Abstract
When immature human myeloid dendritic cells were differentiated in vitro in the presence of aspirin, they were unable to stimulate T-cell proliferation. Aspirin and its major metabolite salicylate changed the surface marker phenotype of dendritic cells. The drugs particularly suppressed the levels of CD83 and the secreted p40 unit of interleukin-12 (IL-12), both markers of mature dendritic cells; 50% inhibitory concentration (IC50) values were 2·5 mm, a concentration more than 100 times greater than the concentration at mid-point inhibition (ID50) value for inhibition of prostaglandin synthesis. Concomitantly, the levels of CD14, a marker of monocytes/macrophages, increased above the levels found in immature dendritic cells. Cyclooxygenase inhibitors ketoprofen, indomethacin and NS-398 had no effect at concentrations more than a thousand-fold higher than their IC50 values. The effects were independent of the presence of prostaglandin E2 in the medium. Salicylates suppressed activation of the nuclear transcription factor κB, which regulates dendritic cell differentiation, but their effects on mature dendritic cells were negligible. Hence, aspirin inhibits dendritic cell function by inhibiting their terminal differentiation at concentrations achieved in the blood of patients chronically treated with high-dose aspirin.
Introduction
Dendritic cells reside in peripheral tissues in an undifferentiated (immature) state; exposure to inflammatory signals such as tumour necrosis factor-α (TNF-α) or interleukin (IL)-1β, stimulates immature dendritic cells to differentiate into mature dendritic cells that can initiate immune responses (reviewed in ref. 1). Mature dendritic cells are located mainly in lymphoid organs where they present antigens and express costimulatory molecules (e.g. CD80, CD86), cytokines (e.g. IL-12) and chemokines (e.g. dendritic cell chemokine 1). The membrane-bound and secreted molecules confer to mature dendritic cells their high immunostimulatory capacity, particularly the ability to recruit naı¨ve T cells.2–4
Dendritic cells are thought to contribute to the pathogenesis of autoimmune diseases by inappropriate presentation of self-antigens. This notion is supported by the findings of unusually high numbers of dendritic cells within tissues involved in inflammatory bowel disease,5 rheumatoid arthritis6 and Graves' disease.7 Evidence from experimental autoimmune thyroiditis,8 insulin-dependent diabetes mellitus in non-obese diabetic mice9,10 and transgenic mice,11 demonstrates that autoimmunity is established and maintained by positive feedback between dendritic cells and the targeted tissue. In other words, once autoimmunity is triggered, tissue destruction leads to an inflammatory environment that attracts dendritic cells and stimulates their maturation. In addition, tissue destruction contributes antigens that are captured and presented by dendritic cells, resulting in expansion of autoimmune effector cells leading to more extensive tissue damage.
The symptoms of autoimmune diseases are often treated with high doses of salicylates.12 However, it is still not clear which cells are important targets of these drugs. Salicylates inhibit T-cell activation in vivo13 and reduce the viability of normal peripheral blood mononuclear cells (PBMC) and B cells in vitro.14 However, the 50% inhibitory concentration (IC50) values for these in vitro effects are high (above 5·0 mm for B cells and above 10 mm for PBMC).14 Because the therapeutic concentration of salicylate in the blood (1·8 mm)15,16 is lower than that required for inhibition of PBMC, we hypothesized that salicylates might act by inhibiting the function of dendritic cells. Inhibition of dendritic cell function might interfere with the positive feedback that maintains autoimmunity.
The cytokines granulocye–macrophage colony-stimulating factor (GM-CSF) and IL-4 promote the transition of monocytes into immature dendritic cells,17 which closely resemble immature dendritic cells found in tissues.1 Inflammatory mediators, infectious agents or stress signals trigger terminal differentiation into mature dendritic cells.18–20 This differentiation process is controlled by the nuclear transcription factor κB (NF-κB).21–23
We studied the effects of aspirin on the ability of immature dendritic cells to respond to a combination of inflammatory mediators (TNF-α, IL-1β and prostaglandin E2[PGE2]). We found that cells treated with aspirin or its major metabolite, salicylate, poorly stimulated T-cell proliferation. The drugs reduced neither dendritic cell viability nor their number, but they suppressed expression of antigen-presenting molecules and costimulatory molecules, secretion of the p40 subunit of IL-12, and the levels of activated NF-κB. On the other hand, cyclooxygenase (COX) inhibitors ketoprofen and indomethacin (COX-1 preference) and NS-398 (COX-2 preference) did not affect dendritic cell maturation up to concentrations more than 1000-fold above their respective concentration at mid-point inhibition (ID50) values. The effects of salicylates on mature dendritic cells were small in comparison to their effects on immature dendritic cells. Our data demonstrate that the process of terminal differentiation of dendritic cells is a sensitive target of inhibition by salicylates through a mechanism independent of COX, but that involves reduction in the levels of activated NF-κB.
Materials and methods
Isolation of CD14-positive cells and culture of dendritic cells
Buffy coats from the venous blood of normal healthy volunteers were obtained by the Division of Transfusion Medicine, Mayo Clinic, according to institutional guidelines. PBMC were isolated by density-gradient separation using Lymphocyte Separation Medium (Organon Teknika, Durham, NC). The cells were washed twice with phosphate-buffered saline (PBS) and once with cold PBS containing 0·5% bovine serum albumin (BSA) and 2·0 mm EDTA, counted, and used as the source for immunomagnetic isolation of CD14-positive cells (Miltenyi Biotec, Auburn, CA). CD14-positive cells were cultured at 1 × 106 cells/ml (3 ml/well in six-well plates) in X-VIVO-15 medium (Bio-Whittaker, Walkersville, MD) supplemented with human AB serum (1·0%; Sigma, St. Louis, MO), penicillin (100 U/ml; Gibco BRL, Gaithersburg, MD), streptomycin (100 µg/ml; Gibco BRL), GM-CSF (800 IU/ml; R & D Systems, Minneapolis, MN) and IL-4 (1000 IU/ml; R & D Systems), as described in ref. 19 except for the omission of IL-6. On days 3 and 5, 1 ml of the medium (modified by an increase of GM-CSF to 1600 IU/ml) was added to each well. On day 7, non-adherent immature dendritic cells were collected by pipetting, counted, centrifuged, resuspended in the medium containing 800 IU/ml of GM-CSF and 1000 IU/ml of IL-4, and plated at 5 × 105 cells/ml in 24-well plates. The ‘cocktail’ of inflammatory mediators – 1100 IU/ml of TNF-α, 1870 IU/ml of IL-1β (both R & D Systems) and 1·0 µg/ml of PGE2 (Sigma) – with or without salicylates, was added to cells at the time of plating. Cells were incubated for 3 days and analysed. Alternatively, the cells were incubated for 3 days with 10 µg/ml of a CD40-specific monoclonal antibody (mAb) (clone EA-5; Biosource, Camarillo, CA) instead of TNF-α, IL-1β or PGE2.
Drugs and incubation conditions
All drugs were from Sigma. Stock solutions were: aspirin, 1·0 m in absolute ethanol; sodium salicylate, 1·0 m in PBS, pH 7·4; ketoprofen and indomethacin, 500 mm in absolute ethanol; and NS-398, 500 mm in dimethylsulphoxide (DMSO). Stock solutions were prepared weekly. The drugs were diluted to final concentrations in the cell culture medium immediately before introduction to cells. Control cells were treated with an equal volume of the solvent; the highest concentrations of ethanol or DMSO in the cell culture medium were 0·1%. Cells were incubated at 37° in a humidified atmosphere containing 5% carbon dioxide.
Allogeneic T-cell proliferation
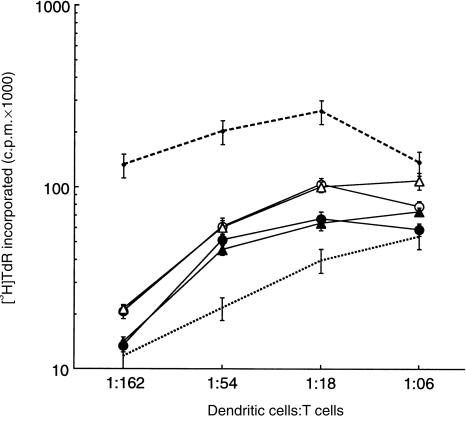
Dendritic cells were washed twice in X-VIVO-15 medium containing 1·0% human AB serum, penicillin, streptomycin and 2-mercaptoethanol (50 µm; Gibco BRL), and irradiated with 3000 rads from a Caesium-137 source. CD3-positive allogeneic (pooled from nine individuals) target leucocytes were prepared from PBMC by immunoadsorption according to the manufacturer's (R & D Systems) instructions. T cells were resuspended at 1 × 105 cells (in a volume of 0·1 ml per well) in X-VIVO-15, supplemented as described above. Allogeneic dendritic cells were added in a volume of 0·1 ml at the ratios indicated in Fig. 1. The cells were incubated for 5 days at 37° in 5% CO2. For the final 12 hr of incubation, the cells were ‘pulsed’ with 1·0 µCi of [3H]thymidine (Amersham, Arlington Heights, IL), then harvested and evaluated for incorporated radioactivity.
Figure 1.
T-cell proliferation stimulated by dendritic cells matured in the presence of aspirin or sodium salicylate. Allogeneic T-cell proliferation (presented as incorporated radioactivity, mean ± SD) was stimulated by immature dendritic cells (dotted line), mature dendritic cells (dashed line), and dendritic cells differentiated for 3 days in the presence of aspirin (2·5 mm, ○ and 5·0 mm, •) and sodium salicylate (2·5 mm, ▵ and 5·0 mm, ▴), and washed free of drugs. The data are representative of three experiments. c.p.m., counts per minute.
Antibodies and flow cytometry
The levels of membrane markers were characterized by flow cytometry using fluorescently labelled antibodies. Cells were harvested by scraping and then rinsing the dishes with PBS, collected by centrifugation and then resuspended in PBS. Antibody was added and the cells were incubated on ice for 20 min in the dark. Cells were washed with 2 ml of PBS, resuspended in 200 µl of PBS and then analysed immediately or fixed by the addition of paraformaldehyde to a final concentration of 1%. Bound antibodies were evaluated on 10 000 cells per assay using a fluorescence-activated flow cytometer (FACScan; Becton-Dickinson, San Jose, CA) and quantified by using cellquest analysis software (Becton-Dickinson). Data were analysed for geometric mean fluorescence intensity or per cent positive cells in comparison with unstained cells or cells stained with isotype controls.
An immunoglobulin G (IgG) isotype control (MOPC21/3421; Biosource) was conjugated to fluorescein isothiocyanate (FITC) or phycoerythrin. Antibodies specific for human leucocyte antigen (HLA)-DR (B-F1; Biosource), CD14 (B-A8; Biosource) and CD86 (BU63; Ancell, Bayport, MN) were conjugated to FITC. Antibodies specific for HLA class I (G46-2.6; Pharmingen, San Diego, CA), CD80 (L307.4; Becton-Dickinson) and CD83 (HB15A; Immunotech, Westbrook, ME) were conjugated to phycoerythrin.
Quantification of apoptosis by flow cytometry
Cells were collected as described above, stained by fluoresceinated annexin-V and propidium iodide (both Roche Diagnostics, Indianapolis, IN), 1·25 µg/ml, and analysed by flow cytometry. Annexin-V staining in test samples was compared with cells treated for 1 day with 500 µm H2O2 as a positive control.
Measurement of the secreted p40 unit of IL-12
Conditioned medium (100 µl) was collected from each well 48 hr after initiation of differentiation, centrifuged to remove debris and stored at −70°. After thawing, 50 µl was withdrawn for p40 measurement by sandwich enzyme-linked immunosorbent assay (ELISA) (Endogen, Westbury, MA), according to the manufacturer's instructions.
Electrophoretic mobility-shift assay
We determined the level of activated NF-κB by using the standard electrophoretic mobility-shift assay.24 Twelve hours after stimulation with the ‘cocktail’ of inflammatory mediators, with or without salicylates, cells were collected by scraping, washed with PBS, and lysed for 30 min on ice using a buffered high-salt detergent (20 mm HEPES, pH 7·9, 350 mm NaCl, 20% w/v glycerol, 1% w/v Nonidet-P40 [NP-40] detergent, 1 mm MgCl2, 0·5 mm EDTA, 0·1 mm EGTA, 0·5 mm dithiothreitol [DTT], 0·1% phenylmethylsulphonyl fluoride [PMSF], 1·0% aprotinin). The lysate was centrifuged at 13 000 g for 5 min at 4°. Protein concentrations were measured with the Micro BCA Protein Assay (Pierce, Rockford, IL). Equal amounts of the lysate, containing 20 µg of protein, were used to assess NF-κB activation by binding to a 32P-labelled NF-κB-specific oligonucleotide, using the manufacturer's protocol (Promega, Madison, WI). For controls, either unlabelled competitor or unlabelled non-competitor oligonucleotide was included in the reaction mixture. Samples were incubated at room temperature for 25 min, electrophoresed on 6% polyacrylamide gels and visualized by autoradiography.
Statistics
All experiments were repeated with samples from two to six individual donors; similar results were obtained on each occasion. All assays were performed at least in triplicate. The probability that the mean values of two experimental groups were identical was tested using the two-tailed t-test for paired samples. The level of significance was set at P < 0·05. Where applicable, data are reported as mean ± standard deviation (SD). Where changes in values are reported as percentage, SD values were calculated by the usual methods.
Results
Aspirin-treated dendritic cells cannot stimulate T cells
Immature dendritic cells stimulate proliferation of T cells much less effectively than mature dendritic cells.1 Accordingly, we used T-cell proliferation to determine the effects of salicylates on dendritic cell differentiation. Data in Fig. 1 show that treatment of dendritic cells with aspirin during the 3-day maturation reduced, in a dose-dependent manner, their ability to stimulate T-cell proliferation.
Aspirin inhibits dendritic cell differentiation
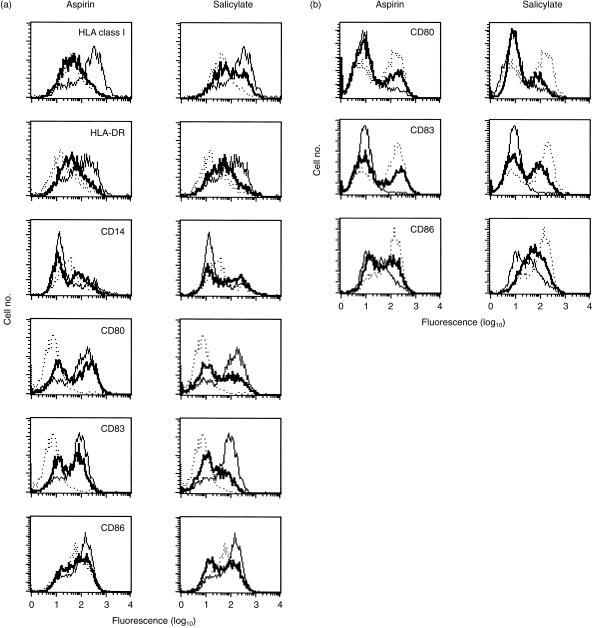
To ascertain if aspirin changed the expression of molecules required for dendritic cell function, we incubated immature dendritic cells with inflammatory mediators TNF-α, IL-1β and PGE2 for 3 days in the presence of aspirin. We measured the levels of HLA class I, HLA-DR, CD14, CD80, CD83 and CD86. At 2·5 mm, aspirin reduced the levels of HLA class I, HLA-DR, CD80, CD83 and CD86 (Fig. 2a). However, aspirin reproducibly increased the levels of CD14 with the same IC50 value (see also the open squares in Fig. 6). Qualitatively similar results were obtained when the cells were differentiated in the presence of the CD40-specific antibody EA-5 (10 µg/ml) instead of TNF-α, IL-β and PGE2 (Fig. 2b).
Figure 2.
Membrane markers in dendritic cells matured by tumour necrosis factor-α (TNF-α), interleukin-β (IL-β) and prostaglandin E2 (PGE2) (a) or by the CD40-specific monoclonal antibody EA-5 (b) in the presence of aspirin or sodium salicylate. Abscissas: Levels of membrane molecules, expressed as logarithms of mean fluorescence intensity (MFI), of antibodies associated with cells. Ordinates: Relative numbers of cells binding any particular number of antibodies. Dotted lines represent the MFI of immature dendritic cells and thin lines represent the MFI of mature dendritic cells in the absence of salicylates; the MFI of cells in the presence of aspirin (2·5 mm) or sodium salicylate (2·5 mm) are shown as thick lines. Data were derived with cells obtained from a single donor. The results are representative of six experiments.
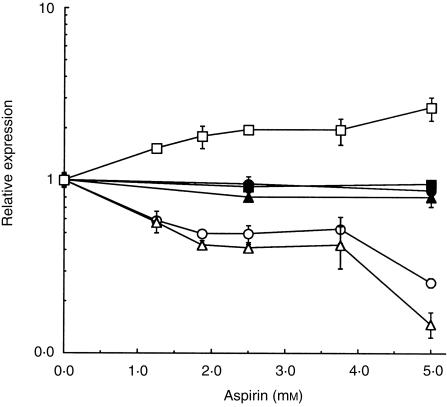
Figure 6.
Relative expression (mean values ± SD) of CD14 (□, ▪), CD80 (○, •) and CD83 (▵, ▴) as a function of aspirin concentration in dendritic cells incubated with inflammatory mediators and aspirin during (open symbols) and after (filled symbols) maturation. Squares, CD14; circles, CD80; triangles, CD83. To compare different absolute levels of mean fluorescence intensity (MFI) for different membrane markers and for cells from different donors, MFI values measured in the presence of aspirin were normalized by the corresponding MFI value determined in the absence of aspirin. The data are representative of two independent experiments.
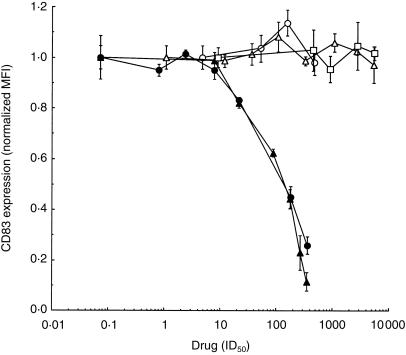
Data presented in Fig. 3 show the changes in expression of CD83 as a function of aspirin concentration; expression of this marker is low in immature dendritic cells, but high in mature dendritic cells.25 With an increasing concentration of the drug, the levels of CD83 decreased with the effective midpoint concentration of aspirin being ≈ 2·5 mm (i.e. some 180 times higher than the IC50 value for COX-2 inhibition of 14 µm).26 Thus, aspirin reduced the expression of antigen-presenting molecules, costimulatory molecules and CD83, a marker of mature dendritic cells, consistent with the notion that it prevented differentiation of immature dendritic cells in response to inflammatory signals.
Figure 3.
Effects of cyclooxygenase (COX) inhibitors on CD83 levels in dendritic cells. The cells were incubated with aspirin (•), sodium salicylate (▴), ketoprofen (□), indomethacin (○) and NS-398 (▵) for 3 days, when the levels of CD83 were measured, and are shown as mean value ± SD. For comparison of drug effectiveness in COX inhibition, the abscissa is shown in units of the respective 50% inhibitory concentration (IC50) values in blood: 26 aspirin and salicylate, 14 µm for COX-2; ketoprofen, 0·11 µm for COX-1; indomethacin, 0·21 µm for COX-1; NS-398, 0·92 for COX-2. To accommodate individual variations among cells from different individuals, the values on the ordinate were normalized by the mean fluorescence intensity (MFI) value measured in the respective dendritic cells matured in the absence of drugs (positive control). The data are representative of two (ketoprofen, indomethacin), three (NS-398) and six (aspirin, sodium salicylate) independent experiments, respectively.
The drug changed neither the cell number nor the percentage of viable cells (as measured by Trypan Blue exclusion) at concentrations up to 5·0 mm (data not shown). To resolve if aspirin induced apoptosis,14,27 we measured the percentage of cells that bound annexin-V, a ligand of phosphatidylserine that is increasingly available for binding in apoptotic cells.28 In cells treated with up to 5·0 mm aspirin, annexin binding did not change relative to untreated cells (data not shown).
Aspirin inhibits secretion of the p40 subunit of IL-12
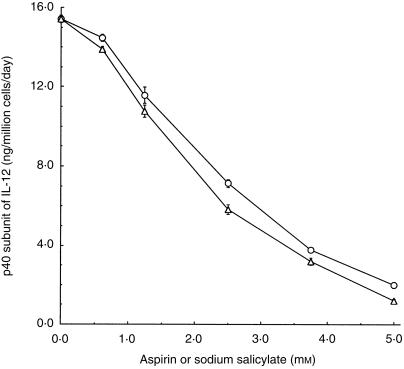
Immature dendritic cells secrete low levels of the p40 subunit of IL-12, while mature dendritic cells secrete high levels of p40.29 Consequently, we used the secreted p40 subunit as another marker of mature dendritic cell phenotype and measured it as a function of aspirin concentration. The amount of p40 secreted in the medium was inversely proportional to drug concentration; the IC50 value was ≈ 2·5 mm (Fig. 4).
Figure 4.
Average levels (mean ± SD) of the p40 unit of interleukin-12 (IL-12), secreted daily per 1 × 106 dendritic cells, as a function of concentration of aspirin (○) or sodium salicylate (▵). The corresponding level in immature dendritic cells was 0·45 ± 0·03 ng of p40. The data are representative of two independent experiments.
Non-acetylated salicylate is as effective as aspirin
Because acetylation-mediated COX inhibition is a key mechanism of aspirin action, we studied the effects of salicylate, the major non-acetylated aspirin metabolite, on dendritic cell function and differentiation. Similarly to aspirin, the presence of salicylate during maturation inhibited the ability of dendritic cells to stimulate T-cell proliferation (Fig. 1). The changes in membrane marker expression on dendritic cells at 2·5 mm salicylate are shown in Fig. 2. The respective data in Fig. 3 indicate that the concentration-dependent effects of salicylate and aspirin were similar. Like aspirin, salicylate had no effect on the cell number and viability (data not shown). Sodium salicylate reduced secretion of the p40 unit of IL-12 by dendritic cells (Fig. 4) with a dose dependence similar to that observed for the suppression of CD83 (Fig. 3).
COX inhibitors do not affect dendritic cell maturation
To determine more precisely the role of COX in dendritic cell maturation, we incubated immature dendritic cells with TNF-α, IL-β and PGE2 as described above, but in the presence of ketoprofen or indomethacin, inhibitors with a preference for COX-1 (IC50 = 0·11 µm and 0·21 µm, respectively),26 or NS-398, an inhibitor with preference for COX-2 (IC50 = 0·92 µm).26 After 3 days in the presence of the drugs at concentrations up to > 1000 times higher than their respective IC50 values, we determined the percentage of cells undergoing apoptosis by annexin-V binding and the levels of dendritic cell maturity by the levels of expressed CD83. We found no effect on annexin binding (data not shown) and on the level of CD83 (Fig. 3). Therefore, inhibition of COX had no apparent effect on dendritic cell maturation. Moreover, dendritic cells were able to mature in the absence of prostaglandins.
PGE2 does not change the effects of COX inhibitor
To ascertain if PGE2 in the maturation medium affected apoptosis or the maturation of dendritic cells, we compared the effects of the drugs on the levels of annexin binding and CD83 in cells differentiated with TNF-α and IL-1β in the absence of PGE2. (To obtain sufficient numbers of mature dendritic cells in the absence of PGE2, concentrations of TNF-α and IL-1β were increased five times above those used elsewhere in this work.) The baseline percentage of annexin-binding cells in the absence of drugs increased from 16·0 ± 0·7 in the presence of PGE2 to 29·5 ± 1·4 in its absence (P = 0·0001), but the drug-induced effects remained identical to those in Fig. 3 (data not shown). Similarly, removal of PGE2 reduced the levels of CD83 in the absence of drugs, but the response to the drugs remained unchanged (Fig. 3). Thus, exogenous PGE2 did stimulate dendritic cell maturation, but the effects of the drugs were independent of PGE2.
Salicylates suppress levels of activated NF-κB in dendritic cells
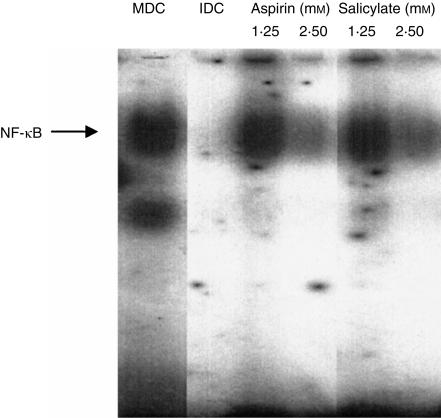
NF-κB regulates the differentiation of dendritic cells22,23,30 and is a target for inhibition by salicylates.31 Consequently, we studied whether NF-κB is inhibited by salicylates in human dendritic cells. By gel-retardation assay, we measured the amount of activated NF-κB in immature dendritic cells, mature dendritic cells and cells differentiated in the presence of salicylates, 12 hr after introduction of inflammatory mediators. Data in Fig. 5 show that the levels of activated NF-κB were undetectable in immature dendritic cells and were high in mature dendritic cells. In the cells treated with salicylates, the levels of activated NF-κB were reduced in a dose-dependent manner. It is noteworthy that, at salicylate concentrations equal to their IC50 value for maturation, activation of NF-κB was significantly inhibited. Again, the effects of aspirin and salicylate were indistinguishable.
Figure 5.
Levels of activated nuclear transcription factor κB (NF-κB) determined by gel-retardation assay in cells treated with salicylates. The lanes from the same gel denote untreated immature dendritic cells (IDC), untreated mature dendritic cells (MDC) and cells treated with aspirin or sodium salicylate. Drug concentrations are as indicated on the figure. The data are representative of three independent experiments.
Mature dendritic cells are less sensitive to salicylates
To determine if mature dendritic cells were affected by salicylates, we differentiated the cells for 2 days. By that time (day 9) at least 80% of the cells were mature, as assessed by CD83 expression (data not shown). Aspirin was then added and the cells were incubated for a further 2 days (i.e. until day 11) when the expression of CD14, CD80 and CD83 was evaluated. Levels of membrane markers remained unchanged in mature dendritic cells treated with aspirin (Fig. 6). However, immature dendritic cells incubated similarly with either aspirin or salicylate were strongly affected. The effects of salicylate on mature dendritic cells were indistinguishable from the effects of aspirin (data not shown).
Discussion
The current models of pathogenesis of autoimmunity indicate that inappropriate dendritic cell function contributes to the positive feedback of inflammation, tissue destruction and representation of tissue-specific self-antigens associated with autoimmunity.10,11 Because high-dose salicylates have been used to control some autoimmune diseases, we investigated the effects of salicylates on the function of human myeloid dendritic cells differentiated by inflammatory mediators. We used primary human monocytes as a convenient in vitro model of inflammation-driven differentiation of myeloid dendritic cells and found that aspirin and other salicylates strongly reduced the ability of dendritic cells to stimulate proliferation of T cells. This finding was accompanied by the observation of dose-sensitive suppression of the levels of antigen-presenting molecules (HLA class I, HLA-DR), costimulatory molecules (CD80, CD86) and CD83, a marker of mature dendritic cells. Because high levels of these molecules on dendritic cells are positively correlated with T-cell stimulation (reviewed in ref. 32), their salicylate-reduced levels are responsible for the suppressed induction of T-cell proliferation.
In our model system, dendritic cell differentiation was induced by inflammatory mediators TNF-α, IL-1β and PGE2. This system is comparable to the one described in ref. 20 except that we omitted IL-6: in preliminary experiments we found that IL-6 had no effect on dendritic cell maturation (data not shown). These conditions strongly favour differentiation and result in differentiation of a high percentage of cells (> 85%) in 3 days. Even under conditions strongly favouring differentiation, aspirin and other salicylates inhibited it. The levels of membrane molecules specific for mature dendritic cells were reduced by salicylates. However, levels of CD14, a molecule not characteristic of mature dendritic cells, were concomitantly up-regulated above the levels found in immature dendritic cells. Fully differentiated cells were not susceptible to inhibition by salicylates, demonstrating that it is the process of differentiation, rather than the function of mature dendritic cells, which is the target of salicylates.
The IC50 value for aspirin and sodium salicylate (≈ 2·5 mm) is similar to the therapeutic concentration of salicylate (1·8 mm)15 in the blood of patients undergoing high-dose aspirin therapy. Thus, pharmacologically relevant levels of salicylates significantly impact differentiation of dendritic cells, even under conditions optimized for differentiation of dendritic cells in vitro. Because such conditions are not likely to be surpassed in tissues, it is possible that the inhibition of dendritic cell maturation by chronically high circulating levels of salicylates is similarly or more effective in vivo.
Aspirin and the non-acetylated salicylate inhibited dendritic cell differentiation at concentrations more than 150 times higher than their IC50 values for COX-2 inhibition (14 µm),26 both in the presence and absence of PGE2. Furthermore, the same IC50 value for inhibition of dendritic cell maturation was obtained for both drugs, indicating that acetylation of COX by aspirin does not contribute to the observed effects. Consequently, the effects of salicylate on dendritic cells must be unrelated to COX inhibition. This conclusion is fully supported by the absence of any effect of ketoprofen, indomethacin and NS-398 at concentrations ranging over four orders of magnitude of their IC50 values.
At concentrations similar to those used in this study, aspirin exercises its effects primarily through inhibition of NF-κB,31,33 although other intracellular signalling pathways are also inhibited.34 NF-κB encompasses a family of five heterodimeric and homodimeric proteins35,36 involved in the transcriptional control of proteins participating in immunity and inflammation.35–37 In most cells, activated NF-κB protects from apoptosis.38–40 Inhibition of NF-κB favours apoptosis in normal and malignant B cells14,41 and T cells.14 In some white blood cells, however, inhibition of NF-κB does not affect cell viability but interferes with expression of inflammatory mediators42 or adhesion molecules.43 We found that pharmacologically active concentrations of salicylates markedly reduced the levels of activated NF-κB in dendritic cells without triggering apoptosis. Similarly, in a murine dendritic cell line, selective inhibition of NF-κB prevented differentiation without triggering apoptosis.22
Activated NF-κB is critical for differentiation of dendritic cells.22,23 Our data show that salicylates inhibit NF-κB function in dendritic cells at pharmacologically relevant concentrations and that they inhibit dendritic cell differentiation with the consequent loss of immunostimulatory function. One potential consequence is that such salicylate effects could reduce the supply of functional mature dendritic cells necessary for maintenance of chronic inflammation in vivo and, thus, break the vicious circle of autoimmunity.
Acknowledgments
This work was supported by Mrs Adelyn L. Luther, Singer Island, Florida, and the Mayo Clinic Cancer Center. A. B. D. is a scholar of the Glen and Florence Voyles Foundation, Terre Haute, Indiana. R. M. is the recipient of a postdoctoral fellowship from the Melvin S. Cohen Foundation, Inc., Eau Claire, Wisconsin. We thank Mrs Peggy A. Bulur and Mr Gaylord J. Knutson for expert assistance, and Dr Franklyn G. Prendergast for continuing interest and support.
Glossary
Abbreviations
- COX
cyclooxygenase
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- ID50
concentration at mid-point inhibition
- IL
interleukin
- NF-κB
nuclear transcription factor κB
- PBMC
peripheral blood mononuclear cells
- PGE2
prostaglandin E2
- TNF-α
tumour necrosis factor-α
- IC50
concentration at 50% inhibition
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Adema GJ, Hartgers F, Verstraten R, et al. A dendritic-cell-derived C-C chemokine that preferentially attracts naive T cells. Nature. 1997;387:713. doi: 10.1038/42716. [DOI] [PubMed] [Google Scholar]
- 3.Sallusto F, Lanzavecchia A, Mackay CR. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol Today. 1998;19:568. doi: 10.1016/s0167-5699(98)01346-2. 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- 4.Sallusto F, Lanzavecchia A. Mobilizing dendritic cells for tolerance, priming, and chronic inflammation. J Exp Med. 1999;189:611. doi: 10.1084/jem.189.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarsfield P, Jones DB, Wright DH. Accessory cells in Crohn's disease of the terminal ileum. Histopathology. 1996;28:213. doi: 10.1046/j.1365-2559.1996.d01-416.x. [DOI] [PubMed] [Google Scholar]
- 6.Thomas R, Quinn C. Functional differentiation of dendritic cells in rheumatoid arthritis: role of CD86 in the synovium. J Immunol. 1996;156:3074. [PubMed] [Google Scholar]
- 7.Molne J, Jansson S, Ericson LE, Nilsson M. Adherence of RFD-1 positive dendritic cells to the basal surface of thyroid follicular cells in Graves' disease. Autoimmunity. 1994;17:59. doi: 10.3109/08916939409014659. [DOI] [PubMed] [Google Scholar]
- 8.Voorby HA, Kabel PJ, de Haan M, et al. Dendritic cells and class II MHC expression on thyrocytes during the autoimmune thyroid disease of the BB rat. Clin Immunol Immunopathol. 1990;55:9. doi: 10.1016/0090-1229(90)90065-x. [DOI] [PubMed] [Google Scholar]
- 9.Lo D, Reilly CR, Scott B, Liblau R, McDewitt HO, Burkly LC. Antigen-presenting cells in adoptively transferred and spontaneous autoimmune diabetes. Eur J Immunol. 1993;23:1693. doi: 10.1002/eji.1830230744. [DOI] [PubMed] [Google Scholar]
- 10.Green EA, Eynon EE, Flavell RA. Local expression of TNF-α in neonatal NOD mice promotes diabetes by enhancing presentation of islet antigens. Immunity. 1998;9:733. doi: 10.1016/s1074-7613(00)80670-6. [DOI] [PubMed] [Google Scholar]
- 11.Ludewig B, Odermatt B, Landmann S, Hengartner H, Zinkernagel RM. Dendritic cells induce autoimmune diabetes and maintain disease via de novo formation of local lymphoid tissue. J Exp Med. 1998;188:1493. doi: 10.1084/jem.188.8.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'brien WM. Pharmacology of nonsteroidal anti-inflammatory drugs. Practical review for clinicians. Am J Med. 1983;75:32. doi: 10.1016/0002-9343(83)90326-1. [DOI] [PubMed] [Google Scholar]
- 13.Crout JE, Hepburn B, Ritts RE., Jr Suppression of lymphocyte transformation after aspirin ingestion. N Engl J Med. 1975;292:221. doi: 10.1056/NEJM197501302920501. [DOI] [PubMed] [Google Scholar]
- 14.Bellosillo B, Pique M, Barragan M, et al. Aspirin and salicylate induce apoptosis and activation of caspases in B-cell chronic lymphocytic leukemia cells. Blood. 1998;92:1406. [PubMed] [Google Scholar]
- 15.United States Pharmacopeial Convention. United States Pharmacopeia/National Formulary, Drug Information for the Health Professional. I. Englewood, CO: Micromedex Inc.; 2000. Salicylates (systemic) p. 2734. [Google Scholar]
- 16.Abramson SB, Weissmann G. The mechanisms of action of nonsteroidal antiinflammatory drugs. Arthritis Rheum. 1989;32:1. doi: 10.1002/anr.1780320102. [DOI] [PubMed] [Google Scholar]
- 17.Hausser G, Burkhard L, Gelderblom HR, Tsunetsugu-Yokota Y, Akagawa K, Meyerhans A. Monocyte-derived dendritic cells represent a transient stage of differentiation in the myeloid lineage. Immunobiology. 1997;197:534. doi: 10.1016/S0171-2985(97)80085-X. [DOI] [PubMed] [Google Scholar]
- 18.Palucka KA, Taquet N, Sanchez-Chapuis F, Gluckman JC. Dendritic cells as the terminal stage of monocyte differentiation. J Immunol. 1998;160:4587. [PubMed] [Google Scholar]
- 19.Reddy A, Sapp M, Feldman M, Subklewe M, Bhardwaj N. A monocyte conditioned medium is more effective than defined cytokines in mediating the terminal maturation of human dendritic cells. Blood. 1997;9:3649. [PubMed] [Google Scholar]
- 20.Jonuleit H, Kuhn U, Muller G, et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 21.Kolb HJ, Schattenberg A, Goldman JM, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86:2041. [PubMed] [Google Scholar]
- 22.Rescigno M, Martino M, Sutherland CL, Gold MR, Ricciardi-Castagnoli P. Dendritic cell survival and maturation are regulated by different signaling pathways. J Exp Med. 1998;188:2175. doi: 10.1084/jem.188.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oyama T, Ran S, Ishida T, et al. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-κB activation in hemopoietic progenitor cells. J Immunol. 1998;160:1224. [PubMed] [Google Scholar]
- 24.Pahl HL, Bauerle PA. Activation of NF-κB by ER stress requires both Ca2+ and reactive oxygen intermediates as messengers. FEBS Lett. 1996;392:129. doi: 10.1016/0014-5793(96)00800-9. [DOI] [PubMed] [Google Scholar]
- 25.Kishimoto T, Kikutani H, von dem Borne AEGK, et al. White cell differentiation antigens. In: Kishimoto T, Kikutani H, editors. Leukocyte Typing. VI. New York: Garland Publishing; 1997. pp. 927–1221. [Google Scholar]
- 26.Cryer B, Feldman M. Cyclooxygenase-1 and cyclooxygenase-2 selectivity of widely used nonsteroidal anti-inflammatory drugs. Am J Med. 1998;104:413. doi: 10.1016/s0002-9343(98)00091-6. [DOI] [PubMed] [Google Scholar]
- 27.Schwenger P, Bellosta P, Vietor I, Basilico C, Skolnik E, Vilcek J. Sodium salicylate induces apoptosis via p38 mitogen-activated protein kinase but inhibits tumor necrosis factor-induced C-Jun N-terminal kinase/stress-activated protein kinase activation. Proc Natl Acad Sci USA. 1997;94:2869. doi: 10.1073/pnas.94.7.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verhoven B, Schlegel RA, Williamson P. Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J Exp Med. 1995;182:1597. doi: 10.1084/jem.182.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rieseries C, Bock G, Klocker H, Bartsch G, Thurnher M. Prostaglandin E2 and tumor necrosis factor α cooperate to activate human dendritic cells: synergistic activation of interleukin 12 production. J Exp Med. 1997;186:1603. doi: 10.1084/jem.186.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burkly L, Hession C, Ogata L, et al. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature. 1995;373:531. doi: 10.1038/373531a0. [DOI] [PubMed] [Google Scholar]
- 31.Kopp E, Ghosh S. Inhibition of NF-κB by sodium salicylate and aspirin. Science. 1994;265:956. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 32.Ni K, O'neill HC. The role of dendritic cells in T cell activation. Immunol Cell Biol. 1997;75:223. doi: 10.1038/icb.1997.35. [DOI] [PubMed] [Google Scholar]
- 33.Yin M, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of IκB kinase-β. Nature. 1998;396:77. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 34.Dong Z, Huang C, Brown RE, Ma W-Y. Inhibition of activator protein 1 activity and neoplastic transformation by aspirin. J Biol Chem. 1997;272:9962. doi: 10.1074/jbc.272.15.9962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grilli M, Chiu JS, Lenardo MJ. NF-κB and rel: participants in a multiform transcriptional regulatory system. Int Rev Cytol. 1993;143:1. doi: 10.1016/s0074-7696(08)61873-2. [DOI] [PubMed] [Google Scholar]
- 36.Baldwin AS. The NF-κB and IκB proteins: new discoveries and insights. Science. 1995;274:782. [Google Scholar]
- 37.Bauerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 38.Beg AA, Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996;274:782. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 39.Wallach D. Cell death induction by TNF: a matter of self-control. Trends Biochem Sci. 1997;22:107. doi: 10.1016/s0968-0004(97)01015-3. [DOI] [PubMed] [Google Scholar]
- 40.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-α-induced apoptosis by NF-κB. Science. 1996;274:787. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 41.Wu M, Lee H, Bellas RE, et al. Inhibition of NF-κB/Rel induces apoptosis of murine B cells. EMBO J. 1996;15:4682. [PMC free article] [PubMed] [Google Scholar]
- 42.Shackelford RE, Alford PB, Xue Y, Thai SF, Adams DO, Pizzo S. Aspirin inhibits tumor necrosis factor α gene expression in murine tissue macrophages. Mol Pharmacol. 1997;52:421. doi: 10.1124/mol.52.3.421. [DOI] [PubMed] [Google Scholar]
- 43.Pillinger MH, Capodici C, Rosenthal P, Kheterpal N, Hanft S, Philips MR. Modes of action of aspirin-like drugs: salicylates inhibit ERK activation and integrin-dependent neutrophil adhesion. Proc Natl Acad Sci USA. 1998;95:14540. doi: 10.1073/pnas.95.24.14540. [DOI] [PMC free article] [PubMed] [Google Scholar]