Abstract
Feline leukaemia virus (FeLV) nucleic acid vaccination of domestic cats affords protection against viraemia and the development of latency without inducing antiviral antibodies.1 To determine the contribution of cell-mediated immunity to the control of virus replication and clearance from the host, FeLV-specific cytotoxic T lymphocyte (CTL) responses were compared in vaccine-protected, transiently viraemic, and persistently viraemic cats. Vaccinal immunity was associated with the detection of higher levels of virus-specific effector CTL in the peripheral blood and lymphoid organs to FeLV Gag/Pro and Env antigens than those observed in unvaccinated control, persistently viraemic cats (P < 0·001). Likewise, higher levels of virus-specific CTLs were also observed in transiently viraemic cats which recovered following exposure to FeLV. In cats that controlled their infection, recognition of Gag/Pro antigens was significantly higher than the recognition of Env antigens. This is the first report highlighting the very significant role that virus-specific CTL have in determining the outcome of FeLV infection in either vaccinated cats or cats recovering naturally from FeLV exposure.
Elucidating the immune mechanisms responsible for the control of retrovirus replication and dissemination within the host remains an important milestone in the development of effective treatments and prophylaxis for retrovirus-associated diseases. Animal model studies have demonstrated that protective vaccination against retrovirus infection is an attainable goal.2–6 However, the immune correlates of protection are less well defined, and virus-specific humoral immunity,7,8 and cell-mediated immunity9–11 either acting alone or in concert,7,12 have been shown to be involved.
Feline leukaemia virus (FeLV) is a naturally occurring mammalian type C retrovirus that causes serious diseases in domestic cats world-wide. Fortunately, the majority of cats exposed to FeLV develop a temporary infection, with or without a transient viraemia, and either completely recover or establish a latent infection.13 A proportion of cats becomes persistently viraemic with subsequent development of fatal FeLV-associated diseases including lymphomas, leukaemias, anaemia, immunodeficiency and reproductive failure. The determinants of susceptibility to FeLV are unknown except that young kittens are fully susceptible, while the majority of cats over 16 weeks of age either recover or develop a latent infection.13 Virus neutralizing antibodies (VNA) are thought to have a role in the recovery of cats from FeLV infection, and protection can be passively transferred by immune serum.14 However, most cats which develop a transient infection recover before VNA appear in the blood, implying that cell mediated immunity may be important in the host's protective immune response. Certainly, cell mediated immune responses to FeLV-induced T-cell lymphomas have been described previously.15 Studies on retroviral infection in man with human immunodeficiency virus (HIV)-1 highlight the importance of strong and persistent cell mediated immune responses in the control of viral replication and in the maintenance of the symptom-free state.16–19 Such observations have resulted in attempts to modify retroviral associated disease in human patients by the adoptive transfer of CD8+ T lymphocytes of predetermined viral antigen specificity.20,21 Immunotherapeutic strategies may prove to be an invaluable adjunct to chemotherapeutic regimes, which recent reports suggest are unlikely to completely eliminate reservoirs of virus within the host.22 Studies on murine retroviruses have also demonstrated the importance of cell-mediated immune responses in protection.23,24
This study aimed to determine the contribution of host virus-specific cellular immune responses, in particular CTLs, in the control of FeLV replication and clearance from the host. The outcome of the FeLV DNA vaccination study afforded us the opportunity to investigate virus-specific cytotoxic T lymphocyte (CTL) function in three well-defined groups of cats. Thus FeLV-specific CTL responses were compared in DNA vaccinated, protected cats, in unvaccinated, persistently viraemic cats, and in FeLV-exposed cats that recovered naturally from their infection.
The 10, 13–15-week-old, outbred, specific pathogen-free (SPF) domestic cats selected for this study were free of FeLV and were serologically negative. Five cats were inoculated intramuscularly (i.m.) on three occasions at intervals of 2 weeks with a FeLV DNA vaccine comprising the gag/pol and env genes of FeLV-A/Glasgow-1 under the control of a cytomegalovirus (CMV) expression vector, together with feline interleukin (IL)-12 and feline IL-18 DNA as genetic adjuvants, as described previously.1 Five control cats were not inoculated with FeLV DNA. All of the cats were challenged intraperitoneally (i.p.) with 2 × 105 FFU of FeLV -A/Glasgow-1 at 20–22 weeks of age and virus isolation was attempted from the peripheral blood at intervals of 3 weeks following challenge by inoculation of plasma onto QN10S cells in vitro.25 The results are shown in Table 1. Virus was not detected in the vaccinated cats (1–5) at any of the time-points examined, indicating that these animals were completely protected. In the control cats, two patterns of response were observed; two of five cats (6 and 7) experienced a transient infection with virus isolated from the peripheral blood only at week 3 p.c., whilst the remaining three cats (8–10) became persistently infected with virus isolated from the peripheral blood at all time-points examined after week 3.
Table 1.
FeLV isolation from peripheral blood
| No. of weeks post challenge | |||||||
|---|---|---|---|---|---|---|---|
| Cat No. | Status | −3 | 0 | 3 | 6 | 9 | 12 |
| 1 | Vaccinated | – | – | – | – | – | – |
| 2 | Vaccinated | – | – | – | – | – | – |
| 3 | Vaccinated | – | – | – | – | – | – |
| 4 | Vaccinated | – | – | – | – | – | – |
| 5 | Vaccinated | – | – | – | – | – | – |
| 6 | Control | – | – | + | – | – | – |
| 7 | Control | – | – | + | – | – | – |
| 8 | Control | – | – | + | + | + | + |
| 9 | Control | – | – | + | + | + | + |
| 10 | Control | – | – | + | + | + | + |
FeLV in peripheral plasma was detected by isolation on QN10S cells which were examined on day 5–7 for specific foci of transformation.
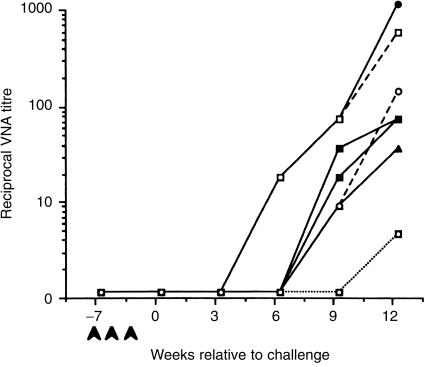
What are the immunological mechanisms responsible for clearance of virus from the vaccinated cats? Neutralizing antibody responses were assayed following vaccination and at 3-week intervals following challenge by focus reduction of FeLV-A/Glasgow-125(Table 1). Despite the obvious importance of VNA, our studies show that FeLV DNA vaccination does not elicit FeLV-specific humoral immunity but does protect cats from challenge. Furthermore, as shown in Fig. 1, the clearance of virus from the infected cats preceded the development of virus-specific antibody1 implying that humoral immunity was not the major factor involved in recovery of the transiently viraemic cats. Recent studies have also shown that it is possible to modulate the viral burden in FeLV-infected cats by adoptive transfer of autologous mitogen-activated lymphoblasts, with or without antiretroviral chemotherapy.26,27 However, the underlying mechanisms and virus-specificity of the transferred cells remain uncharacterized.
Figure 1.
Detection of FeLV-specific antibodies. Neutralising antibodies were assayed by focus reduction of FeLV-A/Glasgow-1.25 Heparinized plasma samples were collected from vaccinated cats (—) transiently viraemic cats (- - -), and persistently viraemic cats (……) prior to vaccination (•), on the day of challenge, and at 3-week intervals until 12 weeks post-challenge.
To assess the involvement of virus-specific cell mediated immunity in the control and clearance of FeLV, mononuclear cells were prepared from all cats at 16 weeks p.c. Single cell suspensions were prepared from the peripheral blood, peripheral (submandibular, retropharyngeal, prescapular and popliteal) and mesenteric lymph nodes, and spleen by centrifugation over Ficoll-Paque (Pharmacia LKB, Biotechnology Inc., Piscataway, NJ) and assayed directly for FeLV-specific cytotoxicity. Target cells were autologous or allogeneic skin fibroblasts derived from skin biopsy samples as described previously.28 The target cells were labelled with 51Cr and infected for 1 hr with recombinant vaccinia viruses expressing the products of either the gag/pro or env of FeLV-A/Glasgow-1 (a kind gift from E. Paoletti, Virogenetics) or wild type vaccinia virus as a control. Alternatively, the skin fibroblast cell lines were infected in vitro with FeLV-A/Glasgow-1. These persistently infected cell lines express all FeLV structural antigens thereby enabling detection of CTL specificities that might be overlooked using recombinant vaccinia viruses to deliver FeLV antigens. Effector cells were added to give a range of effector-to-target (E : T) ratios from 50 : 1 to 6·25 : 1, and lytic activity was measured by monitoring isotope release as described previously.29
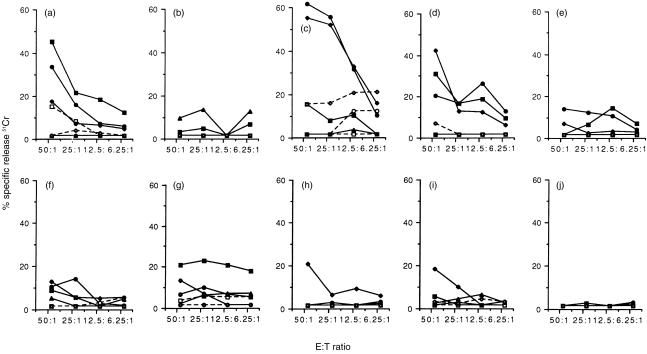
Virus-specific effector CTL responses were detected in the peripheral blood of all five of the vaccinated, protected cats without any prior requirement for in vitro re-stimulation of the effector lymphocytes, implying that virus-specific effector CTLs are present in the circulation at frequencies estimated to be greater than 1 in 100 peripheral blood mononuclear cells (PBMC) 30 (Fig. 2). The magnitude of the responses varied between individual cats reflecting the outbred nature of the group, however, cells of the four vaccinated cats which displayed the highest effector FeLV-specific CTL activities recognized both FeLV-A-infected target cells and target cells infected with recombinant vaccinia viruses expressing either gag/pro or env genes. There was no recognition of target cells infected with wild type vaccinia virus, confirming the specificity of the observed responses. Furthermore allogeneic target cells expressing FeLV antigens were not recognized indicating the major histocompatibility complex (MHC) class I-restricted nature of the response.
Figure 2.
Detection of FeLV-specific effector CTLs. FeLV-specific effector CTL responses were measured directly in the blood of 10 cats, 16 weeks p.c. with 2 × 105 FFU of FeLV-A/Glasgow-1. Five cats were vaccinated with FeLV DNA, and were protected from challenge (1–5; a–e). The remaining five cats were unvaccinated. Two of five cats recovered following challenge (6 and 7; f and g), whereas the other three cats became persistently viraemic (8–10; h–j). Autologous (—) or allogeneic (- - -) skin fibroblasts infected with either recombinant vaccinia viruses expressing FeLV Gag/Pro (▪), FeLV Env (•), or wild type vaccinia virus (▴), or infected with FeLV-A (♦) and labelled with 51Cr were used as target cells in the assay. The release of 51Cr into the culture supernatant from triplicate cultures was detected after 4 hr incubation at 37°.
Three of five unvaccinated cats became persistently viraemic following challenge, and effector FeLV-specific CTL were detected directly in the peripheral blood of two of the three cats in this group. It was notable that CTL activity was observed only when FeLV-infected target cells were used in the assay and there was negligible recognition (< 10% specific lysis) of target cells infected with recombinant vaccinia viruses expressing either FeLV Gag/Pro or Env antigens. This observation suggested that the CTL response in these persistently viraemic cats either recognized a different viral antigen(s), or a different epitope from those expressed by the recombinant vaccinia viruses. In this regard, it may be relevant that the Gag/Pro recombinant vaccinia virus used expresses only the protease of the polyprotein indicating that the CTL elicited in the persistently viraemic cats may be directed towards the reverse transcriptase or integrase. Epitope mapping studies would have to be performed to explain this observation. Alternatively, the low level or absence of virus-specific CTL in the persistently viraemic cats might reflect the activation status of the T cells. To address this possibility, their lymphocytes were stimulated in vitro by FeLV-infected autologous fibroblast cells for 10 days in complete RPMI medium supplemented with 100 IU/ml human recombinant IL-2 (a kind gift from M. Hattori, University of Tokyo). To inactivate the viral particles, the FeLV-infected fibroblasts were irradiated for 10 min in a UV-crosslinker (Stratagene, Netherlands) prior to cocultivation with the lymphocytes. These cells continued to express FeLV antigens, as measured by the presence of p27 antigen in cell lysates by enzyme-linked immunosorbent assay (ELISA), but did not release infectious virus, as assessed by the failure to detect virus in culture supernatant inoculated on to QN10S cells.25 Such re-stimulation and expansion of antigen-specific blasts had no effect on the detection of FeLV-specific CTLs in these cats (data not shown), confirming the absence or low level of FeLV-specific CTL in the peripheral circulation of persistently viraemic cats.
Are similar immune mechanisms responsible for the recovery of cats from transient viraemia? Following exposure to FeLV-A, two of five unvaccinated cats (6 and 7) experienced a transient infection. Virus-specific effector CTLs were detected in the blood of both recovered cats implicating this immune mechanism in the recovery of cats from their infection. Target cells infected with FeLV-A were recognized as well as target cells infected with recombinant vaccinia viruses expressing either FeLV gag/pro or env gene products. Effector CTLs recognising FeLV Gag/Pro were particularly marked in the peripheral blood of one animal (cat 7).
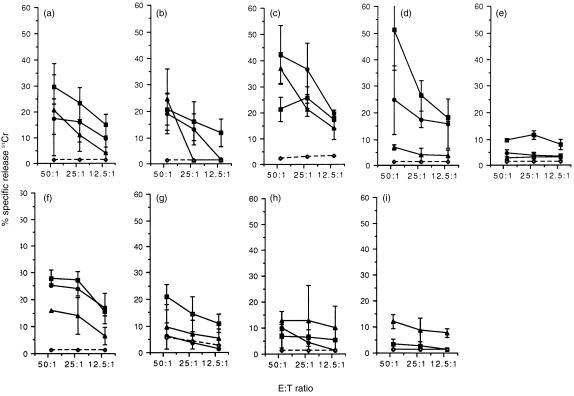
As indicated above, the failure to detect FeLV-specific CTL in some persistently viraemic cats is not due to the inappropriate activation of the T cells in vivo. An alternative explanation is that the CTL may have been sequestered into other lymphoid tissues. To address this possibility, the distribution of the effector FeLV-specific CTL was determined in the peripheral and mesenteric lymph nodes and in the spleen. The results are summarized in Table 2. Analysis of the FeLV-specific CTL responses in the lymph nodes (Fig. 3) emphasized the trends observed in the blood, with high levels of lysis of FeLV-A infected targets and targets expressing Gag/Pro or Env antigens readily detectable in the four of five vaccinated, protected cats. The remaining cat in this group exhibited lower levels of lysis, nevertheless this cat had significant virus-specific CTL in both blood and spleen tissues (Table 2). In the transiently viraemic cats (6 and 7, Fig. 3), although the responses observed in the lymph nodes were similar, when all of the lymphoid tissues were taken into consideration the pattern of CTL responses observed varied markedly between the two cats examined. Whereas in one animal (cat 6) high levels of both FeLV Gag/Pro and Env-specific effector CTL responses were observed in all lymphoid tissues examined, in the other (cat 7) effector CTL responses were only detected in the peripheral lymph node and the levels of virus-specific cytotoxicity observed were lower. A possible reason for this difference was suggested when attempts were made to isolate infectious FeLV from cells cultured from the bone marrow. Although both cats had cleared virus completely from the peripheral blood (Table 1), it was still possible to isolate virus from the bone marrow of cat 6, but not cat 7, indicating that cat 6 had developed a latent FeLV infection. The higher levels of virus specific cytotoxicity in cat 6 may reflect the continued exposure of the host immune system to viral antigens and the requirement of the animal to kill bone marrow cells that express viral proteins. The results of the peripheral and mesenteric lymph nodes also highlight the preferential recognition of Gag/Pro expressing targets in both vaccinated, protected cats and in recovered cats. Further they emphasize the skewing of the response towards FeLV-A-infected targets (Fig. 3), reflecting the putative recognition of other viral antigens including RT and integrase.
Table 2.
FeLV-specific CTL activity
| Cat No. | Status | PBMC | Mesenteric LNC | Peripheral LNC | Spleen |
|---|---|---|---|---|---|
| 1 | Vaccinated/protected | 5/4/2 | 3/2/2 | 4/3/– | 2/1/1 |
| 2 | Vaccinated/protected | 1/–/– | 2/2/3 | 1/–/– | 3/3/4 |
| 3 | Vaccinated/protected | 2/5/5 | 3/5/4 | 2/3/3 | 2/3/3 |
| 4 | Vaccinated/protected | 3/2/2 | 4/4/1 | 4/2/– | 1/2/– |
| 5 | Vaccinated/protected | 2/2/1 | 1/–/– | 2/–/– | 2/1/1 |
| 6 | Exposed/recovered | 1/2/2 | 3/3/2 | 3/3/2 | 5/4/– |
| 7 | Exposed/recovered | 3/1/2 | 1/–/– | 2/1/2 | –/–/– |
| 8 | Persistently viraemic | –/–/2 | –/–/– | 1/–/2 | –/–/2 |
| 9 | Persistently viraemic | –/–/2 | 1/–/2 | 1/–/1 | –/1/2 |
| 10 | Persistently viraemic | –/–/– | n.d. | n.d. | n.d. |
Effector CTL responses were measured directly in the lymphocyte populations indicated. Assays were performed in triplicate at four E : T ratios (50 : 1, 25 : 1, 12·5 : 1, 6·25 : 1). Scores for specific lysis for Gag/Pro/Env/FeLV-A-infected targets: –, 0–5%; 1, 5–10%; 2, 10–20%; 3, 20–30%; 4, 30–40%; 5, 40–50% specific lysis. n.d. not done
Figure 3.
FeLV-specific CTL responses in the peripheral and mesenteric lymph nodes. FeLV-specific effector CTL responses were measured directly in the peripheral and mesenteric lymph nodes of nine cats, 16 weeks p.c. with 2 × 105 FFU of FeLV-A/Glasgow-1. Five cats were vaccinated with FeLV DNA, and were protected from challenge (1–5; a–e). The remaining five cats were unvaccinated. Two of five cats recovered following challenge (6 and 7; f and g), whereas the other two cats became persistently viraemic (8 and 9; h and i). Skin fibroblasts infected with either recombinant vaccinia viruses expressing FeLV Gag/Pro (—, ▪), FeLV Env (—, •), or wild type vaccinia virus (–, ◊), or infected with FeLV-A (—, ▴) and labelled with 51Cr were used as target cells in the assay. The release of 51Cr into the culture supernatant was detected after 4 hr incubation at 37°. The results shown represent the mean values ±2SE from triplicate cultures of mesenteric lymph nodes and peripheral lymph nodes, from which the values for recognition of allogeneic targets have been subtracted.
Statistical analysis of the virus-specific CTL results observed in vaccine-protected cats, recovered cats, and persistently viraemic cats revealed that the difference between groups was highly significant (P < 0·0001 by anova). Pair-wise contrasts between groups were analysed using the Student–Newman–Keuls multiple comparisons test. Analysis of the total CTL responses in all lymphoid tissues examined, to any viral antigen, revealed significantly greater levels of CTL activity in the vaccinated, protected cats than in either the persistently viraemic cats (P < 0·001), or the transiently viraemic cats (P < 0·01). Likewise transiently viraemic cats which recovered following exposure to FeLV had significantly higher CTL responses to viral antigens than persistently viraemic cats (P < 0·01). Recognition of both Gag/Pro and Env viral antigens was higher in the vaccinated, protected group when compared to the persistently viraemic cats (P < 0·01 and P < 0·05, respectively). Therefore, statistical analysis indicated that virus-specific CTLs were a very significant component in determining the outcome of FeLV infection in cats. This interpretation is supported by the detection of significantly higher CTLs recognizing Gag/Pro antigens in transiently viraemic cats when compared to persistently viraemic cats (P < 0·05), whereas the differences in Env-specific CTL responses between these groups was not significant (P > 0·05). This situation contrasts with that observed in feline immunodeficiency virus (FIV) vaccinated protected cats, where the predominant immune response is directed to against the envelope glycoproteins rather than to Gag proteins.9 Indeed, following experimental exposure to FIV the detection of Gag-specific CTLs is often the first indicator that the host is mounting an immune response to the challenge virus.31,32
Following infection with HIV-1 and FIV the host also mounts a very vigorous virus-specific CTL response which accounts for the decline in viraemia;16,17,19,31,32 however, the response fails to effectively clear virus from the host resulting in a long asymptomatic period culminating in the recrudescence of high plasma viraemia and development of immunodeficiency disease. In contrast, the present study demonstrates that the vigorous host FeLV-specific CTL response can effectively clear replicating virus resulting in either protection from challenge in the vaccinates, or in recovery from infection in naïve cats. Why does the very vigorous host immune response fail in lentiviral infections? The reason may be related to differences in the biology of oncoviruses and lentiviruses. Following infection with the FeLV, there is constitutive expression of viral proteins in all oncovirus-infected cells making them highly susceptible targets for the host CTL response. In contrast, infection with HIV-1 or FIV is associated with the development of a reservoir of latently infected cells which express very little or no viral proteins and are consequently not susceptible to CTL clearance. Thus the lentivirus may escape host immune surveillance allowing infection to persist. Only very low levels of FeLV-specific CTLs were detected in persistently viraemic cats, which may account for the inability of these cats to control viral replication in vivo and the persistence of the infectious state.
This report details for the first time the involvement of virus-specific CTLs in the control of feline retroviral disease. Our results suggest that not only is the overall CTL responsiveness compromised in the persistently viraemic cats, but it may be directed to different viral antigens or epitopes from those recognized by cells from either vaccinated, protected cats or transiently viraemic cats which recover following exposure to FeLV. Future studies will examine the temporal association between the evolution of the host cellular immune response and virus replication and dissemination following exposure.
Acknowledgments
J.N.F. is a BBSRC Advanced Fellow. We are grateful to M. Golder, D. Graham, R. Irvine, G. Law, M. McDonald and S. McDonald for excellent technical assistance. This work was in part supported by Q-1 Biotech, UK.
References
- 1.Hanlon L, Argyle DJ, Bain D, et al. FeLV DNA vaccine efficacy is enhanced by co-administration with IL-12 and IL-18 expression vectors. J Virol. 2000 doi: 10.1128/JVI.75.18.8424-8433.2001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almond N, Kent K, Cranage M, Rud E, Clarke B, Stott EJ. Protection by attenuated simian immunodeficiency virus in macaques against challenge with virus-infected cells. Lancet. 1995;345:1342. doi: 10.1016/s0140-6736(95)92540-6. [DOI] [PubMed] [Google Scholar]
- 3.York SM, York CJ. Development of a whole killed feline leukemia virus vaccine. JAVMA. 1991;199:1419. [PubMed] [Google Scholar]
- 4.Hoover EA, Perigo NA, Quackenbush SL, Mathiason-Dubard CK, Overbaugh WS, Kloetzer WS, Elder JH, Mullins JI. Protection against feline leukemia virus infection by use of an inactivated virus vaccine. JAVMA. 1991;199:1392. [PubMed] [Google Scholar]
- 5.Yamamoto JK, Okuda T, Akley CD, Louie H, Pembroke E, Zochlinski H, Munn RJ, Gardner M. Experimental vaccine protection against feline immunodeficiency virus. AIDS Res Hum Retroviruses. 1991;7:911. doi: 10.1089/aid.1991.7.911. [DOI] [PubMed] [Google Scholar]
- 6.Hosie MJ, Flynn JN, Rigby MA, et al. DNA vaccination affords significant protection against feline immunodeficiency virus infection without inducing detectable antiviral antibodies. J Virol. 1998;72:7310. doi: 10.1128/jvi.72.9.7310-7319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hosie MJ, Flynn JN. Feline immunodeficiency virus vaccination: characterization of the immune correlates of protection. J Virol. 1996;70:7561. doi: 10.1128/jvi.70.11.7561-7568.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole KS, Rowles JL, Jagerski BA, et al. Evolution of envelope-specific antibody response in monkeys experimentally infected or immunized with simian immunodeficiency virus and its association with the development of protective immunity. J Virol. 1997;71:5069. doi: 10.1128/jvi.71.7.5069-5079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flynn JN, Keating P, Hosie MJ, MacKett M, Stephens EB, Beatty JA, Neil JC, Jarrett O. Env-specific CTL predominate in cats protected from feline immunodeficiency virus infection by vaccination. J Immunol. 1996;157:3658. [PubMed] [Google Scholar]
- 10.Xu X-N, Screaton GR, Gotch FM, et al. Evasion of cytotoxic T lymphocyte (CTL) responses by nef-dependent induction of Fas ligand (CD95L) expression on simian immunodeficiency virus-infected cells. J Exp Med. 1997;186:7. doi: 10.1084/jem.186.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almond N, Rose J, Sangster R, Silvera P, Stebbings R, Walker B, Stott EJ. Mechanisms of protection induced by attenuated simian immunodeficiency virus. 1. Protection cannot be transferred with immune serum. J Gen Virol. 1997;78:1919. doi: 10.1099/0022-1317-78-8-1919. [DOI] [PubMed] [Google Scholar]
- 12.Flynn JN, Beatty JA, Cannon CA, Stephens EB, Hosie MJ, Neil JC, Jarrett O. Involvement of gag- and env-specific cytotoxic T lymphocytes in protective immunity to feline immunodeficiency virus. AIDS Res Hum Retroviruses. 1995;11:1107. doi: 10.1089/aid.1995.11.1107. [DOI] [PubMed] [Google Scholar]
- 13.Hoover EA, Olsen R, Hardy WDJ, Schaller JP, Mathes LE. Feline leukemia virus infection: age-related variation in response of cats to experimental infection. J Natl Canc Inst. 1976;57:365. doi: 10.1093/jnci/57.2.365. [DOI] [PubMed] [Google Scholar]
- 14.Jarrett O, Russell PH, Stewart MF. Protection of kittens from feline leukaemia virus infection by maternally-derived antibody. Vet Rec. 1977;101:304. doi: 10.1136/vr.101.15.304. [DOI] [PubMed] [Google Scholar]
- 15.Tompkins MB, Tompkins WA. Stimulation of a cell-mediated cytotoxic response to FeLV-induced T cell lymphomas in the cat. J Immunol. 1985;135:2817. [PubMed] [Google Scholar]
- 16.Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, Farthing C, Ho D. Temporal association of cellular immune responses with the initial control of viraemia in primary human immunodeficiency type 1 syndrome. J Virol. 1994;68:4650. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific cytotoxic T-lymphocyte activity associated with control of viremia in primary human immmunodeficiency virus type 1 infection. J Virol. 1994;68:6103. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rinaldo C, Huang X-L, Fan Z, et al. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infected long-term nonprogressors. J Virol. 1995;69:5838. doi: 10.1128/jvi.69.9.5838-5842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogg GS, Jin X, Bonhoeffer S, et al. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 20.Koenig S, Conley AJ, Brewah YA, et al. Transfer of HIV-1-specific cytotoxic T lymphocytes to an AIDS patient leads to selection for mutant HIV variants and subsequent disease progression. Nat Med. 1995;4:330. doi: 10.1038/nm0495-330. [DOI] [PubMed] [Google Scholar]
- 21.Brodie SJ, Lewinson DA, Patterson BK, Jiyampa D, Krieger J, Corey L, Greenberg PD, Riddell SR. In vivo migration and function of transferred HIV-1-specific cytotoxic T cells. Nat Med. 1999;5:34. doi: 10.1038/4716. [DOI] [PubMed] [Google Scholar]
- 22.Finzi D, Blankson J, Siliciano JD, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 23.Sarzotti M, Robbins DS, Hoffman PM. Induction of protective CTL responses in newborn mice by a murine retrovirus. Science. 1996;271:1726. doi: 10.1126/science.271.5256.1726. [DOI] [PubMed] [Google Scholar]
- 24.Dittmer U, Race B, Hasenkrug KJ. Kinetics of the development of protective immunity in mice vaccinated with a live recombinant retrovirus. J Virol. 1999;73:8435. doi: 10.1128/jvi.73.10.8435-8440.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarrett O, Ganiere JP. Comparative studies of the efficacy of a recombinant feline leukaemia virus vaccine. Vet Rec. 1996;138:7. doi: 10.1136/vr.138.1.7. [DOI] [PubMed] [Google Scholar]
- 26.Zeidner NS, Mathiason-Dubard CK, Hoover EA. Reversal of feline leukemia virus infection by adoptive transfer of lectin/interleukin-2-activated lymphocytes, interferon-α, and zidovudine. J Immunotherapy. 1993;14:22. doi: 10.1097/00002371-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Blakeslee J, Noll G, Olsen R, Triozzi PL. Adoptive immunotherapy of feline leukemia virus infection using autologous lymph node lymphocytes. J Aquir Immune Defic Syndr Hum Retrovirol. 1998;18:1. doi: 10.1097/00042560-199805010-00001. [DOI] [PubMed] [Google Scholar]
- 28.Flynn JN, Cannon CA, Beatty JA, MacKett M, Rigby MA, Neil JC, Jarrett O. Induction of feline immunodeficiency virus-specific cytotoxic T cells in vivo with carrier-free synthetic peptide. J Virol. 1994;68:5835. doi: 10.1128/jvi.68.9.5835-5844.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flynn JN, Cannon CA, Reid G, Rigby MA, Neil JC, Jarrett O. Induction of feline immunodeficiency virus-specific cell-mediated and humoral immune responses following immunization with a multiple antigenic peptide from the envelope V3 domain. Immunology. 1995;85:171. [PMC free article] [PubMed] [Google Scholar]
- 30.Gotch FM, Nixon DF, Alp N, McMichael AJ, Borysiewicz LK. High frequency of memory and effector gag specific T lymphocytes in HIV seropositive individuals. Int Immunol. 1990;2:707–712. doi: 10.1093/intimm/2.8.707. [DOI] [PubMed] [Google Scholar]
- 31.Song W, Collisson EW, Billingsley PM, Brown WC. Induction of feline immunodeficiency virus-specific cytolytic T-cell responses from experimentally infected cats. J Virol. 1992;66:5409. doi: 10.1128/jvi.66.9.5409-5417.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beatty JA, Willett BJ, Gault EA, Jarrett O. A longitudinal study of feline immunodeficiency virus-specific cytotoxic T lymphocytes in experimentally infected cats, using antigen-specific induction. J Virol. 1996;70:6199. doi: 10.1128/jvi.70.9.6199-6206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]