Abstract
In this study, we have investigated that after the intraperitoneal infection with murine cytomegalovirus (MCMV), the CD3+ CD4– CD8–(double negative; DN) T-cell receptor (TCR)αβ+ T cells increased in peritoneal cavity, liver and spleen in both resistant C57BL/6 and susceptible BALB/c mice. The total cellular population of these cells showed peak levels around day 5 after infection in all the three investigated organs and the following phenotypical and functional characteristics emerged. The peritoneal DN TCRαβ+ T cells expressed highly skewed TCRVβ8 on day 5 after infection compared with the uninfected mice, but those in spleen and liver showed moderate and low skewed TCRVβ8, respectively. The percentages of NK1.1+ DN TCRαβ+ T cells gradually decreased as did modulation of some of their activation markers consistent with an activated cell phenotype. The peritoneal DN TCRαβ+ T cells on day 5 after infection expressed the genes of interferon-γ (IFN-γ), tumour necrosis factor-α, Eta-1 (early T-cell activation-1) and MCP-1 (monocyte chemoattractant protein 1) but lacked expression of interleukin-4 (IL-4). After in vitro stimulation with phorbol 12-myristate 13-acetate and calcium ionophore in the presence of Brefeldin A, higher frequencies of intracellular IFN-γ+ DN TCRαβ+ T cells were detected in all three investigated organs of infected mice compared with those of uninfected mice. Stimulation of peritoneal DN TCRαβ+ T cells with plate-bound anti-TCRβ monoclonal antibodies showed proliferation and also produced IFN-γ but not IL-4. These results suggest that DN TCRαβ+ T cells were activated and may have an antiviral effect through producing IFN-γ and some macrophage-activating factors during an early phase of MCMV infection.
Introduction
A large proportion of peripheral T cells express T-cell receptor-αβ (TCRαβ) with CD4 or CD8 co-receptors. However, it is also reported that a small population of TCRαβ T cells express neither CD4 nor CD8 as their surface molecules and hence are termed double-negative (DN) TCRαβ T cells.1–3 The DN TCRαβ+ T cells have been shown to be preferentially distributed in the bone marrow, liver and thymus.1,2,4–7 Recently a group from our laboratory showed that the DN TCRαβ+ T cells were generated extrathymically in the peritoneal cavity after the intraperitoneal infection of mice with Listeria monocytogenes.8
The TCR repertoire of these murine CD4– CD8– TCRαβ+ T cells substantially differs from the pool of specificities of mature single-positive (SP) T cells and are usually interleukin-2 receptor-α (IL-2Rα; (CD25)–, IL-2Rβ+, CD44+, CD62L+.1–7 A considerable number of these cells also express the murine natural killer (NK) cell marker NK1.1.4,9 It has been reported that the DN TCRαβ+ T cells express a TCR with limited diversity. A major portion of the thymus DN TCRαβ+ T cells express Vβ8.6,7 The liver DN TCRαβ+ T cells which, it is suggested, develop in the liver also express Vβ8.10 The TCRα chain of the DN TCRαβ+ T cells in thymus and also in liver express a Vα14-Jα281 rearrangement with a conserved junctional amino acid sequence11 and it is suggested that the DN TCRαβ+ T cells with the Vα14-Jα281/Vβ8+ TCR are positively selected in the thymus, liver, or both. Some reports also showed that the DN TCRαβ+ T cells decreased considerably in both thymus and liver in β2-microglobulin-deficient mice10,12,13 and these data supported the hypothesis that the DN TCRαβ+ T cells may be positively selected through the recognition of self major histocompatibility complex (MHC) class I or class Ib molecules.
Previous reports also showed that the DN TCRαβ+ T cell activity is mainly mediated through cytokines14,15 but they can also exhibit natural suppressor activity,16,17 cytotoxic T-cell activity,18 or polyclonal B-cell help.19,20 The experiments into the antigen specificity of human DN TCRαβ+ T cells revealed that they include populations specific for CD1,21,22 allo class I23 and also are mostly self-reactive.24 But the role of the DN TCRαβ+ T cells of different organs against viral infection is almost unknown and only report made by Openshow claimed that after intranasal infection of mice with respiratory syncytial virus (RSV), DN TCRαβ+ T cells increased in the bronchoalveolar lavage associated with other CD4+ and CD8+ conventional T cells.25 In order to investigate the antiviral immune response of the DN TCRαβ+ T cells, we used murine cytomegalovirus (MCMV), a β-herpes virus with a similar pathogenicity pattern to human cytomegalovirus (HCMV), which is a deadly pathogen in organ transplanted patients and in those with acquired immune deficiency syndrome. Here, the immunoregulatory properties of these DN TCRαβ+ T cells that reside in the peritoneal cavity, liver and spleen of mice were extensively investigated after intraperitoneal infection with MCMV. We showed that DN TCRαβ+ T cells increased, developed an activated cell phenotype with skewed TCRV region toward TCRVβ8 repertoire, preferentially produced interferon-γ (IFN-γ) and some macrophage-activating factors during the early phase of intraperitoneal MCMV infection. The implications of the findings are discussed.
Materials and methods
Mice
BALB/c and C57BL/6 mice were purchased from Japan SLC Inc. (Hamamatsu, Japan). They were maintained in a specific pathogen-free environment at our institute. All mice were used at 8–10 weeks of age.
Virus and infection of mice
The Smith strain of MCMV was used in all experiments. Salivary gland-passed MCMV was prepared by homogenizing salivary glands of BALB/c mice infected with 1 × 104 plaque forming units (PFU) of MCMV 3 weeks before and aliquots of homogenized supernatants of salivary glands were stored at −80° in Hanks' balanced salt solution (HBSS) (Life Technology, Grand Island, NY).
Cell preparation
The peritoneal exudate cells (PEC) were harvested by lavage of the peritoneal cavity with 5 ml of ice-cold HBSS, washed and resuspended in RPMI-1640 medium (Life Technology) supplemented with 100 U/ml penicillin G, 100 µg/ml streptomycin, 2 mm l-glutamine, 0·2% sodium bicarbonate, 5 × 10−5 m 2-mercaptoethanol (2-ME), 25 mm HEPES and 10% heat-inactivated fetal calf serum (FCS) (RPMI). The cells were incubated in tissue culture plastic Petri dishes (Greiner, Frickenhausen, Germany) for 60 min at 37° in a humidified atmosphere with 5% CO2 and non-adherent cells were collected by mild washing. The single cell suspensions of spleens were made by passing the spleen through glass slides. The liver mononuclear cells were harvested using Percol (Sigma Chemicals, St Louis, MO) gradient concentrations after passing the liver homogenate tissue through a metal mesh. The nucleated cells were counted by Turk's reagent (Wako Chemicals, Osaka, Japan) using a light microscope.
Flow cytometry assay
Four-colour cytofluorometric analysis was done by FACS Calibur (Becton Dickinson, San Jose, CA). In all experiments, approximately 1 × 106 cells (from the single cell suspensions of non-adherent PEC, processed spleen and liver) were first treated with non-conjugated culture supernatant obtained from the 2.4G2 hybridoma to block FcRγII/III for about 30 min at 4° and red blood cells (RBC) were lysed by treating them with RBC-lysing buffer. After washing with fluorescence-activated cell sorter (FACS) Hanks' washing buffer [Hank's solution ‘Nissui’ (without phenol red) 1%, Na2HCO3 0·03%, NaN3 0·1%, heat-inactivated FCS 5%, and gentamycin 0·1 mg/ml in double distilled H2O], specific stainings are performed as described below.
For CD3+ DN TCRαβ+ T cells
Fluorescein isothiocyamate (FITC)-conjugated anti-TCRβ monoclonal antibody (mAb; H57-597) (Pharmingen, San Diego, CA), phycoerythrin (PE)-conjugated anti-CD4 and anti-CD8 mAb (Life Technology) and allophycocyanin (APC)-conjugated anti-CD3 mAb (Pharmingen). Dead cells were excluded by staining with propidium iodide (PI).
For NK1.1+ CD3+ DN TCRαβ+ T cells
FITC-conjugated anti-CD3 mAb (Pharmingen), PE-conjugated anti-NK1.1 mAb (Pharmingen), biotin-conjugated anti-TCRβ (H57-597) (Pharmingen) followed by streptavidin (SA)- peridin chlorophyll protein (PerCP) antibody (Becton Dickinson) and APC-conjugated anti-CD4 and anti-CD8 mAb (Pharmingen).
For TCRVβ8+ DN TCRαβ+ T cells
FITC-conjugated anti-TCRVβ8.1,8.2 mAb (F32.1) (Pharmingen), PE-conjugated anti-CD4 and anti-CD8 mAb (Life Technology), biotin-conjugated anti-TCRβ followed by SA-PerCP antibody and APC-conjugated anti-CD3 mAb.
For CD3+ DN TCRαβ+ T-cell activation markers
FITC-conjugated anti-TCRβ mAb (H57-597), PE-conjugated anti-CD4 and anti-CD8 mAb, biotin-conjugated anti-CD69, -CD25, -CD44 and -CD62L (Pharmingen) followed by SA-PerCP antibody and APC-conjugated anti-CD3 mAb.
For controls
Cells were stained with FITC-, PE- and APC-conjugated antibodies, except for the biotin-conjugated specific surface marker mAbs when only SA-PerCP was added.
Intracellular IFN-γ FACS analysis
Intracellular IFN-γ was determined as described by Kadena et al.8 Briefly, peritoneal, liver and spleen cells (1 × 106 cells/ml) were incubated for 4 hr at 37° in a humidified atmosphere with 5% CO2 in RPMI containing 10 ng/ml phorbol 12-myristate 13-acetate (PMA) (Sigma), 500 ng/ml calcium ionophore A23187 (Sigma) and 10 µg/ml Brefeldin A (Biomol. Research Lab. Inc., Plymouth Meeting, PA). Cells were then stained with non-conjugated anti-FcRγII/III (2.4G2), PE-conjugated anti-CD4 and anti-CD8 mAb (Life Technology), biotin-conjugated anti-TCRβ (H57-597) (Pharmingen) followed by SA-PerCP antibody (Becton Dickinson) and APC-conjugated anti-CD3 mAb (Pharmingen). The cells were washed and fixed with 0·1 ml Solution A of Cell Perm and Fix (Caltag, San Fransisco, CA) for about 60 min at room temperature (RT), washed and resuspended in 50 µl Solution B of Cell Perm and Fix containing 2 µg/ml FITC-conjugated rat anti-mouse IFN-γ mAb or isotype-matched FITC-conjugated rat immunoglobulin G1k (IgG1k) antibody. All FACS data were analysed using Cell Quest software (Becton Dickinson). The DN TCRαβ+ T-cell populations were identified by gating the CD3+ TCRαβ+ cellular constituents obtained from the lymphocytic area of the side- and forward-scattered diagram and subsequently excluding the CD4+ and CD8+ T cells by setting the quadrants. The total numbers of the DN TCRαβ+ T cells and NK1.1+ DN TCRαβ+ T cells were calculated by multiplying the total percentages of their respective cellular populations with the total nucleated cell number per organ. The CD69-, CD25-, CD44-, CD62L-positive, IFN-γ+ and NK1.1+ DN TCRαβ+ T cells were further gated excluding the CD4+ and CD8+ T cells and then the DN-gated cells positive for each surface marker were determined by using histogram plot analysis.
Enrichment of CD3+ DN TCRαβ+ T cells and CD4+ TCRαβ+ T cells and reverse transcription-polymerase chain reaction (RT-PCR) analysis of cytokine gene expression
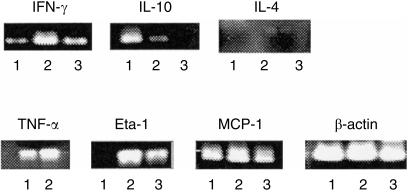
DN TCRαβ+ T cells obtained from the PEC of naive C57BL/6 mice and those from 5 days after MCMV-infected (18–20 mice per group) mice were enriched by two-step magnetic bead treatment. First, CD4+ and CD8+ TCRαβ+ T cells were depleted by sheep anti-rat IgG-coated dynabeads (Oslo, Norway) using anti-CD4 mAb (GK1.5) and anti-CD8 mAb (2.43) and then TCRαβ+ T cells were positively selected and separated by using MACS microbeads and a BS separation column, respectively (Miltenyl Biotec, Gladbach, Germany) following the instruction of the manufacturers with minor modifications. Briefly, after in vitro depletion of CD4+ and CD8+ T cells by dynabeads, PEC were stained with FITC-conjugated anti-TCRβ mAb (Pharmingen) for 15 min at 4°, washed with FACS Hanks' buffer solution and stained with anti-FITC microbeads for 30 min at 4°. After washing, the cells were positively separated by passing the cells through a BS column using FACS Hanks' buffer solution as the elution buffer. The purity of the DN TCRαβ+ T cells was above 92%. The peritoneal CD4+ T cells were similarly enriched by depleting the CD8+ T cells only using the sheep anti-rat IgG-coated dynabeads after treating the cells with anti-CD8 mAb (2.43) and subsequently positively selected and separated by MACS microbeads and the BS column, respectively. The purity of the CD4+ T cells was above 98% as determined by FACS analysis. The mRNA from these separated cells were extracted by mixing the cells with Trizol Reagent (Life Technology) and first strand cDNAs were reverse transcribed using Superscript reverse transcriptase (Life Technology) and random hexamer. The cDNA was amplified by PCR with cytokines or β-actin sense and antisense primers. The amount of cDNA was adjusted by amplification of serially diluted cDNA with β-actin primers after 30 cycles of PCR and compared the intensity of the amplified bands obtained from the ethidium bromide-stained 1·8% gel electrophoresis of the amplified PCR products. The cytokines used were IL-4, IL-10, IFN-γ, TNF-α, Eta-1 (early T-cell activation-1) and MCP-1 (monocyte chemoattractant protein 1) and their respective sense and antisense primers are described by Kadena et al.8
In vitro stimulation of the DN TCRαβ+ T cells
C57BL/6 and BALB/c mice (18–20 mice per group) were intraperitoneally infected with MCMV and their PEC were aseptically harvested on day 5 after infection. The CD4+ and CD8+ T cells of plastic non-adherent cells were magnetically depleted by sheep anti-rat IgG-coated dynabeads (Oslo, Norway) after treatment with anti-CD4 mAb (GK1.5) and anti-CD8 mAb (2.43). The viable cells were counted by trypan blue exclusion and 1 × 105 cells were cultured in 0·2 ml RPMI in 96-well, flat-bottomed tissue culture plates (Greiner) coated 24 hr before with purified anti-TCRβ mAb (H57-597, purified by HiTrap Protein G column, Pharmacia Biotech, Uppsala, Sweden) at a concentration of 50 µg/ml per well in sterile PBS. After 3 days of culture at 37° in a humidified atmosphere with 5% CO2 100-μl supernatants from each well were collected and IFN-γ and IL-4 were measured by conventional enzyme-linked immunosorbent assay (ELISA). The remaining cultured cells were pulsed with 1 µCi/well [3H]thymidine ([3H]TdR) and cultured for another 6 hr at 37° in similar atmospheric conditions. The cells were then harvested by cell harvester and thymidine incorporation was determined by liquid scintillation counter.
Results
Appearance of DN TCRαβ+ T cells in PEC, liver and spleen after MCMV infection
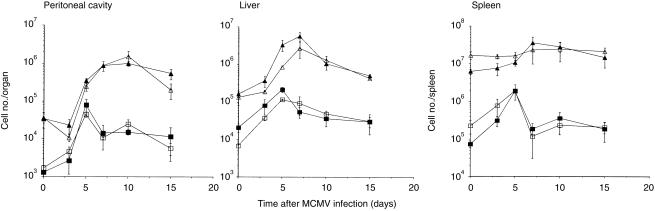
To determine the response of DN TCRαβ+ T cells against intraperitoneal MCMV infection, we first investigated the appearance of these cells in the three major primary virus-infected organs, the peritoneal cavity, liver and spleen, of both resistant C57BL/6 and susceptible BALB/c mice. The kinetic observation of the DN TCRαβ+ T cells showed that the population of these cells increased significantly in all three virus-infected organs and reached their peak levels around day 5 after MCMV infection (Fig. 1). Interestingly, in all three organs, their levels declined rapidly by day 7 after infection. The responses mediated by the DN TCRαβ+ T cells against viral infection are thus far different from those of the conventional CD4+ or CD8+ TCRαβ+ T cells as the later populations reached their peak levels around day 10 after MCMV infection (Fig. 1). Thus, the DN TCRαβ+ T cells increased in number in all three virus-infected organs at the early phase of MCMV infection.
Figure 1.
Appearance of DN TCRαβ T cells in peritoneal cavity, liver and spleen after MCMV infection. C57BL/6 and BALB/c mice were infected intraperitoneally with 1 × 105 and 4 × 103 PFU, respectively. The non-adherent PEC, liver and spleen lymphocytes were harvested on days 0, 3, 5, 7, 10 and 15 after infection and the DN TCRαβ+ T cells were determined by FACS analysis as described in the Materials and Methods. ▪ and □ represent the DN TCRαβ+ T cells of C57BL/6 and BALB/c mice, respectively, and ▴ and ▵ are for the combined populations of CD4+ and CD8+ TCRαβ+ T cells of C57BL/6 and BALB/c mice, respectively. The figure represents one of the three independent experiments with similar results.
Down-regulation of NK1.1+ DN TCRαβ+ T cells in MCMV infection
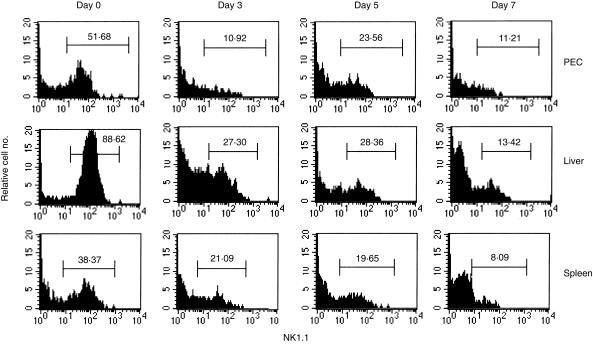
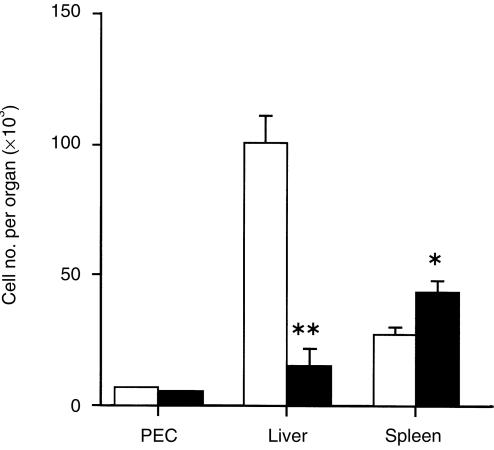
As a good number of DN TCRαβ+ T cells express the NK1.1 surface marker,4,9 we next examined the fate of NK1.1+ DN TCRαβ+ T cells of the peritoneal cavity, liver and spleen by FACS analysis in C57BL/6 mice after intraperitoneal MCMV infection. Down-regulation kinetics of the percentages of the NK1.1+ DN TCRαβ+ T cells were found after MCMV infection in all three organs investigated (Fig. 2). To check whether this down-regulation was due to the increment of the NK1.1– DN TCRαβ+ T cells after MCMV infection or not, we next determined the absolute numbers of the NK1.1+ DN TCRαβ+ T cells in all three investigated organs of both uninfected and infected mice on day 5 after infection as they showed their peak levels on this time point after intraperitoneal infection. Interestingly, the absolute number of the peritoneal NK1.1+ DN TCRαβ+ T cells in infected mice remained at a level similar to that in the uninfected mice, and the liver NK1.1+ DN TCRαβ+ T cells decreased significantly whereas splenic NK1.1+ DN TCRαβ+ T cells increased on day 5 after MCMV infection compared with those in uninfected mice (Fig. 3). Thus, MCMV has down-regulatory effects on the absolute number of NK1.1+ DN TCRαβ+ T cells in the liver but not in the PEC and spleen.
Figure 2.
Kinetics of MCMV-induced down-regulation of the percentages of the NK1.1+ DN TCRαβ+ T cells in C57BL/6 mice. Mice were infected with 1 × 105 PFU intraperitoneally and non-adherent PEC, liver and spleen lymphocytes were harvested on days 0, 3, 5 and 7 after infection. The histogram profiles show the percentages of the NK1.1+ DN TCRαβ+ T cells in DN TCRαβ+ T-cell-gated populations as described in the Materials and Methods. The figure represents one of the three independent experiments with similar results.
Figure 3.
The absolute number of the NK1.1+ DN TCRαβ+ T cells per organs in C57BL/6 mice after MCMV infection. Mice were infected with 1 × 105 PFU intraperitoneally and non-adherent PEC, liver and spleen lymphocytes were harvested on days 0 and 5 after infection. The absolute numbers of the NK1.1+ DN TCRαβ+ T cells per organ were calculated as described in the Materials and Methods. The open and closed bars represent for the total NK1.1+ DN TCRαβ+ T cells per organ on day 0 and day 5 after infection, respectively. The figure represents one of the three independent experiments. **P < 0·005; *P < 0·05 (Student's t-test).
Up-regulation of the Vβ8.1,8.2-bearing DN TCRαβ+ T cells after MCMV infection
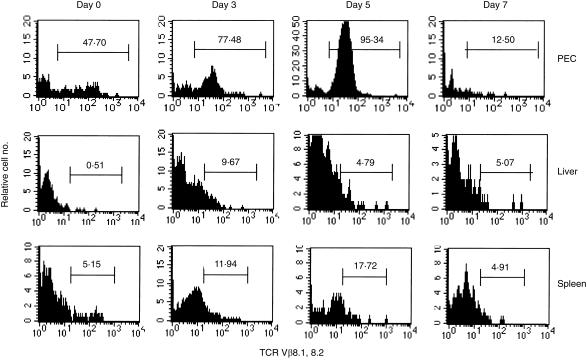
It was reported that DN TCRαβ-bearing thymocytes are distinguishable from the typical TCRαβ+ T cells and have a predominantly restricted TCRVβ8 repertoire.6,9 Moreover, after L. monocytogenes infection more than 80% of the DN TCRαβ+ T cells of the PEC expressed the TCRVβ8 repertoire.27 We next examined the expression of the TCRVβ8 repertoire of DN TCRαβ+ T cells obtained from the peritoneal cavity, liver and spleen of mice after MCMV infection using anti-TCRVβ8.1,8.2 mAb. The FACS analysis data presented in Fig. 4 showed that at day 5 after infection, TCRVβ8.1,8.2+ DN TCRαβ+ T cells increased above two-fold in PEC (> 95%) compared with the uninfected mice but moderate and low-level increments were found in spleen and liver, respectively, in C57BL/6 mice. Similar results were also found for the DN TCRαβ+ T cells in PEC, liver and spleen of BALB/c mice after MCMV infection (data not shown). Thus, after intraperitoneal infection with MCMV, the TCRVβ repertoire of DN TCRαβ+ T cells of peritoneal cavity skewed preferentially towards TCRVβ8.
Figure 4.
Kinetics of MCMV-induced up-regulation of TCRVβ8-bearing DN TCRαβ+ T cells in C57BL/6 mice. Mice were infected with 1 × 105 PFU intraperitoneally and non-adherent PEC, liver and spleen lymphocytes were harvested on days 0, 3, 5 and 7 after infection. The histogram profiles show the percentages of the Vβ8+ DN TCRαβ+ T cells of DN TCRαβ+ T-cell-gated populations as described in the Materials and Methods. The figure represents one of three independent experiments.
Expression of activation markers on DN TCRαβ+ T cells after MCMV infection
To determine the activation state of the DN TCRαβ+ T cells in mice after MCMV infection, we next examined the expressions of CD69 (early activation marker), CD25 (IL-2Rα), CD44 and CD62L (Mel-14) by FACS analysis. As the DN TCRαβ+ T cells reached their peak levels on day 5 in all the investigated organs (Fig. 1), we compared the expression of these surface markers on the DN TCRαβ+ T cells obtained from the mice on day 5 after MCMV infection with those of the uninfected mice. Higher levels of CD69 and CD25 expression were found on the DN TCRαβ+ T cells in PEC, liver and spleen of the virus-infected mice (Table 1). Interestingly, after MCMV infection the expression of CD69 on the PEC and liver DN TCRαβ+ T cells was found to be different from that on spleen; i.e. abruptly increased in PEC and liver but moderately increased (but still statistically significant, P < 0·05) in spleen. But in the case of CD25 expression, an entirely opposite pattern of distribution was observed; i.e. abruptly increased in spleen but moderately increased in PEC and liver after viral infection (Table 1). No major differences were found in the expression of CD44, the memory and activation marker, in the PEC and liver DN TCRαβ+ T cells between the naive and MCMV-infected mice, but the splenic DN TCRαβ+ T cells showed significantly decreased levels of CD44 expression on day 5 after infection (Table 1). The CD62L expression on DN TCRαβ+ T cells also showed a differential pattern in the investigated organs on day 5 after infection. The CD62L+ DN TCRαβ+ T cells decreased in spleen but increased in the PEC and liver (Table 1). The DN TCRαβ+ T cells obtained from the PEC, liver and spleen of BALB/c mice showed the similar results after intraperitoneal infection with MCMV (data not shown). These data suggested that after the viral infection the DN TCRαβ+ T cells of the PEC, liver and spleen became the activated cell phenotype and the activation patterns of the PEC and liver DN TCRαβ+ T cells were different from those of the spleen in both C57BL/6 and BALB/c mice.
Table 1.
Modulation of DN TCRαβ+ T-cell surface markers in naı¨ve and MCMV-infected C57BL/6 mice
| % Surface markers | |||||
|---|---|---|---|---|---|
| Organ | Days after infection | CD69 | CD25 | CD44 | CD62L |
| PEC | Day 0 | 2·41 ± 1·1 | 0·54 ± 0·9 | 85·00 ± 7·7 | 24·45 ± 2·7 |
| Day 5 | 77·17 ± 7·6** | 14·30 ± 3·9** | 75·46 ± 14·3 | 42·81 ± 6·7* | |
| Liver | Day 0 | 8·28 ± 2·0 | 0·14 ± 0·1 | 89·36 ± 4·7 | 2·95 ± 0·4 |
| Day 5 | 51·58 ± 5·1** | 2·75 ± 0·1** | 88·41 ± 0·8 | 7·04 ± 1·9* | |
| Spleen | Day 0 | 7·74 ± 0·5 | 7·30 ± 3·3 | 43·47 ± 4·6 | 6·99 ± 2·7 |
| Day 5 | 12·12 ± 2·4* | 66·21 ± 4·5** | 21·69 ± 1·8* | 0·11 ± 0·2* | |
C57BL/6 mice were infected intraperitoneally with 1 × 105 PFU. PEC, liver and spleen cells were collected and percentages of CD69-, CD25-, CD44- and CD62L-positive DN TCRαβ+ T cells were determined by FACS analysis as described in the Materials and Methods.
P < 0·05
P < 0·005 (Student's t-test).
Cytokine gene expression of the purified DN TCRαβ+ T cells induced by MCMV
To investigate the functional properties of the DN TCRαβ+ T cells against MCMV infection, we next analysed the cytokine gene expression of the DN TCRαβ+ T cells by RT-PCR analysis. As mice were infected intraperitoneally and peritoneal DN TCRαβ+ T cells responded well after viral infection, here we only examined the mRNA expression for some cytokines by the peritoneal DN TCRαβ+ T cells obtained from the mice after viral infection. The DN TCRαβ+ T cells were enriched from the PEC of C57BL/6 mice on days 0 and 5 after MCMV infection by depleting the CD4 and CD8 T cells using dynabeads and subsequently through the positive selection by using MACS microbeads. The PEC CD4+ T cells were similarly enriched on day 5 after infection from the same mice and they were used as the positive control. As shown in Fig. 5, the DN TCRαβ+ T cells expressed higher amounts of IFN-γ, TNF-α, MCP-1 and Eta-1 genes than the uninfected DN TCRαβ+ T cells. Although we could not detect the expression of IL-4 either in infected or uninfected DN TCRαβ+ T cells, the DN TCRαβ+ T cells of infected mice expressed far less IL-10 than did those of uninfected naive mice and a traceable level of IFN-γ was also expressed by the naive DN TCRαβ+ T cells (Fig. 5). These data, thus, suggest that DN TCRαβ+ T cells shifted to T helper type 1 (Th1) type from the Th0 type, presumably resulting in the activation of macrophages through the expression of some macrophage-activating factors and the Th1-type cytokines. Hence, the DN TCRαβ+ T cells may be one of the main effector T-cell populations to activate macrophages and lead to the development of the Th1-type populations, especially during the early stage of viral infection.
Figure 5.
RT-PCR analysis of the cytokine mRNA expression by the DN TCRαβ+ T cells after MCMV infection. C57BL/6 mice (18–20 mice per group) were infected intraperitoneally with 1 × 105 PFU MCMV and plastic non-adherent PEC were harvested on days 0 and 5 after infection. The DN TCRαβ+ T cells were enriched by using dynabeads and MACS microbeads as described in the Materials and Methods. The CD4+ T cells were similarly enriched from the non-adherent PEC of the same mice on day 5 after MCMV infection and were used as a positive control. Total RNA from these purified cells was extracted and the cDNA was amplified by PCR using the specific cytokine sense and antisense primers. The sense and antisense primers of β-actin were used as control. The respective cytokine bands of lanes 1, 2 and 3 are for DN TCRαβ+ T cells on day 0, DN TCRαβ+ T cells on day 5 and CD4+ T cells on day 5 after MCMV infection, respectively. The figure represents one of two independent experiments with similar results.
Analysis of intracellular IFN-γ-positive DN TCRαβ+ T-cell frequencies induced by MCMV infection
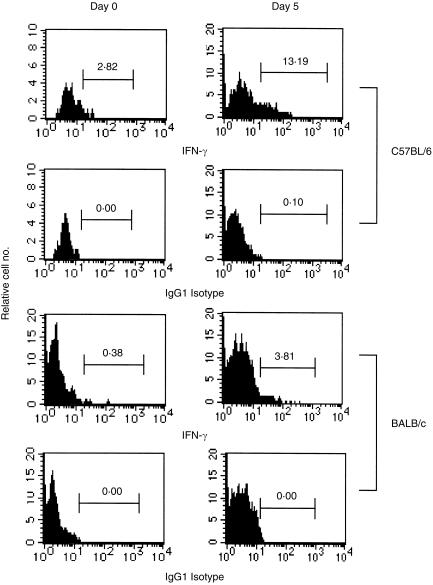
For further confirmation about the higher amount of IFN-γ gene expression by the DN TCRαβ+ T cells, we next determined the frequencies of intracellular IFN-γ+ DN TCRαβ+ T cells harvested from the PEC, liver and spleen of both C57BL/6 and BALB/c mice on days 0, 3, 5 and 7 after MCMV infection. After 4 hr in vitro stimulation with PMA and calcium ionophore in the presence of Brefeldin A, the FACS data showed increased frequencies of intracellular IFN-γ+ DN TCRαβ+ T cells in all three investigated organs of both C57BL/6 and BALB/c mice on days 3, 5 and 7 after MCMV infection. Furthermore, significantly higher frequencies of the intracellular IFN-γ+ DN TCRαβ+ T cells were found in C57BL/6 than in BALB/c mice on day 5 after viral infection in PEC and liver but not in spleen (Fig. 6 and Table 2). We, however, again could not detect IL-4+ DN TCRαβ+ T-cell populations in all the organs investigated from the both strains of mice on day 5 after MCMV infection after similar in vitro stimulation (data not shown). Thus, our results suggest that DN TCRαβ+ T cells from both C57BL/6 and BALB/c mice produced IFN-γ+ DN TCRαβ+ T cells after MCMV infection. The appearance of the higher frequencies of the intracellular IFN-γ+ DN TCRαβ+ T cells in C57BL/6 mice than in BALB/c mice may be one of the resistant parameters of this strain against MCMV infection.
Figure 6.
PEC intracellular IFN-γ+ DN TCRαβ+ T-cell frequencies induced by MCMV infection. C57BL/6 and BALB/c mice were infected intraperitoneally with 1 × 105 and 4 × 103 PFU, respectively. Their peritoneal non-adherent cells (1 × 106/ml) were incubated for 4 hr at 37° with PMA (10 ng/ml) and calcium ionophore (500 ng/ml) in the presence of Brefeldin A (10 mg/ml). Cells were stained with mAbs as described in the Materials and Methods. The histogram profiles show the percentages of the IFN-γ+ DN TCRαβ+ T cells of the DN TCRαβ+ T-cell-gated populations. The figure represents the data of one mouse out of three or four mice per group at each time-point in one of the three independent experiments.
Table 2.
Peritoneal cavity, liver and spleen IFN-γ+ DN TCRαβ+ T-cell frequencies in C57BL/6 and BALB.c mice after MCMV infection
| % IFN-γ+ DN TCRαβ+ T cells | ||||||
|---|---|---|---|---|---|---|
| Liver | Spleen | |||||
| Days after MCMV infection | PEC C57BL/6 | BALB/c | C57BL/6 | BALB/c | C57BL/6 | BALB/c |
| 0 | 2·82 ± 0·0 | 0·05 ± 0·0 | ND | 0·55 ± 0·2 | ND | 0·11 ± 0·01 |
| 3 | 8·70 ± 2·6 | 3·67 ± 0·7 | 2·01 ± 0·9 | 3·55 ± 0·5 | 1·64 ± 0·6 | 0·87 ± 0·4 |
| 5 | 13·87 ± 0·6* | 4·91 ± 1·7 | 21·10 ± 0·5** | 11·13 ± 0·9 | 6·22 ± 1·6 | 2·5 ± 1·0 |
| 7 | 2·13 ± 1·7 | 3·05 ± 0·9 | 3·0 ± 1·7 | 7·62 ± 0·3 | 0·65 ± 0·2 | 0·84 ± 0·5 |
C57BL/6 and BALB/c mice were infected intraperitoneally with 1 × 105 and 4 × 103 PFU, respectively. PEC, liver and spleen were collected and their intracellular IFN-γ was determined by FACS analysis as described in the Materials and Methods.
ND, not determined
P < 0·05
P < 0·005 (Student's t-test).
In vitro stimulation with plate-bound anti-TCRβ mAb for cytokine production and proliferative response of DN TCRαβ+ T cells obtained from the MCMV-infected mice
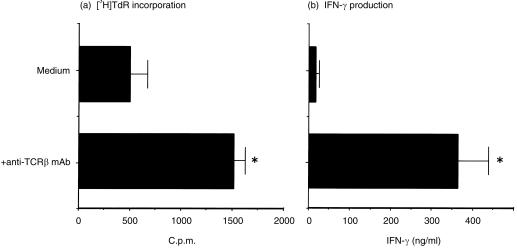
It was reported that after stimulation with plate-bound anti-CD3 mAb, DN TCRαβ+ T cells of thymus and spleen produced large amounts of IL-4, IFN-γ and TNF-α but did not produce detectable amounts of IL-2.15 We next determined their cytokine production and proliferative response after stimulation with the plate-bound immobilized anti-TCRβ mAb. The non-adherent PEC from C57BL/6 mice on day 5 after MCMV infection were enriched for DN TCRαβ+ T cells by depleting the CD4+ and CD8+ T cells with magnetic beads. Then, 105 cells were cultured on the anti-TCRβ mAb-coated 96-well plates for 3 days. From the culture supernatants, a significantly increased amount of IFN-γ production was measured in the anti-TCRβ mAb-coated wells over the uncoated control wells (Fig. 7b). After being pulsed with 1 µCi/well [3H]TdR, the cells cultured in vitro in the anti-TCRβ mAb-coated wells also showed increased proliferation (Fig. 7a). Again, we could not measure any detectable amount of IL-4 from the same cultured supernatants by ELISA (data not shown). Thus, PEC DN TCRαβ+ T cells obtained from the MCMV-infected mice showed functional TCRαβ and developed a Th1 phenotype after viral infection.
Figure 7.
IFN-γ production and proliferative response of the PEC DN TCRαβ+ T cells in C57BL/6 mice. Mice were infected with 1 × 105 PFU intraperitoneally and PEC were aseptically harvested on day 5 after infection. The CD4+ and CD8+ T cells from plastic non-adherent cells were magnetically depleted and 1 × 105 viable cells were cultured in 0·2 ml RPMI in 96-well flat-bottomed tissue culture plates coated 24 hr before with purified anti-TCRβ mAb (H57-597). After 3 days culture at 37° 0·1 ml supernatant from each well was collected and IFN-γ was measured by ELISA. The remaining cultured cells were pulsed with 1 µCi/well [3H]TdR and cultured for another 6 hr at 37°. The cells were then harvested and thymidine incorporation was determined by liquid scintillation counter. The figure represents one of two independent experiments. *P < 0·05 (Student's t-test).
Discussion
Previous studies have shown that the appearance of the DN TCRαβ+ T cells occurred in mice after infection with the RSV.25 In our present study, we mainly focused on the characterization of the DN TCRαβ+ T cells appearing in some major lymphoid organs after intraperitoneal MCMV infection. We showed that DN TCRαβ+ T cells responded well against MCMV infection by increasing their cell number and producing some antiviral cytokines at the early phase of infection. The pattern of their response is thus particularly important as most of the conventional T cells are found effective against viral infection at the late phase of infection. It is well known that in vivo the first-line defence against viral infection is the innate immune response and the innate immune response is mainly mediated by the macrophages and NK cells against MCMV infection at the early phase of infection. Reports are also available that cytokine-mediated antiviral activity (mostly produced by phagocytes and NK cells) is one of the major parts of innate immunity. Hence, the antiviral response mediated by the DN TCRαβ+ T cells through some antiviral cytokine production may thus be considered to be a novel addition to the antiviral innate immune response against MCMV infection.
The response of the DN TCRαβ+ T cells against MCMV infection appeared to be mostly organ-specific, as the PEC and liver DN TCRαβ+ T cells showed activation states different from the T cells of the spleen. After MCMV infection the DN TCRαβ+ T cells of PEC and liver preferentially expressed CD69, that was only moderately expressed by splenic DN TCRαβ+ T cells. It has been reported that DN TCRαβ+ T cells in PEC and liver generated extrathymically8,24 and PEC DN TCRαβ+ T cells showed slightly increased levels of CD44 surface antigen while expressing no IL-2Rα (CD25) or heat-stable antigen after day 5 of L. monocytogenes infection.26 Unlike L. monocytogenes infection we detected less expression of CD25 in the PEC and liver DN TCRαβ+ T cells but higher expression in the spleen. CD44 expression decreased on the splenic DN TCRαβ+ T cells whereas no changes were observed in those of PEC and liver after MCMV infection. Again, the expression of CD62L increased in PEC and liver but decreased in the spleen. These differential activation patterns of the DN TCRαβ+ T cells in different organs may be due to their origin of development and also to their different developmental environment. The origin of the DN TCRαβ+ T cells in the spleen were not well investigated before and thus further studies are necessary to confirm the differential activation phenomena existing in the DN TCRαβ+ T cells of PEC, liver and spleen after viral infection.
About the functional characterization of the TCR complexes of DN TCRαβ+ T cells, several reports are available and show that their TCR are preferentially skewed towards the TCRVβ8 repertoire in normal mice as well as in mice primed with bacterial antigens or after bacterial infection.6,24,26,27 In mice they can recognize bacterial antigen and are self-reactive.27 In humans, they can recognize monomorphic CD1 molecules with bacterial products.27 The human DN TCRαβ+ T-cell lines were found to lyse macrophages infected with Mycobacterium tuberculosis and their cytotoxicity was mediated through CD1-restricted Fas–FasL interaction but had no effect on the viability of the Mycobacteria.28 The prominent expansion of the Vβ8 repertoire in the peritoneal DN TCRαβ+ T cells may show the importance of the recognition of viral protein by the TCR of the DN TCRαβ+ T cells of the peritoneal cavity. Although the actual phenomenon of viral antigen recognition by DN TCRαβ+ T cells is still unknown, here we would like to speculate that, as for other bacterial infections where DN TCRαβ+ T cells can recognize bacterial antigen presented by MHC class I-like non-monophormic CD1 molecules, viral antigens may also be recognized by the DN TCRαβ+ T cells in a similar manner. Further analysis, however, is required to elucidate the antigen specificity of the DN TCRαβ+ T cells induced by MCMV.
Both Eta-1 and IFN-γ can activate macrophages29,30 and it was shown that macrophages showed an early antiviral response against the early phase of MCMV infection.31 IFN-γ itself can exhibit a number of immunoregulatory effects, including the capacity to stimulate the activation of cytotoxic T lymphocytes32,33 and macrophages.34 It is also reported that IFN-γ and TNF-α can inhibit MCMV replication in culture systems, and contribute to in vivo protection during MCMV infection.35–37 The greater IFN-γ and TNF-α production by the DN TCRαβ+ T cells at the early phase of MCMV infection may thus play an important part against the viral replication in vivo and also in the viral clearance, which is mostly mediated by the cytotoxic T cells at the late phase of infection. Again, the increased frequencies of intracellular IFN-γ+ DN TCRαβ+ T cells expressed by the different organs in C57BL/6 mice over BALB/c mice after MCMV infection may also be one of the considerable factors in making this strain resistant against this viral infection. The increased amount of IFN-γ mRNA expression by the peritoneal CD4+ T cells of C57BL/6 mice over BALB/c mice after intraperitoneal MCMV infection was also previously reported by our group.38 It is also worth noting that naive DN TCRαβ+ T cells expressed IL-10 mRNA and also expressed a traceable amount of genes for IFN-γ. Although we could not find detectable amounts of IL-4 mRNA, DN TCRαβ+ T cells of virus-infected mice expressed low but detectable amounts of IL-10 mRNA (Fig. 5). Thus, considering only the production of IFN-γ and IL-10 cytokines, it may be possible to conclude that after MCMV infection, as in the CD4 T cells, the Th1 type of DN TCRαβ+ T cells may be generated from the Th0 section of the DN TCRαβ+ T-cell population.
Unconventional T lymphocytes in the liver and thymus expressing NK1.1+ CD4+ TCRαβintermediate were identified by Emoto et al., who reported that after L. monocyto-genes infection the relative proportion of NK1.1+ CD4+ TCRαβintermediate in the liver disappeared by day 4 after infection with the concomitant abolishing of IL-4 production.39 The down-regulation of NK1.1+ CD4+ TCRαβ+Τ cells after L. monocytogenes infection may facilitate the T-cell activity against listerial infection through increased levels of IFN-γ production induced by the increased secretion of IL-12 from the Listeria-infected-macrophages. Against the viral infection, the response of the peritoneal NK1.1+ DN TCRαβ+Τ cells may be minimal as their total number remained similar to that in uninfected mice. On the other hand the decrease of the liver NK1.1+ TCRαβ+Τ cells may facilitate the generation of the IFN-γ-mediated antiviral responses and at the same time the increment of the splenic NK1.1+ DN TCRαβ+Τ cells may have another, different, antiviral immune response which may persist in the spleen only. Further studies are thus necessary to clarify the differential appearance of the NK1.1+ DN TCRαβ+Τ cells in different organs after MCMV infection.
Finally, the positive response of the DN TCRαβ+ T cells against MCMV infection may have implications for some therapeutic phenomena as some DN TCRαβ+ T-cell clones were reported to be highly autoreactive. For example, after concanavalin A activation and stimulation with irradiated dendritic cell-enriched syngeneic spleen cells, spleen-derived DN TCRαβ+ T-cell clones were found to be highly autoreactive with functional TCRVβ8.1 or Vβ8.2 gene products.19 Others have reported that the DN TCRαβ+ T-cell clones isolated from the rat inguinal lymph nodes of Mycobacterium tuberculosis-induced adjuvant arthritis showed marked proliferative responses to syngenic spleen cells and an intraperitoneal single-dose injection of these autoreactive DN TCRαβ+ T-cell clones resulted in marked suppression of experimental adjuvant arthritis.40 The data recently published also claimed that only the DN TCRαβ+ T-cell populations can effectively protect the non-obese diabetic mice from the spontaneous development of insulin-dependent diabetes mellitus through the Th2-associated cytokine IL-4 and/or IL-10 production.41
Hence, we would like to speculate that these unconventional DN TCRαβ+ T cells may play an important role in the regulation of viral infection and their immune response may be mainly mediated through the activation of macrophages and Th1-type generation against the early phase of MCMV infection.
Acknowledgments
We wish to thank Dr Q-J. Chen, Dr Hideyuki Nukina and Masumi Otsu for their generous help in doing this research. This work was supported in part by a grant from the Ministry of Education, Science and Culture of the Japanese Government.
Glossary
Abbreviations
- DN
double negative
- MCMV
murine cytomegalovirus
- PEC
peritoneal exudate cells
References
- 1.Reimann J. Double-negative (CD4– CD8–), TCRαβ- expressing, peripheral T cells. Scand J Immunol. 1991;34:679. doi: 10.1111/j.1365-3083.1991.tb01592.x. [DOI] [PubMed] [Google Scholar]
- 2.Levitsky HI, Golumbek PT, Pardoll DM. The fate of CD4– CD8– T cell receptor-αβ+ thymocytes. J Immunol. 1991;146:1113. [PubMed] [Google Scholar]
- 3.Kikly K, Dennert G. Evidence for extrathymic development of T NK cells: NK1+ CD3+ cells responsible for acute marrow graft rejection are present in thymus-deficient mice. J Immunol. 1992;149:403. [PubMed] [Google Scholar]
- 4.Sykes M. Unusual T cell populations in adult murine bone marrow. Prevalence of CD3+ CD4– CD8– and αβ TCR+ NK1.1+ cells. J Immunol. 1990;145:3209. [PubMed] [Google Scholar]
- 5.Iiai T, Watanabe H, Seki S, et al. Ontogeny and development of extrathymic T cells in mouse liver. Immunology. 1992;77:556. [PMC free article] [PubMed] [Google Scholar]
- 6.Fowlkes BJ, Kruisbeek AM, Hon-That H, et al. A novel population of T-cell receptor αβ-bearing thymocytes which predominantly express a single Vβ gene family. Nature. 1987;329:251. doi: 10.1038/329251a0. [DOI] [PubMed] [Google Scholar]
- 7.Budd RC, Meischer GC, Howe RC, Lees RK, Born C, MacDonald HR. Developmentally regulated expression of T cell receptor β chain variable domains in immature thymocytes. J Exp Med. 1987;166:577. doi: 10.1084/jem.166.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadena T, Matsuzaki G, Fujise S, et al. TCRαβ+ CD4– CD8– T cells differentiate extrathymically in an lck-independent manner and participate in early response against Listeria monocytogenes infection through interferon-γ production. Immunology. 1997;91:511. doi: 10.1046/j.1365-2567.1997.00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballas Z, Rasmussen W. NK1.1+ thymocytes. Adult murine CD4– CD8– thymocytes contain an NK1.1+ CD3+CD5+hi, CD44hi, TCR-Vβ8+ subset. J Immunol. 1990;145:1039. [PubMed] [Google Scholar]
- 10.Ohteki T, MacDonald HR. Major histocompatibility complex class I related molecules control the development of CD4+8– and CD4– subsets of natural killer 1.1+ T cell receptor-α/β+ cells in the liver of mice. J Exp Med. 1994;180:699. doi: 10.1084/jem.180.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lantz O, Bendelac A. An invariant T cell receptor α chain is used by a unique subset of major histocompatibility complex class-I specific CD4+ and CD4–T cells in mice. J Exp Med. 1994;180:1097. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bix M, Coles M, Raulet D. Positive selection of Vβ8+CD4– thymocytes by class I molecules expressed by hematopoietic cells. J Exp Med. 1993;178:901. doi: 10.1084/jem.178.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bendelac A, Killeen N, Littman DR, Schwartz RH. A subset of CD4+ thymocytes selected by MHC class I molecules. Science. 1994;263:1774. doi: 10.1126/science.7907820. [DOI] [PubMed] [Google Scholar]
- 14.Ceredig R, Lynch F, Newman P. Phenotypic properties, interleukin 2 production, and developmental origin of a ‘mature’ subpopulation of Lyt-2–/L3T4– mouse thymocytes. Proc Natl Acad Sci USA. 1987;84:8578. doi: 10.1073/pnas.84.23.8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zlotnik A, Godfrey DI, Fischer M, Suda T. Cytokine production by mature and immature CD4– CD8– T cells. αβ-T cell receptor+ CD4– CD8– T cells produce IL-4. J Immunol. 1992;149:1211. [PubMed] [Google Scholar]
- 16.Strober S, Dejbachsh S, Van Vlasselaer P, Duwe G, Salini S, Allison JP. Cloned natural suppressor cell lines express the CD3+CD4– CD8– surface phenotype and α, β heterodimer of the T cell antigen receptor. J Immunol. 1989;143:1118. [PubMed] [Google Scholar]
- 17.Palathumpat V, Dejbakhsh-Jones S, Holm B, Wang H, Liang O, Strober S. Studies of CD4– CD8–αβ bone marrow T cells with suppressor activity. J Immunol. 1992;148:373. [PubMed] [Google Scholar]
- 18.Skiner MA, Sambhara SR, Venveniste P, Miller RG. Characterization of αβ+ CD4– CD8– CTL lines isolated from mixed lymphocyte cultures of adult mouse spleen cells. Cell Immunol. 1992;139:375. doi: 10.1016/0008-8749(92)90079-5. [DOI] [PubMed] [Google Scholar]
- 19.Seman M, Boudaly S, Roger T, Morisset J, Pham G. Autoreactive T cells in normal mice: unrestricted recognition of self peptides on dendritic cell I-A molecules by CD4– CD8– T cell receptor α/β+ T cell clones expressing Vβ8.1 gene segments. Eur J Immunol. 1990;20:1265. doi: 10.1002/eji.1830200611. [DOI] [PubMed] [Google Scholar]
- 20.Erad F, Wild M-T, Garcia-Sanz JA, Le Gros G. Switch of CD8 T cells to noncytolytic CD4– CD8– cells that make Th 2 cytokines and help B cells. Science. 1993;260:1802. doi: 10.1126/science.8511588. [DOI] [PubMed] [Google Scholar]
- 21.Porcelli S, Morita CT, Brenner MB. CD1b restricts the response of human CD4– T lymphocytes to a microbial antigen. Nature (London) 1992;360:593. doi: 10.1038/360593a0. [DOI] [PubMed] [Google Scholar]
- 22.Beckman EM, Porcelli S, Morita CT, Behar S, Furlong ST, Brenner MB. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature. 1994;372:691. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 23.Rivas A, Laus R, Engleman EG. Alloantigen-specific cytotoxic clones bearing αβ T cell receptor but not CD4 or CD8 molecules. J Immunol. 1990;145:470. [PubMed] [Google Scholar]
- 24.Abo T, Ohteki T, Seki S, et al. The appearance of T cells bearing self-reactive T cell receptor in the livers of mice injected with bacteria. J Exp Med. 1991;174:417. doi: 10.1084/jem.174.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Openshow JM. Pulmonary epithelial T cells induced by viral infection express T cell receptor α/β. Eur J Immunol. 1991;21:803. doi: 10.1002/eji.1830210338. [DOI] [PubMed] [Google Scholar]
- 26.Matsuzaki G, Li X-Y, Kadena T, et al. Early appearance of T cell receptor αβ+ CD4– CD8– T cells with a skewed variable region repertoire after infection with Listeria monocytogenes. Eur J Immunol. 1995;25:1985.. doi: 10.1002/eji.1830250728. [DOI] [PubMed] [Google Scholar]
- 27.Porcelli S, Brenner MB, Greenstein JL, Balk SP, Terhost C, Bleicherl PA. Recognition of cluster of differentiation 1 antigen by human CD4– CD8– cytolytic T lymphocytes. Nature (London) 1989;341:447. doi: 10.1038/341447a0. [DOI] [PubMed] [Google Scholar]
- 28.Stenger S, Mazzaccaro RJ, Uyemera K, et al. Differential effects of cytolytic T cell subsets on intracellular infection. Science. 1997;276:1684. doi: 10.1126/science.276.5319.1684. [DOI] [PubMed] [Google Scholar]
- 29.Patarca R, Freeman GJ, Singh RP, et al. Structural and functional studies of the early T lymphocyte activation 1 (Eta-1) gene. Definition of a novel T cell-dependent response associated with genetic resistance to bacterial infection. J Exp Med. 1989;170:145. doi: 10.1084/jem.170.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh RP, Patarca R, Schwartz J, Singh P, Cantor H. Definition of a specific interaction between early-T-lymphocyte-activation-1 (Eta-1) protein and murine macrophages in vitro and in vivo. J Exp Med. 1990;171:1931. doi: 10.1084/jem.171.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamano S, Yoshida H, Takimoto H, et al. Role of macrophages in acute murine cytomegalovirus infection. Microbiol Immunol. 1998;42:607. doi: 10.1111/j.1348-0421.1998.tb02331.x. [DOI] [PubMed] [Google Scholar]
- 32.Chen LK, Tourvieille B, Burns GF, et al. Interferon: a cytotoxic T lymphocyte differentiation signal. Eur J Immunol. 1986;16:767. doi: 10.1002/eji.1830160709. [DOI] [PubMed] [Google Scholar]
- 33.Maraskovsky E, Chen WF, Shortman K. IL-2 and IFN-gamma are two necessary lymphokines in the development of cytolytic T cell. J Immunol. 1989;143:1210. [PubMed] [Google Scholar]
- 34.Nathan CF, Hibbs J., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991;3:65. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- 35.Grundy (chalmer) JE, Traman J, Allan JE, Shellam GR, Melier Evidence for a protective role of interferon in resistance to murine cytomegalovirus and its control by non-H-2-linked genes. Infect Immun. 1982;37:143. doi: 10.1128/iai.37.1.143-150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chong KT, Gresser I, Mims CA. Interferon as a defence mechanism in mouse cytomegalovirus infection. J Gen Virol. 1983;64:461. doi: 10.1099/0022-1317-64-2-461. [DOI] [PubMed] [Google Scholar]
- 37.Lucin P, Pavic I, Polic B, Jonijic S, Koszinowski UH. Gamma interferon-dependent clearance of cytomegalovirus infection in salivary glands. J Virol. 1992;66:1977. doi: 10.1128/jvi.66.4.1977-1984.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He X, Yoshida H, Minamishima Y, Nomoto K. Analysis of the role of CD4+ T-cells during murine cytomegalovirus infection in different strains of mice. Virus Res. 1995;36:233. doi: 10.1016/0168-1702(95)00010-n. 10.1016/0168-1702(95)00010-n. [DOI] [PubMed] [Google Scholar]
- 39.Emoto M, Emoto Y, Kaufmann SHE. Interleukin-4-producing CD4+NK1.1+ TCRα/βintermediate liver lymphocytes are down-regulated by Listeria monocytogenes. Eur J Immunol. 1995;25:3321. doi: 10.1002/eji.1830251218. [DOI] [PubMed] [Google Scholar]
- 40.Haque MA, Kimoto M, Inada S, Tokunaga O, Kohashi O. Autoreactive CD4– CD8–αβ T cells to vaccinate adjuvant arthritis. Immunology. 1998;94:536. doi: 10.1046/j.1365-2567.1998.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hammond KJL, Poulton LD, Palmisano LJ, Silveira PA, Godfrey DI, Baxter AG. α/β-T cell receptor (TCR) +CD4– CD8– (NK T) thymocytes prevent insulin-dependent diabetes mellitus in non obese diabetic (NOD) /Lt mice by the influence of interleukin (IL) -4 and /or IL-10. J Exp Med. 1998;187:1047. doi: 10.1084/jem.187.7.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]