Abstract
Listeria monocytogenes is a facultative intracellular pathogen which is internalized by host mammalian cells upon binding to their surface. Further listerial growth occurs in the cytosol after escape from the phagosomal–endosomal compartment. We have previously reported that C1q is able to potentiate L. monocytogenes phagocytosis upon bacterial opsonization by ingestion through C1q-binding structures. In this report, we analysed the post-phagocytic events upon internalization of C1q-opsonized L. monocytogenes and found an induction of macrophage (Mφ)-like IC-21 cell bactericidal mechanisms displayed by the production of oxygen and nitrogen metabolites. Both types of molecules are effective in L. monocytogenes killing. Further analysis of the cellular responses promoted by interaction of C1q with its surface binding structures, leads us to consider C1q as a collaborative molecule involved in Mφ activation. Upon interaction with surface binding structures, C1q was able to trigger and/or amplify the production of reactive oxygen and nitrogen intermediates induced by stimuli such as interferon-γ and L. monocytogenes phagocytosis.
Introduction
Listeria monocytogenes is a facultative intracellular pathogen able to survive and multiply within macrophages (Mφ) 1 as well as in Mφ-like cell lines.2 It can also invade a wide range of normally non-phagocytic cell types, including enterocytes, hepatocytes, fibroblasts and dendritic and endothelial cells.3–5 Listeria monocytogenes is well adapted to the intracellular niche, due to a variety of features: it produces membrane-active exoproteins that mediate phagosomal disruption and bacterial escape to the cytosol; it has regulatory mechanisms for the preferential expression of bacterial genes in infected mammalian cells; and one of its surface proteins, ActA, mediates migration inside and between cells by exploiting the eukaryotic host cell cytoskeletal machinery (reviewed in refs. 2, 6 and 7).
Involvement of C3bi8 and C1q9 complement receptors in L. monocytogenes uptake by phagocytic cells has been documented. Listeria monocytogenes can also be efficiently internalized by phagocytic cells through non-opsonic receptor–ligand interactions. First, the listerial surface proteins InlA (or internalin) and InlB have been shown to mediate penetration into epithelial cells and hepatocytes, both in vitro and in vivo, and to have a role in cell tropism10–13 and second, the listerial surface protein ActA, a major virulence factor primarily involved in actin-based intracellular motility, is also a bacterial ligand in the recognition of the heparan sulphate–proteoglycan receptors on the host cytoplasmic membranes.14
Whereas there has been a general agreement about the contribution of reactive oxygen intermediates (ROI) to listericidal mechanisms,15,16 results on the involvement of reactive nitrogen intermediates (RNI) in the course of listerial infection have been conflicting. For example, some studies suggested that ROI are more important than RNI in the intracellular killing of L. monocytogenes.15,17 However, others clearly indicated the important role of RNI in the contribution to the intracellular elimination of this pathogen.18–20 Many of these disparate results could be explained by differences in the murine strain considered, in the source of host cells, in the conditions for in vitro culture and in the assays of intracellular growth or by a combination of these factors.
In the present report, we attempted to explore the intracellular fate of both unopsonized or C1q-opsonized L. monocytogenes in IC-21 cells as well as to contribute to the elucidation of the involvement of ROI and RNI in the listericidal mechanisms used by this Mφ-like cell line during infection in vitro. We report here that C1q-opsonized L. monocytogenes grow less efficiently intracellularly than the unopsonized organisms. We consequently studied the role of C1q on the expression of those cell functional activities which define an activation state. Experiments were particularly focused on whether C1q would enhance these effector functions. We present evidence that C1q acts as a collaborative molecule for IC-21 cell activation, mainly because it: enhances listericidal capacity and is required to maintain this listericidal function upon induction by other signals; and can amplify ROI and RNI production induced by other stimuli.
Materials and methods
General reagents
The culture medium RPMI-1640 (R0), l-glutamine, fetal calf serum (FCS), Hanks' balanced salt solution (HBSS) and gentamicin sulphate were obtained from Flow (Irvine, UK). Ferricytochrome C, phenol red, sulphanilamide, naphthlylethylene diamine dihydrochloride, phosphoric acid, bovine superoxide dismutase (EC 1.15.1.1; SOD), horseradish peroxidase (EC 1.11.1.7; HRPO), antimycins A1–A4, d-mannitol and 2-mercaptoethylamine (cysteamine) were from Sigma (Sigma Chemical Co, St. Louis, MO). NG-monomethyl-l-arginine (NMMA) and hydrazinecarboximidamide hemisulphate (aminoguanidine) were obtained from Calbiochem (San Diego, CA). Murine recombinant interferon-γ (IFN-γ) was obtained from Stratagene Cloning Systems (La Jolla, CA).
Bacteria
Listeria monocytogenes L028 serovar 1/2c used in this study has been described previously.2 The L. monocytogenes strain was grown in brain–heart infusion broth (Difco Laboratories, Detroit, MI) with aeration at 37°. The bacteria were harvested in logarithmic growth phase and stored at −70° in phosphate-buffered saline (PBS) with 20% glycerol (v/v) until used.
Cells
The murine Mφ-like cells IC-21 (American Type Culture Collection TIB186; ATCC, Rockville, MD) share many functional and phenotypic Mφ characteristics, are phagocytic but not listericidal unless primed with IFN-γ and are useful models for studying the interactions of L. monocytogenes with mammalian phagocytes.2 Cells were plated in 96-well plates and incubated for at least 2 hr at 37°. Cells were maintained in R0 medium supplemented with 10% FCS, 2 mm l-glutamine, and gentamicin (50 µg/ml) (R10 medium). All media and reagents were confirmed to be endotoxin-free (< 0·01 ng/ml) by a chromogenic Limulus amoebocyte lysate microassay from Whittaker M.A. Bioproducts (Walkersville, MD).
Purification of the human C1q complement component
Human C1q and murine C1q display only minor differences in properties, with sequences displaying > 80% identity.21 For these reasons, as well as for the practical ones of economy and simplicity, C1q was isolated from human donor plasma as reported.9 All C1q preparations were homogeneous as determined by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) on 12% gels under reduced conditions and stained with Coomassie brilliant blue. C1q preparations were endotoxin free.
Intracellular growth assay
The assay was performed as previously described.2,9 Briefly, Mφ-like IC-21 cells (at 2 × 106 cells/ml) were plated in 96-well tissue culture plates (Costar, Cambridge, MA) the evening before use and infected, usually, with 2 × 107L. monocytogenes/ml. Infected cells were allowed to ingest bacteria, followed by gentamicin (10 µg/ml) treatment (unless otherwise stated), to eliminate extracellular bacteria. Time 0 was defined as the end of this incubation period. After washing, cells were incubated in R0 for different periods (up to 6 hr) and lysed by several freeze–thawing cycles. The number of viable bacteria per well was determined by quantitative plate counts of colony-forming units (CFU) on blood agar plates (Columbia Blood Agar, Becton Dickinson, San José, CA). Each result presented is the mean of three determinations. In some experiments the effect of different inhibitors of ROI and RNI production (mannitol, cysteamine, NMMA and aminoguanidine) on L. monocytogenes intracellular growth was tested by treating the host cells for 3 hr with inhibitors prior to infection. After washing, the cells were infected as described above. As an intracellular growth measure we defined the replication index (RI) as the number of CFU at a given time divided by the number of CFU at time 0. Values represent the increase in CFU from time 0 being RI > 1.2
Measure of superoxide anion production
O2 − production was measured by the SOD-inhibitable reduction of ferricytochrome C adapted to a microplate format as described previously.22 The appropriate stimuli were: C1q (100 and 300 µg/ml) adsorbed to the plates or in a soluble form; L. monocytogenes (105 and 106) opsonized or not opsonized with C1q (usually at 100 µg/ml) and inhibitors of both ROI and RNI production. Results are expressed as nmol O2– produced per mg of protein. Each result represents the mean of eight determinations.
Quantification of hydrogen peroxide production
H2O2 production was measured by a technique based on the HRPO-dependent conversion of phenol red by H2O2 into a compound with increased absorbance at 600 nm as described.22 Cells were cultured with or without the appropriate stimuli [C1q adsorbed on well bottoms, L. monocytogenes (1 × 105/ml) opsonized or not opsonized with increasing amounts of C1q, or inhibitors]. The quantity of H2O2 produced in each case was interpolated from the standard curve. Results are expressed as nmol H2O2 produced per mg of protein. Each result represents the mean of eight determinations.
Determination of nitrite production
The generation of NO2– by IC-21 cells was determined as described.22 Briefly, cells were incubated in R10 medium in a total volume of 200 µl, on C1q-coated or uncoated plates, in the presence or absence of IFN-γ (100 IU/ml) and/or bacteria (1 × 104/ml) and were opsonized or not with increasing concentrations of C1q. Results of unknown culture fluids were expressed as nmol of NO2– produced per well; which was derived from a sodium nitrite standard curve. Each result represents the mean of eight determinations.
Statistical analysis
Data are expressed as the mean ± standard deviation (SD). The statistical significance of the different experimental conditions was determined by the paired Student's t-test using microstat (Ecosoft Inc., Indianapolis, IN).
Results
Role of exogenous C1q on triggering the listericidal activity of IC-21 cells
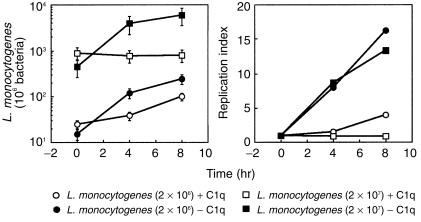
We have described L. monocytogenes binding to C1q, which mediates the enhanced uptake by Mφ-like cell lines through C1q-binding structures.9 We first considered it to be of interest to study the intracellular fate of C1q-opsonized L. monocytogenes in IC-21 cells in parallel with untreated bacteria. Figure 1 shows a typical experiment performed with IC-21 cells. Data from this experiment clearly show that C1q-opsonized L. monocytogenes grow intracellularly less efficiently than when not opsonized. This effect is most evident when the L. monocytogenes replication indices are evaluated: Fig. 1 shows that opsonized bacteria clearly grew more slowly than the others, which could indicate that C1q-opsonized L. monocytogenes are more easily killed by host cells or, alternatively, exhibit a slower growth rate.
Figure 1.
Role of C1q on L. monocytogenes intracellular replication. L. monocytogenes, 2 × 106 and 2 × 107 preopsonized or not with C1q (100 µg/ml) were used to infect host cells, as described in the Materials and methods. Experiments were performed with 3 × 105 IC-21 cells/well. (a) Number of bacteria/well as a function of time; (b) replication index of L. monocytogenes at these times. Data represent the mean intracellular L. monocytogenes/well ± SD of a representative experiment of three performed.
C1q-opsonized L. monocytogenes phagocytosis correlates with ROI and RNI production by IC-21 cells
Hypothetically, the above results suggest that host cell effector activities should have been induced. Such effector processes are usually displayed by both ROI production (including O2– and H2O2), and RNI production (measured by the NO2– release). Both RNI23,24 and ROI23 have been reported to exhibit a potent anti-listerial activity in vitro.
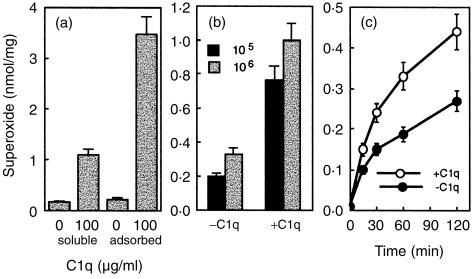
We analysed O2– production first and found it to be triggered mainly by adsorbed C1q (Fig. 2a) in IC-21 cells. Soluble C1q only induced weak production. Further analysis of O2– production upon L. monocytogenes phagocytosis revealed that ingestion of C1q-opsonized L. monocytogenes significantly enhanced the O2– production in IC-21 cells (Fig. 2b). Such increase in O2– production induced by C1q-opsonized L. monocytogenes, was evident early in the reaction and remained constant or even increased over time (Fig. 2c).
Figure 2.
C1q-opsonized L. monocytogenes and adsorbed C1q onto the wells induce O2– production and correlates with the bacterial ingestion. (a) O2– production was measured in IC-21 cells (at 3 × 105/well) incubated with the following stimuli: C1q (100 µg/ml) adsorbed to plates or added in solution. (b) In this experiment IC-21 cells (at 3 × 105/well) were exposed to 1 × 105 or 1 × 106L. monocytogenes previously opsonized or not with C1q (100 µg/ml). This shows O2– production measured as described after 60 min incubation with each stimulus. (c) A kinetic study of O2– production by IFN-γ-treated IC-21 cells infected with 1 × 105L. monocytogenes opsonized with or without C1q. Data are presented as the mean ± SD nmol of O2– produced per mg of protein in three different experiments.
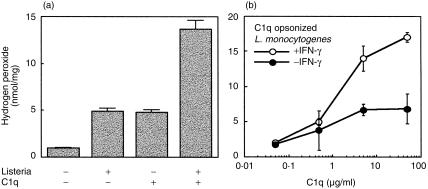
Accordingly, H2O2 production was also induced by immobilized C1q as well as by L. monocytogenes phagocytosis (Fig. 3a). However, the addition of L. monocytogenes stimuli generated two-fold more H2O2. Similarly, in other experiments, H2O2 generation was significantly increased by phagocytosis of C1q-opsonized L. monocytogenes (Fig. 3b). As expected, this effect was considerably enhanced in the presence of IFN-γ (Fig. 3b).
Figure 3.
Enhancement of H2O2 production by C1q-opsonized L. monocytogenes phagocytosis as well as by C1q adsorbed onto the wells. (a) H2O2 production was quantified in IC-21 cells. Approximately 3 × 105 cells/well were incubated for 60 min with different stimuli: C1q adsorbed onto the plate wells (5 µg/ml) and/or L. monocytogenes (1 × 106). (b) Enhancement of H2O2 production in response to L. monocytogenes opsonized with increasing amounts of C1q in the presence or not of IFN-γ (100 U/ml). Results are expressed as the mean ± SD nmol of H2O2 produced per mg of protein of eight determinations.
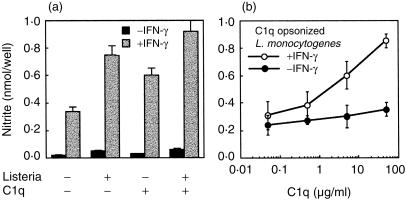
Mφ release of RNI is induced by a combination of IFN-γ and soluble (e.g. lipopolysaccharide) or particulate (e.g. L. monocytogenes) stimuli as amplifier co-signals. We have studied the role of C1q on the RNI production by IC-21 cells triggered by IFN-γ and listerial uptake. Listeria monocytogenes opsonized or not with C1q, in the presence or absence of IFN-γ, was used to trigger NO2– release (Fig. 4), As shown in Fig. 4(a), immobilized C1q is able to enhance the NO2– production induced by signals such as L. monocytogenes plus IFN-γ. In a different experiment, analysis of NO2– production induced in IFN-γ-treated IC-21 cells by L. monocytogenes phagocytosis also revealed clear enhancement when bacteria were opsonized with C1q (Fig. 4b).
Figure 4.
C1q potentiates the NO2– production induced by different stimuli. (a) NO2– production is shown for IFN-γ-treated IC-21 cells (at 3 × 105/well) incubated for 48 hr (with or without IFN-γ[100 U/ml]) in plates with (5 µg/ml) or without C1q adsorbed on wells in the presence or absence of bacteria (1 × 105). (b) Comparison of the NO2– response of IC-21 cells to varying concentrations of C1q used to opsonize L. monocytogenes (1 × 105 bacteria) in the presence or not of IFN-γ (100 U/ml). Results are expressed as the mean ± SD of eight determinations of nmol of NO2– produced/well.
These data seem to indicate that upon phagocytosis of C1q-opsonized bacteria, ROI and RNI production in IC-21 cells was clearly stimulated in comparison with the production induced by unopsonized micro-organisms.
Inhibitors of ROI and RNI abrogate the listericidal activity induced by phagocytosis of C1q opsonized L. monocytogenes
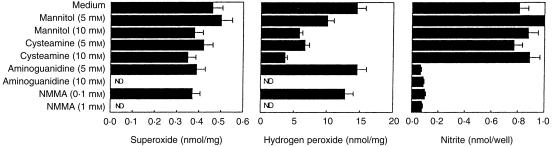
Selective inhibitors are extremely useful probes for characterizing target molecules or defining function, particularly when the targets are critical in intracellular networks. The effects of several putative scavengers on both ROI and RNI generation by IC-21 cells were first titrated in each case. It is important to mention that the IC-21 viability was unaffected by these inhibitors, neither at the concentrations tested, nor over the experimental time–course. However, experiments with inhibitors were only considered valid if the degree of inhibition produced correlated with the rate constants for scavenger reaction with ·OH. Inhibition of H2O2 by both mannitol (an ·OH scavenger) and cysteamine (an ·OH and HOCl scavenger) was dose dependent but these scavengers did not affect O2– production. Antioxidant enzymes, catalase or SOD, did not affect the H2O2 generation, probably because they cannot easily diffuse into the IC-21 cells (data not shown). It is generally assumed that RNI are also triggered in addition to the ROI generated with the respiratory burst. Aminoguanidine, an inducible nitric oxide-synthase (iNO-synthase) inhibitor25 and NMMA only inhibited RNI generation as expected (Fig. 5).
Figure 5.
Effect of scavengers and inhibitors on the generation of both oxygen and nitrogen intermediates. Parallel sets of IC-21 cells incubated with L. monocytogenes (104−105 bacteria/ml) were assayed for H2O2, O2– and NO2– production, in the absence (medium) or presence of the indicated reagents, as described in the Materials and methods. Results are expressed as the mean ± SD of triplicate determinations.
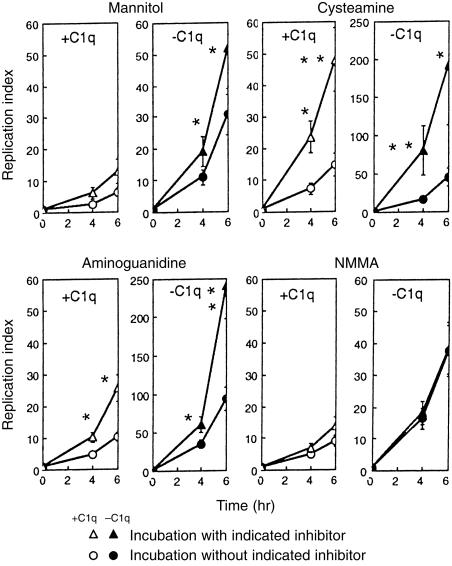
In order to asses the role of both ROI and RNI in the Mφ listericidal activity induced by C1q-opsonization, the following experiment was performed. IC-21 Mφ-like cells were preincubated for 3 hr in the presence or absence of ROI and RNI inhibitors and then infected with C1q-opsonized L. monocytogenes or unopsonized bacteria as described. Figure 6 shows firstly, C1q-opsonized L. monocytogenes ingestion by IC-21 cells exhibited a significant reduction in intracellular bacteria relative to control (unopsonized L. monocytogenes) (as also shown in Fig. 1). Second, unopsonized L. monocytogenes growth in IC-21 cells previously treated with inhibitors (except when NMMA was used) was enhanced and third, cell treatment with cysteamine and aminoguanidine (mannitol and NMMA were without effect) significantly enhanced replication indices when intracellular growth of C1q-opsonized bacteria was considered. These observations suggest that ROI and RNI participate in intracellular L. monocytogenes killing.
Figure 6.
IC-21 cells kill intracellular C1q-opsonized L. monocytogenes by an oxygen and nitrogen-dependent mechanism. IC-21 cells were incubated for 3 hr with or without the inhibitors indicated and then, infected with bacteria previously opsonized with (100 µg/ml) or without C1q for 60 min at 4°. Gentamicin (5 µg/ml) was added after 1 hr. Values are expressed as replication index of L. monocytogenes at different times. Data represent the mean replication index of three experiments. *P < 0·05; **P < 0·01.
Finally, in order to test the role of the mitochondrial respiration indirectly, L. monocytogenes phagocytosis by IC-21 cells was performed in the presence of antimycins (these are inhibitors of the electron transport along the respiratory chain). As expected, L. monocytogenes phagocytosis, opsonized or not with C1q, was not affected by antimycin treatment (10−3−10−4 m) (data not shown). These observations agree with our previous study in both virulent L. monocytogenes and its non-haemolytic mutant, demonstrating that interference with respiration or oxidative phosphorylation has no effect.2
Discussion
Previous studies in both IC-21 and P388D1 cells demonstrated that C1q was able to enhance L. monocytogenes phagocytosis by direct binding to the bacterium and ingestion through C1q-binding structures.9 In this report we show that those bacteria with C1q on their surface are not as sucessful in establishing optimal intracellular replication as unopsonized bacteria (in spite of C1q-opsonized L. monocytogenes being bound and taken up by IC-21 cells in higher numbers than those which are not [ref. 9 and present report]). It has been proposed that individual, or more probably the combined production of both ROI and RNI molecules is responsible for direct intracellular L. monocytogenes killing.18 The unsuccessful growth when C1q-opsonized L. monocytogenes are taken up by Mφ could hypothetically be due to enhanced triggering of both ROI and RNI production by C1q-opsonized L. monocytogenes. Support for this hypothesis comes from the following observations reported in the present study: the C1q interaction with structures present on the cell membrane triggers production of potentially harmful ROI (H2O2 and O2–); C1q is able to amplify RNI production; and L. monocytogenes intracellular growth was enhanced by pretreatment of host cells with ROI as well as RNI inhibitors.
Although unopsonized L. monocytogenes is able to induce a respiratory burst this is not as important as that induced by C1q-opsonized bacteria. The intracellular replication rate of unopsonized bacteria is enhanced by both ROI and aminoguanidine (NMMA was ineffective).
Induction of ROI and RNI production by C1q probably requires a conformational change in this molecule. Soluble C1q only provokes a weak cellular response, unlike immobilized C1q. These results agree with those previously reported by others.26 In addition, we found a correlation between phagocytosis of opsonized bacteria and enhanced production of reactive molecules, indicating that the C1q conformational change required for cellular responses could be stabilized by immobilization to plastic, by coating latex beads with C1q (unpublished observations) or by opsonization of whole L. monocytogenes. The increased ROI and RNI generation upon phagocytosis of C1q-opsonized L. monocytogenes could explain the somewhat unsuccessful intracellular replication of these opsonized bacteria. In this respect, analysis of the phagocytosis of C1q-opsonized L. monocytogenes revealed that the slower L. monocytogenes intracellular growth observed was in part reversed by ROI and RNI inhibitors. These data indicate that scavenging ·OH radicals and HOCl (cysteamine or mannitol treatment) were effective in avoiding L. monocytogenes killing. Our observations agree with the generally accepted assumption that the combination of O2– and H2O2 is one of the most potent mechanisms of bacterial killing. Intracellular listericidal ability elicited by ingestion of C1q opsonized bacteria was also impaired by aminoguanidine (a selective inhibitor of the cytokine-inducible form of NO-synthase) or by NMMA (another competitive inhibitor of NO-synthase). Interestingly, aminoguanidine was shown to stimulate IFN-γ production18 as well as antigen presentation22 but the significance of these observations is not clear. It has recently been shown that mice deficient in both the gp91 subunit of NADPH oxidase and NO-synthase could not kill L. monocytogenes.16 However, the killing of attenuated L. monocytogenes as well as Salmonella typhimurium and Escherichia coli was diminished but demonstrable, suggesting the existence of alternative anti-bacterial pathways.16 In our model therefore listericidal activity induced by C1q could be exerted by both ROI and RNI molecules and could be considered an amplified signal of that induced by IFN-γ in Mφ and in the Mφ-like IC-21 cell line (refs 27,28 and present report). In conclusion, the listericidal mechanisms displayed by IC-21 cells seem to depend on both ROI and RNI generation, as previously suggested by others.18
Complement components have been postulated to participate in the phagocytosis of many intracellular pathogens mainly via CR1, CR3 and/or CR4,28–30 as well as through C1q-binding structures.9,31,32 However, it seems that C3 does not trigger an appreciable oxidative burst and hence its surface receptors constitute a safe passage into host cells for the parasites that use them, in spite of enhanced parasite phagocytosis (as with Leishmania spp.29). It has been described that L. monocytogenes are able to use CR3 to enter Mφ33 and further studies have shown that L. monocytogenes uptake through CR3 leads to bacterial killing.8,34 Nevertheless, the nature of the listericidal mechanisms triggered by such an entry route are unknown and no correlation between bacterial killing through CR3 phagocytosis and triggering of oxidative mechanisms was provided. Probably, entry through C1q-binding molecules or through CR3 could lead to induction of different cellular signals. Recently, it was reported that C1q binds specifically to human CR1 (CD35), the leucocyte C3b/C4b receptor.35 However, CR1 is not the only C1q receptor, since C1q does bind and activate cells which do not express CR1 (e.g. endothelial cells).36 The specific role of CR1, other proposed C1q receptors (classified into two groups: intracellular C1q-binding molecules and cell surface C1q receptors)37,38 or structures that are associated with them (e.g. CD21) 39 remain to be clearly defined.
Finally, C1q-coated latex beads (but not soluble C1q or latex beads alone) enhance the antigen presentation capacity of peritoneal Mφ along with tumoricidal activity (unpublished data), supporting the idea that C1q is a co-stimulatory signal for Mφ activation. These observations are in accordance with those indicating that exogenous C1q added to Mφ is important for lipid A-mediated tumour cytotoxicity.40,41 In summary, our data suggest that the route of L. monocytogenes ingestion into IC-21 cells (preferentially if it is C1q-mediated) can lead to different cellular signals, some of which induce the expression of functions such as bactericidal activity exerted by ROI and RNI generation, as well as enhanced abilities triggered by other stimuli. Together, these results suggest that C1q behaves as a collaborative molecule or a second signal for Mφ activation. Nevertheless, the possible role for C1q opsonization during an in vivo listerial infection remains to be elucidated. Future studies in the C1q-deficient mouse model42 should help to place the role of C1q-dependent listerial uptake in its physiological context.
Acknowledgments
This paper is dedicated to Priscilla Ann Campbell deceased on January 3, 1998. C. A.-D. performed C1q opsonization and intracellular growth assays. E. C.-M. and P. L.-M. contributed to functional assays; F. L.-C. and the two first authors contributed intellectually; F. L.-C. also provided funding and was the general advisor. This research was supported by programme FIS 94/0933. We are indebted to P. Sánchez-Velasco for statistical analysis of data and to I. M. Outschoorn (Instituto Carlos III, Madrid) for critical reading of the manuscript.
References
- 1.Sheehan B, Kocks C, Dramsi S, Gouin E, Klarsfeld AD, Mengaud J, Cossart P. Molecular and genetic determinants of the Listeria monocytogenes infectious process. Curr Top Microbiol Immunol. 1994;192:187–216. doi: 10.1007/978-3-642-78624-2_9. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Domı´nguez C, Carrasco-Marı´n E, Pérez-Dı´az JC, Baquero F, Leyva-Cobián F. Phagocytosis of Listeria monocytogenes by macrophages: effect of different agents on the structural and metabolic requirements of the host cell. Immunol Infect Dis. 1995;5:127–37. [Google Scholar]
- 3.Drevets DA, Sawyer RT, Potter TA, Campbell PA. Listeria monocytogenes infects human endothelial cells by two distinct mechanisms. Infect Immun. 1995;63:4268–76. doi: 10.1128/iai.63.11.4268-4276.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guzmán C, Rohde M, Chakraborty T, Domann E, Hudel M, Wehland J, Timmis K. Interaction of Listeria monocytogenes with mouse dendritic cells. Infect Immun. 1995;63:3665–73. doi: 10.1128/iai.63.9.3665-3673.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood S, Maroushek N, Czuprynski C. Multiplication of Listeria monocytogenes in a murine hepatocyte cell line. Infect Immun. 1993;61:3068–72. doi: 10.1128/iai.61.7.3068-3072.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lasa I, Cossart P. Actin-based bacterial motility: towards a definition of the minimal requirements. Trends Cell Biol. 1996;6:109–14. doi: 10.1016/0962-8924(96)81001-4. [DOI] [PubMed] [Google Scholar]
- 7.Portnoy DA, Chakraborty T, Goebel W, Cossart P. Molecular determinants of Listeria monocytogenes pathogenesis. Infect Immun. 1992;60:1263–7. doi: 10.1128/iai.60.4.1263-1267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drevets DA, Campbell PA. Roles of complement and complement receptor type 3 in phagocytosis of Listeria monocytogenes by inflammatory mouse peritoneal macrophages. Infect Immun. 1991;59:2645–52. doi: 10.1128/iai.59.8.2645-2652.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alvarez-Domı´nguez C, Carrasco-Marı´n E, Leyva-Cobián F. Role of complement component C1q in phagocytosis of Listeria monocytogenes by murine macrophage-like cell lines. Infect Immun. 1993;61:3664–72. doi: 10.1128/iai.61.9.3664-3672.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dramsi S, Biswas I, Maguin E, Braun L, Mastroeni P, Cossart P. Entry of Listeria monocytogenes into hepatocytes requires expression of InlB a surface protein of the internalin multigene family. Mol Microbiol. 1995;16:251–61. doi: 10.1111/j.1365-2958.1995.tb02297.x. [DOI] [PubMed] [Google Scholar]
- 11.Gaillard J-L, Berche P, Frehel C, Gouin E, Cossart P. Entry of L. monocytogenes into cells is mediated by internalin a repeat protein reminiscent of surface antigens from Gram-positive cocci. Cell. 1991;65:1127–41. doi: 10.1016/0092-8674(91)90009-n. [DOI] [PubMed] [Google Scholar]
- 12.Gaillard J-L, Finlay BB. Effect of cell polarization and differentiation on entry of Listeria monocytogenes into the enterocyte-like caco-2 cell line. Infect Immun. 1996;64:1299–308. doi: 10.1128/iai.64.4.1299-1308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mengaud J, Ohayon H, Gounon P, Mège RM, Cossart P. E-cadherin is the receptor for internalin a surface protein required for entry of L monocytogenes into epithelial cells. Cell. 1996;84:923–32. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 14.Alvarez-Domı´nguez C, Vázquez-Boland JA, Carrasco-Marı´n E, López-Mato P, Leyva-Cobián F. Host cell heparan sulfate proteoglycans mediate attachment and entry of Listeria monocytogenes and the listerial surface protein ActA is involved in heparan sulfate receptor recognition. Infect Immun. 1997;65:78–88. doi: 10.1128/iai.65.1.78-88.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohya S, Tanabe Y, Makino M, Nomura T, Xiong H, Arakawa M, Mitsuyama M. The contributions of reactive oxygen intermediates and reactive nitrogen intermediates to listericidal mechanisms differ in macrophages activated pre- and postinfection. Infect Immun. 1998;66:4043–9. doi: 10.1128/iai.66.9.4043-4049.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiloh MU, MacMicking JD, Nicholson S, et al. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity. 1999;10:29–38. doi: 10.1016/s1074-7613(00)80004-7. [DOI] [PubMed] [Google Scholar]
- 17.Inoue S, Itagaki S-I, Amano F. Intracellular killing of Listeria monocytogenes in the J7741 macrophage-like cell line and the lipopolysaccharide LPS-resistant mutant LPS1916 cell line defective in the generation of reactive oxygen intermediates after LPS treatment. Infect Immun. 1995;63:1876–86. doi: 10.1128/iai.63.5.1876-1886.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beckerman KP, Rogers HW, Corbett JA, Schreiber RD, McDaniel ML, Unanue ER. Release of nitric oxide during the T cell-independent pathway of macrophage activation: its role in resistance to Listeria monocytogenes. J Immunol. 1993;150:888–95. [PubMed] [Google Scholar]
- 19.Boockvar KS, Granger DL, Poston RM, Maybodi M, Washington MK, Hibbs Jb, Jr, Kurlander RL. Nitric oxide produced during murine listeriosis is protective. Infect Immun. 1994;62:1089–100. doi: 10.1128/iai.62.3.1089-1100.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacMicking JD, Nathan C, Hom G, et al. Altered response to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–50. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 21.Dodds AW, Petry F. The phylogeny and evolution of the first component of complement C1. Behring Inst Mitt. 1993;93:87–102. [PubMed] [Google Scholar]
- 22.Carrasco-Marı´n E, Paz-Miguel JE, López-Mato P, Alvarez-Domı´nguez C, Leyva-Cobián F. Oxidation of defined antigens allows protein unfolding and increases both proteolytic processing and exposes peptide epitopes which are recognized by specific T cells. Immunology. 1998;95:314–21. doi: 10.1046/j.1365-2567.1998.00618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bortolussi R, Vanderbroucke-Grauls CMJE, Asbeck BS, Verhoef J. Relation of bacterial growth phase to killing of Listeria monocytogenes by oxidative agents generated by neutrophils and enzyme systems. Infect Immun. 1987;55:3197–203. doi: 10.1128/iai.55.12.3197-3203.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gregory SH, Wing EJ, Hoffman RA, Simmons RK. Reactive nitrogen intermediates suppress the primary immunological response to Listeria. J Immunol. 1993;150:2901–9. [PubMed] [Google Scholar]
- 25.Misko TP, Moore WM, Kasten TP, et al. Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur J Pharmacol. 1993;233:119–25. doi: 10.1016/0014-2999(93)90357-n. [DOI] [PubMed] [Google Scholar]
- 26.Goodman EB, Tenner A. Signal transduction mechanism of C1q-mediated superoxide production Evidence for the involvement of temporally distinct staurosporine-insensitive and -sensitive pathways. J Immunol. 1992;148:3920–8. [PubMed] [Google Scholar]
- 27.Buchmeier N, Schreiber RD. Requirement of endogenous interferon-γ production for resolution of Listeria monocytogenes infection. Proc Natl Acad Sci USA. 1985;82:7404–8. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlesinger LS, Bellinger-Kawashara CG, Payne NR, Horwitz HA. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement component C3. J Immunol. 1990;144:2771–80. [PubMed] [Google Scholar]
- 29.Mosser DM, Edelson PJ. The third component of complement C3 is responsible for the intracellular survival of Leishmania major. Nature. 1987;327:329–31. doi: 10.1038/327329b0. [DOI] [PubMed] [Google Scholar]
- 30.Schlesinger LS, Horwitz HA. Phagocytosis of Mycobacterium leprae by human monocyte-derived macrophages is mediated by complement receptors CR1, CD35, CR3 CD11b/CD18 and CR4 CD11c/CD18 and IFN–γ activation inhibits complement receptor function and phagocytosis of this bacterium. J Immunol. 1991;147:1983–94. [PubMed] [Google Scholar]
- 31.Rimoldi MT, Tenner AJ, Bobak DA, Joiner KA. Complement component C1q enhances invasion of human mononuclear phagocytes and fibroblasts by Trypanosoma cruzi trypomastigotes. J Clin Invest. 1989;84:1982–9. doi: 10.1172/JCI114388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryan US, Schultz DR, Goodwin JD, Vann JM, Selvaraj MP, Hart MA. Role of C1q in phagocytosis of Salmonella minnesota by pulmonary endothelial cells. Infect Immun. 1989;57:1356–62. doi: 10.1128/iai.57.5.1356-1362.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drevets DA, Leenen PJM, Campbell PA. Complement receptor type 3 CD11b/CD18 involvement is essential for killing of Listeria monocytogenes by mouse macrophages. J Immunol. 1993;151:5431–9. [PubMed] [Google Scholar]
- 34.Drevets DA, Canono BP, Campbell PA. Listericidal and nonlistericidal mouse macrophages differ in complement receptor type 3-mediated phagocytosis of L monocytogenes and in preventing escape of the bacteria into cytoplasm. J Leukocyte Biol. 1992;52:70–9. doi: 10.1002/jlb.52.1.70. [DOI] [PubMed] [Google Scholar]
- 35.Klickstein LB, Barbashov SF, Liu T, Jack RM, Nicholson-Weller A. Complement receptor type 1, CR1, CD35 is a receptor for C1q. Immunity. 1997;7:345–55. doi: 10.1016/s1074-7613(00)80356-8. [DOI] [PubMed] [Google Scholar]
- 36.Nepomuceno RR, Tenner AJ. C1qRP the C1q receptor that enhances phagocytosis is detected specifically in human cells of myeloid lineage endothelial cells and platelets. J Immunol. 1998;160:1929–35. [PubMed] [Google Scholar]
- 37.Nicholson-Weller A, Klickstein LB. C1q-binding and C1q receptors. Curr Opin Immunol. 1999;11:42–6. doi: 10.1016/s0952-7915(99)80008-9. [DOI] [PubMed] [Google Scholar]
- 38.Tenner AJ. C1q receptors: regulating specific functions of phagocytic cells. Immunobiology. 1998;199:250–64. doi: 10.1016/S0171-2985(98)80031-4. [DOI] [PubMed] [Google Scholar]
- 39.Matsumoto AK, Martin DR, Carter RH, Klickstein LB, Ahearn JM, Fearon DT. Fuctional dissection of the CD21/CD19/TAPA-1/Leu-13 complex of B lymphocytes. J Exp Med. 1993;178:1407–17. doi: 10.1084/jem.178.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leu RW, Zhou A, Shannon BJ, Herriot MJ. Inhibitors of C1q biosynthesis suppress activation of murine macrophages for both antibody-dependent and antibody-independent tumor cytotoxicity. J Immunol. 1990;144:2281–6. [PubMed] [Google Scholar]
- 41.Leu RW, Zhou A, Rummage J, Fast DJ, Shannon BJ. Reconstitution of a deficiency of AKR mouse macrophages for their response to lipid A activation for tumor cytotoxicity by complement subcomponent C1q: role of IFN-γ. J Immunol. 1991;147:1315–21. [PubMed] [Google Scholar]
- 42.Botto M, Dell'agnola C, Bygrave AE, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–9. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]