Abstract
Whether CD5 on B cells marks a subset functionally distinct from the conventional CD5 negative (CD5neg) adult population or is more an indicator of activation, remains contentious. Here we have investigated whether CD5 positive (CD5pos) and CD5neg B cells can be distinguished in terms of their response to surrogate signals aimed to model, in vitro, T-cell dependent (TD) and T-independent (TI) encounters with antigen in vivo: the predominantly CD5pos B-cell population found in cord blood, CD5 B cells positively selected from tonsils and their CD5neg counterparts, were compared. Neonatal B cells displayed a near-identical phenotype to that of adult CD5pos B cells, being characterized by uniform immunoglobulin M (IgM), immunoglobulin D (IgD), CD23 and CD44 coexpression. When cultured with anti-IgM maintained at high density on CD32-tranfected mouse L cells to model TI responses or on CD40 ligand (CD40L)-bearing L cells (with or without captured anti-IgM) to model TD encounters, DNA synthesis was stimulated to a similar extent in all three populations. Focusing on CD5 and CD23, we found that – although the signals delivered promoted distinct profiles of expression – under each condition of activation, the phenotypes that emerged for adult CD5pos and CD5neg B cells were remarkably similar. Neonatal B cells displayed a greater diminution in CD5 expression than adult CD5pos B cells following CD40 signals but otherwise the two populations again behaved similarly. The inclusion of interleukin-4 (IL-4) to cultures where cells were costimulated via surface (s)IgM and CD40 resulted in a complete loss of CD5 expression and a corresponding hyperexpression of CD23, irrespective of the population studied. The near-identical response of CD5pos and CD5neg B cells to surrogate TD or TI signals in vitro and their convergence to indistinguishable phenotypes is wholly supportive of CD5 being a fluctuating marker of activation rather than it delineating functionally distinct subsets.
INTRODUCTION
The existence of discrete subsets of B cells that can be segregated primarily on their presence or absence of CD5 is now well established in mice.1 However, the origin of the subpopulations – and whether they truly represent distinct lineages – remains a matter of controversy. Some believe that CD5 positivity defines a so-called ‘B-1a’ subset of cells, distinct from the conventional adult B2 population by: (i) appearing early in ontogeny; (ii) displaying bone marrow-independent self-renewal capacity; (iii) preferentially localizing in adults to peritoneal and pleural cavities; (iv) expressing immunoglobulins that are more polyreactive in their binding capacity; and (v) constitutively expressing signal transducer and activator of transcription-3 (STAT3). A minor ‘sister’ population possessing the above characteristics but lacking CD5 have been classified as B-1b.2,3 Other investigators – and major exponents here are Wortis and colleagues – hypothesize that CD5-positive (CD5pos) B cells arise as a result of any newly differentiated B cell undergoing extensive, and possibly chronic, cross-linking of its B-cell receptors (BCR).4
Evidence has been presented to indicate that CD5pos B cells respond to T-cell independent (TI) antigens, participate primarily in natural immunity and are associated with autoimmunity, whereas CD5-negative (CD5neg) B cells respond to T-cell dependent (TD) antigens and have a dominant role in acquired immunity.5 In contrast, it is interesting to note that in humans, neonatal life is associated with deficient humoral responses to TI-2 antigens and that, when compared to adults, this is accompanied with a surplus of B cells expressing CD5: the fall in CD5pos B cells in the circulation as a child matures coincides with the emergence of intact functional responses to TI signals.6–8
Exploration of ensuing alterations in function and phenotype on exposure of B-cell populations to TI-2 and TD antigens has been modelled in vitro by the provision of signals delivered using cross-linked anti-immunoglobulin M (IgM) and CD40 monoclonal antibodies (mAb), respectively, the latter mimicking the essential CD40–CD40 ligand (CD40L) pairing that accompanies cognate B–T interactions during TD responses.9,10 In mice, small resting B cells differentially respond to surface (s)IgM cross-linking and CD40 stimulation by producing populations with distinct phenotypes, the hallmark changes being the reciprocal induction/disappearance of CD5 and CD23.11 For human adult B cells, triggering via CD40 results not only in a marked up-regulation of CD23 but also in the appearance of CD5 on a minor subset of cells.12 Moreover, the induction of CD5 can be readily effected with polyclonal stimulators such as phorbol 12-myristate 13-acetate (PMA) or Staphylococcus aureus Cowan strain I (SAC) – a TI-2 superantigen surrogate.13–15 These observations, coupled with the finding that CD5pos B cells can be encouraged to become CD5 negative on culture with interleukin (IL)-4, have provided further argument against the notion of human CD5pos B cells representing a subset distinct from that of the CD5neg population.16
While the function of CD5 is not yet fully resolved, it has been shown that its engagement sequesters the pseudo-immunoreceptor tyrosine-based activation motif (ITIM)- containing molecule away from surface immunoglobulin, consequently preventing the blockade of BCR-mediated signals that would otherwise arise.17 This proposed negative role of the CD5 molecule in antigen receptor-mediated proliferation makes it important to assess whether constitutive or induced expression of CD5 influences the responsiveness of human B cells to TD and TI signal mimetics. To address this, and to provide further insight into the mutability (or otherwise) of CD5 positivity on different human B-cell populations, we have compared the functional responses and emergent phenotypes of CD5-rich umbilical cord blood B cells with purified CD5pos and CD5neg adult B cells following their receipt of signals delivered via cell membrane-presented CD40L and/or anti-IgM.
MATERIALS AND METHODS
Reagents
The mAbs OKT1 (anti-CD5, immunoglobulin G1 [IgG1]), UCHT2 (anti-CD5, IgG1), OKT3 (anti-CD3, IgG1), 61D3 or UCHM-1 (anti-CD14, IgG1) and OKT10 (anti-CD38, IgG1) were produced from hybridomas in the Medical Research Council Centre for Immune Regulation, University of Birmingham, and purified by ion-exchange chromatography on DE52 (Whatman Ltd, Maidstone, Kent, UK). For fluorescence-activated cell sorter (FACS®) analysis, we used fluorescein isothiocyanate (FITC)-conjugated immunoglobulin D (IgD), IgG, CD5, CD14, CD19, CD21, CD23, CD40, CD44, CD56, CD77, Ki-67 and phycoerythrin (PE) -conjugated CD2 and IgM (Dako Ltd, High Wycombe, Bucks., UK), FITC-conjugated CD10, CD25 and CD71, PE-conjugated CD3, CD23, and CD38, and PerCP-conjugated CD20 (Becton-Dickinson, Oxford, Oxon, UK), PE-conjugated IgM (PharMingen, San Diego, CA) and PE-Cy5-conjugated CD5 (Immunotech, Marseille, France). Human IL-4 was purchased from R & D Systems Ltd (Oxford, Oxon, UK).
Isolation of B cells from human tonsils
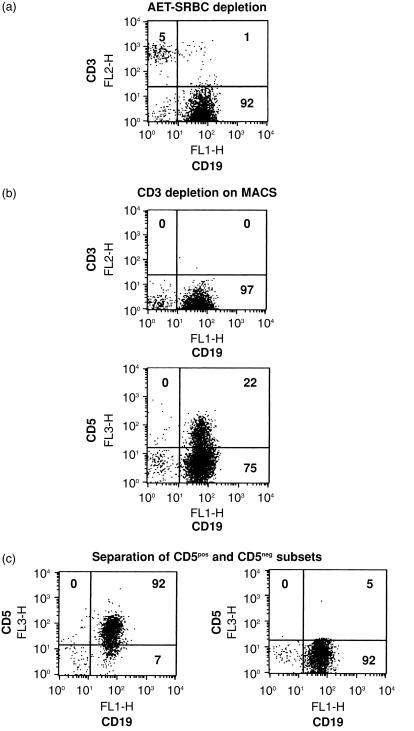
Tonsils were obtained from patients undergoing routine tonsillectomy. Cells were extracted by dissection and dispersal in RPMI-1640 (Gibco Ltd, Paisley, Strathclyde, UK). Mononuclear cells were layered onto Ficoll-Paque® (Pharmacia Biotech, Uppsala, Sweden) and centrifuged at 450 g for 20 min at room temperature. Interface cells were washed in RPMI-1640, and T cells were depleted by E-rosetting with amino ethyl isothiouronium bromide (AET)-treated sheep red blood cells (SRBC), with rosettes removed by centrifugation on Ficoll-Paque. Enriched B cells were depleted of remaining T cells using depletion columns on a magnetic cell separator (VarioMACS; Miltenyi BiotecGmbH, Bergisch Gladbach, Germany). Briefly, non-rosetting cells were cultured with 20 µg/ml of anti-CD3 (OKT3) and UCHM-1 (anti-CD14) mAbs for 15 min at 4°. On washing with phosphate-buffered saline (PBS) containing 0·5% bovine serum albumin (BSA) and 5 mm EDTA (MACS buffer), cells were subsequently incubated with goat anti-mouse IgG Microbeads® (Miltenyi Biotec). After washing with MACS buffer, magnetically unlabelled cells were collected as highly purified B cells, as assessed by immunofluorescence labelling with CD3, CD19, CD56 and CD14. These cells were used for experiments either as unfractionated B cells or were subsequently incubated with 50 µg/ml of anti-CD5 mAb (BL1a; IgG2a, Immunotech) for 15 min at 4°, washed and incubated with goat anti-mouse IgG Microbeads for an additional 15 min at 4°. Preliminary experiments showed that BL1a mAb did not affect the growth response of unfractionated tonsillar B cells at the concentration used for cell separation (see the Results). After washing, both positively selected (CD5pos) and negatively selected cells (CD5neg) were collected after separation on a positive selection column. For preparations used in this study, the purity of CD5pos and CD5neg B cells was >90% and >95%, respectively. These procedures and the resultant populations generated are given in Fig. 1.
Figure 1.
Separation of CD5-positive (CD5pos) and CD5-negative (CD5neg) B-cell subsets from tonsils. Tonsils initially depleted of T cells by amino ethyl isothiouronium bromide-sheep red blood cell (AET-SRBC) rosetting (a) were then depleted of the remaining T cells by using depletion columns on a magnetic cell separator (MACS) (b). Highly purified B cells were separated into CD5pos and CD5neg B cells on a positive selection column (c). Each separation step was accompanied with flow cytometry.
Isolation of B cells from cord blood
Positive selection on a magnetic cell separator with anti-CD19 coupled Microbeads (Miltenyi Biotec) was used to isolate B cells from mononuclear cells separated from cord blood on Ficoll-Paque. Following this procedure, >98% of the resulting cell population was CD19pos.
Culture of B cells
Tonsillar or cord blood B cells (106/ml) were cultured in flat-bottom 96-well microtitre plates to measure DNA synthesis or in 48-well plates (Becton-Dickinson Labware) to determine changes in phenotype, in a total volume of 200 µl or 0·5 ml, respectively, in RPMI-1640 containing penicillin (100 IU/ml), streptomycin (100 µg/ml), 2 mmol/l of glutamine (Gibco, Grand Island, NY) and 10% fetal calf serum (FCS; Sera Lab Ltd, Crawley Down, Sussex, UK) at 37° in a humidified incubator in 5% CO2. Where indicated, non-transfected mouse L cells, mouse L cells transfected with the human CD32 gene (CD32-L cells), or mouse cells co-transfected with CD32 and CD40L (CD32/CD40L-L cells), were used as described previously.18 Briefly, adherent L cells cultured in RPMI-1640 with 10% FCS and antibiotics (CM) were recovered using 0·02% disodium EDTA in PBS, pH 7·2, resuspended in CM and γ-irradiated (7000 rads) before addition to B cells at a ratio of 1 : 10 (L cells : B cells). The influence of sIgM ligation with or without ligation of CD40 was assessed by using CD32/CD40L-transfected L cells and CD32-transfected L cells, respectively, and 0·5 µg/ml of anti-IgM antibody AF6 (IgG1). Where indicated, IL-4 (100 ng/ml) was added at the start of cell culture.
Flow cytometric analysis
The cells were analysed immediately after separation or after different times in culture, as indicated in the Results. Cultured cells were harvested by incubation for 5 min with 0·02% disodium EDTA to disperse aggregates, washed with RPMI-1640 and than stained prior to analysis on a FACScan flow cytometer (Becton-Dickinson, Mountain View, CA). Cell suspensions were stained using standard direct two- or three-colour immunofluorescence staining methods as previously described.19 Briefly, after harvesting, cells were washed in PBS supplemented with 5% goat serum (Harlaan Sera-Lab Limited, Loughborough, Leicestershire, UK) and 0·1% sodium azide (Sigma, Poole, Dorset, UK) and then incubated using at least 2 × 105 cells per sample with previously determined optimal concentrations of mAbs conjugated to different fluorochromes (FITC, PE and PE-cyanin 5.1 (CY5) or FITC, PE and peredinin-chlorophyll protein (PerCP) for 15 min in the dark at room temperature. Cells were then washed and subsequently resuspended in 0·5% formaldehyde (Sigma) in PBS containing 5% goat serum and 0·1% sodium azide, and analysed within 24 hr of staining on a flow cytometer.
Measurement of DNA synthesis
Cells cultured in triplicate in flat-bottom microtitre plates for 3 days were pulsed with [3H]thymidine ([3H]Tdr; Amersham International, Amersham, Bucks, UK; 10 µCi/ml in CM, 50 µl per well) for the final 16–18 hr of culture. The cells were harvested onto glass fibre filters on a Skatron (Helis Bio Ltd, Newmarket, Suffolk, UK) cell harvester, and incorporation of [3H]Tdr was measured by liquid scintillation spectroscopy. The activity of anti-CD5 antibodies (UCHT2, BL1a and OKT1) in such assays was assessed following their addition at the beginning of culture at the following concentrations: 0·5, 5 and 50 µg/ml.
Simultaneous DNA content and cell-surface immunofluorescence analysis
CD5neg B cells were harvested after 3 days of culture, as described above, and then processed for simultaneous analysis of DNA content and CD5 expression. Briefly, cell suspensions were stained with anti-CD5 FITC for 15 min in the dark at room temperature. After washing, cells were fixed with fixation buffer (containing 4% formaldehyde in Dulbecco's PBS) for 20 min at 4°, washed and permeabilized with permeabilization buffer (containing 1% FCS, 0·1% NaN3, 0·1% saponin in Dulbecco's PBS) for 10 min prior to addition of 50 µg/ml of propidium iodide (PI). Cells were then cultured for 20 min at 4°, washed with permeabilization buffer and analysed immediately on a flow cytometer.
RESULTS
Phenotypic characterization of cord blood and fractionated tonsillar B cells
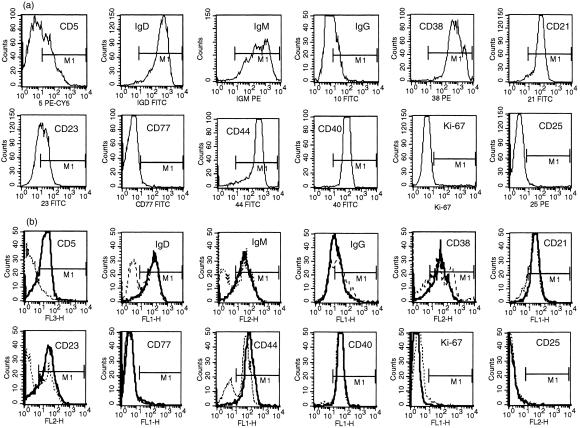
Given that the aim of our study was to explore and compare the consequences of signalling the different B-cell populations through sIgM and CD40, it was necessary to establish in some detail the starting phenotype of the subsets used. In terms of the expression of sIgD, sIgM, sIgG, CD21, CD23, CD25, CD38, CD40, CD44 and CD77, unseparated cord blood B cells and fractionated CD5pos tonsillar B cells were remarkably similar (Fig. 2). Consistent with this, it could be seen that – as expected – the majority of cord blood B cells were CD5pos, although the actual numbers in individual samples ranged from 45 to 77%. The universally high CD23 expression associated with both cord blood B cells and fractionated CD5pos tonsillar B cells is at odds with the mutual exclusivity of these markers on murine B-cell subsets but fully consistent with what has been reported previously for humans, especially the CD5/CD23 double positivity that provides the hallmark phenotype of B-chronic lymphocytic leukaemia.1,20 A notable difference between the CD5-rich population found in cord blood and the CD5pos B cells of tonsils was the substantially higher level of CD38 and IgM expression on the former, as reported previously.21,22
Figure 2.
Comparison of phenotypic markers in a representative example of freshly isolated umbilical cord blood (a) and CD5-positive (CD5pos) and CD5-negative (CD5neg) B cells (b). In (b), dotted lines represent the level of expression by CD5neg B cells and solid lines the level of expression by CD5pos B cells. The ranges (M1) are set with an irrelevant isotype-matched fluorescent conjugate. FITC, fluorescein isothiocyanate; IgD, immunoglobulin D; IgG, immunoglobulin G; IgM, immunoglobulin M.
Importantly, fractionated CD5pos and CD5neg tonsillar B cells expressed identical levels of CD40. While they differed in the proportion of cells expressing sIgM – all CD5pos cells were sIgMpos while only ≈ 70% of CD5neg cells expressed sIgM – the level of expression on positive cells was the same, irrespective of CD5 status (Fig. 2b). The sIgMneg subset contained within the CD5neg fraction was accounted for by isotype-switched cells not present within the CD5pos populations from either tonsil or cord blood (Fig. 2 and refs 23,24). CD25, an activation antigen for human B cells,25 was almost completely absent from all populations analysed: similarly, the small minority of cells displaying intracellular staining for the cell cycle-associated nuclear antigen, Ki-67, confirmed that most cells were residing in the resting state (Fig. 2). The bimodal staining profiles obtained for sIgD, CD38 and CD44 with the CD5neg tonsillar B-cell population compared with their homogeneous patterns of expression on the CD5pos fraction probably reflected the almost exclusive extrafollicular localization of the latter; CD5neg B cells, by contrast, can be located in all major compartments of secondary lymphoid tissues.26 The extremely low numbers of CD77pos cells found, even in the CD5neg tonsillar fraction, indicated that there was no significant contribution from centroblasts to these preparations, again consistent with the predominantly resting configuration of the subsets isolated.
Induction of DNA synthesis in B-cell subsets in response to BCR and CD40 signals
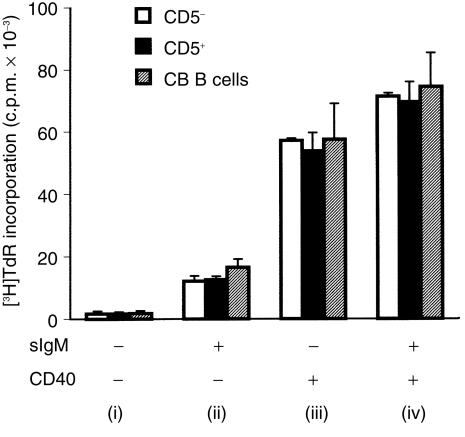
Next we studied how the B-cell populations under investigation compared in their response to signals generated through BCR and/or CD40. This was achieved by plating the B cells onto irradiated mouse L cells as follows: unmodified L cells, serving as a control; CD32-tranfected L cells carrying IgG1 mAb (AF6) to sIgM, providing ‘TI-like’ signals; and CD32/CD40L co-tranfectants, either with or without captured anti-IgM, to model TD signalling. For each set of signals, all three populations studied – neonatal B cells as well as CD5pos and CD5neg tonsillar B cells – responded almost identically (Fig. 3). In keeping with the lack of activation markers noted above, each population displayed a negligible spontaneous uptake of [3H]Tdr. There was a significant response to the L cell-captured anti-IgM and an even greater one to membrane-expressed CD40L, which was slightly boosted on co-engagement of BCR.
Figure 3.
Comparison of DNA synthesis in cord blood (CB) B cells and different fractions of tonsillar B cells (CD5-positive [CD5pos] and CD5-negative [CD5neg]) after the engagement of B-cell receptors (BCR) and/or CD40. B cells (105 cells/well) were cultured for 3 days with: (i) non-transfected mouse L cells, (ii) CD32-transfected mouse L cells and anti-immunoglobulin M (IgM) antibody at 0·5 µg/ml (BCR), (iii) CD40 ligand (CD40L)/CD32-transfected cells (CD40) and (iv) CD40L/CD32-transfected L cells and anti-IgM antibody. [3H]Thymidine ([3H]Tdr) incorporation (in counts per minute [c.p.m.])was measured during the last 18 hr of culture. Values for [3H]Tdr uptake by mouse L cells alone were always less than 1000 counts per minute (c.p.m.) (results not shown). Data are given as the mean + SD of three experiments.
It was considered important to establish whether the BL1a antibody used to isolate the CD5pos fraction from tonsil was, in itself, capable of modulating DNA synthesis. This antibody was compared with two others – OKT1 and UCHT2 – each directed against different epitopes. As seen in Table 1, none of the mAbs were directly mitogenic to unseparated (but CD5-containing) tonsillar B cells and they did not influence the signal delivered by cell-bound anti-IgM. However, at the lowest concentration tested (0·5 µg/ml), the UCHT2 mAb increased the proliferation of unfractionated B cells in response to a CD40 signal. This same antibody, as well as OKT1, induced a dose-dependent decrease in DNA synthesis when B cells received signals jointly through BCR and CD40. In summary, although there is evidence for epitope-dependent modulation of some BCR and CD40 responses with CD5 antibodies, BL1a – the mAb selected for isolating the CD5pos fractions in this study – was found to be inert in this regard.
Table 1.
DNA synthesis in unfractionated tonsillar B cells in response to anti-CD5 monoclonal antibodies (mAbs) after engagement of CD40 or CD40 and surface immunoglobulin M (sIgM)
| BL1a (μg/ml) | OKT1 (μg/ml) | UCHT2 (μg/ml) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stimulus | 0 | 0·5 | 5 | 50 | 0·5 | 5 | 50 | 0·5 | 5 | 50 |
| Experiment 1 | ||||||||||
| Control | 995 ± 401 | 2250 ± 600 | 2188 ± 648 | 2734 ± 1106 | 1448 ± 230 | 1449 ± 465 | 1540 ± 548 | 1606 ± 877 | 2138 ± 218 | 1912 ± 523 |
| sIgM | 3021 ± 334 | 5005 ± 1154 | 5751 ± 1504 | 8495 ± 1824 | 5563 ± 1298 | 4068 ± 1265 | 4306 ± 1906 | 2843 ± 693 | 5690 ± 1862 | 4647 ± 1517 |
| CD40 | 18 325 ± 6428 | 20 204 ± 2974 | 19 880 ± 6592 | 20 841 ± 2727 | 18 502 ± 3771 | 18 133 ± 4833 | 14 689 ± 5474 | 30 099 ± 4016 | 26 428 ± 4423 | 17 529 ± 6674 |
| sIgM + CD40 | 40 504 ± 4364 | 38 464 ± 4772 | 36 575 ± 4836 | 40 931 ± 4569 | 42 701 ± 4367 | 36 709 ± 4715 | 26796 ± 3890 | 49 168 ± 5760 | 32 125 ± 5341 | 22 905 ± 4122 |
| Experiment 2 | ||||||||||
| Control | 1474 ± 933 | 2027 ± 604 | 2047 ± 361 | 2634 ± 674 | 2031 ± 464 | 1834 ± 101 | 1459 ± 598 | 1721 ± 733 | 1884 ± 800 | 2127 ± 807 |
| sIgM | 8354 ± 2526 | 6374 ± 1827 | 7058 ± 1258 | 7185 ± 1096 | 7629 ± 1385 | 4995 ± 2853 | 2555 ± 915 | 3372 ± 1318 | 5641 ± 2198 | 5163 ± 1495 |
| CD40 | 23 291 ± 5754 | 17 383 ± 1573 | 18 462 ± 2437 | 22 029 ± 4992 | 23 533 ± 3467 | 225 851 ± 5231 | 21 252 ± 3784 | 28 242 ± 5763 | 22 009 ± 4461 | 20 633 ± 5898 |
| sIgM + CD40 | 40 921 ± 6836 | 45 891 ± 8802 | 43 891 ± 3656 | 49 934 ± 8253 | 39 469 ± 6212 | 46 012 ± 5242 | 28 001 ± 3558 | 38 852 ± 8529 | 37 125 ± 3242 | 32 997 ± 3247 |
B cells (105 cells/well) were cultured for 3 days with: non-transfected mouse L cells (‘Control’); CD32-transfected mouse L cells and anti-IgM antibody at 0·5 µg/ml (‘sIgM’); CD40 ligand (CD40L)/CD32-transfected cells (‘CD40’); and CD40L/CD32-transfected L cells and anti-IgM antibody (‘sIgM + CD40’) in the presence or absence of CD5 mAbs. [3H]Thymidine ([3H]Tdr) incorporation was measured during the final 18 hr of culture. Values for [3H]Tdr uptake by mouse L cells alone were always less than 1000 counts per minute (c.p.m.) (results not shown).
Changes in surface phenotype after BCR and CD40 stimulation of B-cell populations
The hallmark of the phenotypic changes on murine B cells engaged in BCR and CD40 signalling is the reciprocal induction/disappearance of CD5 and CD23. We investigated how signals generated through BCR and/or CD40 influenced the coexpression of CD5 and CD23 on human neonatal B cells and tonsillar B-cell fractions. Despite the remarkably similar levels of DNA synthesis seen in each of the populations with these stimuli, it remained possible that the differentiation pathways followed by CD5pos and CD5neg B cells in response to TD and TI surrogate signals were distinct.
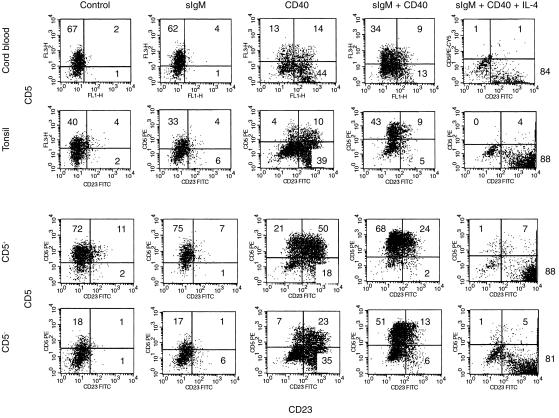
It has been previously reported for total resting tonsillar B cells that BCR co-ligation increases the expression and proportion of CD40-induced CD5pos cells, while CD40-stimulated up-regulation of CD23 is partially reversed.12,27 Here we showed a similar outcome for purified CD5neg B cells, demonstrating that the alterations observed in unfractionated populations are not caused by selective subset outgrowth. In contrast to the situation in the mouse, we found that CD40 ligation – and not BCR triggering – led, in the CD5neg population, to an enhancement of CD5 expression on cells that were also induced to express CD23 (Fig. 4). However, BCR cross-linking did significantly augment the CD40-dependent induction of CD5 on cells previously CD5neg such that, within 3 days, approximately two-thirds were CD5pos: under these conditions, the predominant CD5pos subset was now CD23neg (Fig. 4).
Figure 4.
Flow cytometric analysis of neonatal B cells (‘Cord blood’) and different fractions of tonsillar B cells (total B cells designated as ‘Tonsil’). Two-colour fluorescence-activated cell sorter (FACScan®) analysis shows staining of cells with CD23 monoclonal antibodies (mAb) conjugated to fluorescein isothiocyanate (FITC) (x-axis) and CD5 mAb conjugated to phycoerythrin (PE) or PE-Cy5 (y-axis). Cells were cultured for 3 days in the presence of non-transfected L cells (‘Control’), anti-immunoglobulin M (anti-IgM) antibody (0·5 µg/ml) cross-linked on CD32-transfected L cells (surface [s]IgM) and dual CD32/CD40 ligand (CD40L)-transfected L cells, with (sIgM + CD40) and without (CD40) anti-IgM antibodies, in the presence or absence of 100 ng/ml of recombinant interleukin-4 (rIL-4). The quadrants were drawn on the basis of control cells stained with isotypic antibody of irrelevant specificity. The numbers given are the percentages of cells in each quadrant. The data given are from a representative example of four similar experiments, each of which demonstrated the same trends shown here.
For CD5-rich neonatal B cells particularly – but also to some extent for fractionated CD5pos tonsillar B cells – culture with CD40L resulted in a down-regulation of CD5 expression (Fig. 4). Co-ligation with BCR abrogated such CD40-dependent down-regulation of CD5, especially on the CD5pos tonsillar B-cell starting population. Regarding CD5/CD23 coexpression, the patterns obtained following stimulation of the CD5pos subset via CD40 and/or BCR were remarkably similar to those generated in the CD5neg fraction (Fig. 4) and not greatly different from those arising in cord blood B cells.
It should be noted that on all cultured – but unstimulated – populations, CD23 expression was lost by day 3: presumably this reflects its known turnover from the cell surface by endogenous proteolytic cleavage.28 IL-4 has been reported not only to potently up-regulate CD23, but also to selectively down-regulate CD5 on unfractionated tonsillar B cells either under basal conditions or following PMA-stimulated CD5 expression.16 We confirmed this effect of IL-4 on basal CD5 levels for both cord blood and isolated CD5pos tonsillar B cells (results not shown) and also showed that, for each subset engaged in signalling via BCR and CD40, the presence of IL-4 resulted in a virtual loss of constitutive or induced CD5 with a corresponding hyperexpression of CD23 (Fig. 4).
Cell cycle status of CD5pos cells generated from CD5negB cells on BCR/CD40 ligation
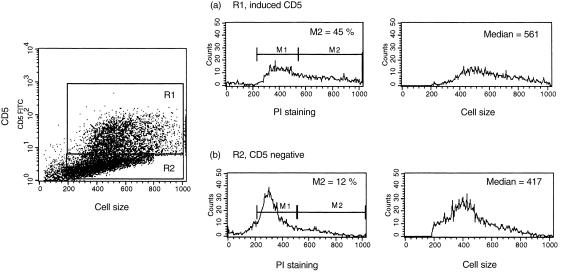
Studies on peripheral blood B cells activated with PMA were supportive of the notion that CD5 behaves more as an activation antigen on human B cells rather than as a marker for a discrete lineage of cells.15 Moreover, CD5pos and CD5neg B cells have been reported to express similar percentages of Ki-67, the cycle-related nuclear antigen, when activated with anti-IgM and IL-2.29 We investigated whether the induced expression of CD5 following the activation of CD5neg B cells through co-engagement of BCR and CD40 was related to their position within the cell cycle. As seen in Fig. 5, when assessed against forward scatter, cells induced to express CD5 on BCR/CD40 co-ligation tended to be larger than those that remained CD5neg. When subjected to simultaneous analysis of DNA content and CD5 expression, almost 50% of the previously CD5neg B cells that had been induced to become CD5pos upon stimulation through BCR and CD40 were found to be in active cell cycle: this contrasted with cells that remained CD5 negative, where only a small minority displayed S or G2/M DNA.
Figure 5.
Simultaneous detection of DNA content and CD5 expression in CD5-negative (CD5neg) B cells following B-cell receptor (BCR) and CD40 engagement. CD5neg B cells were cultured for 3 days with irradiated CD40 ligand (CD40L)/CD32-transfected L cells and anti-immunoglobulin M (anti-IgM) antibody. Cell-surface staining with fluorescein isothiocyanate (FITC)-conjugated anti-CD5 monoclonal antibody (mAb) was followed by fixation, permeabilization and addition of propidium iodide (PI). DNA content was measured in cells that up-regulated CD5 (region 1, R1) and those that remained CD5 negative (region 2; R2). Histogram sets (a) and (b) show PI staining and forward scatter (relative cell size) in cells that induced CD5 and those that remained CD5 negative, respectively. Histogram marker 1 (M1) shows the cells in the G0 and G1 stages, and M2 shows the percentage of cells in the G2 + M and S phases of the cell cycle. The median forward scatter for cells in each region is also indicated.
DISCUSSION
The origin of CD5pos B cells continues to fuel debate and controversy: the current state of the opposing viewpoints – with particular reference to human B cells – was elegantly reviewed in a recent article by Youinou et al.30 Here, the authors proposed a reconciliation of the divergent theses by postulating two different classes of CD5pos B cells:
Those where CD5 expression was ‘constitutive’, i.e. B-1a cells with their distinctive properties of self-renewal, polyreactive antigen specificity, selective tissue localization and early appearance in ontogeny, as outlined above.
CD5neg conventional B cells that have been induced to express CD5 on appropriate activation.
Cord blood B cells are generally considered to represent the former, while a substantial proportion of CD5pos B cells found in tonsils may be accounted for by the latter.7,30 From studies in the mouse, it has been suggested that stimulation with TI-2 type antigens is the major route to inducible CD5 expression, leading to the postulate that CD5 B cells found in vivo are the result of such activation by conventional environmental antigens; conversely, TD signals purportedly down-regulate CD5 in favour of CD23 up-regulation on murine B-cell subsets.11
In the present study, we found that – irrespective of either their CD5 status or source of origin – the way human B cells performed in response to individual modes of stimulation was quite similar. Contrasting with the situation described for murine B cells, we also found that induction of CD5 was CD40 dependent and was certainly not mutually exclusive with CD23 expression. Whilst BCR cross-linking alone (to model TI-2 signalling) resulted in a significant induction of DNA synthesis in all populations under study, there was no induction of CD5 de novo on CD5neg cells and no substantial increase was noted on the level of CD5 expression on CD5pos cells. However, BCR signals substantially augmented CD40-mediated up-regulation of CD5, demonstrating a clear contribution of this pathway to the inducible CD5 phenotype.
For all populations studied, BCR co-ligation yielded a substantial diminution in the CD40-induced CD23 population that otherwise emerged: loss of CD23pos cells was evident in both CD5pos and CD5neg subsets. Loss of CD23 is a feature associated with B cells entering germinal centre (GC) responses.12,27 Dual occupancy of sIgM and CD40 on resting tonsillar B cells has previously been described to generate a blast population with features reminiscent of GC B cells, although more recently it was demonstrated that high critical threshold occupancy of CD40 by its cognate ligand in the absence of a BCR signal is most effective at inducing the expression of CD77, a hallmark phenotype of the GC.31 It is a contentious issue as to whether B-1 cells can participate in GC responses, although a rare population of CD5pos cells has been described within the GC B-cell-enriched fraction from tonsils.24 Conversely, CD5pos peritoneal B cells in mice do not appear to be capable of generating GC and memory responses in vivo. 32 Caligaris-Cappio et al. suggested that CD5pos cord blood B cells exposed to IL-1 and IL-2 acquire features of B blasts proliferating in the GC of secondary follicles.33 More recent data argue against a role for IL-1 and IL-2 in promoting this phenotype but, rather, highlight interferon (IFN)-α and IFN-γ as key cytokines in the development of cells with GC features.27 Our own preliminary experiments investigating a range of phenotypic markers have indicated that, among tonsillar B cells, CD5pos and CD5neg populations are equally prone to develop GC-like features in response to BCR/CD40 co-ligation, consistent with the notion that CD5 is a fluctuating marker of activation in this environment rather than delineating a functionally distinct subset.
Induced expression of CD5 – exemplified in this study post-BCR/CD40 co-engagement – was associated with an activated phenotype, as evidenced by the high percentage of CD5pos cells that were in the active cell cycle. However, efficient stimulation per se does not necessarily result in CD5 positivity, as dramatically demonstrated by the addition of IL-4 to these conditions. While IL-4-promoted down-regulation of both constitutive and induced CD5 expression has been noted previously,16 it was remarkable to observe the near complete loss of the BCR/CD40-dependent CD5 induction/maintenance for all populations under study. Indeed, together with the hyperexpression of CD23, in terms of these two putative subset markers, the different populations stimulated via sIgM and CD40 converged to a totally indistinguishable phenotype in the presence of IL-4.
Our results in toto are supportive of CD5 being a fluctuating marker of human B cells responding to selective stimuli, regardless of their source of origin: its presence or absence reflecting the previous experience of a cell. What the functional consequence to the B cell of modulating CD5 might be is, however, unclear.34 Sen et al. recently suggested that CD5 negatively regulates sIgM-mediated signals by recruiting src homology 2 domain-containing protein tyrosine phosphatase-1 (SHP-1), a cytosolic protein tyrosine phosphatase, into the B-cell receptor complex in B-1 cells.35 In this context, it has also been shown that CD5 ligation of resting tonsillar B cells results in apoptosis.36 In the present study, we found that extensive cross-linking of CD5 with two of three anti-CD5 mAbs used (UCHT2 and OKT1) delivered an antiproliferative signal to B cells engaged in signalling via sIgM and CD40. As these experiments were performed using unseparated tonsillar B cells we have yet to determine whether constitutively expressed and induced CD5 function similarly in this regard. The appearance of CD5 might thus provide a means of dampening, or redirecting, B-cell responses to selective activation signals: this could be triggered by external ligands or through its intrinsic association with VH framework determinants, as characterized in the rabbit.37–40 The ability to modulate readily, both up and down, CD5 expression on normal B-cell populations via defined physiological receptors should facilitate the further exploration of its function in respect to these candidate ligands.
Acknowledgments
This work was supported by a Wellcome Travel Fellowship (A.G.) and a Medical Research Council Programme Grant; J.G. is an MRC non-Clinical Research Professor. We are very grateful to Peter Lydyard for his detailed evaluation and criticism of this paper.
Abbreviations
- AET
amino ethyl isothiouronium bromide
- BCR
B-cell receptor
- CD40L
CD40 ligand
- MACS
magnetic cell sorting
- PMA
phorbol 12-myristate 13-acetate
- SAC
Staphylococcus aureus Cowan strain I
- sIg
surface immunoglobulin
- TD
T-cell dependent
- TI
T-cell independent
REFERENCES
- 1.Hardy RR, Hayakawa K. CD5 B cells, a fetal B cell lineag. Adv Immunol. 1994;55:297–339. doi: 10.1016/s0065-2776(08)60512-x. [DOI] [PubMed] [Google Scholar]
- 2.Hardy RR, Hayakawa K. A developmental switch in B lymphopoiesi. Proc Natl Acad Sci USA. 1991;88:11550–4. doi: 10.1073/pnas.88.24.11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karras JG, Wang Z, Huo L, Howard RG, Frank DA, Rothstein TL. Signal transducer and activator of transcription-3 (STAT3) is constitutively activated in normal, self-renewing B-1 cells but only inducibly expressed in conventional B lymphocyte. J Exp Med. 1997;185:1035–42. doi: 10.1084/jem.185.6.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wortis HH. Surface markers, heavy chain sequences, and B cell lineage. Int Rev Immunol. 1992;8:235–46. doi: 10.3109/08830189209055576. [DOI] [PubMed] [Google Scholar]
- 5.Stall AM, Wells SM, Lam K-P. B-1 cells: unique origins and function. Semin Immunol. 1996;8:45–59. doi: 10.1006/smim.1996.0007. [DOI] [PubMed] [Google Scholar]
- 6.Anderson U, Bird AG, Britton BS, Palacios R. Humoral and cellular immunity in humans studied at the cell leve. Immunol Rev. 1981;57:1–38. doi: 10.1111/j.1600-065x.1981.tb00440.x. [DOI] [PubMed] [Google Scholar]
- 7.Hardy RR, Hayakawa K, Shimizu M, Yamasaki K, Kishimoto T. Rheumatoid factor secretion from human Leu-1+ B cells. Science. 1987;236:81–3. doi: 10.1126/science.3105057. [DOI] [PubMed] [Google Scholar]
- 8.Bhat NM, Kantor AB, Bieber MM, Stall AM, Herzenberg LA, Teng NNH. The ontogeny and functional characteristics of human B-1 (CD5+) B cells. Int Immunol. 1992;4:243–52. doi: 10.1093/intimm/4.2.243. [DOI] [PubMed] [Google Scholar]
- 9.Parker DC, Wadsworth DC, Schneider GB. Activation of murine B lymphocytes by anti-immunoglobulin is an inductive signal leading to immunoglobulin secretio. J Exp Med. 1980;152:138–51. doi: 10.1084/jem.152.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tony HP, Phillips NE, Parker DC. Role of membrane immunoglobulin (Ig) crosslinking in membrane Ig-mediated, major histocompatibility-restricted T cell–B cell interaction. J Exp Med. 1985;162:1695–708. doi: 10.1084/jem.162.5.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wortis HH, Teutsch M, Higer M, Zheng J, Parker DC. B-cell activation by crosslinking of surface IgM or ligation of CD40 involves alternative signal pathways and results in different B-cell phenotype. Proc Natl Acad Sci USA. 1995;92:3348–52. doi: 10.1073/pnas.92.8.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wheeler K, Gordon J. Co-ligation of surface IgM and CD40 on naïve B lymphocytes generates a blast population with an ambiguous extrafollicular/germinal centre cell phenotyp. Int Immunol. 1996;8:815–28. doi: 10.1093/intimm/8.6.815. [DOI] [PubMed] [Google Scholar]
- 13.Miller RA, Gralow J. The induction of leu-1 antigen expression in human malignant and normal B cells by phorbol myristic acetate (PMA) J Immunol. 1984;133:3408–14. [PubMed] [Google Scholar]
- 14.Morikawa K, Oseko F, Morikawa S. Induction of CD5 antigen on human CD5- B cells by stimulation with Staphyloccocus aureus Cowan strain. Int Immunol. 1993;5:809–16. doi: 10.1093/intimm/5.8.809. [DOI] [PubMed] [Google Scholar]
- 15.Vernino L, Pisetsky DS, Lipsky PE. Analysis of the expression of CD5 by human B cells and correlation with functional activit. Cell Immunol. 1992;139:185–97. doi: 10.1016/0008-8749(92)90111-2. [DOI] [PubMed] [Google Scholar]
- 16.Defrance T, Vanbervliet B, Durand I, Banchereau J. Human interleukin 4 down-regulates the surface expression of CD5 on normal and leukemic B cells. Eur J Immunol. 1989;19:293–9. doi: 10.1002/eji.1830190212. [DOI] [PubMed] [Google Scholar]
- 17.Bikah G, Carey J, Ciallella JR, Tarakhovsky A, Bondada S. CD5-mediated negative regulation of antigen receptor-induced growth signals in B-1 cells. Science. 1996;274:1906–9. doi: 10.1126/science.274.5294.1906. [DOI] [PubMed] [Google Scholar]
- 18.Gagro A, Gordon J. The interplay between T helper subset cytokines and IL-12 in directing human B lymphocyte differentiatio. Eur J Immunol. 1999;29:3369–79. doi: 10.1002/(SICI)1521-4141(199910)29:10<3369::AID-IMMU3369>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 19.Gagro A, Rabatic S. Allergen-induced CD23 on CD4+ T lymphocytes and CD21 on B lymphocytes in patients with allergic asthma: evidence and regulatio. Eur J Immunol. 1994;24:1109–14. doi: 10.1002/eji.1830240515. [DOI] [PubMed] [Google Scholar]
- 20.Caligaris-Cappio F, Janossy G. Surface markers in chronic lymphoid leukemias of B cell typ. Semin Hematol. 1985;22:1–15. [PubMed] [Google Scholar]
- 21.Malavasi F, Funaro A, Roggero S, Horenstein A, Calosso L, Mehta K. Human CD38: a glycoprotein in a search of a functio. Immunol Today. 1994;15:95–7. doi: 10.1016/0167-5699(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 22.Macardle PJ, Weedon H, Fusco M, Nobbs S, Ridings J, Flego L, Roberton DM, Zola H. The antigen receptor complex on cord B lymphocyte. Immunology. 1997;90:376–82. doi: 10.1111/j.1365-2567.1997.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tucci A, Mouzaki A, James H, Bonnefoy J-Y, Zubler RH. Are cord blood B cells functionally mature. Clin Exp Immunol. 1991;84:389–94. [PMC free article] [PubMed] [Google Scholar]
- 24.Holder MJ, Abbot SD, Milner AE, Gregory CD, Casamayor M, Johnson GD, MacLennan ICM, Gordon J. Interleukin-2 expands and maintains IgM plasmablasts from a CD5+ subset contained within the germinal center cell-enriched (surface IgD¯/CD39¯ buoyant) fraction of human tonsi. Int Immunol. 1993;5:1059–66. doi: 10.1093/intimm/5.9.1059. [DOI] [PubMed] [Google Scholar]
- 25.Butcher RD, McGarvie GM, Cushley W. Recombinant interleukin-4 promotes expression of the CD25 (Tac) antigen at the plasma membrane of high-density human tonsillar B lymphocyte. Immunology. 1990;69:57–64. [PMC free article] [PubMed] [Google Scholar]
- 26.Dono M, Burgio VL, Tacchetti C, et al. Subepithelial B cells in the human palatine tonsil. I. Morphologic, cytochemical and phenotypic characterizatio. Eur J Immunol. 1996;26:2035–42. doi: 10.1002/eji.1830260911. [DOI] [PubMed] [Google Scholar]
- 27.Galibert L, Burdin N, de Saint-Vis B, Garrone P, Van Kooten C, Banchereau J, Rousset F. CD40 and B cell antigen receptor dual triggering of resting B lymphocytes turns on a partial germinal center phenotyp. J Exp Med. 1996;183:77–85. doi: 10.1084/jem.183.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon J, Cairns JA, Liu Y-J, Flores-Romo L, MacLennan ICM, Jansen KU, Bonnefoy J-Y. Role of membrane and soluble CD23 in lymphocyte physiolog. Monogr Allergy. 1991;29:156–68. [PubMed] [Google Scholar]
- 29.Jamin C, Le Corre R, Lydyard PM, Youinou P. Anti-CD5 extends the proliferative response of human CD5+ B cells activated with anti-IgM and interleukin- Eur J Immunol. 1996;26:57–62. doi: 10.1002/eji.1830260109. [DOI] [PubMed] [Google Scholar]
- 30.Youinou P, Jamin C, Lydyard PM. CD5 expression in human B-cell population. Immunol Today. 1999;20:312–6. doi: 10.1016/s0167-5699(99)01476-0. [DOI] [PubMed] [Google Scholar]
- 31.McCloskey N, Pound JD, Holder MJ, Williams JM, Roberts LM, Lord JM, Gordon J. The extrafollicular-to-follicular transition of human B lymphocytes: induction of functional globotriaosylceramide (CD77) on high threshold occupancy of CD4. Eur J Immunol. 1999;29:3236–44. doi: 10.1002/(SICI)1521-4141(199910)29:10<3236::AID-IMMU3236>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 32.Linton P-J, Lo D, Lai L, Thorbecke GJ, Klinman NR. Among naïve precursor cell subpopulations only progenitors of memory B cells originate germinal centre. Eur J Immunol. 1992;22:1293–7. doi: 10.1002/eji.1830220526. [DOI] [PubMed] [Google Scholar]
- 33.Caligaris-Cappio F, Riva M, Tesio L, Schena M, Gaidano G, Begui L. Human normal CD5+ B lymphocytes can be induced to differentiate to CD5– B lymphocytes with germinal center cell feature. Blood. 1989;73:1259–63. [PubMed] [Google Scholar]
- 34.Arnold LW, McCray SK, Tatu C, Clarke SH. Identification of a precursor to phosphatidylcholine-specific B-1 cells suggesting that B-1 cells differentiate from splenic conventional B cells in vivo: cyclosporin A blocks differentiation to B- J Immunol. 2000;164:2924–30. doi: 10.4049/jimmunol.164.6.2924. [DOI] [PubMed] [Google Scholar]
- 35.Sen G, Bikah G, Venkatamaran C, Bondada S. Negative regulation of antigen receptor-mediated signalling by constitutive association of CD5 with the SHP-1 protein tyrosine phosphatase in B-1 B cells. Eur J Immunol. 1999;29:3319–28. doi: 10.1002/(SICI)1521-4141(199910)29:10<3319::AID-IMMU3319>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 36.Pers JO, Jamin C, Le Corre R, Lydyard PM, Youinou P. Ligation of CD5 on resting B cells, but not on resting T cells, results in apoptosi. Eur J Immunol. 1998;28:4170–6. doi: 10.1002/(SICI)1521-4141(199812)28:12<4170::AID-IMMU4170>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 37.Van de Velde HI, von Hoegen W, Luo W, Parnes JR, Thielemans K. The B-cell surface protein CD72/Lyb-2 is the ligand for CD. Nature. 1991;351:662–5. doi: 10.1038/351662a0. [DOI] [PubMed] [Google Scholar]
- 38.Biancone L, Bowen MA, Lim A, Aruffo A, Andres G, Stamenkovic I. Identification of a novel inducible cell-surface ligand of CD5 on activated lymphocyte. J Exp Med. 1996;184:811–9. doi: 10.1084/jem.184.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pospisil R, Fitts MG, Mage RG. CD5 is a potential selecting ligand for B cell surface immunoglobulin framework region sequence. J Exp Med. 1996;184:1279–84. doi: 10.1084/jem.184.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hippen K, Tze LE, Behrens TW. CD5 maintains tolerance in anergic cells. J Exp Med. 2000;191:883–9. doi: 10.1084/jem.191.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]