Abstract
Human monocyte-derived dendritic cells (MoDCs) obtained from peripheral blood monocytes (PBMC) cultured with granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) can be activated in vitro by a variety of simple chemicals such as haptens and several metals. Recently, it has been demonstrated that transforming growth factor-β1 (TGF-β1) can induce further differentiation of MoDCs to the cells that share some characteristics with epidermal Langerhans cells, i.e. they contain Birbeck granules and express E-cadherin. In this study, using such TGF-β1-treated dendritic cells (TGF-β1+ DCs), we examined the in vitro effects of representative haptens, i.e. NiCl2 and dinitrochlorobenzene (DNCB), on their phenotypic and functional characteristics, comparing with those reported in vivo in epidermal Langerhans cells during the sensitization phase of a contact sensitivity reaction. Treatment of TGF-β1+ DCs with NiCl2 increased their expression of the molecules related to antigen presentation such as CD86, major histocompatibility complex class I and class II, and CD83, although weakly, in addition to that of those essential for their migration to the regional lymph nodes, such as CD49e, CD44 and its variant 6, while it down-regulated the expression of the molecules required for homing to the skin and staying in the epidermis, such as cutaneous leucocyte antigen (CLA) and E-cadherin. It also increased the production of tumour necrosis factor-α, but not that of IL-1β or IL-12. DNCB also increased their CD86 expression and down-regulated E-cadherin and CLA, but did not affect other phenotypic changes that were observed in TGF-β1+ DCs treated with NiCl2. TGF-β1+ DCs treated with either NiCl2 or DNCB increased their allogeneic T-cell stimulatory function. In addition, reverse transcribed polymerase chain reaction revealed augmented expression of chemokine receptor 7 mRNA by TGF-β1+ DCs when treated with either NiCl2 or DNCB. Moreover, consistent with this data, TGF-β1+ DCs treated with these chemicals chemotactically responded to macrophage inflammatory protein-3β. These data suggest the possibility that TGF-β1+ DCs present a good in vitro model to study the biology of epidermal Langerhans cells.
Introduction
We found that murine Langerhans cells (LCs) up-regulate their expression of class II major histocompatibility complex (MHC) antigen and antigen-presenting function after hapten painting on the skin, whereas the chemicals that simply irritate the skin rather than sensitize animals cannot induce this phenomenon.1 Later, we demonstrated that the application of haptens to murine skin was accompanied by the up-regulation of several co-stimulatory molecules on LCs, i.e. CD40, CD54, CD80 and CD86.2 Thus, haptens can induce the activation of LCs in vivo. In addition, Enk & Katz3 indicated a crucial role played by interleukin-1β (IL-1β) secreted by LCs themselves in their increased expression of class II MHC antigen after hapten application.
Recently, using monocyte-derived dendritic cells (MoDCs), which were obtained from the culture of purified monocytes in the presence of granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-4, we examined similar effects of simple chemicals on their activation.4 The obtained results clearly demonstrated that purified DCs responded to such chemicals as NiCl2 and dinitrochlorobenzene (DNCB) in vitro by significantly augmenting their expression of CD54, CD86 and human leucocyte antigen (HLA)-DR and by increasing their production of pro-inflammatory cytokines. Furthermore, the augmented expression of CD86 on DCs treated with DNCB was suppressed by either anti-IL-1β or anti-tumour necrosis factor-α (TNF-α) antibody, while that induced with NiCl2 was relatively insensitive to these antibody treatments, suggesting that different chemicals use different signal transduction pathway to stimulate DCs.
Geissmann et al.5 reported that transforming growth factor-β1 (TGF-β1), could induce LCs from peripheral blood monocytes (PBMC) in vitro in the presence of GM-CSF and IL-4. Recently, however, Jaksits et al.6 have demonstrated several phenotypic changes between epidermal LCs and DCs derived from the culture of monocytes in the presence of GM-CSF, IL-4 and TGF-β1. Namely, although both share CD1a, E-cadherin and Birbeck granules, MoDCs with or without TGF-β1 express CD11b and factor XIIIa.
The first purpose of this study was to examine whether these TGF-β1-treated DCs (TGF-β1+ DCs) also respond to the simple chemicals like MoDCs do, since there were several papers demonstrating that TGF-β1 affects the intracellular signal transduction pathway in various cells, such as mitogen-activated protein kinase (MAP) kinases.7,8 If TGF-β1+ DCs can respond to simple chemicals, the next purpose was to reveal whether haptens can induce phenotypic and functional changes in these TGF-β1+ DCs that have been reported to occur in epidermal LCs during the initiation phase of the contact hypersensitivity reaction in vivo, such as the down-regulation of E-cadherin9 and cutaneous leucocyte antigen (CLA),8 the induction of matrix metalloproteinase-9 (MMP-9) expression,10 and the augmentation of some of β1-integrins,11,12 CD44 and some of its variants,13 as well as the expression of chemokine receptor 7 (CCR7) mRNA that is involved in their chemotactic response to macrophage inflammatory protein-3β (MIP-3β).14–16 In addition, since Enk & Katz3 have suggested that the maturation of epidermal LCs stimulated by cutaneous application of haptens is triggered by IL-1β secreted by LCs themselves, we examined whether the maturation of TGF-β1+ DCs induced by haptens is also mediated by IL-1β.
Materials and methods
Media and reagents
The medium used in the study was RPMI-1640 including 25 mm HEPES buffer (Sigma Chemical Co., St. Louis, MO) supplemented with 2 mm l-glutamine, 1 mm sodium pyruvate, 1% penicillin, streptomycin and fungizone antibiotic solution (Sigma) and 10% fetal calf serum (FCS; Bioserum, Canterbury, Victoria, Australia) (complete medium). The buffer used for purification of CD14+ monocytes from (PBMC) was phosphate-buffered saline (PBS) supplemented with 1% bovine serum albumin (less than 1 ng/mg of detectable endotoxin) (Sigma) and 5 mm ethylenediaminetetraacetic acid (EDTA; MACS buffer). NiCl2 (Sigma), and 2,4-dinitrochlorobenzene (DNCB) (Wako Pure Chemicals, Osaka, Japan) were used for the stimulation of DCs. The endotoxin content of the final dilution used was < 30 pg/ml, as determined by the Limulus amoebocyte lysate assay (Seikagaku Co Inc. Tokyo, Japan). We used the following monoclonal antibodies (mAbs) for immunostaining: fluorescein isothiocyanate (FITC)-anti-CLA, anti-CD40, anti-CD80, anti-CD86 antibodies, phycoerythrin (PE)-conjugated-anti-CD29, FITC or PE-conjugated isotype-matched mouse control antibodies [immunoglobulin G2a (IgG2a) and G2b; PharMingen, San Diego, CA], FITC- or PE-conjugated anti-HLA-DR antibody (Becton-Dickinson, San Jose, CA), FITC-conjugated anti-CD49e and -CD49f (Serotec Ltd, Oxford, UK), FITC-conjugated anti-CD49d, PE-conjugated anti-CD83 antibody (Immunotech, Marseilles, France), FITC-conjugated anti-CD54 antibody (Ancell, Bayport, MN), FITC-conjugated anti-CD44 antibody (Caltag Laboratories, Burlingame, CA), FITC-conjugated anti-HLA I-ABC antibody (Biosource, Camarillo, CA), PE-conjugated anti-CD1a antibody (Coulter, Hialeah, FL), monoclonal anti-human E-cadherin antibody (Takara Biomedicals, Tokyo, Japan), Lag (gift of Dr F. Furukawa, Hamamatsu University, Sizuoka, Japan) and isotype control antibody (IgG1) (Sigma). For examining the effects of cytokines on surface molecule expression by TGF-β1+ DCs, we used mouse anti-IL-1β mAb, anti-TNF-α antibody (Genzyme Corporation, Cambridge, MA), and isotype matched control antibodies (PharMingen). Magnetic activated cell sorter (MACS) colloidal supermagnetic microbeads conjugated with anti-human CD14 mAb (CD14 microbeads) were purchased from Miltenyi Biotec Inc., Sunnyvale, CA. Recombinant human (rh) GM-CSF was a gift from Kirin Brewery Co., Tokyo, Japan, and rhIL-4 and rhTGF-β1 were purchased from Genzyme Corporation, Cambridge, MA and R & D Systems, Minneapolis, MN. NiCl2 was solubilized in distilled water, while DNCB was solubilized in dimethyl sulfphoxide (DMSO) at a concentration of 1 m. The final concentration of DMSO was always less than 0·1% and cultures of DCs with 0·1% DMSO were also examined as a control.
Culture of DCs from PBMC
PBMC were isolated from heparinized fresh leucocyte-enriched buffy coats from different donors using Lymphoprep (Nycomed Pharma As, Oslo, Norway). After several washes with PBS, 1 × 108 PBMC were treated with 150 µl of CD14 microbeads in 600 µl of MACS buffer at 4° for 30 min. After washing with MACS buffer, the cells coated with CD14 microbeads were separated by a magnetic cell separator, MACS (Miltenyi Biotech), according to the manufacturer's protocol. Before culturing we examined the percentage of CD14+ cells in these preparations by flow cytometry and used cell specimens containing more than 98% of CD14+ cells in the experiments.
CD14+ monocytes (2 × 106/ml) were cultured in complete medium containing 100 ng/ml rhGM-CSF and 100 ng/ml rhIL-4 with or without 100 ng/ml TGF-β1 for 6 days. On the 5th day the cells were subcultured to expand the volume twice with the same combination of cytokines.
Chemical treatment of cultured TGF-β1+ DCs
Six days after the culture with GM-CSF and IL-4 with or without TGF-β1, the cultured cells were treated with various concentrations of NiCl2, or DNCB for 48 hr. In some experiments, in addition to the chemicals, neutralizing antibody for IL-1β or TNF-α at each optimal concentration or control isotype-matched antibody was added to the culture.
Flow cytometry and immunocytological analysis
Forty-eight hours after treatment with the chemicals, the phenotypic changes of TGF-β1+ DCs were analysed by flow cytometry. Cell staining was performed using a combination of FITC-conjugated antibody (25 µg/ml) and PE-conjugated antibody (25 µg/ml). For E-cadherin expression on TGF-β1+ DCs, the TGF-β1+ DCs were first incubated with anti-E-cadherin antibody and revealed by PE-conjugated anti-mouse immunoglobulins (Tago Immunologicals, Camarillo, CA). After washing with PBS supplemented with 1% BSA and 0·02% NaN3 (FACS buffer), the cells were analysed by FACScan using cellquest software (Becton Dickinson). Dead cells were gated out after staining with 0·5 µg/ml propidium iodide solution.
Immunohistochemical analysis of LAG expression was performed on cytospin slides for MoDCs and TGF-β1+ DCs. The slides were at first fixed and permeabilized by Fix & Perm Cell Permeabilization Kit (Caltag Laboratories, An Der Grub, Austria). After blocking with goat serum, the slides were incubated with Langerhans cell-associated granule (LAG) or non-reactive mouse IgG1 antibody, MOPC 21 and then stained with FITC-conjugated anti-mouse immunoglobulins (Tago Immunologicals, Camarillo, CA). The slides were mounted in permafluor (Lipshaw Immunon, Pittsburgh, PA) containing 0·5 µg/ml propidium iodide, and were observed under a confocal microscope (Leica microscope and system GmbH, Wetzlar, Germany).
Enzyme-linked immunosorbent assay (ELISA) for cytokine production
The culture supernatants of the TGF-β1+ DCs treated with various chemicals were recovered 24 and 48 hr after culture. The production of IL-1β, TNF-α, IL-10, IL-12p70 and MMP-9 was measured by ELISA kit obtained from R & D Systems for IL-1β, Genzyme for TNF-α and IL-10, Endogen Inc., Soburn, MA for IL-12p70 and Amersham Life Science, Buckinghamshire, UK for MMP-9, respectively, using 96-well microtitre plates, according to each manufacturer's instructions. The levels of IL-1β, TNF-α, IL-10, IL-12p70, or MMP-9 were calculated by using a standard curve obtained with recombinant IL-1β (from 0 to 125 pg/ml), recombinant TNF-α (from 0·5 to 32·0 pg/ml), recombinant IL-10 (from 0 to 256 pg/ml), recombinant IL-12p70 (from 0 to 600 pg/ml), and recombinant MMP-9 (from 0 to 64 ng/ml).
Primary allogeneic T-cell stimulation by TGF-β1+ DCs
PBMC were obtained by Ficoll–Paque (Pharmacia) gradient centrifugation of heparinized blood. These PBMC were treated with a pan-T-cell isolation kit (Miltenyi Biotec), according to the manufacturer's protocol. These enriched T cells consisted of more than 95% CD3+ cells. T cells (2 × 105 cells/well) were co-cultured in 96-well flat-bottom microtitre plates with various numbers of LCs, which were precultured with studied chemicals for 2 days and washed. After 4 days of culture at 37° in a 5% CO2 humidified atmosphere, the cells were pulsed with 10 µm bromodeoxyuridine (BrdU) (Boehringer Mannheim GmbH, Germany) during the last 16 hr of culture. The immunoenzymatic measurement of BrdU uptake by T cells was performed by 5-bromo-2′-deoxy-uridine labelling and detection kit III (Boehringer Mannheim). The intensity of the colour reaction was read in an automatic ELISA plate reader (Titertek Multiskan MC) using a 492-nm filter. The mean values ± SE from three replicates were taken from each sample culture. To decrease the background proliferation of LCs, LCs were irradiated with 3000 rads with a Softex (Softex Co. Ltd, Tokyo, Japan).
Reverse transcription–polymerase chain reaction (RT-PCR)
Total RNA was extracted from TGF-β1+ DCs using the guanidinium thiocyanate method mentioned by the manufacturer (ISOGEN; Nippon gene Inc., Toyama, Japan), and RNA was quantified by spectrophotometry. First-strand cDNA was synthesized from total RNA extracted in RNAse-free conditions. The reaction was performed with 1 µg of total RNA using TaKaRa RNA PCR kit (AMV) (Takara Biochemicals, Osaka, Japan), as described by the manufacturer. PCR was performed in a Perkin Elmer 2400 thermal cycler, in a final volume of 100 µl reaction mixture containing 1 µm of each forward and reverse primer, using TaKaRa RNA PCR kit, according to the manufacturer's protocol. CCR7 primers, which were described by Dieu et al.,14 were designed. The + 154/CCR7 5′-GATTACATCGGAGACAACACC-3′ forward primer and −1202/CCR7 5′-TAGTCCAGGCAGAAGAGTCG-3′ reverse primer, and the primer set for human GAPDH (Maxim Biotech, Inc., San Francisco, CA) were used for RT-PCR. The reaction mixture was subjected to 30 cycles of PCR with the following conditions: 94° for 1 min, 55° for 1 min and 72° for 1 min. PCR products were visualized on 1·2% agarose gels containing 0·1 µg/ml ethidium bromide.
Chemotactic assay
Cell migration was evaluated using a chemotaxis microchamber technique (48-well Boyden microchamber; Neuroprobe, Pleasanton, CA). In brief, MIP-3β was diluted to concentrations ranging from 1 to 1000 ng/ml in RPMI-1640 medium, and was added to the lower wells of the chemotaxis chamber. Then, 5 × 104 cells/well in 50 µl of RPMI-1640 medium were applied to the upper wells of the chamber, with a standard 5-µm pore polyvinylpyrrolidone-free polycarbonate filter (Neuroprobe) separating the lower wells. The chamber was incubated at 37° in humidified air with 5% CO2 for 90 min. Then, cells that had migrated to the underside of the filter were stained with May–Gruenwald's solution and Giemsa's solution and were counted microscopically at × 200 magnification in five randomly selected fields per well. Each assay was performed in duplicate and the results were expressed as the mean ± SD of migrating cells per field.
Statistical analysis
The statistical significance of the effects of simple chemicals on allogeneic T-cell stimulatory function was analysed by using the Mann–Whitney test between non-treated TGF-β1+ DCs and TGF-β1+ DCs treated with simple chemicals.
Results
TGF-β1+ DCs expressing E-cadherin and CLA can be induced from peripheral blood CD14+ cells in the presence of GM-CSF, IL-4 and TGF-β1
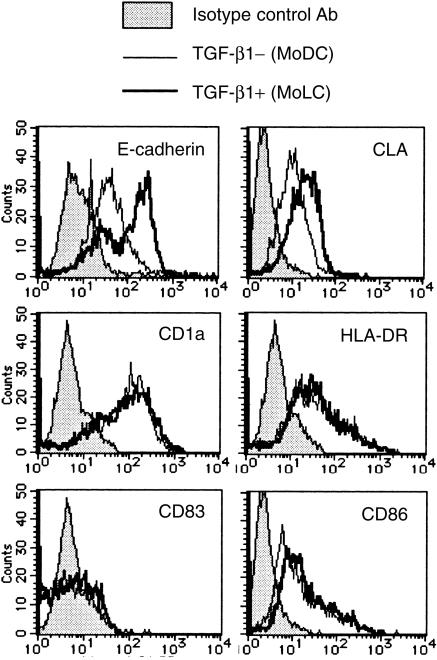
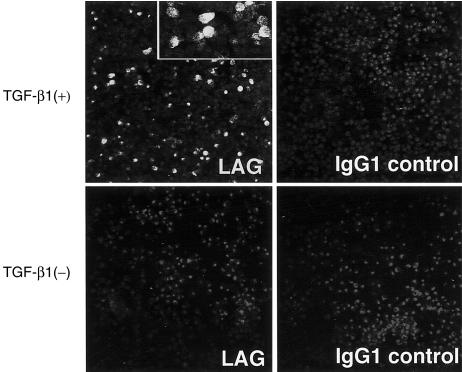
According to the procedure reported by Geissmann et al.,5 we induced peripheral blood CD14+ monocytes to differentiate into TGF-β1+ DCs, which were characterized by the expression of CD1a, HLA-DR antigen, CD86, E-cadherin and CLA (Fig. 1). The expression of Lag antigen, a Birbeck granule-related molecule, was observed in 10–20% of TGF-β1+ DCs, while no MoDCs expressed Lag antigen (Fig. 2). These TGF-β1+ DCs significantly differ phenotypically from MoDCs by the expression of E-cadherin and CLA. They did not express the maturation marker of DCs, CD83.
Figure 1.
Monocyte-derived DCs treated with TGF-β1 induce E-cadherin and CLA. Peripheral blood CD14+ monocytes were cultured with GM-CSF and IL-4 (MoDCs) or with GM-CSF, IL-4 and TGF-β1 (TGF-β1+ DCs) for 6 days. The surface expression of several phenotypic markers for DCs or LCs were examined by flow cytometry. These are representative data from five different experiments that reproduced a similar staining pattern.
Figure 2.
Monocyte-derived DCs treated with TGF-β1 induce LAG expression. The cytospin slides were at first fixed and permeabilized. After blocking with goat serum, the slides were incubated with LAG or non-reactive mouse IgG1 antibody, MOPC 21, and then stained with FITC-conjugated anti-mouse immunoglobulins. The slides were mounted in permafluor containing 0·5 µg/ml propidium iodide, and observed under a confocal microscope. Original magnification: × 200. Inset: original magnification: × 400.
Haptens can induce phenotypic changes in TGF-β1+ DCs in vitro
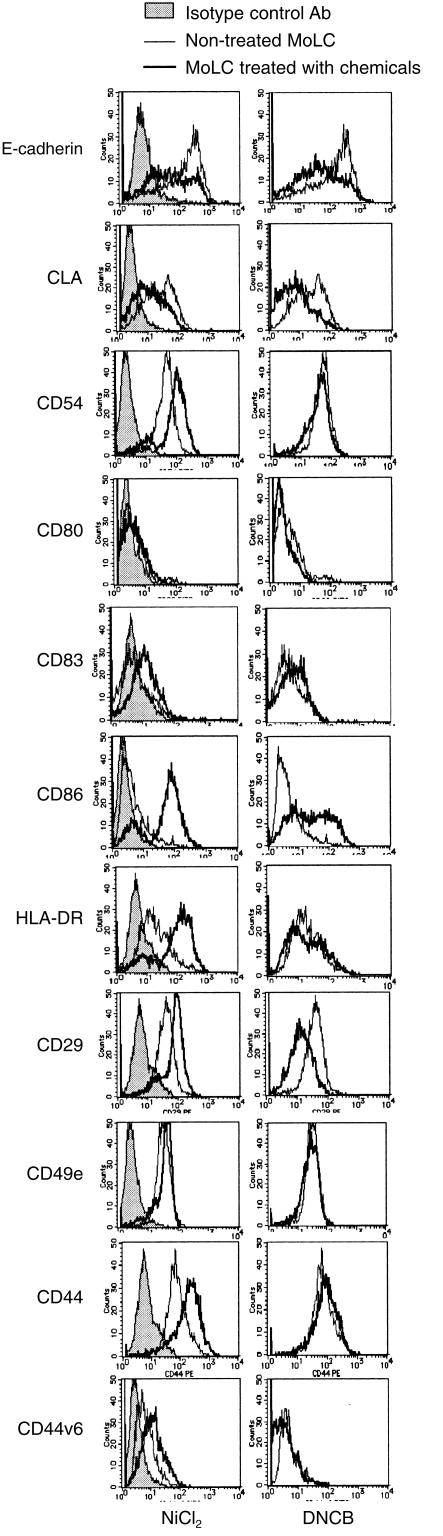
We reported that NiCl2, the representative hapten, increased the expression of CD54, CD86 and HLA-DR antigen on MoDCs, while DNCB augmented only the expression of CD86.4 Therefore, at first, we examined whether these chemicals could induce similar phenotypic changes on TGF-β1+ DCs. In the following experiments, we adjusted concentrations of these chemicals at a three-fold dilution from their respective lethal doses (more than 1000 µm of NiCl2 and more than 100 µm of DNCB were lethal for TGF-β1+ DCs), and demonstrated only the data obtained with the concentrations that induced a maximum effect. Figure 3, which is one of the representative data from five different experiments, clearly demonstrates that 300 µm of NiCl2 and 30 µm of DNCB induced phenotypic changes in TGF-β1+ DCs similar to those noted in MoDCs. In addition, they increased CD83 expression on TGF-β1+ DCs weakly. Various in vivo studies on epidermal LCs have demonstrated that LCs showed several phenotypic changes, such as the down-regulation of E-cadherin,9 and CLA,8 and the augmentation of some β1-integrins,11,12 CD44 and some of its variants,13 in addition to the augmentation of CD54, CD86 and HLA-DR antigen in the initiation phase of the allergic contact hypersensitivity reaction. Thus, we next examined whether these phenotypic changes would also occur in TGF-β1+ DCs treated with the haptens. As for E-cadherin and CLA expression, TGF-β1+ DCs stimulated with NiCl2 or DNCB down-regulated their expression of these two molecules. When we examined the expression of CD49e, -f and -g and CD29, NiCl2 increased the expression of CD49f and CD29 integrins, while DNCB rather down-regulated CD29 expression. Furthermore, we found that again only TGF-β1+ DCs treated with NiCl2 increased the expression of pan CD44 epitope and its variant exon V6.
Figure 3.
The treatment with NiCl2 and DNCB induced several phenotypic changes on TGF-β1+ DCs. TGF-β1+ DCs differentiated from peripheral blood CD14+ monocytes by GM-CSF, IL-4 and TGF-β1 were treated with 300 µm of NiCl2 or 30 µm of DNCB for 48 hr. These hapten-treated TGF-β1+ DCs were analysed by flow cytometry for their expression of the molecules related to the function of LCs. These are representative data from five different experiments that showed similar results.
TGF-β1+ DCs treated with NiCl2 increases the secretion of TNF-α, while MMP-9 is constitutively secreted by TGF-β1+ DCs irrespective of stimulation
We examined the production of several cytokines and MMP-9. TGF-β1+ DCs could not secrete IL-1β or IL-12 p70 with any kinds of stimulation (Table 1). Only TNF-α and IL-10 were detected in the culture supernatants of TGF-β1+ DCs stimulated with NiCl2. In contrast, TGF-β1+ DCs constitutively secreted MMP-9, but this was not augmented by any stimulation.
Table 1.
Production of cytokines and MMP-9 by MoDCs (ng/ml)
| Treatment | IL-1β | TNF-α | IL-10 | IL-12 | MMP-9 |
|---|---|---|---|---|---|
| Exp. 1 | |||||
| Non-treated | 0·0 | 0·0 | 0·0 | 0·0 | 22·4 |
| 300 μm Ni | 0·0 | 18·1 | 0·0 | 0·0 | 21·1 |
| 30 μm DNCB | 0·0 | 0·0 | 0·0 | 0·0 | 20·2 |
| 100 μm DNCB | 0·0 | 0·0 | 0·0 | 0·0 | 21·4 |
| 10 μm DNFB | 0·0 | 0·0 | 0·0 | 0·0 | 25·8 |
| 30 μm DNFB | 0·0 | 0·0 | 0·0 | 0·0 | 24·9 |
| exp. 2 | |||||
| Non-treated | 0·0 | 36·8 | 23·0 | 0·0 | 61·2 |
| 300 μm Ni | 0·0 | 57·9 | 36·3 | 0·0 | 57·4 |
| Exp. 3 | |||||
| Non-treated | 0·0 | 0·0 | 0·0 | 0·0 | 10·9 |
| 30 μm DNCB | 0·0 | 49·0 | 0·0 | 0·0 | 10·4 |
| 30 μm DNCB | 0·0 | 0·0 | 0·0 | 0·0 | 14·9 |
| 30 μm DNCB | 0·0 | 0·0 | 0·0 | 0·0 | 14·5 |
| Exp. 4 | |||||
| Non-treated | ND | 0·0 | 0·0 | ND | ND |
| 100 μm Ni | ND | 10·0 | 0·0 | ND | ND |
| 300 μm Ni | ND | 10·0 | 0·0 | ND | ND |
| 1000 μm Ni | ND | 80·0 | 0·0 | ND | ND |
| 30 μm DNCB | nd | 0·0 | 0·0 | ND | ND |
| 100 μm DNCB | ND | 5·0 | 0·0 | ND | ND |
nd, not determined.
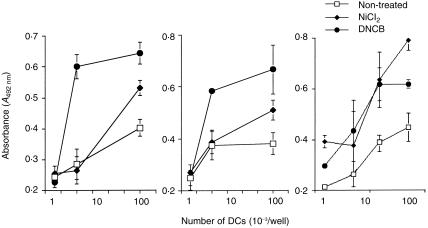
TGF-β1+ DCs treated with haptens increase their allogeneic T-cell stimulatory activity
Concomitantly with the augmented CD86 expression, TGF-β1+ DCs pretreated with NiCl2 or DNCB stimulated allogeneic T cells more vigorously than non-treated TGF-β1+ DCs (Fig. 4).
Figure 4.
TGF-β1+ DCs treated with haptens increase their allogeneic T-cell stimulatory function. Allogeneic T cells (2 × 105 cells/well) were co-cultured in 96-well flat-bottom microtitre plates with various numbers of TGF-β1+ DCs, which were precultured with 300 µm of NiCl2 or 30 µm of DNCB for 2 days and washed. After 4 days of culture at 37° in a 5% CO2 humidified atmosphere, the cells were pulsed with 10 µm bromodeoxyuridine (BrdU) during the last 16 hr of culture. The allogeneic T-cell stimulation by TGF-β1+ DCs was evaluated by the immunoenzymatic measurement of BrdU uptake by T cells.
TGF-β1+ DCs treated with haptens augment CCR7 mRNA expression and respond chemotactically to MIP-3β
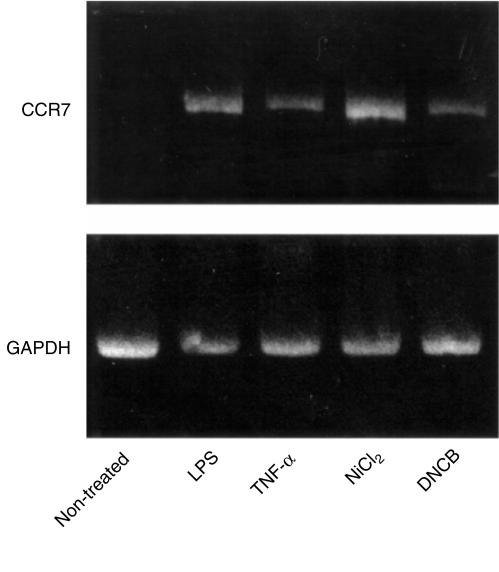
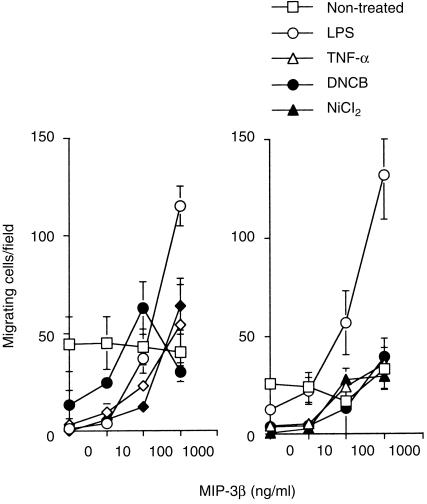
Mature DCs stimulated by lipopolysaccharide (LPS), TNF-α, or CD40L have been reported to respond to MIP-3β, probably with CCR7.14,17 Furthermore, Saeki et al.15 presented evidence suggesting that MIP-3β and CCR7 participate in the emigration of LCs from epidermis to the regional lymph nodes via lymphatics. Therefore, in this study, we examined CCR7 mRNA expression on TGF-β1+ DCs under the treatment with the haptens and their chemotactic response to MIP-3β. As shown in Fig. 5, TGF-β1+ DCs treated with either NiCl2 or DNCB in addition to those treated with LPS and TNF-α, up-regulated CCR7 mRNA. Parallel to this data, TGF-β1+ DCs treated with the haptens demonstrated a significant chemotactic response to MIP-3β, which was comparable to that of the response of TGF-β1+ DCs treated with 100 ng/ml of TNF-α, but much weaker than that of TGF-β1+ DCs treated with 10 ng/ml of LPS (Fig. 6). Unexpectedly, we recognized the migration of significant numbers of non-treated TGF-β1+ DCs which did not express CCR7 mRNA. As this migration was totally independent on the concentration of MIP-3β, we considered that their migration was a random migration rather than a chemotactic response.
Figure 5.
TGF-β1+ DCs treated with haptens increase CCR7 mRNA expression. Total RNA was isolated from TGF-β1+ DCs, which were treated with 10 ng/ml of LPS, 100 ng/ml of TNF-α, 300 µm of NiCl2 or 30 µm of DNCB, for 2 days, and used for RT-PCR with CCR7- and glyceraldehyde-3-phosphate dehydrogenase (G3PDH)-(loading control) specific primers. (a) Non-treated TGF-β1+ DCs; (b) TGF-β1+ DCs treated with 10 ng/ml of LPS; (c) TGF-β1+ DCs treated with 100 ng/ml of TNF-α; (d)TGF-β1+ DCs treated with 300 µm of NiCl2; (e) TGF-β1+ DCs treated with 30 µm of DNCB.
Figure 6.
TGF-β1+ DCs treated with haptens respond to MIP-3β. TGF-β1+ DCs, which were precultured with 10 ng/ml of LPS, 100 ng/ml of TNF-α, 300 µm of NiCl2, or 30 µm of DNCB, for 2 days and washed, were assessed by a chemotaxis microchamber technique for their chemotactic responses to MIP-3β; 5 × 104 cells/well in 50 µl of RPMI 1640 medium were applied to the upper wells of the chamber, with a standard 5-µm pore polyvinylpyrrolidone-free polycarbonate filter separating the lower wells. The chamber was incubated at 37° in humidified air with 5% CO2 for 90 min. Then, cells that had migrated to the underside of the filter were counted microscopically at × 200 magnification in five randomly selected fields per well. Each assay was performed in duplicate and the results were expressed as the mean ± SD of migrating cells per field. Shown are two of three experiments with similar results. In these experiments, significant numbers of non-treated TGF-β1+ DCs which did not express CCR7 mRNA and migrated independent of the concentration of MIP-3β, which suggested that their migration was a random migration rather than a chemotactic response.
The activation of TGF-β1+ DCs mediated by NiCl2 is mediated by mechanisms different from those in that by DNCB
Since Enk& Katz3 suggested that the cutaneous application of haptens triggered the maturation of epidermal LCs via IL-1β secreted by LCs themselves, and our data indicated that NiCl2 and DNCB induced phenotypic changes and cytokine production in TGF-β1+ DCs in a different fashion, we finally examined the mechanism involved in the hapten-induced activation of TGF-β1+ DCs (Table 2). When we added anti-TNF-α or anti-IL-1β antibody to the culture of TGF-β1+ DCs stimulated with the haptens, they suppressed significantly (P < 0·05), although partially, the augmentation of CD86 induced by DNCB, whereas they could not suppress that induced by NiCl2 at all. In a control experiment where we added the relevant neutralizing antibody to the culture of TGF-β1+ DCs stimulated with recombinant TNF-α or IL-1β, each antibody completely abrogated the activation of DCs induced by the corresponding cytokine.
Table 2.
The suppression of hapten-induced CD86 augmentation on MoDCs by anti-cytokine antibodies (%suppression)
| Antibodies | |||
|---|---|---|---|
| Chemicals | Control | Anti-TNF-α | Anti-IL-1β |
| Exp.1 | |||
| DNCB | 0 | 43 | 80 |
| NiCl2 | 0 | 13 | 7·3 |
| Exp. 2 | |||
| DNCB | 0 | 96 | 26 |
| NiCl2 | 0 | 23 | 28 |
| Exp. 3 | |||
| DNCB | ND | 57 | 57 |
| NiCl2 | ND | 0 | 0 |
| Exp. 4 | |||
| DNCB | ND | 2 | 60 |
| NiCl2 | ND | 0 | 0 |
| Exp. 5 | |||
| DNCB | 0 | 19 | 22 |
| NiCl2 | 0 | 19 | 0 |
Per cent suppression of mean fluorescence (MFI) was defined as [(MFI of hapten-stimulated DCs) − (MFI of hapten-stimulated DCs treated with cytokine antibody)] / [(MFI of hapten-stimulated DCs) − (MFI of non-treated controls)] × 100.
Discussion
The initial purpose of this study was to answer the question whether TGF-β1+ DCs are also stimulated directly by haptens like MoDCs. Our present study demonstrated that haptens such as NiCl2 and DNCB directly stimulated TGF-β1+ DCs to induce increased CD86 expression and augmented antigen presenting function, as noted in MoDCs.1
The second purpose was to characterize the phenotypic and functional changes occurring in TGF-β1+ DCs treated with the haptens. Several lines of evidence have demonstrated that DCs, including LCs, show two stages of maturation.18 Namely, the first is the differentiation from bone marrow precursor cells to immature DCs and the second is the maturation from immature DCs to mature DCs that have potent antigen-presenting function. Furthermore, recent progress on DC research has shown that the latter maturation process of DCs is characterized by their augmented expression of various co-stimulatory molecules, class II MHC antigens,2,19–22 and CD83,23,24 down-regulation of c-fms,25 production of several pro-inflammatory cytokines and IL-1226–28 and chemotactic response to MIP-3β, probably via CCR7.14,17 In addition to these changes associated with DC maturation, epidermal LCs have been reported to acquire the following phenotypic and functional changes in the maturation process during the induction phase of allergic contact hypersensitivity, i.e. the down-regulation of E-cadherin9 and CLA,8 expression of MMP-9,10 and augmentation of β1-integrin and some α-integrins,11,12 and CD44 and some of its variants.13 In our experiments, we found that TGF-β1+ DCs stimulated by haptens lacked several characteristics of fully matured DCs or LCs, because their expression of CD83 was weak in addition to the lack of IL-12 production. In contrast, however, they down-regulated E-cadherin and CLA, and chemotactically responded to MIP-3β, which paralleled their CCR7 mRNA expression. Furthermore, TGF-β1+ DCs stimulated with NiCl2 induced augmentation of β1-integrin and α5-integrin, CD44 and some of its variants, which are expected to play a role in the migration of DCs through the dermis to draining lymph nodes. They also produced MMP-9, although its production was not augmented by the hapten treatment.
These unique characteristics of hapten-stimulated TGF-β1+ DCs indicate that haptens trigger maturation of TGF-β1+ DCs, but not to a full extent, while they can supply the signals necessary for them to emigrate from the epidermis to regional lymph nodes via the lymphatics. In future, these observations made in TGF-β1+ DCs should also be confirmed in epidermal LCs.
In this study, we demonstrated that inducibility of phenotypic changes of TGF-β1+ DCs differs between different haptens. Namely, only the TGF-β1+ DCs stimulated with NiCl2, but none of those treated with DNCB, showed augmentation of CD54, HLA-DR, α5β1-integrin, CD44 and CD44v6. Consistent with these differences, the experiments using anti-TNF-α and anti-IL-1β antibodies demonstrated such a difference between NiCl2 and DNCB in their stimulatory effects on TGF-β1+ DCs. Namely, DNCB seemed to at first stimulate TGF-β1+ DCs to secrete IL-1β or TNF-α, which then induced their CD86 expression. On the other hand, NiCl2 directly induced the augmentation of CD86 expression on TGF-β1+ DCs. These data indicate that haptens trigger maturation of TGF-β1+ DCs at least via two different mechanisms. It has been demonstrated that the two transition metals, NiCl2 and CoCl2, directly induce the formation of reactive oxygen intermediates that activate NF-κB.29–32 On the other hand, Arner et al.33 reported that DNCB was an irreversible inhibitor of human thioredoxin reductase, which was accompanied by a large increase in NADPH oxidase activity. This increased NADPH oxidase activity produces reactive oxygen intermediates, which may activate NF-κB. Indeed, recently, Rescigno et al.34 demonstrated that LPS induced DC maturation through NF-kB activation. However, our present study demonstrating the difference between DNCB and NiCl2 in their stimulatory effects on TGF-β1+ DCs, indicates the importance of the signals other than NF-kB in their activation of TGF-β1+ DCs. We are currently investigating the signal transduction pathways involved in activation by these chemicals.
Finally, it is well known that the reaction of epidermal LCs to simple chemicals such as haptens or metals is the initial step in allergic contact sensitivity. Therefore, it is crucial to clarify the precise underlying mechanisms. For the observation of the direct effects of these chemicals on LCs, however, it is essential to culture large numbers of purified immature LCs. Although we want to examine human LCs in such a way, it is impossible to fulfil the required conditions. Thus our present data suggest that TGF-β1+ DCs can be substituted for the immature epidermal LCs and provide a good in vitro model to study the biological role of epidermal LCs in allergic contact sensitivity.
Acknowledgments
This study was supported in part by grant 12670803 from the Ministry of Education, Science and Culture of Japan, by Special Co-ordination Funds for Promoting Science and Technology from the Science and Technology Agency of the Japanese Government, and by the Cell Science Research Foundation.
Glossary
Abbreviations
- DC
dendritic cell
- MoDC
monocyte-derived dendritic cell
- MoLC
monocyte-derived Langerhans cell
References
- 1.Aiba S, Katz SI. Phenotypic and functional characteristics of in vivo-activated Langerhans cells. J Immunol. 1990;145:2791. [PubMed] [Google Scholar]
- 2.Ozawa H, Nakagawa S, Tagami H, Aiba S. Interleukin-1 beta and granulocyte-macrophage colony-stimulating factor mediate Langerhans cell maturation differently. J Invest Dermatol. 1996;106:441. doi: 10.1111/1523-1747.ep12343589. [DOI] [PubMed] [Google Scholar]
- 3.Enk AH, Katz SI. Early molecular events in the induction phase of contact sensitivity. Proc Natl Acad Sci USA. 1992;89:1398. doi: 10.1073/pnas.89.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aiba S, Terunuma A, Manome H, Tagami H. Dendritic cells differently respond to haptens and irritants by their production of cytokines and expression of co-stimulatory molecules. Eur J Immunol. 1997;27:3031. doi: 10.1002/eji.1830271141. [DOI] [PubMed] [Google Scholar]
- 5.Geissmann F, Prost C, Monnet JP, Dy M, Brousse N, Hermine O. Transforming growth factor beta1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic Langerhans cells. J Exp Med. 1998;187:961. doi: 10.1084/jem.187.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaksits S, Kriehuber E, Charbonnier AS, Rappersberger K, Stingl G, Maurer D. CD34+ cell-derived CD14+ precursor cells develop into Langerhans cells in a TGF-beta 1-dependent manner. J Immunol. 1999;163:4869. [PubMed] [Google Scholar]
- 7.Caux C, Vanbervliet B, Massacrier C, et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNFalpha. J Exp Med. 1996;184:695. doi: 10.1084/jem.184.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebner S, Lenz A, Reider D, Fritsch P, Schuler G, Romani N. Expression of maturation-/migration-related molecules on human dendritic cells from blood and skin. Immunobiology. 1998;198:568. doi: 10.1016/S0171-2985(98)80079-X. [DOI] [PubMed] [Google Scholar]
- 9.Schwarzenberger K, Udey MC. Contact allergens and epidermal proinflammatory cytokines modulate Langerhans cell E-cadherin expression in situ. J Invest Dermatol. 1996;106:553. doi: 10.1111/1523-1747.ep12344019. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi Y. Langerhans' cells produce type IV collagenase (MMP-9) following epicutaneous stimulation with haptens. Immunology. 1997;90:496. doi: 10.1046/j.1365-2567.1997.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staquet MJ, Levarlet B, Dezutter-Dambuyant C, Schmitt D. Human epidermal Langerhans cells express beta 1 integrins that mediate their adhesion to laminin and fibronectin. J Invest Dermatol. 1992;99:12S. doi: 10.1111/1523-1747.ep12668241. [DOI] [PubMed] [Google Scholar]
- 12.Aiba S, Nakagawa S, Ozawa H, Miyake K, Yagita H, Tagami H. Up-regulation of alpha 4 integrin on activated Langerhans cells: analysis of adhesion molecules on Langerhans cells relating to their migration from skin to draining lymph nodes. J Invest Dermatol. 1993;100:143. doi: 10.1111/1523-1747.ep12462783. [DOI] [PubMed] [Google Scholar]
- 13.Weiss JM, Sleeman J, Renkl AC, et al. An essential role for CD44 variant isoforms in epidermal Langerhans cell and blood dendritic cell function. J Cell Biol. 1997;137:1137. doi: 10.1083/jcb.137.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dieu MC, Vanbervliet B, Vicari A, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saeki H, Moore AM, Brown MJ, Hwang ST. Cutting edge: secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J Immunol. 1999;162:2472. [PubMed] [Google Scholar]
- 16.Sallusto F, Schaerli P, Loetscher P, Schaniel C, Lenig D, Mackay CR, Qin S, Lanzavecchia A. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol. 1998;28:2760. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. 10.1002/(sici)1521-4141(199809)28:09<2760::aid-immu2760>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 17.Luster AD. Chemokines – chemotactic cytokines that mediate inflammation. New Eng J Med. 1998;338:436. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 18.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 19.Romani N, Lenz A, Glassel H, Stossel H, Stanzl U, Majdie O, Fritsch P, Schuler G. Cultured human Langerhans cells resemble lymphoid dendritic cells in phenotype and function. J Invest Dermatol. 1989;93:600. doi: 10.1111/1523-1747.ep12319727. [DOI] [PubMed] [Google Scholar]
- 20.Symington FW, Brady W, Linsley PS. Expression and function of B7 on human epidermal Langerhans cells. J Immunol. 1993;150:1286. [PubMed] [Google Scholar]
- 21.Girolomoni G, Zambruno G, Manfredini R, Zacchi V, Ferrari S, Cossarizza A, Giannetti A. Expression of B7 costimulatory molecule in cultured human epidermal Langerhans cells is regulated at the mRNA level. J Invest Dermatol. 1994;103:54. doi: 10.1111/1523-1747.ep12389619. [DOI] [PubMed] [Google Scholar]
- 22.Yokozeki H, Katayama I, Ohki O, et al. Functional CD86 (B7–2/B80) on cultured human Langerhans cells. J Invest Dermatol. 1996;106:147. doi: 10.1111/1523-1747.ep12329735. [DOI] [PubMed] [Google Scholar]
- 23.Zhou LJ, Schwarting R, Smith HM, Tedder TF. A novel cell-surface molecule expressed by human interdigitating reticulum cells, Langerhans cells, and activated lymphocytes is a new member of the Ig superfamily. J Immunol. 1992;149:735. [PubMed] [Google Scholar]
- 24.Zhou LJ, Tedder TF. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J Immunol. 1995;154:3821. [PubMed] [Google Scholar]
- 25.Akagawa KS, Takasuka N, Nozaki Y, Komuro I, Azuma M, Ueda M, Naito M, Takahashi K. Generation of CD1+RelB+ dendritic cells and tartrate-resistant acid phosphatase-positive osteoclast-like multinucleated giant cells from human monocytes. Blood. 1996;88:4029. [PubMed] [Google Scholar]
- 26.Heufler C, Koch F, Stanzl U, et al. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur J Immunol. 1996;26:659. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- 27.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang K, Kubin M, Cooper KD, Lessin SR, Trinchieri G, Rook AH. IL-12 synthesis by human Langerhans cells. J Immunol. 1996;156:1402. [PubMed] [Google Scholar]
- 29.Klein CB, Frenkel K, Costa M. The role of oxidative processes in metal carcinogenesis. Chem Res Toxicol. 1991;4:592. doi: 10.1021/tx00024a001. [DOI] [PubMed] [Google Scholar]
- 30.Huang X, Frenkel K, Klein CB, Costa M. Nickel induces increased oxidants in intact cultured mammalian cells as detected by dichlorofluorescein fluorescence. Toxicol Appl Pharmacol. 1993;120:29. doi: 10.1006/taap.1993.1083. 10.1006/taap.1993.1083. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Yokoi I, Liu J, Mori A. Cobalt (II) and nickel (II) ions as promoters of free radicals in vivo: detected directly using electron spin resonance spectrometry in circulating blood in rats. Arch Biochem Biophys. 1993;306:402. doi: 10.1006/abbi.1993.1529. 10.1006/abbi.1993.1529. [DOI] [PubMed] [Google Scholar]
- 32.Huang X, Zhuang Z, Frenkel K, Klein CB, Costa M. The role of nickel and nickel-mediated reactive oxygen species in the mechanism of nickel carcinogenesis. Env Health Perspect. 1994;102:281. doi: 10.1289/ehp.94102s3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arner ES, Bjornstedt M, Holmgren A. 1-Chloro-2,4-dinitrobenzene is an irreversible inhibitor of human thioredoxin reductase. Loss of thioredoxin disulfide reductase activity is accompanied by a large increase in NADPH oxidase activity. J Biol Chem. 1995;270:3479. doi: 10.1074/jbc.270.8.3479. 10.1074/jbc.270.8.3479. [DOI] [PubMed] [Google Scholar]
- 34.Rescigno M, Martino M, Sutherland CL, Gold MR, Ricciardi-Castagnoli P. Dendritic cell survival and maturation are regulated by different signaling pathways. J Exp Med. 1998;188:2175. doi: 10.1084/jem.188.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]