Abstract
An immunodominant epitope of human immunodeficiency virus-1 (HIV-1) gp160 recognized by Dd class I major histocompatibility complex (MHC) molecule-restricted, CD8+ cytotoxic T lymphocytes (CTL) was originally identified as a peptide composed of 15 amino acids (P18IIIB: RIQRGPGRAFVTIGK). However, further study has indicated that a 10-mer peptide, I-10 (RGPGRAFVTI), within P18IIIB is the minimal-sized epitope and the trimming step(s) of two carboxyl terminal amino acids (GK) is essential to produce I-10 from P18IIIB. In the processing, angiotensin-1-converting enzyme (ACE), found in sera, plays a central role in generating I-10. Target cells could be sensitized with I-10 under conditions where ACE activity in the sera was abrogated. In contrast, in the case of P18IIIB, requiring further processing to delete the C-terminus of two amino acids in order to act, sensitization of target cells was completely abrogated under the conditions. Pretreatment of target cells with brefeldin A (BFA), preventing the presentation of endogenous antigens from the class I MHC molecule pathway, did not inhibit the presentation of P18IIIB. Moreover, glutaraldehyde-fixed cells, which can not process native protein, though they could present the exogenously added peptides, were also sensitized by P18IIIB. These results clearly demonstrate that the fine processing to produce I-10 occurred in the extracellular milieu. Furthermore, our result suggests that the longer P18IIIB can bind to the class I molecules on the cell surface, and then be trimmed by ACE while it is bound. The mechanisms behind the extracellular processing outlined in this paper will offer important information for designing peptide-based vaccines to elicit MHC molecule-restricted effectors.
Introduction
Antigen-specific T lymphocytes recognize processed or fragmented antigenic peptides in association with major histocompatibility complex (MHC) molecules presented on the surface of antigen-presenting cells (APC) by their specific T-cell receptors.1–3 In general, exogenous antigens can be taken up by APC and presented to CD4+ T lymphocytes in conjunction with class II MHC molecules, whereas endogenously synthesized antigens are presented to CD8+ cytotoxic T lymphocytes (CTL) with class I MHC molecules.4 By sequencing analysis of endogenously processed, MHC molecule-associated peptides, it has been shown that the epitopic peptides fitting in the groove of class I MHC molecules are usually composed of eight to 10 amino acids.5 To generate such a minimal-sized epitope peptide from native antigens, there must be a number of processing steps conducted inside of the cells, but little is known about the final trimming step(s).
We have identified an immunodominant epitope of human immunodeficiency virus-1 (HIV-1) IIIB isolate envelope glycoprotein, gp160, for Dd class I MHC molecule-restricted murine CD8+ CTL as a 15-mer peptide P18IIIB (RIQRGPGRAFVTIGK).6,7 Further detailed studies have demonstrated that a 10-mer peptide, I-10 (RGPGRAFVTI), within P18IIIB is the minimal sized epitope for those gp160 specific-CD8+ CTL8,9 and trimming step(s) to remove two carboxyl-terminal amino acids (GK) from the longer P18IIIB is essential to produce the I-10.10 Kozlowski et al. have reported that angiotensin-I-converting enzyme (ACE), a carboxypeptidase found in serum, plays a central role in producing the active peptide I-10.10 Furthermore, we have suggested the possibility of extracellular processing to trim the peptide using an in vitro cell-free antigen-presenting system with soluble H-2Dd class I MHC molecule-coated plates.
Recently, it has been reported that newly synthesized viral polypeptides generated in the cytoplasm are fragmented into peptides and transported into the lumen of the endoplasmic reticulum (ER) by the transporter associated with antigen processing (TAP),11,12 where they are trimmed to minimal-length antigenic peptides to fit with class I MHC molecules in the ER when appropriate proteases are present.13,14 It has also been speculated that the trimming of peptides in the ER is triggered by aminopeptidases.14 However, Eisenlohr et al. showed that an increase in the expression of ACE on the ER membrane in cells enhances the antigen presentation of endogenously synthesized peptide to CD8+ CTL by coinfection experiments with ACE gene-expressing recombinant vaccinia virus and vaccinia virus recombinants encoding influenza virus nucleoprotein-derived peptide gene.15 Therefore, we cannot completely rule out the possibility that an ACE-like enzyme within the cell participates in the production of the minimal sized epitopic peptide I-10 from P18IIIB.
In this paper, based on the findings to date, we attempted to analyse the actual features of extracellular processing and to clarify the site where the processing occurs when P18IIIB is used as antigenic peptide in our CTL assay system.
Materials and methods
CTL line
The CTL line (LINE-IIIB) specific for the HIV-1 envelope protein of the IIIB-strain was generated by repetitive restimulation of vSC-25 (HIV-1 gp160 IIIB-expressing recombinant vaccinia virus)16 immune BALB/c spleen cells in 24-well culture plates in complete T-cell medium (CTM) (RPMI-1640 medium containing 10% heat inactivated fetal calf serum (FCS), 2 mm l-glutamine, 10 mm HEPES, 100 µm non-essential amino acids, 10 mm sodium pyruvate, 100 U/ml penicillin, 100 µg/ml streptomycin, 50 µm 2-mercaptoethanol) with mitomycin C (MMC)-treated gp160 gene-transfected BALB/c.3T3 fibroblasts (15–12)6 in the presence of 10% rat concanavalin A (Con A)-stimulated spleen cell culture supernatant (Rat T-STIM without Con A, Collaborative Research Inc., Bedford, MA) as described previously.6 LINE-IIIB was restricted by the Dd class I MHC molecule and specific for an immunodominant epitope of HIV-1 gp160 (P18IIIB: RIQRGPGRAFVTIGK).6,7
Synthetic peptides
Peptides were synthesized on an Applied Biosystems (Foster City, CA) Model 430A peptide synthesizer, using conventional t-butoxycarbonyl (t-Boc) chemistry and cleaved from the resin by liquid HF. Synthetic peptides were purified by gel filtration on Bio-gel P-4 and analysed by high pressure liquid chromatography (HPLC) on a C18 reverse-phase column. The peptides that contained more than 90% of the desired product were used for experiments.
CTL assay
Cytolytic activity of the CTL line was measured as previously described6 using a standard 5 hr 51Cr release assay with various 51Cr-labelled targets (mainly BALB/c.3T3 fibroblasts), as indicated in the figure legends. In several experiments, effector CTL and 51Cr-labelled target cells were mixed with various concentrations of target peptide at the beginning of the assay. The percentage specific 51Cr release was calculated as 100 × (experimental release − spontaneous release)/(maximum release − spontaneous release). Maximum release was determined from supernatants of target cells that were lysed by addition of 5% Triton-X-100. Spontaneous release was determined from target cells incubated without added effector cells.
Depletion of FCS from culture medium
BALB/c.3T3 fibroblast cells (H-2d) were washed three times with serum-free RPMI-1640 medium and resuspended in the same serum-free RPMI-1640. Then the cells were incubated for 30 min at 37° to remove remaining FCS completely. After the incubation, the cells were washed again and resuspended in CTM without FCS. Then the target peptides and CrO4 were added to the cell suspension and further incubated overnight. After the incubation, the cells were washed three times and used as target cells in CTL assay.
Treatment of target cells with ACE inhibitor
The ACE EC3.4.15.1 peptidyl/dipeptide hydrolase-specific inhibitor captopril (Sigma Chemical Co., St Louis, MO) was dissolved in 10 mm phosphate-buffered saline (PBS). BALB/c.3T3 fibroblast target cells were preincubated with or without 400 µm of captopril at 37° for 30 min. Then 3 µm of peptides and CrO4 were added to the target cells and incubated overnight in the presence of captopril. After the incubation, cells were washed three times and used as target cells for CTL assay.
In some of the experiments, captopril-pretreated target cells were directly added to U-bottomed 96-well CTL assay plates after extensive washing together with effector cells and peptides to see the difference of inhibitory effect of captopril for each peptide.
Treatment of target cells with brefeldin A (BFA), an inhibitor of protein transport from endoplasmic reticulum to Golgi apparatus
BFA (Sigma) was dissolved in methanol at 1 mg/ml. Chromium-51-labelled BALB/c.3T3 fibroblast cells were incubated with or without 6 µg/ml of BFA at 37° for 3 hr. Then peptides were added to target cells and further incubated for 2 hr in the presence of BFA. After the incubation, the cells were washed three times with RPMI-1640 containing 5% FCS and 0·4 µg/ml of BFA, and used as target cells. Because the inhibitory activity disappeared on removal of BFA from the medium, BFA-treated target cells should be incubated with effector CTL continuously in the presence of 0·4 µg/ml of BFA during the 5 hr CTL assay.
Treatment of vSC25-infected cells with BFA
BALB/c.3T3 fibroblast cells were infected with vSC25 at 1 × 107 plaque-forming units (p.f.u.)/ml for 2 hr at 37° and washed three times. Cells were then incubated with or without 6 µg/ml of BFA for 2 hr at 37°. Peptides and CrO4 were added and cells were further incubated overnight. The cells were washed three times and then used as target cells.
Glutaraldehyde fixation of target cells
Glutaraldehyde fixation was done by the procedure described by Hosken et al.17 Briefly, 51Cr-labelled BALB/c.3T3 fibroblast cells were washed three times with RPMI-1640 medium and resuspended in 1 ml of RPMI-1640.
Freshly prepared 2% glutaraldehyde (Sigma) in PBS was added to the cells at a final concentration of 0·05%. The cells were gently mixed for 15 s. Then an equal volume of 0·2 m l-lysine (Sigma) was added and the cells were mixed for an additional minute to stop the fixation reaction. After being washed twice with RPMI-1640 containing 5% FCS, the cells were resuspended with 10% FCS containing RPMI-1640. They were incubated with or without peptides and captopril to see their ability to process the externally added peptides.
Results
Effect of inhibition of ACE-like activity on target sensitization by epitope peptide
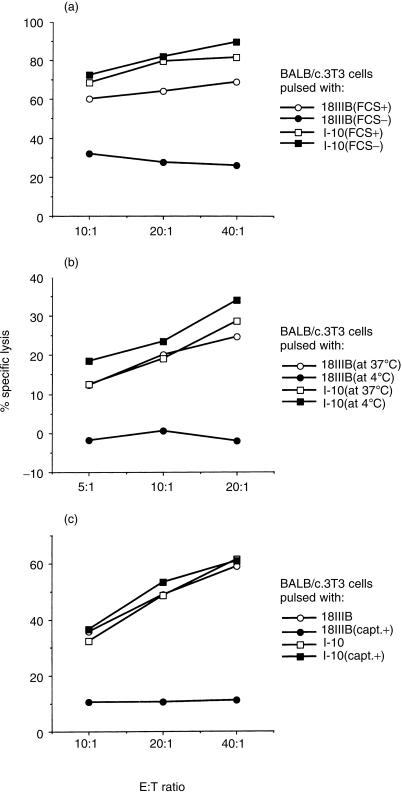
It has been suggested that ACE, an exopeptidase cutting two carboxyl-terminal amino acids in peptide, exists in serum.18 To test whether the activity of ACE was required to produce the active minimal epitopic peptide I-10 (RGPGRAFVTI) from P18IIIB (RIQRGPGRAFVTIGK), we examined the effect of abrogating ACE activity in the serum on the sensitization of fibroblast target cells with peptides in various conditions. We first studied the effect of depletion of FCS from the culture medium. BALB/c.3T3 fibroblast cells (H-2d) were pulsed with either I-10 or P18IIIB for 3 hr in normal culture medium (CTM) or in serum-free culture medium. As shown in Fig. 1(a), target cells could be sensitized with peptide I-10 obtained by removing two carboxyl-terminal residues from P18IIIB and thus could be lysed by the specific CTL line in the absence of serum. In contrast, in the case of P18IIIB, sensitization of target cells was greatly reduced under the same condition.
Figure 1.
Inhibition of ACE-like activity in culture medium abrogates sensitization of target cells with the longer peptide P18IIIB. (a) BALB/c.3T3 fibroblast cells were incubated with 3 µm of peptide P18IIIB (circles) or I-10 (squares) in CTM (open symbols) or in serum-free CTM (closed symbols) overnight in the presence of 51Cr. Then they were washed three times and used as target cells. (b) Chromium 51-labelled BALB/c.3T3 cells were incubated with 3 µm of peptide P18IIIB (circles) or I-10 (squares) for two hours at 37° (open symbols) or 4° (closed symbols), respectively. Then they were washed and used as target cells. (c) BALB/c.3T3 cells were incubated with 3 µm of peptide P18IIIB (circles) or I-10 (squares) in the presence (closed symbols) or absence (open symbols) of 400 µm of ACE inhibitor, captopril. Then they were washed three times to remove all free peptide and used as target cells. LINE-IIIB cells were used as effector cells. The SEM of triplicate cultures was always < 5% of the mean.
Because the most suitable temperature for ACE to act is 37°, we next tested whether the sensitization of targets with P18IIIB was affected at lower temperature (4°). We added P18IIIB or I-10–51Cr-labelled target cells and incubated then in the presence of serum for 2 hr at either 37° or 4°. Then, we assayed the cytotoxicity for these peptide-pulsed target cells of antigen-specific Dd-restricted CTL line (LINE-IIIB). As indicated in Fig. 1(b), target cells were sensitized with I-10 at both temperatures, whereas sensitization with the longer peptide P18IIIB was completely abrogated at the lower temperature. Therefore, sensitization of target cells with P18IIIB must require enzymatic activity at 37° to process peptide P18IIIB.
It has been reported that the activity of ACE in the serum is inhibited by its specific inhibitor, captopril.19 Thus, to examine the role of ACE in the generation of active peptide I-10, we added captopril together with epitope peptides to target cells in the presence of serum at 37°. As clearly shown in Fig. 1(c), although the sensitization of target cells with I-10 was not inhibited in the presence of captopril at all, the sensitization with P18IIIB was completely abrogated. These results strongly indicate that abrogation of ACE activity inhibits the processing of the longer peptide P18IIIB.
Effect of the number of carboxyl-terminal and amino-terminal residues in P18IIIB analogue peptides on sensitivity to captopril
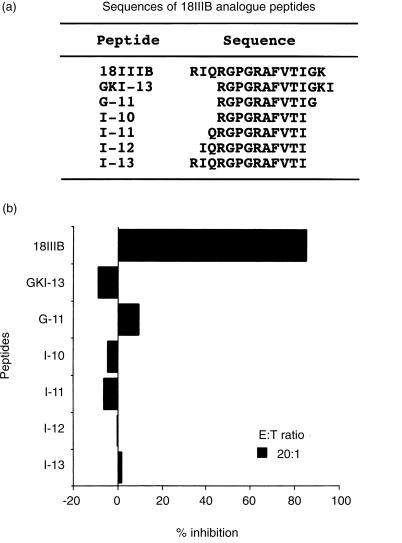
ACE has carboxypeptidase activity, which removes C-terminal dipeptides from a variety of polypeptide substrates. Thus, to confirm whether ACE is the major c-terminal processor of P18IIIB, we analysed the effect of the number of amino acid residues following the C-terminus of I-10 on target sensitization. As shown in Fig. 2, when captopril was added to inhibit dipeptidase activity in serum ACE, target BALB/c.3T3 cells were not sensitized with P18IIIB having two extended residues (GK) from the C-terminus of I-10, whereas they could be sensitized with G-11 or GKI-13 having one or three extended residues from I-10, respectively. In contrast, ACE does not participate in amino (N)-terminal trimming step(s) of P18IIIB, since the target cells were effectively sensitized in the presence of captopril with I-11, I-12 and I-13 having one, two, and three extended residues from the N-terminus of I-10, respectively (Fig. 2). These results demonstrate that ACE plays a critical role in the C-terminal trimming of P18IIIB to produce active minimal peptide, I-10.
Figure 2.
Effect of number of carboxyl-terminal and amino-terminal residues in P18IIIB analogue peptides on target cell sensitization in the presence of captopril. (a) Amino acid sequences of synthetic P18IIIB analogue peptides used in the experiment. (b) BALB/c.3T3 cells were incubated with 3 µm of various P18IIIB analogue peptides in the presence or absence of the ACE inhibitor, captopril (400 µm) and used as target cells in CTL assay. LINE-IIIB cells were used as effector cells. Data are expressed as percent inhibition calculated from the percentage specific lysis of target cells in the absence of the inhibitor.
Pretreatment with BFA did not inhibit antigen presentation of P18IIIB
The above results suggest that serum ACE removes two C-terminal residues from P18IIIB to produce an active class I-restricted epitope peptide I-10. However, intracellular processing with ACE-like enzyme15 for P18IIIB trimming is still possible. Thus, we tried to exclude such a possibility by treatment with fungal metabolite BFA, an inhibitor of protein transport from the ER to Golgi apparatus, which inhibits the presentation of endogenous antigens by class I MHC molecules.20
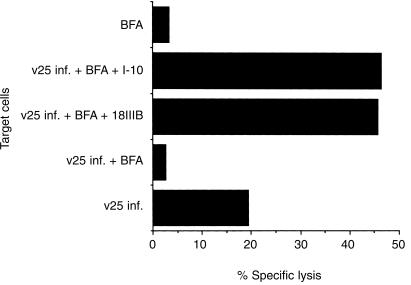
Three hours pretreatment of BALB/c.3T3 fibroblast target cells with effective amounts of BFA (6 µg/ml) did not alter the antigen presentation of P18IIIB at all as compared with I-10, as long as target cells were incubated with serum-containing medium (data not shown). On the other hand, vSC25 (HIV-1 gp160 IIIB expressing recombinant vaccinia virus)-infection to generate intracellular processing of I-10 was completely abolished with BFA treatment (Fig. 3). Also, BFA-treated vSC25-infected cells could still be sensitized with P18IIIB as well as I-10 in the presence of FCS at 37° (Fig. 3). The results strongly indicate that the fine trimming step(s) to remove two carboxyl-terminal amino acids for presentation to class I MHC molecules takes place outside of the cell.
Figure 3.
BFA treatment inhibits antigen processing and presentation of vSC25-infected cells but not the presentation of exogenously added peptide P18IIIB.vSC25 (HIV-IIIB gp160 expressing recombinant vaccinia virus) infected, BFA-treated BALB/c.3T3 cells were incubated with 3 µm of peptide P18IIIB or I-10 in the presence of 51Cr. Then, they were washed three times to remove free peptide and 51Cr and used as target cells. LINE-IIIB cells were used as effector cells. The SEM of triplicate cultures was always < 5% of the mean.
Effect of glutaraldehyde fixation on target cell sensitization with epitope peptide
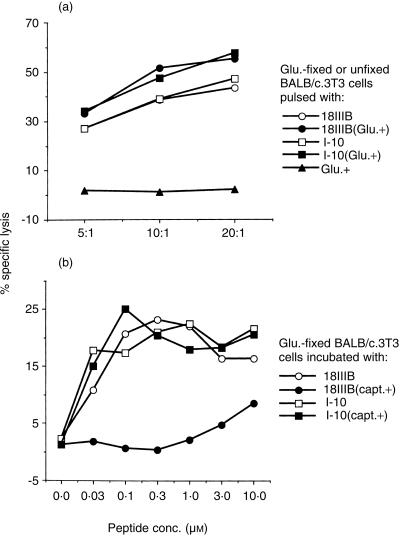
We tried to confirm the extracellular processing of epitope peptide P18IIIB using glutaraldehyde-fixed cells that can not process native protein, but can present exogenously added peptides in association with class I MHC molecules for specific CTL recognition.17 Shimonkevitz et al. showed that the fixed cells were not viable and unable to process antigens intracellularly.21 As shown in Fig. 4(a), glutaraldehyde-fixed cells could be sensitized by both P18IIIB and I-10 for specific CTL recognition. In contrast, the processing with P18IIIB of these fixed cells was inhibited by adding captopril to the culture medium (Fig. 4b). Taken together, the results demonstrate that the fine processing for producing active peptide I-10 from P18IIIB occurs in the extracellular milieu.
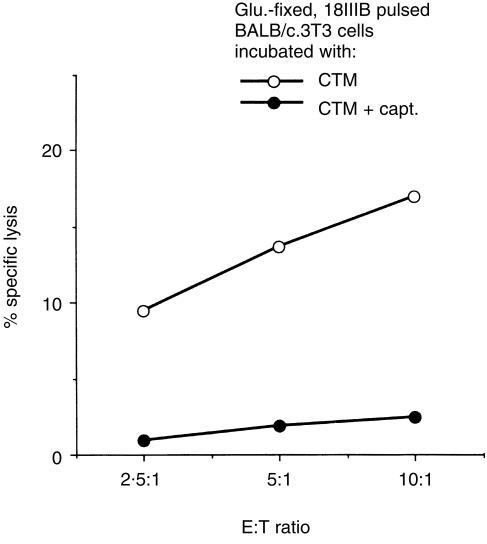
Figure 4.
Effect of glutaraldehyde fixation on the sensitization of target cells by epitope peptides. (a) 51Cr-labelled, glutaraldehyde-fixed (closed symbols) or unfixed (open symbols) BALB/c.3T3 cells were incubated with 3 µm of peptide P18IIIB (circles) or I-10 (squares) for 2 hr at 37°. They were then washed three times and used as target cells. LINE-IIIB cells were used as effector cells. (b) 51Cr-labelled, glutaraldehyde-fixed BALB/c.3T3 cells were incubated with or without captopril for 30 min. Then 5 × 103 captopril-treated (closed symbols) or untreated (open symbols) cells were incubated with 5 × 104 LINE-IIIB cells in the presence of various amounts of peptide P18IIIB (circles) or I-10 (squares) in 96-well round-bottom microtitre plates during the CTL assay. Captopril (400 µm) was present throughout the assay.
It is shown that the class I MHC molecules themselves are involved in determining the antigenic peptides produced intracellularly.22 Thus, we tried to confirm whether class I MHC molecules on the cell surface membrane also participate in the extracellular processing of P18IIIB. In preliminary experiments, we have found that most of the peptide P18IIIB has already bound to class I MHC molecules on glutaraldehyde-fixed target cells within 10 min incubation, while cleavage of P18IIIB to minimal I-10 by enzymes in the serum starts 20 min after the peptide is added (data not shown). Based on these findings, glutaraldehyde-fixed target cells were pulsed with P18IIIB in CTM for 10 min and then washed three times to remove the remaining free peptide. Under this condition, the binding of P18IIIB to class I MHC molecules is thought to occur, whereas the processing to produce a minimum-sized peptide that fits to a groove on the class I MHC molecules should start. As shown in Fig. 5, when the glutaraldehyde-fixed, P18IIIB-bound target cells were further incubated in CTM for 2 hr, the percentage specific lysis by LINE-IIIB was much greater than when incubated in the presence of captopril. The result suggests that the longer P18IIIB bound to class I MHC molecules expressed on the cell surface membrane is cut off by soluble-formed ACE existing in the neighbourhood of the membrane.
Figure 5.
Class I MHC molecules participate in extracellular processing of longer peptide P18IIIB. 51Cr-labelled, glutaraldehyde-fixed, P18IIIB-pulsed BALB/c.3T3 cells were washed three times to remove free peptide. Then, the fixed cells were incubated in the presence (closed symbols) or absence (open symbols) of captopril for two hours and used as target cells. LINE-IIIB cells were used as effector cells. The SEM of triplicate cultures was always < 5% of the mean.
Inhibition of CTL activity by free antigenic peptide occurs even in BFA-treated CTL
Recently, we reported that the cytotoxic activity of a CTL line, LINE-IIIB, is strongly inhibited on as little as 10 min exposure to minimal epitope peptide I-10 free in solution.23 We also found that an ACE inhibitor, captopril, did not affect the inhibitory activity of I-10, but almost completely abrogated the activity of P18IIIB. Thus, the inhibitory phenomenon is generated with only the minimal peptide I-10, which may bind directly to the surface MHC molecules of CTL, but in the case of P18IIIB, processing to produce I-10 in the serum is required for the inhibitory activity. Therefore, we examined whether the inhibition could be observed with minimal peptide produced by extracellular processing. To test this, BFA-treated or untreated CTL were incubated with I-10 or P18IIIB for 3 hr in an FCS-containing culture medium. After the incubation, the cells were washed three times and tested for their CTL activity against target cells. As shown in Fig. 6, the BFA-treated CTL line was also inhibited by free peptide I-10 produced on the surface of CTL by C-terminal proteolytic cleavage of P18IIIB.
Figure 6.
Inhibition of CTL activity by free antigenic peptide is induced in a BFA-treated CTL line. The CD8+ CTL line, LINE-IIIB was incubated with 6 µg/ml of BFA for 3 hr. Peptides were added and incubation continued for three hours. After the incubation, cells were washed three times to remove free peptide and used as effector cells. P18IIIB-pulsed BALB/c.3T3 cells were used as target cells.
Discussion
In this study, we confirmed that ACE in serum was necessary for the final trimming step(s) to produce minimal active epitope peptide I-10 from the longer peptide P18IIIB in our experimental systems using HIV-1 gp160-specific CD8+ CTL. Furthermore, we attempted to show that this trimming step(s) by ACE occurs extracellularly and in association with class I MHC molecules expressed on the cell surface. Kozlowski et al. have reported similar processing generated by the enzymes in the serum using their cell-free system.10,24 However, it has recently been reported that class I MHC molecule-restricted peptides are finely trimmed in the ER.13,14 Moreover, Eisenlohr et al. showed the possibility that trimming of peptides could be triggered by ACE-like enzymes artificially expressed on the membrane of the ER.15 In their experiments, the trimming process was inhibited by BFA treatment, so the processing might occur in an intracellular class I MHC molecule-mediated antigen-processing pathway. In addition, they showed that a unique trimming phenomenon occurred in some intracellular compartment inaccessible to captopril. However, as we have demonstrated here that BFA-treated or glutaraldehyde-fixed target cells could be sensitized with the longer P18IIIB in the presence of serum ACE to almost the same extent as I-10, the processing is likely to have proceeded extracellularly, distinct from the conventional class I MHC molecule-mediated pathway.
ACE exists as a membrane-bound form in a variety of cells and also as a water-soluble form in serum, the latter produced by proteolytic cleavage of the C-terminal region.18,25 Kozlowski et al. have showed that peptide P18IIIB can be processed, using a cell-free system, into shorter peptides by the soluble form of ACE in the absence of class I MHC molecules; but it is not yet clear whether trimming of the peptide by the cell-associated form of ACE is also involved. To examine which type of ACE trims the peptide P18IIIB extracellularly, we performed experiments using glutaraldehyde-fixed target cells in which the enzymatic activity of cell-surface ACE was greatly reduced and class I MHC molecules on the cell surface membrane were immobilized. The fact that the glutaraldehyde-fixed cells in the sera could be sensitized with the longer P18IIIB and lysed by the specific CTL line equally as well as with I-10 strongly suggested the importance of the serum-soluble form of ACE for the trimming of P18IIIB into the active form I-10. Conversely, the lack of processing even by unfixed cells in the absence of serum (Fig. 1) implies that cell surface and intracellular proteases do not play a significant role.
It was reported that the class I MHC molecules themselves participate in the trimming of peptides derived from cellular proteins.22 In the present study, the class I MHC molecules seemed to contribute to the production of the antigenic peptide and protect the peptide from further degradation. Therefore, we examined the role of MHC molecules in the fine trimming of P18IIIB in our experimental systems using glutaraldehyde-fixed, peptide P18IIIB-bound target cells. Because peptide P18IIIB already bound to target cells could be trimmed by incubation in serum-containing medium, we speculated that the trapping of peptide P18IIIB by the class I MHC molecules is more efficient for fine trimming. However, Kozlowski et al. showed that processing of P18IIIB to I-10 occurred also in the absence of H-2Dd class I MHC molecules, though the activity was not efficient.24 Accordingly, when longer peptides, such as P18IIIB are added, to sensitize target cells free in solution, there could be two mechanisms of extracellular processing. The minimal epitopic peptide I-10 produced in the sera by serum ACE may immediately fit to class I MHC molecules preventing further degradation. Alternatively, antigenic peptides such as P18IIIB may first bind to the class I MHC molecules expressed on the cell surface and then be trimmed into minimal-sized epitopic peptides by soluble-form ACE-like enzymes nearby in the serum for efficient processing. The latter mechanism might be an another form of processing distinct from conventional types.
Recently, we have found that antigen-specific CTL activity is strongly inhibited following brief exposure to minimal epitope peptide I-10 free in solution.23 Although this phenomenon was also inducible by the treatment with peptide P18IIIB, more than 2 hr was necessary when P18IIIB was used as an inhibitor. Because the inhibitory activity of P18IIIB was abrogated by captopril and was greatly decreased in the absence of serum (data not shown), the processing of P18IIIB to produce a minimal sized epitope peptide in serum must be necessary for inducing such CTL inhibition. In the present study, we found that inhibition by the longer peptide P18IIIB could also be achieved in BFA-treated CTL, in which the ability of class I MHC molecule-mediated intracellular antigen presentation was greatly reduced. As demonstrated in Fig. 6, extracellular trimming appears to be sufficient for inducing the CTL inhibition with P18IIIB containing minimal antigenic epitopes. In the case that the CTL destroy the virus-infected cells and large amounts of digested intracellular proteins and their fragments, as well as virus-derived peptides are released from the cells locally, such CTL inhibition would interfere with the clearance of the virus. Because it is widely known that antigen-specific CTL play a central role in preventing the spread of virus and growth of tumour in vivo, the analysis of the mechanisms behind the extracellular processing outlined in this paper will offer important information for designing peptide-based vaccines to elicit MHC molecule-restricted effectors.
Acknowledgments
We thank Dr Taku Tsukui for critical reading of the manuscript and helpful suggestions. This work was supported in part by grants from the Ministry of Education, Culture, and Science, from the Ministry of Health, and CREST, JST, Japan.
References
- 1.Townsend ARM, Rothbard J, Gotch FM, Bahadur G, Wraith D, McMichael AJ. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986;44:959. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- 2.Van Bleek GM, Nathenson SG. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature. 1990;348:213. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]
- 3.Rotzschke O, Falk K, Deres K, Schild H, Norda M, Metzger J, Jung G, Rammensee H-G. Isolation and analysis of naturally processed viral peptides as recognized by cytotoxic T cells. Nature. 1990;348:252. doi: 10.1038/348252a0. [DOI] [PubMed] [Google Scholar]
- 4.Germain RN. The ins and outs of antigen processing and presentation. Nature. 1986;322:687. doi: 10.1038/322687a0. [DOI] [PubMed] [Google Scholar]
- 5.Rammensee H-G, Falk K, Rotzschke O. Peptides naturally presented by MHC class I molecules. Annu Rev Immunol. 1993;11:213. doi: 10.1146/annurev.iy.11.040193.001241. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi H, Cohen J, Hosmalin A, et al. An immunodominant epitope of the human immunodeficiency virus envelope glycoprotein gp160 recognized by class I major histocompatibility complex molecule-restricted murine cytotoxic T lymphocytes. Proc Natl Acad Sci USA. 1988;85:3105. doi: 10.1073/pnas.85.9.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi H, Houghten R, Putney SD, Margulies DH, Moss B, Germain RN, Berzofsky JA. Structural requirements for class I MHC molecule-mediated antigen presentation and cytotoxic T cell recognition of an immunodominant determinant of the human immunodeficiency virus envelope protein. J Exp Med. 1989;170:2023. doi: 10.1084/jem.170.6.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeshita T, Kozlowski S, England RD, et al. Role of conserved regions of class I MHC molecules in the activation of CD8+cytotoxic T lymphocytes by peptide and purified cell-free class I molecules. Int Immunol. 1993;5:1129. doi: 10.1093/intimm/5.9.1129. [DOI] [PubMed] [Google Scholar]
- 9.Takeshita T, Takahashi H, Kozlowski S, et al. Molecular analysis of the same HIV peptide fuctionally binding to both a class I and a class II MHC molecule. J Immunol. 1995;154:1973. [PubMed] [Google Scholar]
- 10.Kozlowski S, Corr M, Takeshita T, et al. Serum angiotensin-1 converting enzyme activity processes a human immunodeficiency virus 1 gp160 peptide for presentation by major histocompatibility complex class I molecules. J Exp Med. 1992;175:1417. doi: 10.1084/jem.175.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neefjes JJ, Momburg F, Hammerling GJ. Selective and ATP-dependent translocation of peptides by the MHC-encoded transporter. Science. 1993;261:769. doi: 10.1126/science.8342042. [DOI] [PubMed] [Google Scholar]
- 12.Momburg F, Roelse J, Hammerling GJ, Neefjes JJ. Peptide size selection by the major histocompatibility complex-encoded peptide transporter. J Exp Med. 1994;179:1613. doi: 10.1084/jem.179.5.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elliott T, Willis A, Cerundolo V, Townsend A. Processing of major histocompatibility class I-restricted antigens in the endoplasmic reticulum. J Exp Med. 1995;181:1481. doi: 10.1084/jem.181.4.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snyder HL, Yewdell JW, Bennink JR. Trimming of antigenic peptides in an early secretory compartment. J Exp Med. 1994;180:2389. doi: 10.1084/jem.180.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenlohr LC, Bacik I, Bennink JR, Bernstein K, Yewdell JW. Expression of a membrane protease enhances presentation of endogenous antigens to MHC class I-restricted T lymphocytes. Cell. 1992;71:963. doi: 10.1016/0092-8674(92)90392-p. [DOI] [PubMed] [Google Scholar]
- 16.Chakrabarti S, Robert-Guroff M, Wong-Staal F, Gallo RC, Moss B. Expression of the HTLV-III envelope gene by a recombinant vaccinia virus. Nature. 1986;320:535. doi: 10.1038/320535a0. [DOI] [PubMed] [Google Scholar]
- 17.Hosken NA, Bevan MJ, Carbone FR. Class I-restricted presentation occurs without internalization or processing of exogenous antigenic peptides. J Immunol. 1989;142:1079. [PubMed] [Google Scholar]
- 18.Lanzillo JJ, Fanburg BL. Angiotensin I converting enzyme from human plasma. Biochemistry. 1977;16:5491. doi: 10.1021/bi00644a015. [DOI] [PubMed] [Google Scholar]
- 19.Cushman DW, Cheung HS, Sabo EF, Ondetti MA. Design of potent competitive inhibitors of angiotensin-converting enzyme. Carboxyalkanoyl and mercaptoalkanoyl amino acids. Biochemistry. 1977;16:5484. doi: 10.1021/bi00644a014. [DOI] [PubMed] [Google Scholar]
- 20.Yewdell JW, Bennink JR. Brefeldin A specifically inhibits presentation of protein antigens to cytotoxic T lymphocytes. Science. 1989;244:1072. doi: 10.1126/science.2471266. [DOI] [PubMed] [Google Scholar]
- 21.Shimonkevitz R, Kappler J, Marrack P, Grey H. Antigen recognition by H-2-restricted T cells. I. Cell-free antigen processing. J Exp Med. 1983;158:303. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falk K, Rotzschke O, Rammensee H-G. Cellular peptide composition governed by major histocompatibility complex class I molecules. Nature. 1990;348:248. doi: 10.1038/348248a0. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi H, Nakagawa Y, Leggatt GR, Ishida Y, Saito T, Yokomuro K, Berzofsky JA. Inactivation of human immunodeficiency virus (HIV)-1 envelope-specific CD8+ cytotoxic T lymphocytes by free antigenic peptide: a self-veto mechanism? J Exp Med. 1996;183:879. doi: 10.1084/jem.183.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozlowski S, Corr M, Shirai M, Boyd LF, Pendleton CD, Berzofsky JA, Margulies DH. Multiple pathways are involved in the extracellular processing of MHC class I-restricted peptides. J Immunol. 1993;151:4033. [PubMed] [Google Scholar]
- 25.Wei L, Alhenc-Gelas F, Soubrier F, Michaud A, Corvol P, Clauser E. Expression and characterization of recombinant human angiotensin I-converting enzyme. J Biol Chem. 1991;266:5540. [PubMed] [Google Scholar]