Abstract
We investigated the involvement of antibody in protection against vaginal herpes simplex virus type-2 (HSV-2) infection by comparing intact and B-cell knockout (KO) mice. Vaginal immunization of intact mice with attenuated HSV-2 markedly reduced an HSV-2 challenge infection in the vagina. In contrast, immunization of B-cell KO mice produced less immunity against the challenge infection and that immunity occurred in a different pattern. At 20 hr after challenge, immunostaining of virus proteins in the vaginal epithelium and shed virus protein titres in the vaginal secretions were not significantly different between immunized and non-immunized B-cell KO mice and were much greater than in immunized intact mice. At 48 hr after challenge, the vaginal infection in immunized B-cell KO mice was markedly less than at 20 hr but remained ≈ sevenfold higher than in intact mice. This pattern of challenge infection in the vagina indicates that B cells, and probably the antibody derived from them, provided significant protection against reinfection in intact mice, especially during the first 20 hr after challenge, while other effector mechanisms became important between 20 and 48 hr after challenge. To determine whether T-cell immunity in immunized B-cell KO mice was equal to that in intact mice, we assessed interferon-γ (IFN-γ) secretion by memory T cells in vivo in the vagina at 20 hr after challenge. We found no significant differences in the up-regulation of major histocompatibility complex (MHC) class II antigens in the epithelium, up-regulation of vascular cell adhesion molecule-1 (VCAM-1) in vascular endothelium, or recruitment of T cells to the mucosa, indicating that the memory T-cell response to virus challenge was the same in intact and B-cell KO mice.
Introduction
Immune resistance to vaginal infection by herpes simplex virus type-2 (HSV-2) has been studied in mice.1–4 Vaginal immunization with attenuated virus induces strong immunity that either prevents or markedly reduces infection of the vaginal epithelium and blocks the development of neurological disease after challenge with wild-type virus.4, 5 This immunity involves T lymphocytes, 6–9 interferon-γ (IFN-γ) 7–10 and possibly immunoglobulin G (IgG) antibody.11
At present, the significance of antibody for protection of the female genital tract against infection remains controversial.12 In the particular case of HSV-2, passive transfer of immune serum or anti-HSV monoclonal antibody (mAb) failed to protect against challenge infections, 3, 13 and specific antibody was not detected in vaginal secretions after a parenteral immunization that produced high antibody titres in serum.3 Little specific secretory immunoglobulin A (SIgA) was detected in vaginal secretions, even after local immunization in the vagina, and immunity against vaginal HSV-2 infection was comparable in intact and IgA knockout (KO) mice.11–14 Moreover, Kuklin et al.9 reported that intranasal immunization of mice with recombinant vaccinia virus vectors expressing HSV glycoproteins B or D produced high titres of specific IgA and IgG in the vagina but failed to prevent epithelial infection. They concluded that protective immunity in these mice depended mainly on CD4+ lymphocytes. Similarly, Milligan et al.7 concluded that T-cell immunity is required for protection of the vaginal mucosa, even in the presence of high titres of specific HSV-2 antibody, and Sin et al.15 reported that a T helper 1 (Th1)-type immune response provided protective immunity against vaginal herpes infection whereas a T helper 2 (Th2)-type immune response worsened the disease.
On the other hand, passive transfer of a mAb to HSV glycoprotein B protected mice against vaginal challenge infection, even though it was not detected in vaginal secretions, 13 and passive transfer of serum IgG from immune mice diminished a vaginal challenge infection even though the mean antibody titre in vaginal secretions of recipient mice was only 8% of that measured in actively immunized mice.11 In the latter study, purified IgG from vaginal secretions of immunized mice neutralized HSV-2 in vitro when used at its in vivo concentration, and removal of the vaginal secretions from immune mice minutes before vaginal virus challenge increased vaginal infection, suggesting a protective role of secreted antibodies.
To further elucidate the protective role of secreted antibody in the female genital tract, we evaluated the relative contributions of humoral and cell-mediated immunity in protection against vaginal HSV-2 infection among intact, B-cell deficient and T-cell deficient mice.
Materials and methods
Animals and virus
Fifty-four C57BL/6 (intact) and 45 Igh-6tm 1Cgn (B-cell KO) female mice were purchased from Jackson Laboratories (Bar Harbor, ME) and were 16 weeks old at the beginning of treatment. Wild-type HSV-2 and attenuated HSV-2, a strain that contains a partial deletion of the thymidine kinase gene, were generously provided by Dr Mark McDermott (McMaster University, Hamilton, Canada).1, 2
Vaginal immunization and challenge
Age-matched intact and B-cell KO mice were placed into each of the following three treatment groups:
Non-immune/challenged mice.
Immune/challenged mice.
Ascites-treated immune/challenged mice.
Initially, all mice were injected with 2·5 mg of Depo-Provera® (DP; Upjohn Co., Kalamazoo, MI) diluted in phosphate-buffered saline (PBS). The hormone-treated mice were susceptible to vaginal HSV-2 infection from 5 to at least 20 days after DP treatment.4 Six days after DP treatment, the mice were anesthetized with tribromoethanol and either not immunized or immunized by intravaginal inoculation of 20 µl of attenuated HSV-2 at 1·5 × 106 plaque-forming units (PFU)/ml. These mice were referred to as immune or non-immune mice, respectively. Five weeks later the mice were again treated with DP, and both immune and non-immune mice were challenged intravaginally with 20 µl of wild-type HSV-2 at 5 × 106 PFU/ml. These mice were referred to as immune/challenged or non-immune/challenged mice, respectively. Some immune mice were injected intraperitoneally (i.p.) with 0·5 ml of anti-IFN-γ ascites10 17 hr before challenge, and with 1·0 and 0·5 ml of anti-Thy-1.2 ascites8 6 and 2 days before challenge, respectively (ascites-treated immune/challenged mice).
Sample collection and processing
At 24 hr before challenge, vaginal washes were collected from intact immune and B-cell KO immune mice for measurement of IgG. At 20 hr after challenge, vaginal washes were collected from some of the mice in each group for measurements of IFN-γ and shed virus protein. These mice were then killed and the vaginae removed and processed (as described below) for measurements of HSV-2 infection, lymphocytes, major histocompatibility complex (MHC) class II and vascular cell adhesion molecule-1 (VCAM-1). Vaginal washes were collected from the remaining mice 48 hr after challenge for measurement of shed virus proteins, and these mice were examined for signs of illness up to 14 days after challenge, as described previously.4 Each experimental group contained nine mice (Table 1). Vaginal washes from progestin-treated mice contained 10–15 µl of mucus, which was extracted (as described previously) to obtain the vaginal secretion.10 Vaginal tissues were fixed in 2% paraformaldehyde and processed for immunostaining, as described previously.10
Table 1.
Effects of immunization on vaginal epithelial infection, shed virus protein and neurological disease in intact and immunodeficient mice
| Shed virus protein titres§ | |||||
|---|---|---|---|---|---|
| Mice* | Immunity† | Mean % epithelial infection ± SEM‡ | 20 hr | 48 hr | Illness scores |
| Intact | Non-immune | 7.1 ± 1.4 | 211 (4.87 ± 0.33) | 1330 (6.55 ± 0.11) | 1.2 |
| [8/8] | [9/9] | [9/9] | |||
| Intact | Immunized | 0.03 ± 0.02 | 20.3 (2.74 ± 0.24) | 1.85 (0.56 ± 0.39) | 0 |
| [2/9] | [9/9] | [2/9] | |||
| B-cell KO¶ | Non-immune | 3.9 ± 0.9 | 208 (4.86 ± 0.36) | 1790 (6.82 ± 0.14) | 1.9 |
| [9/9] | [9/9] | [9/9] | |||
| B-cell KO | Immunized | 1.9 ± 0.6 | 277 (5.12 ± 0.14) | 12.4 (2.29 ± 0.43) | 0 |
| [9/9] | [9/9] | [8/9] | |||
| Intact | Immunized | 1.2 ± 0.3 | 123 (4.38 ± 0.31) | 251 (5.03 ± 0.43) | 0 |
| T-cell depleted | [8/9] | [9/9] | [9/9] | ||
| B-cell KO | Immunized | 4.5 ± 1.0 | 323 (5.26 ± 0.29) | NA | NA |
| T-cell depleted | [9/9] | [9/9] | |||
T-cell-depleted mice were treated with anti-Thy-1.2 ascites 6 and 2 days before challenge and with anti-interferon-γ (IFN-γ) ascites 17 hr before challenge.
Mice were immunized with attenuated virus 6 weeks before vaginal challenge with wild-type herpes simplex virus type-2 (HSV-2).
The number of mice infected/total no. of mice is given in brackets.
Geometric mean titres are followed in parenthesis by the log3 geometric mean titres ± SEM measured 20 hr and 48 hr after challenge. The number of mice infected/total no. of mice is given in brackets.
KO, knockout.
Shed virus protein and IFN-γ
Shed virus proteins were measured by chemiluminescence enzyme-linked immunosorbent assay (ELISA), as described previously, 10 in vaginal secretions that were collected 20 hr and 48 hr after vaginal challenge with wild-type virus. Shed virus proteins were not detected in vaginal washes of highly immune mice after challenge (sterilizing immunity), 8 indicating that this method did not detect residual challenge virus in the vagina. When virus proteins were present in the vagina their titres were closely correlated with the percentage of the vaginal epithelium that was infected with the virus, indicating that this method measured virus protein that is shed into the vaginal lumen from infected epithelial cells.8 IFN-γ was measured as previously described10 in vaginal secretions that were collected 20 hr after challenge.
Evaluation of vaginal tissues by immunofluorescence labelling
Quantification of epithelial infection was carried out as previously described.4, 8 The lengths of HSV-2-stained segments and total lengths of vaginal epithelium were measured in histological sections sampled from four areas of each vagina. Immunolabelling of lymphocytes, plasma cells and MHC class II was carried out as previously described.5 Lymphocytes were counted in four randomly selected high-power fields (× 40) from each of two separate regions of vagina. Plasma cells were counted in one complete cross-section from each of four separate regions of vagina from each mouse. The staining of MHC class II antigens in the vaginal epithelium was evaluated in two coded sections from each mouse as nil (0), weak (1), moderate (2), or bright (3), along with the proportion of epithelium with each kind of staining. The average staining in each mouse was calculated as: half the sum of all the products of staining intensity multiplied by the corresponding proportion of epithelium showing that staining in the two sections. The group mean staining was the average score of the nine mice in each group, and ranged from 0·0 for no epithelial staining in any mice to 3·0 for bright staining in all the epithelia of all mice. For VCAM-1 labelling, sections of vagina were blocked in 2% fetal calf serum (FCS), incubated in monoclonal rat anti-mouse CD106 (VCAM-1; PharMingen, San Diego, CA), washed in PBS, treated with 0·5% hydrogen peroxide in methanol, washed in PBS, incubated in biotinylated goat anti-rat IgG (Vector Laboratories Inc., Burlingame, CA) followed by streptavidin-peroxidase (Zymed Laboratories Inc., San Francisco, CA), and exposed to substrate (AEC kit; Zymed). The numbers of labelled blood vessels in the vaginal stroma of non-immune/challenged and immune/challenged intact and B-cell KO mice were counted in one complete cross-section from each of two separate regions of vagina from each mouse. The intensity of labelling in the blood vessels was also recorded as weak (1), moderate (2), or bright (3).
Illness scores
Illness was evaluated as previously described.10 A maximum illness score of 3·0 was assigned to mice that died or became so ill that euthanasia was desirable by 9 days after inoculation of wild-type virus. Mice that never showed signs of illness were scored as 0·0.
Statistics
The statistical significance of the results was evaluated using the Mann–Whitney U-test.
Results
Immunity to vaginal HSV-2 infection
Immunization of intact mice markedly reduced an HSV-2 challenge infection in the vagina, as indicated by reduced immunostaining of virus proteins in the vaginal epithelium and by reduced concentrations of shed virus proteins in the vaginal secretions (Table 1: P = 0·0002 for epithelial infection; P = 0·0003 for shed virus protein 20 hr after challenge; P < 0·0001 for shed virus protein 48 hr after challenge). In contrast, immunization of B-cell KO mice produced less immunity against the challenge infection, and that immunity occurred in a different pattern. At 20 hr after challenge, epithelial infection in the immunized B-cell KO mice was much greater than in immunized intact mice (P < 0·0001), but only marginally and not significantly less than in non-immunized B-cell KO mice (P = 0·094). Similarly, the shed virus protein titre in immunized B-cell KO mice 20 hr after challenge was more than 10-fold higher than in immunized intact mice (P < 0·0001) and was marginally, but not significantly, higher than in the non-immune reference group (P = 0·60). Nevertheless, by 48 hr after challenge the vaginal infection was largely cleared from immunized B-cell KO mice, whose mean shed virus protein titre was less than 5% of that found in such mice at 20 hr after challenge (P < 0·0001) and less than 1% of that found in the non-immune reference group at 48 hr (P < 0·0001). This pattern of challenge infection in the vagina indicates that B cells, and probably the antibody derived from them, provided significant protection against reinfection in intact mice during the first 20 hr after challenge, and that other effector mechanisms became important between 20 and 48 hr after challenge. The legacy of the early protection by B cells and antibody in intact mice can be seen in the shed virus protein titres 48 hr after challenge, where the mean titre in B-cell KO mice remained ≈ sevenfold higher than that of the intact mice (P = 0·011).
T cells and/or IFN-γ also contributed to immunity against vaginal HSV-2 challenge, as intact immune mice whose T cells and IFN-γ were depleted in vivo with mAbs had greater epithelial infection (P = 0·0008) and higher shed virus protein titres at 20 hr (P = 0·0019) and 48 hr (P < 0·0001) than did the comparable, non-depleted immune group (Table 1). Initially, the intact mice that were depleted of T cells and IFN-γ showed marginally better resistance to challenge infection than B-cell KO mice, although the differences were not statistically significant (P = 0·44 epithelial infection, P = 0·094 shed virus protein at 20 hr). Subsequently, B-cell KO mice resisted challenge infection substantially better than the intact mice that were depleted of T cells and IFN-γ (P = 0·0008 shed virus protein at 48 hr). This comparison again indicates that B cells and antibody were important in the protection seen at 20 hr after challenge, whereas T cells and IFN-γ were important in the clearance of virus that occurred between 20 and 48 hr after challenge. The involvement of antibody in early protection is further indicated by our finding that the B-cell KO mice that were depleted of T cells/IFN-γ had nearly fourfold higher epithelial infection (P = 0·050) and ≈ 2·5-fold higher shed virus protein titres at 20 hr after challenge (P = 0·063) than did the comparable intact mice (Table 1).
Humoral immunity in B-cell KO mice
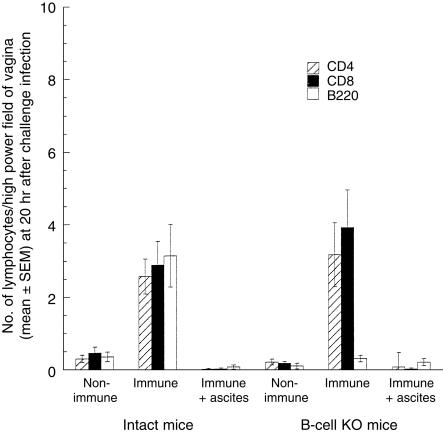
To confirm that the B-cell KO mice lacked humoral immunity, we assessed B cells, IgA plasma cells and IgG plasma cells in vaginal sections and IgG in vaginal secretions of intact and B-cell KO mice (Fig. 1, Table 2). As expected, B-cell KO mice lacked plasma cells and IgG. The small numbers of B220+ cells in each group of B-cell KO mice would appear to be the result of non-specific staining.
Figure 1.
Lymphocytes in vaginae of non-immune, immune and T-cell-depleted immune intact and B-cell knockout (KO) mice 20 hr after vaginal challenge with wild-type virus. The numbers of CD4+ and CD8+ cells in the vaginae of immune/challenged intact and B-cell KO mice were not significantly different (CD4, P = 0·93; CD8, P = 0·49). The small numbers of B cells observed in the B-cell KO groups are the result of non-specific staining, as staining with an isotype-matched control antibody in the intact immune group yielded a similar value of 0·2 cells per high power field. These counts arise because it is occasionally difficult to distinguish the membrane staining of lymphocytes from the endogenous fluorescence of granulocytes.
Table 2.
Immune parameters in the vagina of intact and B-cell knockout (KO) mice
| No. of plasma cells* | |||||||
|---|---|---|---|---|---|---|---|
| Mice | Immunity | IgA | IgG | IgG (ng/ml)† | IFN-γ‡ | MHC class II§ | VCAM-1¶ |
| Intact | Non-immune | 0.21 ± 0.1 | 0.29 ± 0.3 | — | −1.26 ± 0.43 | 0.0 | 6.1 ± 1.2 |
| B-cell KO | Non-immune | 0 | 0 | — | −0.49 ± 0.33 | 0.0 | 6.0 ± 1.4 |
| Intact | Immunized | 5.4 ± 1.8 | 14.6 ± 4.9 | 590 ± 83 | −0.86 ± 0.67 | 0.75 ± 0.16 | 119 ± 11 |
| B-cell KO | Immunized | 0 | 0 | 0.18 ± 2.2 | −0.20 ± 0.35 | 0.69 ± 0.16 | 113 ± 16 |
| Intact | Immunized | — | — | — | — | 0.0 | 3.1 ± 1.3 |
| T-cell depleted | |||||||
| B-cell KO | Immunized | — | — | — | — | 0.0 | 5.2 ± 1.4 |
| T-cell depleted | |||||||
Mean ± SEM. Plasma cells were counted in complete cross-sections of vaginae at 20 hr after challenge.
Mean ± SEM. Concentration of immunoglobulin G (IgG) (ng/ml) in vaginal secretions collected from immunized mice 24 hr before vaginal challenge.
Concentration of interferon-γ (IFN-γ) (pg/ml) in vaginal secretions 20 hr after challenge, mean ± SEM. The limit of detectability in our assay, defined as the mean of blank measurements plus 3 SD, was 5 pg/ml. The mean IFN-γ measurement in each group was well below the limit of detectability, indicating that little, if any, IFN-γ was present in the vaginal secretions.
Staining of vaginal epithelial cells on a scale of 0–3 at 20 hr after challenge. One section from each of two tissue blocks from each mouse was examined. The difference between immunized intact and B-cell KO groups was not statistically significant (P = 0.74). Major histocompatibility complex (MHC) class II was not up-regulated after challenge in the T-cell-depleted groups.
Number of stained blood vessels per section in the vaginal stroma at 20 hr after challenge. One section from each of two tissue blocks from each mouse was examined. The difference between immunized intact and B-cell KO groups was not statistically significant (P > 0.99). Vascular cell adhesion molecule-1 (VCAM-1) expression was not up-regulated after challenge in the T-cell-depleted groups.
Effectiveness of T-cell depletion
To confirm that in vivo depletion of T cells from intact and B-cell KO mice was effective, we assessed CD4+ and CD8+ cells in vaginal sections at 20 hr after challenge (Fig. 1). As expected, the ascites treatments effectively depleted T cells from the mice.
T-cell immunity in intact and B-cell KO mice
To determine whether T-cell immunity was equivalent in immunized intact and B-cell KO mice, we assessed IFN-γ secretion by memory T cells in vivo in the vagina at 20 hr after challenge. In addition to a direct measurement of IFN-γ in vaginal secretions, we measured three parameters that are dependent on local IFN-γ secretion: up-regulation of MHC class II expression in the vaginal epithelium; up-regulation of VCAM-1 expression in vascular endothelial cells; and recruitment of T lymphocytes into the vagina (Table 2, Fig. 1). No IFN-γ was detected in the vaginal secretions of any mice. However, weak staining of MHC class II antigens was observed mainly in the basal cells of the epithelium in immunized mice after challenge. This staining varied over the length of the epithelium and from animal to animal in both intact and B-cell KO mice, and the mean staining intensity was not significantly different between the two groups (Table 2). MHC class II antigens were not detectable by immunostaining in the vaginal epithelial cells of non-immune mice after challenge or in T-cell/IFN-γ-depleted mice. Labelling of VCAM-1 was observed in the endothelium of a few blood vessels in the vaginal stroma of non-immune/challenged intact and B-cell KO mice. Much larger numbers of labelled blood vessels were present in the vagina of immune/challenged mice, and the difference between intact and B-cell KO groups was not statistically significant (Table 2). The intensity of VCAM-1 staining in individual blood vessels varied from 1 to 3 in both intact and B-cell KO mice and did not differ between the two groups.
Few lymphocytes were detected in the vaginae of non-immune/challenged intact and B-cell KO mice, but CD4+ and CD8+ lymphocytes were vigorously recruited to the vaginae of immunized intact and B-cell KO mice after challenge (Fig. 1). The numbers of CD4+ and CD8+ lymphocytes recruited to the vagina were not significantly different among the intact and B-cell KO groups. The combined data indicate that memory T cells in the vaginae of immunized intact and B-cell KO mice secreted IFN-γ in response to vaginal challenge with virus, and that this secretion was not significantly different between the two groups.
Discussion
The present study of intact and B-cell KO mice demonstrates that B cells and probably antibody play a significant role in immunity against HSV-2 infection of the vaginal epithelium. We found that antibody mainly acts early during immune resistance to challenge infection, whereas cell-mediated immunity mainly acts later. An early involvement of antibody was indicated by the observation that the shedding of viral proteins from infected vaginal epithelial cells at 20 hr after challenge was markedly reduced by immunization in intact mice, but not in B-cell KO mice. A later involvement of cell-mediated immunity was indicated by the observation that while B-cell KO mice showed little resistance to the challenge infection at 20 hr they had largely cleared the infection by 48 hr after challenge. The sequential importance of antibody followed by cell-mediated immunity was similarly indicated by data from immunized intact and B-cell KO mice that were depleted of T cells and IFN-γin vivo shortly before the challenge infection.
The results of this study are consistent with several earlier reports on the involvement of antibody11 and T cells/ IFN-γ2, 7–10 in immunity against vaginal HSV-2 infection. An early role for antibody is consistent with the presence of neutralizing IgG antibody in vaginal secretions of immunized mice11 and with widely accepted views concerning the role of secreted antibodies at mucosal surfaces. When present, secreted antibodies typically neutralize challenge organisms in the lumen and thereby reduce infection of the epithelium. A subsequent role for antibody is possible, but it appears to be minor in comparison to cell-mediated immunity in the vaginal HSV-2 model used here. Memory T cells, on the other hand, require 6–8 hr to secrete substantial amounts of IFN-γ in the vagina in response to the challenge antigen, 10 and may require even longer to develop cytolytic activity. Thus, an early involvement of antibody followed subsequently by cell-mediated immunity, as indicated by our data, is not unexpected.
Antibody in vaginal secretions may be especially important for immune protection against sexually transmitted pathogens such as human immunodeficiency virus-1 (HIV-1), whose infection should if possible be prevented rather than cleared, and for extracellular pathogens such as Trichomonas and Candida, whose binding to the vaginal epithelium can be blocked by antibody.12 It appears, however, that the role of antibody in vaginal immunity can be obscured by small details of the experimental procedure. For example, antibody-mediated neutralization of challenge organisms was apparent, in the present study, early after challenge but was masked by cell-mediated immunity 48 hr after challenge. Similarly, antibody-mediated protection was revealed by direct measurements of epithelial infection but not by observations on the development of neurological illness. Moreover, secreted antibody was probably washed off the mucosal surface of immune mice by a challenge inoculum whose volume was greater than that of the mucosal secretions, 12 and it was probably removed by the treatment with sodium hydroxide that was used to permeabilize the vaginal epithelium prior to challenge so that susceptibility to infection was increased.15 The route of immunization also influences antibody-mediated protection. Secreted antibody is relatively more protective in vaginally immunized mice than in parenterally immunized mice because local immunization leads to accumulation of plasma cells in the vagina and local production of specific antibody, resulting in secreted antibody titres and specific activities that are several-fold higher than in parenterally immunized mice.5
Previous studies using B-cell KO mice have raised concern about whether cell-mediated immunity in the KO strain, including IFN-γ production by memory T cells, is equal to that of the C57BL/6 parent strain.16 We addressed this concern in the present study by making both direct and indirect measurements of IFN-γ secretion from memory T cells in vivo in the vagina of immunized mice after local antigen challenge. Previous studies using BALB/c mice revealed that IFN-γ appeared in the vaginal secretions of immune mice as early as 8 hr after vaginal challenge, 10 but was not detectable in vaginal secretions of non-immune mice until 48 hr after challenge, indicating that its secretion required memory T cells. In support of this interpretation, IFN-γ secretion in immune/challenged mice was abolished by in vivo depletion of T cells with mAb.7 The IFN-γ that was secreted in immune/challenged BALB/c mice up-regulated the expression both of MHC class II antigens in the vaginal epithelium10 and VCAM-1 in vascular endothelial cells, 17 and it recruited large numbers of T and B lymphocytes to the vaginal mucosa.10 These responses were blocked by pretreatment in vivo with mAb to IFN-γ. In the present study, IFN-γ was not detectable in the vaginal secretions of either intact or B-cell KO immune mice after virus challenge. However, the indirect measurements of IFN-γ in vaginal tissue revealed up-regulation of VCAM-1 and MHC class II antigens and recruitment of lymphocytes in both intact and B-cell KO mice. These three responses to IFN-γin vivo in the vaginal mucosa were not significantly different between intact and B-cell KO mice, indicating that the memory T-cell response to antigen challenge was essentially the same in the two strains. Our observations are thus consistent with those of Asano & Ahmed, 18 who reported that the numbers of CD8+ memory T cells were the same in intact and B-cell-deficient mice after infection with lymphocytic choriomeningitis virus.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (HD 17337). The authors thank Dr Mark McDermott for supplying attenuated and wild-type HSV-2 and Sheila Scillufo and Maureen Doran for their excellent technical assistance.
Glossary
Abbreviations
- AEC
aminoethyl carbazole
- DP
Depo-Provera®
- ELISA
enzyme-linked immunosorbent assay
- GMT
geometric mean titre
- HSV-2
herpes simplex virus-type 2
- IFN-γ
interferon gamma
- IgA
immunoglobulin A
- IgG
immunoglobulin G
- KO
knockout
- MHC
major histocompatibility complex
- PBS
phosphate-buffered saline
- PFU
plaque-forming units
- VCAM-1
vascular cell adhesion molecule-1
References
- 1.McDermott MR, Smiley BJ, Brais PLJ, Rudzroga H, Bienenstock J. Immunity in the female genital tract after intravaginal vaccination of mice with an attenuated strain of herpes simplex virus type 2. J Virol. 1984;51:247. doi: 10.1128/jvi.51.3.747-753.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDermott MR, Brais PLJ, Goettsche GC, Evelegh MJ, Goldsmith CH. Expression of immunity to intravaginal herpes simplex virus type 2 infection in the genital tract and associated lymph nodes. Arch Virol. 1987;93:51. doi: 10.1007/BF01313893. [DOI] [PubMed] [Google Scholar]
- 3.McDermott MR, Brais LJ, Evelegh MJ. Mucosal and systemic antiviral antibodies in mice inoculated intravaginally with herpes simplex virus type 2. J Gen Virol. 1990;71:1497. doi: 10.1099/0022-1317-71-7-1497. [DOI] [PubMed] [Google Scholar]
- 4.Parr MB, Kepple L, McDermott MR, Drew MD, Bozzola JJ, Parr EL. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab Invest. 1994;70:369. [PubMed] [Google Scholar]
- 5.Parr EL, Parr MB. Immunoglobulin G, plasma cells, and lymphocytes in the murine vagina after vaginal or parenteral immunization with attenuated herpes simplex virus type 2. J Virol. 1998;72:5137. doi: 10.1128/jvi.72.6.5137-5145.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDermott MR, Goldsmith CH, Rosenthal KL, Brais LJ. T lymphocytes in genital lymph nodes protect mice from intravaginal infection with herpes simplex virus type 2. J Infect Dis. 1989;159:460. doi: 10.1093/infdis/159.3.460. [DOI] [PubMed] [Google Scholar]
- 7.Milligan GN, Bernstein DI, Bourne N. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J Immunol. 1998;160:6093. [PubMed] [Google Scholar]
- 8.Parr MB, Parr EL. Mucosal immunity to herpes simplex virus type 2 infection in the mouse vagina is impaired by in vivo depletion of T lymphocytes. J Virol. 1998;72:2677. doi: 10.1128/jvi.72.4.2677-2685.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuklin NA, Daeshia M, Chun SJ, Rouse BT. Role of mucosal immunity in herpes simplex virus infection. J Immunol. 1998;160:5998. [PubMed] [Google Scholar]
- 10.Parr MB, Parr EL. The role of gamma interferon in immune resistance to vaginal infection by herpes simplex virus type 2 in mice. Virology. 1999;258:282. doi: 10.1006/viro.1999.9739. 10.1006/viro.1999.9739. [DOI] [PubMed] [Google Scholar]
- 11.Parr EL, Parr MB. Immunoglobulin G is the main protective antibody in mouse vaginal secretions after vaginal immunization with attenuated herpes simplex virus type 2. J Virol. 1997;71:8109. doi: 10.1128/jvi.71.11.8109-8115.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parr MB, Parr EL. Female genital tract immunity in animal models. In: Ogra PL, Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, editors. Mucosal Immunology. 2. San Diego, CA: Academic Press, Inc.; 1999. p. 1395. [Google Scholar]
- 13.Eis-Hubinger AM, Schmidt DS, Schneweis KE. Anti-glycoprotein B monoclonal antibody protects T cell depleted mice against herpes simplex virus infection by inhibition of virus replication at the inoculated mucous membrane. J Gen Virol. 1993;74:379. doi: 10.1099/0022-1317-74-3-379. [DOI] [PubMed] [Google Scholar]
- 14.Parr MB, Harriman GR, Parr EL. Immunity to vaginal HSV-2 infection in immunoglobulin A knockout mice. Immunology. 1998;95:208. doi: 10.1046/j.1365-2567.1998.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sin JI, Kim JJ, Boyer JD, Ciccarelli RB, Higgins TJ, Weiner DB. In vivo modulation of vaccine-induced immune responses toward a Th 1 phenotype increases potency and vaccine effectiveness in a herpes simplex virus type 2 mouse model. J Virol. 1999;73:501. doi: 10.1128/jvi.73.1.501-509.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Homann D, Tishon A, Berger DP, Wiegle WO, von Herrath MG, Oldstone MBA. Evidence for an underlying CD4 helper and CD8 T-cell defect in B-cell-deficient mice: failure to clear persistent virus infection after adoptive immunotherapy with virus-specific memory cells from mMT/mMT mice. J Virol. 1998;72:9208. doi: 10.1128/jvi.72.11.9208-9216.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parr MB, Parr EL. Interferon-γ upregulates intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 and recruits lymphocytes into the vagina of immune mice challenged with herpes simplex virus-2. Immunology. 2000;99:540. doi: 10.1046/j.1365-2567.2000.00980.x. 10.1046/j.1365-2567.2000.00980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asano MS, Ahmed R. CD8 T cell memory in B cell-deficient mice. J Exp Med. 1996;183:2165. doi: 10.1084/jem.183.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]