Abstract
We previously reported delayed expression of monocyte chemoattractant protein-1 (MCP-1) in human neutrophils cultured with a cytokine-rich crude supernatant of phytohaemagglutinin-stimulated peripheral blood mononuclear cells (PHA-sup). Tumour necrosis factor-α (TNF-α) contained in the PHA-sup played a key role in this event, but there appeared to be another factor(s) in the same supernatant that co-operated with TNF-α for maximal MCP-1 expression. In the present study, we reduced TNF-α concentrations in the PHA-sup to minimal levels using anti-TNF-α affinity columns (TNF-depleted-sup) and investigated the co-operation between TNF-α and TNF-depleted-sup. Nine hours of preincubation with TNF-depleted-sup altered the responsiveness of neutrophils to TNF-α and enabled TNF-α to increase the level of MCP-1 expression to a maximal level within 4 hr. The priming effect was not due to the increased expression of cell-surface TNF receptors. However, the activation of primed cells by TNF-α was clearly through TNF receptor–p55. Finally, the activity in the TNF-depleted-sup that co-operated with TNF-α was eluted at 60 000 MW on high-performance liquid chromatography–gel filtration. Thus, delayed neutrophil expression of MCP-1 is regulated by a cytokine-dependent mechanism that induces neutrophils to enter a ‘mature’ stage.
Introduction
Infiltration of leucocytes is a hallmark of the inflammatory response. Among leucocyte populations, polymorphonuclear neutrophils (PMN) are the first cells to infiltrate into sites of inflammation after invasion by pathogens. PMN have been considered to be terminally differentiated cells with a variety of important functions, including phagocytosis, generation of reactive oxygen intermediates, and release of proteolytic enzymes. PMN also produce many pro-inflammatory cytokines as well as chemokines, and influence the direction and evolution of immune processes.1 For example, PMN stimulated with lipopolysaccharide (LPS) or granulocyte–macrophage colony-stimulating factor (GM-CSF) produce interleukin-8 (IL-8), one of the CXC-subfamily of the chemokines, and PMN-derived IL-8 can contribute to the further recruitment of PMN in vivo.2 Stimulated PMN also produce macrophage inflammatory protein-1α (MIP-1α), one of the CC-subfamily of the chemokines. PMN-derived MIP-1α may play a role in the infiltration of mononuclear leucocytes that follows the infiltration of PMN during the inflammatory reactions.3 In a cutaneous delayed-type hypersensitivity (DTH) reaction in rats, immunoreactive monocyte chemoattractant protein-1 (MCP-1) was mainly associated with infiltrating PMN and neutralization of MCP-1 activity inhibited the infiltration of T cells and monocytes, suggesting an important role of PMN-derived MCP-1 in DTH.4
Cytokines or chemokines produced by epithelial cells and infiltrating leucocytes at inflammatory sites act in a complex network of interacting mediators. Cytokines such as tumour necrosis factor-α (TNF-α), IL-1 and interferon-γ (IFN-γ) are primary inducers of chemokines in this network. With regard to PMN-derived chemokines, stimulation with IFN-γ in combination with either TNF-α or LPS synergistically induces IFN-γ-inducible protein of 10 000 MW (IP-10) mRNA expression.5 In our previous study, a high level of MCP-1 expression was induced in PMN cultured with the crude supernatants of phytohaemagglutinin (PHA)-stimulated peripheral blood mononuclear cells (PBMC) (PHA-sup).6 From 3 to 5 ng of MCP-1 was secreted from 5 million PMN stimulated with the PHA-sup. MCP-1 mRNA was one of the most abundant cytokine/chemokine mRNA along with IL-8 and MIP-1 in PMN cultured overnight in the presence of the PHA-sup. TNF-α contained in the PHA-sup was a critical factor for the maximal expression of MCP-1. However, recombinant human TNF-α (rhTNF-α) alone induced only a low level of MCP-1 mRNA expression and addition of neutralizing antibody against TNF-α failed to completely abolish MCP-1 expression inducing activity in the PHA-sup. There appeared to be another factor that primed neutrophils to produce maximal levels of MCP-1.
In the present study, we further analysed the mechanisms of MCP-1 expression in human PMN and found that there was a factor(s) in the PHA-sup that co-operated with TNF-α. This factor(s) maximized the responsiveness of PMN to TNF-α in the expression of MCP-1, and the priming co-factor activity in the PHA-sup was eluted around 60 000 MW by high-performance liquid chromatography (HPLC)–gel filtration.
Materials and methods
Reagents
RPMI-1640 and TRIzol reagent were purchased from Life Technologies (Gaithersburg, MD). Fetal bovine serum (FBS) was from HyClone (Logan, UT). PHA was from Sigma (St Louis, MO). Dextran T-500 and Protein G–Sepharose Fast Flow were from Pharmacia Biotech, Inc. (Piscataway, NJ). Accu-Prep was from Accurate Chemical & Scientific Corp. (Westbury, NY). Recombinant human TNF-α (rhTNF-α), mouse monoclonal antibodies against human TNF-α, TNF receptor (TNFR)-p55 (Type I), TNFR-p75 (Type II) and human IFN-γ were from R & D Systems, Inc. (Minneapolis, MN). Neutralizing activity of anti-TNF-α was confirmed in our previous study.6 Anti-IFN-γ immunoglobulin G (IgG) inhibited the expression of IP-10 mRNA in PMN after stimulation with 1 ng/ml of TNF-α and 10 ng/ml of IFN-γ (data not shown). Recombinant TNF-α mutant proteins, Trp32Thr86TNF-α (specific for TNFR-p55) and Asn143 Arg145TNF-α (specific for TNFR-p75), were kindly provided by Dr L. Loetscher (F. Hoffmann-La Roche Ltd, Basel, Switzerland).7 Fluorescein isothiocyanate (FITC)-conjugated sheep anti-mouse IgG was from Novocastra Laboratories Ltd. (Newcastle, UK). Human β-actin cDNA was from Clontech (Palo Alto, CA) and [α-32P]dCTP was from ICN (Costa Mesa, CA).
Preparation of PHA-sup and control-sup
PBMC were isolated from leukapheresis preparations obtained from the National Institutes of Health Blood Bank, Bethesda, MD, as described previously.6 PBMC were cultured at 37° for 4–6 hr at a density of 5 × 106 cells/ml in RPMI-1640 containing 1% FBS with 5 µg/ml PHA, and the cell-free supernatants were obtained (PHA-sup). PBMC were also cultured in the absence of PHA for the same period. Just before the termination of the culture, PHA was added to 5 µg/ml, and the cell-free culture supernatants were obtained (control-sup).
Isolation and culture of PMN
PMN were obtained from heparinized blood from healthy volunteers (10 U/ml of heparin to the blood). One volume of 5% Dextran-T500 in phosphate-buffered saline (PBS) was added to three volumes of blood in 50-ml tubes for sedimentation of red blood cells. After 30 min incubation at room temperature, the leucocyte-rich plasma was overlaid onto Accu-prep and centrifuged at 800 g for 20 min. PMN were separated from erythrocytes by lysis in 0·2% NaCl, washed three times with RPMI-1640 containing 10% FBS at 4° and resuspended in the complete medium at a density of 1 × 107 cells/ml. Contamination of PBMC in PMN preparations obtained by this method was less than 0·5% by morphological examination. Cell viability was higher than 99% by trypan blue staining.
One and a half millilitres of PMN suspension (1 × 107 cells/ml) was mixed with the same volume of PHA-sup, TNF-depleted-sup (described below), or medium in the presence or absence of appropriate stimuli in a six-well cluster (Coster, Cambridge, MA). Final PMN density in the culture was 5 × 106 cells/ml of PMN. The cells were cultured at 37°, and then total RNA was extracted by using TRIzol Reagent. To inhibit the activity of TNF-α or IFN-γ in the PHA-sup, PHA-sup was preincubated with each neutralizing IgG at 37° for 30 min
Absorption of TNF-α on anti-TNF-α immunoabsorbent columns
To reduce TNF-α concentrations in the PHA-sup, immunoabsorbent minicolumns were used.8 Briefly, Pasteur pipettes were cut so that 15–20 mm length of the large bore portion remained. A small amount of glass wool was plugged into the narrow bore portion of each column. Protein G–Sepharose was added to each column (50 µl in packed volume). Columns were washed twice with 0·15 m NaCl. Five microgrammes of anti-human TNF-α mouse monoclonal IgG was added to each column. The columns were washed with 40 µl of PBS. Two and a half millilitres of PHA-sup was applied onto a column and pass-through fractions were collected as TNF-depleted-sup. Concentrations of TNF-α in the TNF-depleted-sup were quantified by an enzyme-linked immunosorbent assay (ELISA) kit (R & D Systems, detection limit was 15·6 pg/ml).
Northern blot analysis
Northen blot analysis was performed as previously described.9 Blots were hybridized with 32P-labelled human MCP-1 cDNA,10 IP-10, or β-actin cDNA probe. Levels of MCP-1 mRNA expression were quantified by Scion ImagePC (Scion Corp., Frederick, MD), standardized against the levels of β-actin expression, and shown as relative density to the MCP-1 expression level induced by PHA-sup.
Flow cytometric analysis of TNFRs
PMN were washed with ice-cold PBS containing 0·2% bovine serum albumin (BSA) and 0·1% NaN3, and resuspended in 40 µl of PBS–BSA with mouse IgG against human TNFR-p55 or TNFR-p75, or normal mouse IgG. After incubation on ice for 30 min, the cells were washed twice and incubated with 40 µl of FITC-conjugated sheep anti-mouse IgG diluted to 1 : 100. The cells were washed and analysed for the expression of TNFR-p55 or TNFR-p75, by using FACScan (Becton Dickinson, San Jose, CA).
HPLC–gel filtration
HPLC–gel filtration was performed at room temperature on a TSK-3000 column (Toyo Soda, Tokyo, Japan) equilibrated with RPMI-1640 medium. One hundred millilitres of TNF-depleted-sup was concentrated to 1 ml, and 100 µl of the concentrated material was injected into the column. Fractions of 1 ml were collected at 1 ml/min flow rate. Injection of the sample and collection of each fraction were repeated several times to obtain enough volume for assays. The column was calibrated with BSA (67 000 MW), ovalbumin (OVA, 43 000 MW), and cytochrome c (12 500 MW).
Results
Reduction of TNF-α concentration from PHA-sup decreases MCP-1 mRNA-inducing activity (MCP-1-IA) of the PHA-Sup
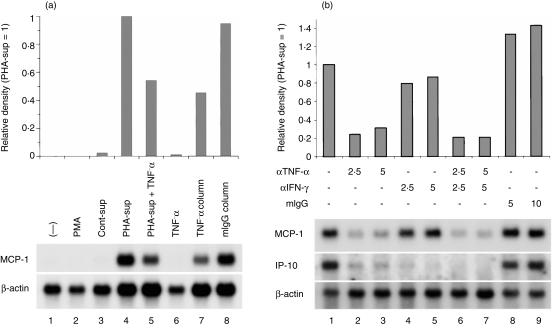
To reduce TNF-α concentrations in the PHA-sup to minimal levels (less than 0·09 ng/ml), we passed PHA-sup through two consecutive anti-TNF-α columns, and then tested the TNF-depleted-sup for its MCP-1-IA. As shown in Fig. 1(a), medium alone, 5 µg/ml of PHA, control-sup, or 5 µg of anti-TNF-α IgG did not induce significant amounts of MCP-1 mRNA (lanes 1–3, 6). Addition of anti-TNF-α IgG depleted about 45% of MCP-1-IA in the PHA-sup (lanes 4, 5). TNF-depleted-sup containing 0·04 ng/ml of TNF-α exhibited about 43% of MCP-1-IA detected in the PHA-sup (lane 7), whereas normal mouse IgG columns did not affect either TNF-α concentration or MCP-1-IA (lane 8). Since 0·04 ng/ml of TNF-α did not induce significant MCP-1 mRNA expression in PMN,6 the present data clearly indicated that there was another factor(s) responsible for the remaining MCP-1-IA in the TNF-depleted-sup.
Figure 1.
Effects of anti-TNF-α or anti-IFN-γ on the expression of MCP-1 mRNA by PHA-sup-stimulated human PMN. (a) PMN (5 × 106 cells/ml) were cultured in the medium alone (lane 1), in the presence of PHA (lane 2), control-sup (lane 3), PHA-sup (lane 4), PHA-sup and anti-TNF-α-neutralizing IgG (5 µg) (lane 5), anti-TNF-α-neutralizing IgG (5 µg) (lane 6), TNF-depleted-sup (lane 7), or PHA-sup passed through normal mouse-IgG-conjugated columns (lane 8) for 18 hr at 37°. Total RNA was isolated and loaded onto a 1·2% agarose gel. The blot was hybridized with 32P-labelled human MCP-1 and β-actin cDNA probes. Autoradiographic signals were analysed by densitometry as described in the Materials and Methods. (b) PMN (5 × 106 cells/ml) were cultured with PHA-sup in the presence of anti-TNF-α IgG (µg/well) (lanes 2, 3, 6, 7), anti-IFN-γ (µg/well) (lanes 4–7), or normal mouse IgG (µg/well) (lanes 8, 9). Total RNA isolated from each well were subjected to Northern blot analysis. The blot was hybridized with 32P-labelled human MCP-1, IP-10 and β-actin cDNA probes. Autoradiographic signals were analysed by densitometry as described in the Material and Methods. Representative of two independent experiments with identical results.
We investigated the effect of anti-IFN-γ neutralizing IgG on the MCP-1-IA in the PHA-sup, because low levels of IFN-γ (less than 2·5 U/ml) were detected in the PHA-sup by ELISA. Anti-TNF-α IgG significantly inhibited the expression of MCP-1 as previously reported (Fig. 1b, lanes 2, 3).6 In contrast, anti-IFN-γ IgG had no effect on the MCP-1-IA in the PHA-sup by itself (lanes 4, 5). There was also no additional decrease in MCP-1-IA when anti-IFN-γ IgG was added in combination with anti-TNF-α IgG (lanes 6, 7). We also investigated the effects of these antibodies on PHA-sup-induced IP-10 mRNA expression. Addition of either anti-TNF-α IgG or anti-IFN-γ IgG reduced PHA-sup-induced IP-10 mRNA expression to minimal levels (lanes 2–5), and addition of both antibodies completely inhibited the expression of IP-10 mRNA (lanes 6, 7). These results indicated that IFN-γ contained in the PHA-sup played a critical role in IP-10 mRNA expression but not in MCP-1 mRNA expression.
TNF-α and TNF-depleted-sup co-operate for the maximal induction of MCP-1
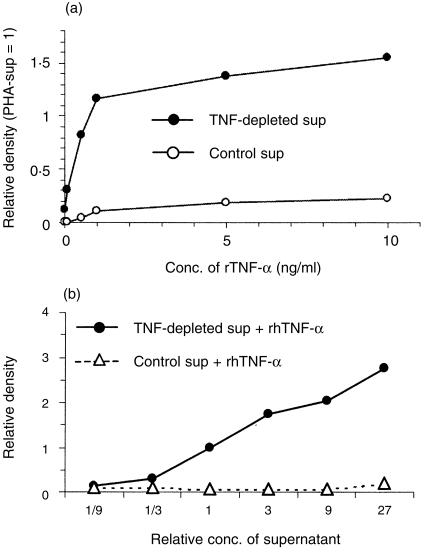
As shown in Fig. 2(a), PMN cultured in the medium or control-sup did not express significant amounts of MCP-1 mRNA without addition of rhTNF-α. Addition of 1–10 ng/ml of rhTNF-α induced low levels of MCP-1 mRNA. When PMN were cultured with TNF-depleted-sup, the cells expressed low levels of MCP-1 mRNA. Interestingly, when 0·1 or 0·5 ng/ml of rhTNF-α was added to the culture, the expression of MCP-1 mRNA was markedly increased and the peak expression level was detected when 1 ng/ml of rhTNF-α was added. This peak level was almost identical to the level induced by the PHA-sup. This result indicated that TNF-depleted-sup and rhTNF-α co-operated for the maximal expression of MCP-1 mRNA and that rhTNF-α was as effective as native TNF-α as an inducer of MCP-1 mRNA in PMN.
Figure 2.
Co-operative effects between TNF-α and TNF-depleted-sup for maximal MCP-1 mRNA expression. (a) Synergistic induction of MCP-1 mRNA by PMN cultured with TNF-depleted-sup in the presence of various concentrations of TNF-α (0·1–10 ng/ml). TNF-α concentration in the TNF-depleted-sup was 63 pg/ml. PMN were cultured for 18 hr at 37°. Total RNA from PMN were subjected to Northern blot analysis. Autoradiographic signals were analysed by densitometry as described in the Materials and Methods. (b) Synergistic induction of MCP-1 mRNA by PMN cultured with various concentrations of TNF-depleted-sup (1/9–27 times) with 1 ng/ml of TNF-α. TNF-depleted-sup or control-sup were concentrated 27 times and serially diluted every three times. The concentration of TNF-α in the concentrated TNF-depleted-sup was 137 pg/ml. The concentrations of TNF-α in each TNF-depleted-sup after dilution or control-sup were adjusted with rhTNF-α so that the final TNF-α concentration in each culture well was 1 ng/ml. PMN were cultured for 18 hr at 37°. Total RNA from PMN were subjected to Northern blot analysis. Autoradiographic signals were analysed by densitometry as described in the Materials and Methods. Representative of multiple independent experiments with similar results.
TNF-depleted-sup increased the level of MCP-1 expression in PMN dose dependently
TNF-depleted-sup and control-sup were concentrated 27-fold and the concentrated supernatants were serially diluted at 1 : 3. The concentration of TNF-α in the concentrated TNF-depleted-sup was measured by ELISA and then rhTNF-α was added so that each culture well contained 1 ng/ml of total TNF-α. As shown in Fig. 2(b), the level of MCP-1 expression was dependent on the concentration of TNF-depleted-sup, suggesting the presence of a factor(s) in the TNF-depleted-sup that co-operated with TNF-α. There was no co-operation between control-sup and rhTNF-α.
Preincubation with the TNF-depleted-sup alters the responsiveness of PMN to TNF-α for the maximal expression of MCP-1 mRNA
TNF-α is known to up-regulate MCP-1 expression rapidly in other types of cells, including monocytes/macrophages, fibroblasts, endothelial cells and tumour cells.11 However, it took about 8 hr for PMN to express detectable levels of MCP-1 mRNA and 16 hr to express the maximal level after stimulation with the PHA-sup.6 We hypothesized that there might be a factor in the TNF-depleted-sup that altered the responsiveness of PMN to TNF-α during the culture and once the responsiveness was changed TNF-α would rapidly induce MCP-1 expression.
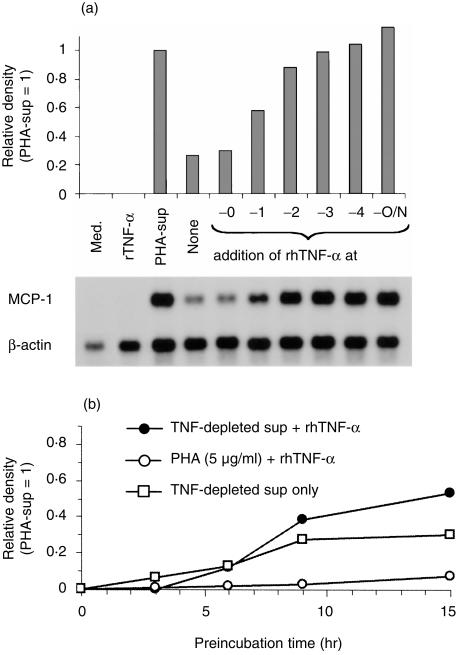
To test our hypothesis, PMN were incubated in the presence of PHA-sup or TNF-depleted-sup overnight. Between 1 and 4 hr before the end of culture 1 ng/ml of rhTNF-α was added to the cells cultured with TNF-depleted-sup (Fig. 3a). Medium or TNF-α alone did not induce significant MCP-1 expression (lanes 1, 2). Overnight incubation with the PHA-sup induced a high level of MCP-1 mRNA expression (lane 3). TNF-depleted-sup induced only a low level MCP-1 expression (lane 4). However, addition of rhTNF-α during the last few hours of the culture markedly increased the level of MCP-1 expression in the cells cultured overnight with the TNF-depleted-sup (lanes 6–9). The maximal level, that was identical to the level induced with the PHA-sup (lane 3) or a combination of the TNF-depleted-sup and rhTNF-α (lane 10), was detected when the cells were incubated with rhTNF-α for 3–4 hr (lanes 8, 9). PMN were also incubated with 1 ng/ml of rhTNF-α in complete medium for 18 hr, and TNF-depleted-sup concentrated 10-fold was added 3 hr prior to the end of the culture. The level of MCP-1 expression was much lower than that induced by PHA-sup (data not shown).
Figure 3.
Effect of preincubation with TNF-depleted-sup on MCP-1 mRNA expression in PMN. (a) PMN were cultured with TNF-depleted-sup for 18 hr and 1 ng/ml of rhTNF-α was added 4, 3, 2, or 1 hr prior to the end of the culture. PHA-sup-induced MCP-1 mRNA expression was also examined for comparison. Total RNA from PMN were subjected to Northern blot analysis. (b) PMN were cultured with TNF-depleted-sup for various periods of time and rhTNF-α was added to 1 ng/ml 3 hr prior to the end of culture. Total RNA from PMN were subjected to Northern blot analysis. Representative of three independent experiments with similar results.
We next investigated how many hours of preincubation with the TNF-depleted-sup were necessary to alter the responsiveness to TNF-α (Fig. 3b). Preincubation of PMN with PHA for 3, 6, 9, or 15 hr and another 3 hr incubation in the presence of rhTNF-α did not change the responsiveness of PMN to rhTNF-α. However, when PMN were preincubated with the TNF-depleted-sup for 9 hr, addition of rhTNF-α markedly increased the level of MCP-1 expression in 3 hr.
To test our hypothesis further, 5 µg of anti-TNF-α neutralizing IgG was added at different time-points after initiation of culture with the PHA-sup. The inhibitory effect of anti-TNF-α IgG was detected up to 18 hr (data not shown). These results indicated that TNF-depleted-sup altered the responsiveness of PMN to rhTNF-α during the overnight incubation.
PHA-sup does not increase the expression of TNFR-p55 and TNFR-p75 on PMN
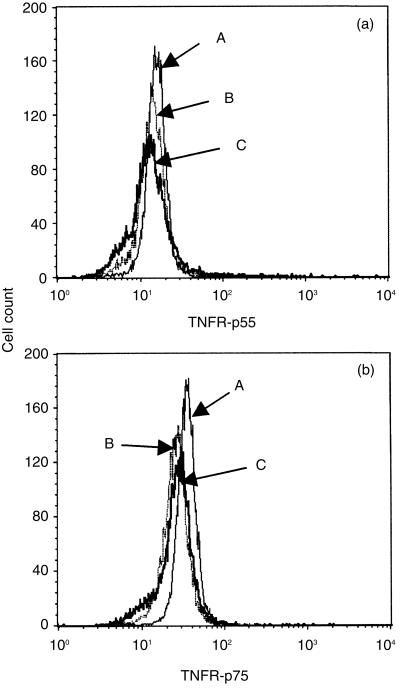
Since the responsiveness of PMN to rhTNF-α was altered, we speculated that the expression level of TNFR on PMN might be up-regulated after overnight culture with the PHA-sup. As shown in Fig. 4, freshly isolated PMN expressed both types of TNFR, as previously reported.12 Expression of neither receptor was increased after overnight culture with PHA-sup. Therefore, the alteration induced with the TNF-depleted-sup was not due to the increased number of TNFR on the cell surface.
Figure 4.
Flow cytometric analysis of TNFR-p55 and TNFR-p75 expression on PMN. Histograms represent profiles of cell count against log fluorescence showing expression of TNFR-p55 (a) or TNFR-p75 (b). (A) freshly isolated PMN; (B) PMN cultured with PHA-sup for 18 hr; (C) PMN cultured with control-sup for 18 hr. Representative of two independent experiments with similar results.
TNF-α induces MCP-1 expression in PMN through TNFR-p55
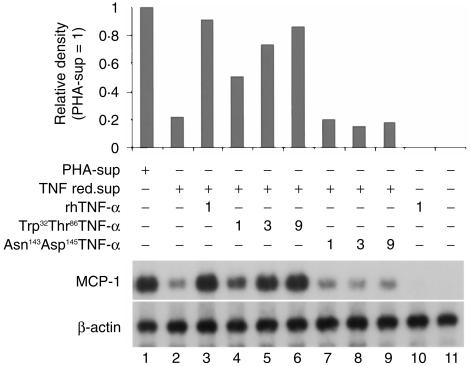
To determine which type of TNFR is involved in the process, PMN were cultured overnight with the TNF-depleted-sup in the presence of rhTNF-α, Trp32Thr86TNF-α (specific for TNFRp55), or Asn143Arg145TNF-α (specific for TNFRp75).7 As shown in Fig. 5, Trp32Thr86TNF-α (lanes 4–6), but not Asn143Arg145TNF-α (lanes 7–9), dose-dependently increased MCP-1 mRNA expression, indicating that TNF-α induced MCP-1 mRNA expression in PMN through TNFR-p55.
Figure 5.
Determination of TNFR involved in MCP-1 mRNA expression in PMN. PMN were cultured with TNF-depleted-sup in the presence of Trp32Thr86TNF-α and Asn143Arg145TNF-α, kindly provided by Dr L. Loetscher (F. Hoffmann-La Roche Ltd, Basel, Switzerland).7 Total RNA from PMN were subjected to Northern blot analysis. PHA-sup- and TNF-depleted-sup-induced MCP-1 mRNA expression were used as positive control. Representative of three independent experiments with similar results.
Co-factor activity is eluted around 60 000 MW by HPLC–gel filtration
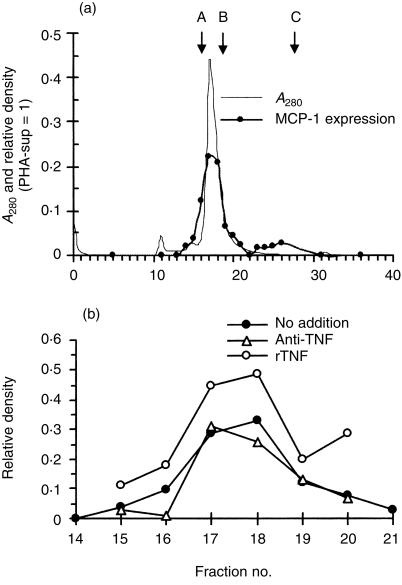
The molecular mass of the factor in the TNF-depleted-sup was analysed on a TSK-3000 column. Figure 6(a) shows that the fraction 17 and 18, corresponding to approximately 60 000 MW, induced low levels of MCP-1 mRNA expression similar to that induced with the TNF-depleted-sup. There was a minor peak between fraction 23 and 30. To confirm that this low level MCP-1 expression was not due to the existing low concentration of TNF-α, PMN were cultured with these fractions in the presence of anti-TNF-α neutralizing IgG. As shown in Fig. 6(b), MCP-1 expression was not inhibited by anti-TNF-α antibody. In contrast, addition of rhTNF-α enhanced the expression of MCP-1, suggesting that these fractions may contain the factor(s) that co-operated with TNF-α for the maximal expression of MCP-1 in PMN.
Figure 6.
Elution of MCP-1-IA in the TNF-depleted-sup by HPLC-gel filtration. (a) Concentrated TNF-depleted-sup was injected into a TSK 3000 column. MCP-1-IA in each fraction was assayed by Northern blot analysis. Data were analysed by densitometry and normalized against the levels of β-actin expression. Protein concentration (A280) and the level of MCP-1 mRNA expression in each fraction are presented. Molecular mass markers (arrows): A, BSA (67 000); B, OVA (43 000); C, cytochrome c (12·5 000). Representative of four independent experiments with similar results. (b) Five microgrammes of anti-TNF-α-neutralizing antibody or 1 ng/ml of rhTNF-α was added to the fraction 14–21, and MCP-1 mRNA expression was analysed by Northern blot analysis. Data were analysed by densitometry and normalized against the levels of β-actin expression. Representative of two independent experiments with similar results.
Discussion
We previously reported that the culture supernatant of PHA-stimulated PBMC induced a high level expression of MCP-1 mRNA in PMN, and TNF-α in the PHA-sup was partially responsible for the expression of MCP-1.6 We also speculated the involvement of another factor because a part of MCP-1 mRNA expression induced by the PHA-sup remained after neutralizing TNF-α activity with an anti-TNF-α-neutralizing antibody. In the present study, we confirmed our previous results by showing that reducing TNF-α concentration from the PHA-sup to a minimal level resulted in only a partial inhibition of MCP-1 mRNA expression. Furthermore, the maximal MCP-1 mRNA expression was completely restored by addition of rhTNF-α to TNF-depleted-sup, despite the fact that rhTNF-α by itself never induced high levels of MCP-1 mRNA expression in PMN. TNF-α dose-dependently induced MCP-1 mRNA in combination with TNF-depleted-sup and conversely TNF-depleted-sup also dose-dependently induced MCP-1 mRNA expression in the presence of TNF-α. These results clearly indicated the presence of a factor(s) in the TNF-depleted-sup that co-operated with TNF-α for maximal expression of MCP-1 mRNA.
The PHA-sup we used in this study was collected 4–6 hr after stimulation of PBMC with PHA. Therefore, it contained only a few cytokines at significant levels. These cytokines included TNF-α (1–2 ng/ml), IL-1β (≈100 pg/ml), IL-8 (> 10 ng/ml) and IFN-γ (less than 250 pg/ml). There have been several studies reporting a synergistic effect between TNF-α and IFN-γ on the transcription of various genes,13 suggesting that IFN-γ might be the contributing factor in the TNF-depleted-sup. In fact, IP-10 mRNA, previously reported to be induced in PMN by a synergistic effect between TNF-α and IFN-γ,5 was also induced with the PHA-sup, and the pretreatment of the PHA-sup with antibodies against TNF-α and IFN-γ completely inhibited the expression of IP-10 mRNA (Fig. 1b). However, anti-IFN-γ had no effect on PHA-sup-induced MCP-1 mRNA expression, indicating that IFN-γ was not involved in MCP-1 mRNA expression. Neither IL-1β nor IL-8 co-operated with TNF-α (data not shown). We finally analysed the molecular mass of the MCP-1-IA in the TNF-depleted-sup by HPLC–gel filtration, and detected a major activity around 60 000 MW and a minor activity in a smaller molecular mass range. Since proteins with small molecular masses could bind to serum proteins and could be eluted around 60 000 MW, we cannot completely exclude the possibility that the MCP-1-IA in the TNF-depleted-sup was due to proteins with smaller molecular weights. Further studies are necessary to identify this co-operating factor(s) in the TNF-depleted-sup.
As previously reported, MCP-1 produced by early infiltrating PMN appears to play a role in the development of DTH.4 Our current hypothesis is that the factor(s) eluted around 60 000 MW plays a critical role in the maturation of PMN and ‘mature’ PMN-derived MCP-1 regulates the transition from acute inflammation to DTH. This led us to investigate whether specific antigens could induce secretion of MCP-1-IA from sensitized T cells. We stimulated PBMC from purified protein derivative (PPD)-positive human donors with PPD and used the culture supernatants to stimulate PMN. Although the supernatants themselves did not induce MCP-1 mRNA expression in PMN, these supernatants significantly increased the level of MCP-1 expression in co-operation with rhTNF-α (data not shown), suggesting that antigen-stimulated T cells also produce the co-factor(s).
At least 9 hr of preincubation with the TNF-depleted-sup was necessary for the subsequent TNF-α-induced maximal MCP-1 mRNA expression. During this preincubation time, the responsiveness of PMN to TNF-α was altered. It has been known that several cytokines, such as TNF-α or GM-CSF, can prime PMN for the activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase.14 In the primed state, there is no increase in oxidase activity, but subsequent stimulation provokes a response that is larger than that in non-primed cells. Meda et al. reported that PMN stimulated in the presence of IFN-γ for 18 hr demonstrated an enhanced LPS-induced IL-1β and TNF-α production as well as TNF-α-induced IL-1β production.2 Several findings suggest that tyrosine phosphorylation plays an important role in priming.14 Since TNF-depleted-sup did not induce the maximal MCP-1 mRNA expression by itself and tyrosine phosphorylation was important for the expression of MCP-1,6 the mechanisms involved in MCP-1 expression resemble the activation of NADPH oxidase in PMN.
Alteration in the responsiveness to TNF-α suggested that the expression of cell-surface TNFRs might be up-regulated by pretreatment with the TNF-depleted-sup. Several groups previously reported that up-regulation of TNFR expression by IFN-γ was responsible for the increased TNF-dependent cytotoxicity in some tumour cell lines.15,16 However, in our study up-regulation of cell-surface TNFR expression is not the mechanism whereby the factor(s) in the TNF-depleted-sup co-operates with TNF-α to induce maximal MCP-1 expression in PMN. As shown in Fig. 5, the activation signal of TNF-α to induce MCP-1 mRNA expression in PMN is transmitted through TNFR-p55. Since TNF-α is capable of activating freshly isolated PMN to synthesize RNA anew,17 probably through TNFR-p55, we hypothesize that a change that occurs during the priming stage must be involved in the pathway regulating the transcription of the MCP-1 gene. We previously reported that early protein synthesis and tyrosine phosphorylation were involved in the PHA-sup-induced MCP-1 mRNA expression in PMN.6 Thus, it is likely that a protein that plays a role in this pathway is synthesized during the process.
Finally, the present study suggests that under appropriate conditions, circulating PMN have the capacity to mature further to acquire features previously known for other types of cells. Recently, Oehler et al.18 showed that lactoferrin-positive immediate precursors of end-stage PMN differentiate into dendritic cells in the presence of GM-CSF, IL-4 and TNF-α. GM-CSF-stimulated PMN also expressed human leucocyte antigen (HLA)-DR and functioned as antigen-presenting cells.19 Expression of HLA-DR was up-regulated after overnight incubation of PMN with the PHA-sup (data not shown). Thus, PMN may play broader roles in the immune response than was previously thought. Our goal is to understand better the functions of PMN that comprise two-thirds of all peripheral blood leucocytes.
Acknowledgments
We thank Dr Hansruedi Loetscher (F. Hoffmann-La Roche Ltd, Basel, Switzerland) for providing recombinant TNF-α mutant proteins. We also thank Dr Joost J. Oppenheim for reviewing this manuscript.
Glossary
Abbreviations
- DTH
delayed-type hypersensitivity
- MCP-1
monocyte chemoattractant protein-1
- PHA-sup
supernatants of phytohaemagglutinin-stimulated peripheral blood mononuclear cells
- PMN
polymorphonuclear neutrophil
References
- 1.Cassatella MA. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–6. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 2.Meda L, Gasperini S, Ceska M, Cassatella MA. Modulation of proinflammatory cytokine release from human polymorphonuclear leukocytes by gamma interferon. Cell Immunol. 1994;157:448–61. doi: 10.1006/cimm.1994.1241. 10.1006/cimm.1994.1241. [DOI] [PubMed] [Google Scholar]
- 3.Kasama T, Strieter RM, Standiford TJ, Burdick MD, Kunkel SL. Expression and regulation of human neutrophil-derived macrophage inflammatory protein 1 alpha. J Exp Med. 1993;178:63–72. doi: 10.1084/jem.178.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rand ML, Warren JS, Mansour MK, Newman W, Ringler DJ. Inhibition of T cell recruitment and cutaneous delayed-type hypersensitivity-induced inflammation with antibodies to monocyte chemoattractant protein-1. Am J Pathol. 1996;148:855–64. [PMC free article] [PubMed] [Google Scholar]
- 5.Cassatella MA, Gasperin S, Calzetti F, Bertagnin A, Luster AD, McDonald PP. Regulated production of the interferon-gamma-inducible protein-10 (IP-10) chemokine by human neutrophils. Eur J Immunol. 1997;27:111–15. doi: 10.1002/eji.1830270117. [DOI] [PubMed] [Google Scholar]
- 6.Yamashiro S, Kamohara H, Yoshimura T. MCP-1 is selectively expressed in the late phase by cytokine-stimulated human neutrophils: TNF-α plays a role in the maximal MCP-1 mRNA expression. J Leukoc Biol. 1999;165:671–9. doi: 10.1002/jlb.65.5.671. [DOI] [PubMed] [Google Scholar]
- 7.Loetscher H, Stueber D, Banner D, Mackay F, Lesslauer W. Human tumor necrosis factor alpha (TNFalpha) mutants with exclusive specificity for the 55-kDa or 75-kDa TNF receptors. J Biol Chem. 1993;268:26350–7. [PubMed] [Google Scholar]
- 8.Leonard EJ, Skeel A. Disposable microliter immunoabsorbent columns: construction and operation. J Immunol Methods. 1985;82:341–5. doi: 10.1016/0022-1759(85)90366-7. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimura T. cDNA cloning of guinea pig monocyte chemoattractant protein-1 and expression of the recombinant protein. J Immunol. 1993;150:5025–32. [PubMed] [Google Scholar]
- 10.Yoshimura T, Yuhki N, Moore SK, Appella E, Lerman MI, Leonard EJ. Human monocyte chemoattractant protein-1 (MCP-1): full length cDNA cloning, expression in mitogen-stimulated blood mononuclear leukocytes, and sequence similarity to mouse competence gene JE. FEBS Lett. 1989;244:487–93. doi: 10.1016/0014-5793(89)80590-3. [DOI] [PubMed] [Google Scholar]
- 11.Yoshimura T, Ueda A. Monocyte chemoattractant protein-1. In: Aggarwal BB, Gutterman JU, editors. Human Cytokines: Handbook for Basic and Clinical Research, II. Cambridge: Blackwell Science; 1996. pp. 198–221. [Google Scholar]
- 12.Murray J, Barbara JAJ, Dunkley SA, et al. Regulation of neutrophil apoptosis by tumor necrosis factor-alpha: Requirement for TNFR55 and TNFR75 for induction of apoptosis in vitro. Blood. 1997;90:2772–83. [PubMed] [Google Scholar]
- 13.Paludan SR. Synergistic action of pro-inflammatory agents: cellular and molecular aspects. J Leukoc Biol. 2000;67:18–25. doi: 10.1002/jlb.67.1.18. [DOI] [PubMed] [Google Scholar]
- 14.Hallett MB, Lloyds D. Neutrophil priming: the cellular signals that say ‘amber’ but not ‘green’. Immunol Today. 1995;16:264–8. doi: 10.1016/0167-5699(95)80178-2. [DOI] [PubMed] [Google Scholar]
- 15.Kost ER, Mutch DG, Herzog TJ. Interferon-gamma and tumor necrosis factor-alpha induce synergistic cytolytic effects in ovarian cancer cell lines-Roles of the TR60 and TR80 tumor necrosis factor receptors. Gynecol Oncol. 1999;72:392–401. doi: 10.1006/gyno.1998.5257. 10.1006/gyno.1998.5257. [DOI] [PubMed] [Google Scholar]
- 16.Tsujimoto M, Yip YK, Vilcek J. Interferon-gamma enhances expression of cellular receptors for tumor necrosis factor. J Immunol. 1986;136:2441–4. [PubMed] [Google Scholar]
- 17.Beaulieu AD, Paquin R, Rathanaswami P, McColl SR. Nuclear signaling in human neutrophils. J Biol Chem. 1992;267:426–32. [PubMed] [Google Scholar]
- 18.Oehler L, Majdic O, Pickl WF, et al. Neutrophil granulocyte-committed cells can be driven to acquire dendritic cell characteristics. J Exp Med. 1998;187:1019–28. doi: 10.1084/jem.187.7.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gosselin EJ, Wardwell K, Rigby WFC, Guyre M. Induction of MHC class II on human polymorphonuclear neutrophils by granulocyte/macrophage colony-stimulating factor, IFN-γ, and IL-3. J Immunol. 1993;151:1482–90. [PubMed] [Google Scholar]