characterization of the calcitonin gene-related peptide in mouse T lymphocytes. Neuropeptides. 1989;14:237–42. doi: 10.1016/0143-4179(89)90052-8. [DOI] [PubMed] [Google Scholar]
- 13.McGillis JP, Humphreys S, Reid S. Characterization of functional calcitonin gene-related peptide receptors on lymphocyte membrane properties. J Immunol. 1991;147:3482–9. [PubMed] [Google Scholar]
- 14.Owan I, Ibaraki K. The role of calcitonin gene-related peptide (CGRP) on macrophages: the presence of functional receptors and effects on proliferation and differentiation into osteoclast-like cells. Bone Mineral. 1994;24:151–64. doi: 10.1016/s0169-6009(08)80152-3. [DOI] [PubMed] [Google Scholar]
- 15.Umeda Y, Takamiya M, Yoshizaki H, Arisawa M. Inhibition of mitogen-stimulated T lymphocyte proliferation by calcitonin gene-related peptide. Biochem Biophys Res Commun. 1988;154:227–35. doi: 10.1016/0006-291x(88)90674-2. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Fiscus RR, Tang Z, Yang L, Wu J, Fan S, Mathews HL. CGRP in the serum of endotoxin-treated rats suppresses lymphoproliferation. Brain Behav Immun. 1994;8:282–92. doi: 10.1006/brbi.1994.1027. [DOI] [PubMed] [Google Scholar]
- 17.Casini A, Geppetti P, Maggi CA, Surrenti C. Effects of calcitonin gene-related peptide (CGRP), NKA and NKA (4–10) on the mitogen response of human peripheral blood mononuclear cells. Naunyn-Schmiedeberg's Arch Pharmacol. 1989;339:354–8. doi: 10.1007/BF00173591. [DOI] [PubMed] [Google Scholar]
- 18.Umeda Y. Inhibition of immune responses by calcitonin gene-related peptide. Ann NY Acad Sci. 1992;657:552–4. doi: 10.1111/j.1749-6632.1992.tb22832.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Fiscus RR, Yang L, Mathews HL. Suppression of the functional activity of IL-2-activated lymphocytes by CGRP. Cell Immunol. 1995;162:105–13. doi: 10.1006/cimm.1995.1057. [DOI] [PubMed] [Google Scholar]
- 20.Feng Y, Tang Y, Guo JX, Wang X. Inhibition of LPS-induced TNF-α production by calcitonin gene-related peptide (CGRP) in cultured mouse peritoneal macrophages. Life Sci. 1997;61:281–7. doi: 10.1016/s0024-3205(97)00866-7. [DOI] [PubMed] [Google Scholar]
- 21.Tang Y, Feng Y, Wang X. Calcitonin gene-related peptide potentiates LPS-induced IL-6 release from mouse peritoneal macrophages. J Neuroimmunol. 1998;84:207–12. doi: 10.1016/s0165-5728(97)00257-9. [DOI] [PubMed] [Google Scholar]
- 22.Torri H, Hosoi J, Beissert S, et al. Regulation of cytokine expression in macrophages and the Langerhans' cell-like line XS52 by calcitonin gene-related peptide. J Leukoc Biol. 1997;61:216–23. doi: 10.1002/jlb.61.2.216. [DOI] [PubMed] [Google Scholar]
- 23.Nong YH, Titus RG, Ribeiro JMC, Remold HG. Peptides encoded by the calcitonin gene inhibit macrophage function. J Immunol. 1989;143:45–9. [PubMed] [Google Scholar]
- 24.Fox FE, Kubin M, Cassin M. Calcitonin gene-related peptide inhibits proliferation and antigen presentation by human peripheral blood mononuclear cells: effects on B7, interleukin 10, and interleukin 12. J Invest Dermatol. 1997;108:43–8. doi: 10.1111/1523-1747.ep12285627. [DOI] [PubMed] [Google Scholar]
- 25.Tang Y, Han C, Fiscus RR, Wang X. Increase of calcitonin gene-related peptide (CGRP) release and mRNA levels in endotoxic rats. Shock. 1997;7:225–9. doi: 10.1097/00024382-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 27.Suzumura A, Sawada M, Takayanagi T. Production of interleukin-12 and expression of its receptors by murine microglia. Brain Res. 1998;787:139–42. doi: 10.1016/s0006-8993(97)01166-9. [DOI] [PubMed] [Google Scholar]
- 28.Chijiwa T, Mishima A, Hagiwara M, et al. Inhibition of forskolin-induced meurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-p-bromocinnamylamino)ethyl]-5-isoquinoleinsulfonamide (H89), of PC12 D pheochromocytoma cells. J Biol Chem. 1990;265:5267–72. [PubMed] [Google Scholar]
- 29.Vignery A, Wang F, Ganz MB. Macrophages express functional receptors for calcitonin gene-related peptide. J Cell Physiol. 1991;149:301–6. doi: 10.1002/jcp.1041490217. [DOI] [PubMed] [Google Scholar]
- 30.Abello J, Kaiserlian D, Cuber JC, Revilard JP, Chayvialle JA. Characterization of calcitonin gene-related peptide receptors and adenylate cyclase response in the murine macrophage cell line P388D1. Neuropeptides. 1991;19:43–9. doi: 10.1016/0143-4179(91)90072-q. [DOI] [PubMed] [Google Scholar]
- 31.Van-der-Pouw-Kraan TC, Snijders A, Boeije LC, De-Groot ER, Alewijnse AE, Leurs R, Aarden LA. Histamine inhibits the production of interleukin−12 through interaction with H2 receptors. J Clin Invest. 1998;102:1866–73. doi: 10.1172/JCI3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu CY, Wang K, McDyer JF, Seder RA. Prostaglandin E2 and dexamethasone inhibit IL-12 receptor expression and IL-12 responsiveness. J Immunol. 1998;161:2723–30. [PubMed] [Google Scholar]
- 33.Elenkov IJ, Webster E, Papanicolaou DA, Fleisher TA, Chrousos GP, Wilder RL. Histamine potently suppresses human IL-12 and stimulates IL-10 production via H2 receptors. J Immunol. 1998;161:2586–93. [PubMed] [Google Scholar]
- 34.Bordignon P, Mazzeo D, Lucia PD, D'ambrosio D, Lang R, Fabbri L, Selfb C, Sinigaglia F. Beta2-agonist prevents Th1 development by selective inhibition of interleukin-12. J Clin Invest. 1997;100:1513–9. doi: 10.1172/JCI119674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.RieSeries C, Bock G, Klocker H, Bartsch G, Thurnher M. Prostaglandin E2 and tumor necrosis factor alpha cooperate to activate human dendritic cells: synergistic activation of interleukin 12 production. J Exp Med. 1997;186:1603–8. doi: 10.1084/jem.186.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez GA, Montiminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–80. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Jones SB, Zhou Z, Han C, Fiscus RR. Calcitonin gene-related peptide (CGRP) and neuropeptide Y (NPY) levels are elevated in plasma and decreased in vena cava during endotoxin shock in the rat. Circ Shock. 1992;36:21–30. [PubMed] [Google Scholar]
- 38.Skeen MJ, Miller MA, Shinnich TM, Ziegler HK. Regulation of murine macrophage IL-12 production. Activation of macrophages in vivo, restimulation in vitro, and modulation by other cytokines. J Immunol. 1996;156:1196–206. [PubMed] [Google Scholar]
- 39.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–76. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]