Abstract
Proinflammatory cytokines have been shown to activate endothelial cells. To investigate the effect of cytokines on the interaction of human umbilical vein endothelial cells (HUVEC) with Pseudomonas aeruginosa, cells were treated with interferon-γ (IFN-γ) plus tumour necrosis factor-α (TNF-α) for 24 hr and exposed to P. aeruginosa suspension for 1 hr. Light microscopy showed that activated cells internalized significantly more bacteria than control cells. To ascertain the effect of cytokines on the microbicidal activity of HUVEC, the concentrations of viable intracellular (IC) bacteria in control and activated cells were determined, at 1 and 5 hr postinfection, by the gentamicin exclusion assay. In control cells, no significant decrease in the concentration of bacteria was detected 5 hr postinfection. In contrast, in activated cells the concentration of viable bacteria at 5 hr was significantly lower. Concentrations of superoxide and hydrogen peroxide detected in supernatants of activated cells were significantly higher than in control cell supernatants. HUVEC anti-P. aeruginosa activity was insensitive to the antioxidants superoxide dismutase, dimethylthiourea and allopurinol as well as to the l-arginine analogues aminoguanidine and NG-monomethyl-l-arginine (l-NMMA), but was significantly inhibited by catalase. Our results indicate that HUVEC can be activated by IFN-γ plus TNF-α to kill IC P. aeruginosa and suggest a role for reactive oxygen radicals, notably hydrogen peroxide, in HUVEC antibacterial activity.
INTRODUCTION
Scattered infectious vasculitis and microscopic abscesses involving many organs are common consequences of Pseudomonas aeruginosa bloodstream infections, mainly in neutropenic patients.1 It is believed that to enter the tissue parenchyma from blood vessels, blood-borne micro-organisms must adhere to and penetrate the endothelial lining of the vasculature. Therefore, endothelial cells may be considered as a defence against haematogenously disseminated P. aeruginosa infections. In vitro studies have shown that human umbilical vein endothelial cells (HUVEC) actively phagocytized P. aeruginosa.2,3 However, HUVEC were not efficient at killing intracellular (IC) bacteria as after 6 hr of infection there was only a 14% decrease in the counts of viable IC micro-organisms. In addition, an increasing concentration of bacteria was recovered from the culture medium with time, suggesting that the internalized micro-organisms were released from endothelial cells.2 These results led to the speculation that in vivo, P. aeruginosa internalization by endothelial cells, rather than conferring protection against disseminated infection, would protect the bacteria from host defences and antibiotics. Release of viable micro-organisms into the bloodstream would play an important role in the pathogenesis of bacteraemia.
Endothelial cells have been viewed as a passive structural lining of blood vessels but it is now evident that upon activation by cytokines, such as interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α), they can influence inflammatory reactions and the immune response.4,5 Although endothelial cells are not professional phagocytes, they share a number of other functional similarities with macrophage (Mφ). Upon stimulation they present Fc receptor-mediated phagocytosis, can express class II major histocompatibility complex (MHC) antigens and present antigens to lymphocytes.5,6
IFN-γ is a proinflammatory cytokine that enhances host defences against a variety of pathogens. It has been shown to augment the microbicidal activity of neutrophils and Mφ by stimulating microbial uptake and by increasing the production of reactive oxygen intermediates (ROI) and nitric oxide (NO). IFN-γ may also activate endothelial cells. It was shown to upregulate the release of ROI7 and NO by HUVEC,8 and to stimulate mouse endothelial cells to kill intravascular Schistosoma mansoni9 and human cells to inhibit the IC replication of Toxoplasma gondii.10 However, it has not yet been elucidated whether IFN-γ can enhance the microbicidal activity of endothelial cells against IC bacteria.
In the present study we demonstrated that HUVEC can be activated by IFN-γ plus TNF-α to kill IC P. aeruginosa through production of ROI.
MATERIALS AND METHODS
Reagents
All reagents used were from Sigma Chemical Co (St. Louis, MO), unless indicated otherwise.
Bacteria
Piliated P. aeruginosa PAK were grown overnight at 37° in trypticase soy broth (Difco Laboratories, Detroit, MI), harvested by centrifugation and resuspended in M-199 medium containing 25 mm HEPES (N,2 hydroxyethylpiperazine-N′2 ethanesulphonic acid) to an optical density (OD) at 640 nm of 0·1, corresponding to 108 colony-forming units (CFU)/ml.
Cell culture
Cells were isolated from human umbilical veins as previously described2,3 and seeded in culture flasks (coated with 1% gelatin) in M-199 medium containing 10% fetal calf serum (FCS) and antibiotics (complete culture medium). Confluent primary cultures were trypsinized and 2·5 × 104 cells in 100 µl of complete culture medium were cultured until confluency, either on glass coverslips or in wells of 96-well tissue culture plates. Cell cultures were treated with IFN-γ (6507; lot 113H10211) at 100 U/ml plus TNF-α (0157; lot 74H00921) at 1000 U/ml in complete culture medium for 24 hr, as described by Oswald et al.9 Control monolayers were incubated with complete culture medium only. Human monocyte-derived Mφ were obtained by culturing adherent peripheral blood mononuclear cells for 7 days in complete culture medium, as described previously,11 and were used as positive controls in experiments assessing the effect of cytokine activation on superoxide and hydrogen peroxide production by cultured cells.
P. aeruginosa adherence to and internalization by HUVEC
Confluent monolayers of control or cytokine-activated HUVEC cultured on glass coverslips were incubated with P. aeruginosa suspensions for 1 hr at 37°, washed three times with phosphate buffered saline (PBS), pH 7·2, and fixed with 4% paraformaldehyde in PBS for 1 hr at 4°. In order to distinguish extracellular (EC) bacteria, adherent to endothelial cells, from IC bacteria, fixed monolayers were treated successively with a polyclonal anti-P. aeruginosa antibody raised in rabbits (diluted 1 : 200) for 1 hr, with a 1 : 1000 dilution of biotinylated anti-rabbit immunoglobulin G (IgG) (Amersham Corp., Arlington Heights, IL), with a 1 : 1000 dilution of streptavidin complexed to horseradish peroxidase (Amersham) and then with diaminobenzidine-imidazol-H2O2 solution at 0·1%. Cultures were then counterstained with May-Grunwald-Giemsa stain, which stained IC bacteria violet in endothelial cells. The number of IC and EC bacteria in 130 different control and 130 activated cells, in two different assays, was determined by light microscopy.
P. aeruginosa survival in activated HUVEC
Control and activated confluent monolayers in 96-well microtitre plates were incubated with P. aeruginosa suspensions for 1 hr, and then washed and treated with 300 µg/ml of gentamicin in culture medium for 1 or 5 hr, to kill EC bacteria, as previously described.2 After each incubation period, cells from at least three wells were rinsed and lysed with 0·1% Triton-X-100 in PBS. Cell lysates were diluted and plated in trypticase soy agar (TSA; Difco) to quantify viable IC bacteria. In all experiments, aliquots of the antibiotic-containing medium were collected and plated in TSA to confirm the efficacy of gentamicin for killing EC-adherent P. aeruginosa.
Viability of cytokine-activated HUVEC
As activation may lead to cell injury,4 the viability of activated HUVEC was evaluated using the methylthiazole tetrazolium (MTT) test.12 Briefly, control non-activated or cytokine-activated cells were exposed to MTT solution at 1 mg/ml in M-199 culture medium for 1 hr at 37°. Cells were then rinsed and the insoluble formazan crystals formed in viable metabolic active cells were solubilized in isopropanol. The absorbances of supernatants from control and activated cells were measured using an automatic microplate scanning spectrophotometer (Bio-Rad, Richmond, CA), with a 570-nm test wavelength and a 690-nm reference wavelength. Viability of control and activated HUVEC harbouring IC P. aeruginosa was also evaluated (after 1 and 5 hr) by the MTT assay. To ascertain that IC bacteria had not contributed to MTT oxidation in infected HUVEC, as a control 1 ml of a 106 CFU/ml P. aeruginosa suspension was centrifuged and the bacterial pellet was incubated with MTT solution for 1 hr, rinsed and treated with isopropanol, as described above.
Effect of antioxidants and of l-arginine analogues on HUVEC bactericidal activity
Cells were incubated for 24 hr with IFN-γ plus TNF-α in complete culture medium in the presence or absence of superoxide dismutase (SOD) at 300 U/ml, catalase at 10 U/ml, dimethyl thiourea (DMTU) at 10 mm, allopurinol at 0·1 mm, aminoguanidine 200 µm or NG-monomethyl-l-arginine (l-NMMA) at 200 µm. Control cultures were incubated with complete culture medium only. After the incubation period, the supernatants from cell cultures were removed, cells were exposed to P. aeruginosa suspension for 1 hr, washed and then incubated with the gentamicin-containing medium. SOD, catalase, DMTU, allopurinol, aminoguanidine or l-NMMA, at the same concentrations used during the activation period, were included both in the bacterial suspension and in the gentamicin-containing medium. The concentration of viable IC bacteria was determined after 1 and 5 hr, as described above.
Superoxide and hydrogen peroxide production
The concentration of superoxide and hydrogen peroxide in supernatants from control and cytokine-activated cells was determined by the reduction of ferricytochrome C assay and by the phenol red method, respectively, as described by Pick & Mizel.13
NO production
NO reacts with water in culture medium to produce nitrite, the concentration of which can be taken as a measure of NO production. The nitrite concentration in the supernatants of 2·0 × 105 control and activated cells cultured in 24-well tissue culture plates was determined by a colorimetric assay, based on the Griess reaction.14
Statistical analysis
Results are expressed as mean ± SEM of data obtained in experiments carried out at least in triplicate. Statistical differences were determined using the Student's t-test or Mann–Whitney rank sum test. A P-value below 0·05 was considered statistically significant.
RESULTS
Cytokine activation enhanced HUVEC endocytic and microbicidal activities
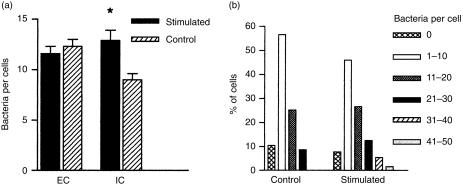
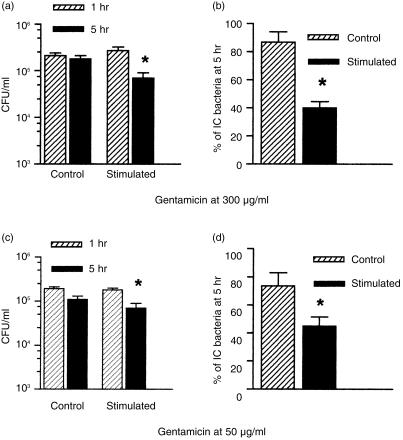
P. aeruginosa adherence to and internalization by endothelial cells was assessed by light microscopy after immunoperoxidase staining of EC micro-organisms. As shown in Fig. 1(a), control and cytokine-stimulated HUVEC did not differ in the mean number of adherent EC bacteria. In contrast, stimulated cells internalized significantly more bacteria than control cells (P < 0·05). Figure 1(b) shows the range of IC bacteria in control and stimulated cells. While no control cell contained more than 30 IC P. aeruginosa, 7·0% of stimulated cells contained from 31 to up 50 IC micro-organisms. Figure 2 provides visual evidence of both EC and IC bacteria in cytokine-stimulated HUVEC. To investigate the effect of cytokine activation on the fate of IC bacteria, control and activated cells were exposed to bacterial suspensions for 1 hr, and the concentrations of viable IC micro-organisms 1 and 5 hr postinfection were determined using the gentamicin exclusion assay. Figure 3(a) shows that in control cells bacterial concentration at 5 hr postinfection was not significantly different from the concentration at 1 hr. In contrast, in activated cells the concentration at 5 hr was significantly lower than at 1 hr (P < 0·02). Figure 3(b) shows the percentage of bacteria detected in control and in activated cells at 5 hr postinfection, relative to bacterial concentration at 1 hr as 100%. The P. aeruginosa concentration in control cells at 5 hr represented 86·8 ± 7·4% of the concentration detected at 1 hr. The percentage of residual bacteria in activated cells (40·2 ± 4·4%) was significantly lower (P < 0·001). However, as cytokine activation enhanced HUVEC endocytic activity, we wondered whether the reduction in P. aeruginosa survival in activated cells could be caused by a simultaneous increase in the uptake of gentamicin. Cells were therefore exposed to culture medium containing gentamicin at 50 µg/ml for duration of the assay. The rationale of this approach was that the amount of the antibiotic taken up by the cells would be decreased to a sub-minimum inhibitory concentration for the P. aeruginosa PAK strain used in this study. Figures 3(c) and 3(d) show the results obtained in assays carried out with gentamicin at 50 µg/ml. In activated cells, the concentration of IC bacteria at 5 hr was significantly lower than at 1 hr (P < 0·02) whereas in control cells the difference between bacterial concentrations at 1 and 5 hr was not statistically significant. Figure 3(d) shows that the P. aeruginosa concentration in control cells at 5 hr represented 73·6 ± 9·4% of the concentration at 1 hr. The percentage of residual bacteria in activated cells (44·8 ± 6·6%) was significantly lower (P = 0·02).
Figure 1.
(a) Effect of cytokine stimulation on Pseudomonas aeruginosa adherence to and internalization by human umbilical vein endothelial cells (HUVEC). Data represent mean ± SEM of adherent (EC) and intracellular (IC) bacteria in 130 control and 130 stimulated cells, as assessed by light microscopy in two different assays. *P < 0·05 by the Mann–Whitney test. (b) Range of IC P. aeruginosa in control and stimulated cells.
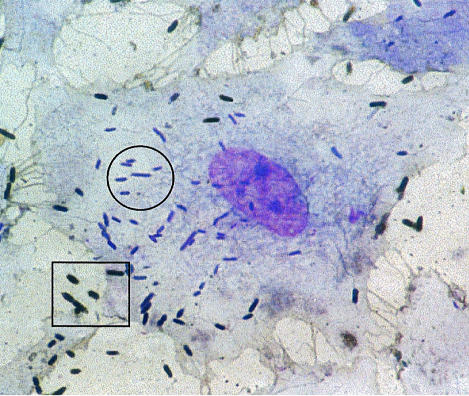
Figure 2.
Light microscopy view of a cytokine-activated endothelial cell showing intracellular bacteria stained violet (surrounded by a circle) and adherent extracellular bacteria, stained brown (surrounded by a rectangle). Magnification × 4300.
Figure 3.
Effect of cytokine stimulation of human umbilical vein endothelial cells (HUVEC) on the fate of intracellular (IC) Pseudomonas aeruginosa: (a) and (b), cells treated with gentamicin at 300 µg/ml; (c) and (d), cells treated with gentamicin at 50 µg/ml. (a) and (c): IC concentrations of P. aeruginosa in stimulated HUVEC at 5 hr postinfection were significantly lower than at 1 hr postinfection (*P < 0·02). (b) and (d): percentage of IC bacteria in control and in stimulated HUVEC at 5 hr postinfection, regarding the bacterial concentration at 1 hr postinfection as 100%. *P < 0·001 and P = 0·02 for (b) and (d), respectively. Data are expressed as mean ± SEM, and values were obtained from at least three independent assays carried out in triplicate.
Effect of cytokine activation on HUVEC viability
In the MTT test, the amount of formazan that cells produce is proportional to their metabolic activity and viability.12 The absorbance of solubilized formazan produced by non-infected activated cells represented 105 ± 7·6% of the absorbance of solubilized formazan obtained from control, non-infected HUVEC. Therefore, the cytokine activation did not lead to damage of endothelial cells. In contrast, the absorbance values of HUVEC activated for 24 hr and harbouring IC P. aeruginosa for 1 and 5 hr represented 85·9 ± 4·2% and 85·5 ± 10·8%, respectively, of the absorbance values of non-infected activated cells. These results suggest that activated HUVEC were somehow damaged by bacterial products or by the oxidative stress secondary to simultaneous stimulation by cytokine and bacterial infection.
Role of ROI production in the bactericidal activity of cytokine-activated HUVEC
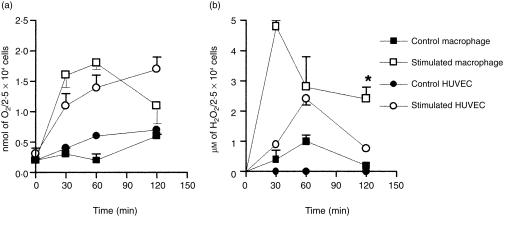
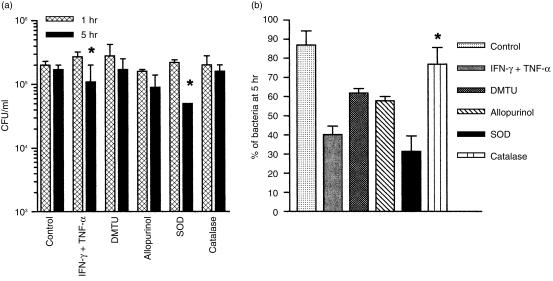
Two different approaches were used to determine whether ROI may have accounted for the anti-P. aeruginosa activity of cytokine-treated cells: (i) comparative evaluation of the release of superoxide and hydrogen peroxide by control and activated cells; or (ii) evaluation of the effect of antioxidants added to the culture medium in the HUVEC bactericidal activity. The rationale of the latter approach was that exogenous antioxidants will probably gain entry into phagocytic cells, as previously demonstrated.15,16 Fig. 4(a) shows that the concentrations of superoxide produced by activated HUVEC and by human monocyte-derived Mφ (positive control) were significantly higher (P < 0·05 and < 0·01 for HUVEC and Mφ, respectively) than the concentrations produced by control resting cells. As shown in Fig. 4(b), no hydrogen peroxide could be detected in the supernatant of control HUVEC whereas up to 2·4 ± 0·2 µm was detected in activated endothelial cell supernatants. Release of hydrogen peroxide by activated Mφ was significantly higher (P < 0·01), and occurred earlier, than release by activated HUVEC. The effect of different antioxidants relative to the bactericidal activity of activated HUVEC is shown in Fig. 5. Bacterial concentrations at 5 hr postinfection in activated cells treated with DMTU and allopurinol, two scavengers of hydroxyl radicals, and in activated cells treated with catalase, which decomposes hydrogen peroxide, were not statistically different from the concentrations at 1 hr. In contrast, the P. aeruginosa concentration at 5 hr in activated HUVEC treated with SOD, which dismutates superoxide in hydrogen peroxide, was significantly lower than at 1 hr (P < 0·01). This finding led to speculation as to whether the increase in concentration of hydrogen peroxide in SOD-treated cells may have accounted for the reduction in bacterial concentration at 5 hr postinfection. The decrease in the difference between P. aeruginosa concentrations at 1 and 5 hr postinfection in catalase-treated activated cells, which is shown in Fig. 5(a) and is visualized better in Fig. 5(b), seemed to corroborate this hypothesis. Figure 5(b) shows that the percentage of bacteria detected in catalase-treated activated cells at 5 hr postinfection (76·9 ± 5·3%) was significantly higher (P < 0·01) than the percentage of residual bacteria in untreated activated cells (40·2 ± 4·4%) and was similar to the percentage in control cells (86·8 ± 7·4%). Figure 5(b) shows also that treatment of activated cells with DMTU and allopurinol did not modify significantly the HUVEC anti-P. aeruginosa activity. However, whereas the percentage of bacteria in cells treated with the two hydroxyl radical scavengers was slightly higher than the percentage in activated cells, the percentage of bacteria at 5 hr in cells treated with SOD was slightly lower. This finding further indicates that dismutation of superoxide in hydrogen peroxide may have somehow enhanced HUVEC bactericidal activity. Taken together, these results suggest that hydrogen peroxide may be a putative effector molecule of the bactericidal activity of cytokine-treated HUVEC.
Figure 4.
Release of superoxide (a) and hydrogen peroxide (b) by control and stimulated human umbilical vein endothelial cells (HUVEC) and human monocyte-derived macrophage (Mφ). *P < 0·01 when results obtained with stimulated Mφ were compared with results from stimulated HUVEC. Data are expressed as mean ± SEM, and values were obtained from at least two independent assays carried out in quadruplicate.
Figure 5.
(a) Effect of different antioxidants on the fate of intracellular (IC) Pseudomonas aeruginosa in cytokine-activated human umbilical vein endothelial cells (HUVEC). The bacterial concentration at 5 hr postinfection in activated cells treated with superoxide dismutase (SOD) was significantly lower than the concentration at 1 hr postinfection (*P < 0·01). (b) Percentage of residual IC bacteria at 5 hr postinfection in control cells, in cytokine-activated cells and in cytokine-activated cells treated simultaneously with different antioxidants. Percentages were calculated regarding bacterial concentrations at 1 hr postinfection as 100%. *P = 0·001 when the bacterial percentage in catalase-treated, activated cells was compared with the percentage in untreated, activated cells. Data are expressed as mean ± SEM, and values were obtained from at least three independent assays carried out in triplicate. DMTU, dimethyl thiourea; IFN-γ, interferon-γ; TNF-α, tumour necrosis factor-α.
Role of NO in HUVEC bactericidal activity
Two different approaches were used to assess the role of NO in HUVEC anti-P. aeruginosa activity: (i) comparative evaluation of the release of NO by control and activated cells; and (ii) evaluation of the effect of l-arginine analogues on the bactericidal activity of activated cells. The concentration of nitrite detected in the supernatants of activated cells (7·5 ± 0·4 µm) did not differ from the concentration detected in the supernatants of control cells (7·8 ± 1·3 µm), as determined in two different assays carried out in triplicate. On the other hand, although the percentages of residual bacteria at 5 hr postinfection in activated cells treated with aminoguanidine (54·1 ± 6·1%) or l-NMMA (53·1 ± 8·9%) were slightly higher than the percentage in untreated activated cells (43·8 ± 6·9%), the differences between these values were not statistically significant.
DISCUSSION
Classically, endothelial cells have been viewed as passive members of the immune system, acting as barriers to the influx of microbes and secreting factors that bring professional phagocytes to sites of bacterial invasion. In recent years, however, a body of evidence has surfaced documenting the ability of endothelial cells to phagocytose17 and kill18,19 IC bacteria and, under stimulation, to express phenotypic characteristics of Mφ-like cells.6 Mφ activated by proinflamatory cytokines kill internalized micro-organisms basically by action of granule proteins and by two oxidative pathways, involving the synthesis of ROI and NO.
We have previously shown that human endothelial cells actively internalize P. aeruginosa but they did not efficiently eliminate internalized bacteria.2 This probably stems, at least partially, from both the low index of endosome–lysosome fusion observed in P. aeruginosa-infected HUVEC and the lysis of membranes of P. aeruginosa-containing endosomes.3 As IFN-γ and TNF-α have been shown to activate the microbicidal activity of Mφ, and also to modulate different properties of human endothelial cells,4,5 in this study we investigated the synergic effect of these cytokines on the HUVEC anti-P. aeruginosa activity. Activated cells killed ≈ 60·0% of IC bacteria in 5 hr, whereas control non-activated cells killed approximately four times fewer bacteria (≈ 15·0%). We demonstrate therefore that IFN-γ and TNF-α can also modulate the bactericidal activity of HUVEC.
Endothelial cells are known to synthesize and release superoxide anions during their phagocytic activity.20,21 Accordingly, potential mechanisms by which activated HUVEC could have influenced the survival of IC P. aeruginosa include enhancement of ROI production. As previously described by Matsubara et al.,7 we observed that the release of superoxide by HUVEC was significantly augmented upon stimulation by IFN-γ plus TNF-α. However, the anti-P. aeruginosa activity detected in activated cells does not seem to depend on superoxide itself as it could not be inhibited by SOD, an enzyme that accelerates the dismutation of superoxide to form hydrogen peroxide. Rather, upon the action of SOD, HUVEC bactericidal activity was slightly increased. In contrast, the bactericidal activity of cytokine-treated HUVEC was significantly inhibited by catalase, which converts hydrogen peroxide to H2O and ½O2. Hydrogen peroxide is a more reactive oxidant than superoxide and its potential cytotoxicity is increased by its capacity to readily diffuse across biological membranes. As we also observed that activated HUVEC produced significantly more hydrogen peroxide than control cells, it is tempting to speculate that hydrogen peroxide production and HUVEC anti-P. aeruginosa activity may relate to one another as cause and effect. However, as P. aeruginosa is known to produce different catalases,22 further experiments are necessary to determine the precise role of hydrogen peroxide and/or of products secondary to the reduction of hydrogen peroxide in the microbicidal activity of activated HUVEC. Even though cytokine-stimulated HUVEC produced significantly more superoxide and hydrogen peroxide than resting cells, the concentration of ROI they produced was significantly lower than that produced by cytokine-stimulated monocyte-derived Mφ. This different level of ROI production may contribute to the inability of endothelial cells to effectively kill IC P. aeruginosa.
Another potential mechanism by which HUVEC activation may have influenced the survival of IC P. aeruginosa is by up-regulation of the NO output.23 NO is produced in human cells by NOS isoforms that have been categorized into inducible (iNOS) and constitutive (cNOS) isoenzymes. Endothelial cells produce cNOS, which generates low NO output and is related to the physiological control of vascular tone. In contrast, other cells, such as Mφ, exhibit NOS activity only after activation by cytokines.24 The relationship between proinflammatory cytokines and increased NO output has generally been attributed to cytokine-induced iNOS expression but studies have shown that NO production by HUVEC can also be enhanced by augmenting the specific activity of the cNOS isoform.25,26 Although cytokines have been reported to increase the release of NO by HUVEC, in this study, control non-activated cells did not differ from activated cells in the nitrite concentration detected in their supernatants. Studies have suggested that NO accounted for the anti-S. mansoni activity of cytokine-activated murine endothelial cells9 and also for the anti-rickettsial activity of bovine endothelial cells.27 However, because in this study the treatment of activated HUVEC with l-arginine analogues did not increase the percentage of residual bacteria to levels detected in control cells, it seems that NO alone is not the major molecule accounting for the anti-P. aeruginosa activity detected in activated HUVEC.
The activation of phagocytic cells to produce NO often also induces the production of ROIs, creating the possibility of an interaction between these molecules.28 The interaction between NO and superoxide produces peroxynitrite, which upon protonation forms peroxynitrous acid. The final effect of these reactions is probably the mutual scavenging of NO and ROIs.29 The intermediate peroxynitrite, however, is a stronger oxidant than NO and has been referred to as a potent microbicidal molecule.30 Therefore, it is possible that besides ROIs, species generated second to their interaction with NO may have contributed to the anti-P. aeruginosa activity of cytokine-treated HUVEC. Alternatively, NO may have potentiated the hydrogen peroxide-induced killing of bacteria, as described by Pacelli et al.31 Enhancement of the hydrogen peroxide antibacterial activity by NO may explain the slight increase, in our study, in the percentage of residual bacteria in cells treated with l-arginine analogues. Experiments studying combination treatment with different antioxidants are currently underway in an attempt to elucidate the actual mechanism of activated HUVEC anti-P. aeruginosa activity.
The generation and IC accumulation of hydrogen peroxide and NO have been associated with oxidant-mediated damage of HUVEC32,33 and bovine endothelial cells infected with rickettsial micro-organisms,27 probably secondary to peroxidation of internal membrane lipids. In our study, the viability of activated HUVEC infected with P. aeruginosa, as assessed by the MTT test, was decreased by 15% in comparison with non-infected activated cells, but this difference between infected and non-infected cells did not change during the 5-hr duration of the assays. These results suggest that scavenger systems within HUVEC34 may have prevented ROIs and NO from causing permanent cell damage. The observation that activated HUVEC remains metabolically active, together with other results presented in this study, suggests that, upon activation, endothelial cells may play at least a partial role against dissemination of P. aeruginosa infections.
Even though data reported in this work and elsewhere may suggest a similar role for endothelial cells and Mφ in the control of infectious agents, one has to bear in mind the striking structural and functional differences between these two cell types. Mφ are motile cells that migrate to sites of infection or injury where they play a pivotal role in the destruction and removal of micro-organisms. Mφ overproduction of reactive oxygen and nitrogen intermediates in response to cytokine activation enhances substantially their microbicidal activity. On the other hand, the physiological role of endothelial cells is to provide an effective barrier separating the blood from tissues and a non-thrombogenic surface over which blood can flow. Physiologically, ROI generation by and release from endothelial cells will probably favour angiogenesis, by altering supporting basement membrane and the surrounding connective tissue,7 whereas NO production is a critical homeostatic regulator of vasodilator tone. Endothelium-derived NO regulates other physiological actions within the vasculature, including platelet aggregation and leucocyte adhesion.35 Although under normal conditions endothelial cells are well equipped with scavenger systems,34 the extent of oxidant radical production by cytokine-primed cells after bacterial infection may be too high and lead to endothelium injury.27,32,33 There is compelling evidence to suggest that endothelial damage secondary to oxidative stress facilitates adhesion of platelets and other blood cells to the vascular wall and can thus be considered a primary cause of atherogenesis and thrombosis.36 Accordingly, although oxidant production by cytokine-activated endothelial cells may contribute to the killing of P. aeruginosa, the final effects of cell activation may be deleterious to the host organism. We are currently evaluating this possibility.
Acknowledgments
This research was supported by grants no. 520 375-5 from CNPq and no. 41 960881 00 from FINEP/MCT/PRONEX. M.C.A. was supported by a scholarship from FAPERJ (E-26/150 635/95).
REFERENCES
- 1.Teplitz C. Pathogenesis of Pseudomonas aeruginosa vasculitis and septic lesions. Arch Pathol. 1965;80:297–314. [PubMed] [Google Scholar]
- 2.Plotkowski MC, Saliba AM, Pereira SHM, Cervante MP, Bajolet-Laudinat O. Pseudomonas aeruginosa selective adherence to and entry into human endothelial cells. Infect Immun. 1994;62:5456–63. doi: 10.1128/iai.62.12.5456-5463.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plotkowski MC, Meirelles MN. Concomitant endosome–phagosome fusion and lysis of endosomal membranes account for Pseudomonas aeruginosa survival in human endothelial cells. J Submicrosc Cytol Pathol. 1997;29:229–37. [PubMed] [Google Scholar]
- 4.Pober JS, Cotran RS. Cytokines and endothelial cell biology. Physiol Rev. 1990;70:427–51. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- 5.Mantovani A, Bussolino F, Dejana E. Cytokine regulation of endothelial cell functions. FASEB J. 1992;6:2591–9. doi: 10.1096/fasebj.6.8.1592209. [DOI] [PubMed] [Google Scholar]
- 6.Robinson DH, Warren MK, Liang LT, Oprendy JJ, Nielsen TB, Kang YH. Retroviral transformation of cerebral microvasculature endothelial cells: macrophage-like and microvascular endothelial cell properties. Blood. 1991;77:294–305. [PubMed] [Google Scholar]
- 7.Matsubara T, Ziff M. Increased superoxide anion release from human endothelial cells in response to cytokines. J Immunol. 1986;137:3295–8. [PubMed] [Google Scholar]
- 8.Kilbourn RG, Belloni P. Endothelial cell production of nitrogen oxides in response to interferon-γ in combination with tumor necrosis factor, interleukin-1 or endotoxin. J Natl Cancer Inst. 1990;82:772–6. doi: 10.1093/jnci/82.9.772. [DOI] [PubMed] [Google Scholar]
- 9.Oswald IP, Eltoum I, Wynn TA, Schwartz B, Caspar P, Paulin D, Sher A, James SL. Endothelial cells are activated by cytokine treatment to kill an intravascular parasite, Schistosoma mansoni, through the production of nitric oxide. Proc Natl Acad Sci USA. 1994;91:999–1003. doi: 10.1073/pnas.91.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodman JP, Dimier IH, Bout DT. Human endothelial cells are activated by IFN-γ to inhibit Toxoplasma gondii replication. J Immunol. 1991;147:2019–23. [PubMed] [Google Scholar]
- 11.Murray HW, Cartelli DM. Killing of intracellular Leishmania donovani by human mononuclear phagocytes. J Clin Invest. 1983;72:32–44. doi: 10.1172/JCI110972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denizot F, Lange R. Rapid colorimetric assay for cell growth and survival. Modification of the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–5. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 13.Pick E, Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol. 1981;46:211–26. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- 14.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannebaum SR. Analysis of nitrate, nitrite and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 15.Root RK, Metcalf J, Oshino N, Chance B. H2O2 release from human granulocytes during phagocytosis. J Clin Invest. 1975;55:945–55. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mcripley RJ, Sbarra AJ. Role of the phagocyte in host–parasite interactions. Relationship between stimulated oxidative metabolism and hydrogen peroxidase formation, and intracellular killing. J Bacteriol. 1967;94:1417–24. doi: 10.1128/jb.94.5.1417-1424.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryan US. Phagocytic properties of endothelial cells. In: Ryan US, editor. Endothelial Cells. Vol. 3. Boca Raton: CRC Press; 1988. pp. 33–49. [Google Scholar]
- 18.Zhang B, Cebtra M, Cao GL, Taylor RM, Ratych RE, Rosen GM. Penicillin G-induced microbicidal activity of endothelial cells cultured on gelfoan blocks. J Infect Dis. 1996;174:1001–9. doi: 10.1093/infdis/174.5.1001. [DOI] [PubMed] [Google Scholar]
- 19.Zhang B, Cebtra M, Cao GL, Taylor RM, Ratych RE, Domachwske JB, Malech HL, Rosen GM. Are free radicals responsible for endothelial cell killing of Staphylococcus aureus? Immunol Lett. 1997;58:113–20. doi: 10.1016/s0165-2478(97)00035-7. [DOI] [PubMed] [Google Scholar]
- 20.Garcia JGN, Dodson RF, Callhan KS. Effect of environmental particulates on cultured human and bovine endothelium. Cellular injury via an oxidant-dependent pathway. Lab Invest. 1989;57:37–44. [PubMed] [Google Scholar]
- 21.Jones AS, O'donnell VB, Wood JD, Bronghton JP, Hughes EJ, Jones OTG. Expression of phagocyte NADPH oxidase components in human endothelial cells. Am J Physiol. 1996;271:1626–34. doi: 10.1152/ajpheart.1996.271.4.H1626. [DOI] [PubMed] [Google Scholar]
- 22.Miller RA, Britingan BE. Role of oxidants in microbial pathophysiology. Clin Microbiol Rev. 1997;10:1–18. doi: 10.1128/cmr.10.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gross SS, Jaffe EA, Levi EA, Kilbourn RG. Cytokine-activated endothelial cells express an isotype of nitric oxide synthase which is tetrahydrobiopterin-dependent, calmodulin-independent and inhibited by arginine analogs with a rank-order of potency characteristic of activated macrophages. Biochem Biophys Res Commun. 1991;178:823–9. doi: 10.1016/0006-291x(91)90965-a. [DOI] [PubMed] [Google Scholar]
- 24.Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol Rev. 1991;43:109–42. [PubMed] [Google Scholar]
- 25.Werner-Felmayer G, Werner ER, Fuchs D, Hansen G, Reibnegger G, Schmidt K, Weiss G, Wachter H. Pteridine biosynthesis in human endothelial cells. J Biol Chem. 1993;268:1842–6. [PubMed] [Google Scholar]
- 26.Rosenkranz-Weiss P, Sessa WC, Milstien S, Kaufman S, Watson CA, Pober JS. Regulation of nitric oxide synthesis by proinflamatory cytokines in human umbilical vein endothelial cells. Elevations in tetrahydrobiopterin levels enhance endothelial nitric oxide synthase specific activity. J Clin Invest. 1994;93:2236–43. doi: 10.1172/JCI117221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mutunga M, Preston PM, Sumption KJ. Nitric oxide is produced by Cowdria ruminantium-infected bovine pulmonary endothelial cells in vitro and is stimulated by gamma interferon. Infect Immun. 1998;66:2115–21. doi: 10.1128/iai.66.5.2115-2121.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carreras MC, Pargament GA, Catz SD, Poderoso JJ, Boveris A. Kinetics of nitric oxide and hydrogen peroxide production and formation of peroxynitrite during the respiratory burst of human neutrophils. FEBS Lett. 1994;341:65–8. doi: 10.1016/0014-5793(94)80241-6. [DOI] [PubMed] [Google Scholar]
- 29.Wink DA, Hambauer I, Krisna MC, Degraff W, Gramson J, Mitchell JB. Nitric oxide protects against cellular damage and cytotoxicity from reactive oxygen species. Proc Natl Acad Sci USA. 1993;90:9813–7. doi: 10.1073/pnas.90.21.9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu L, Gunn C, Bekman JS. Bactericidal activity of peroxynitrite. Arch Biochem Biophys. 1992;298:452–7. doi: 10.1016/0003-9861(92)90434-x. [DOI] [PubMed] [Google Scholar]
- 31.Pacelli R, Wink DA, Cook JA, et al. Nitric oxide potentiates hydrogen peroxide-induced killing of Escherichia coli. J Exp Med. 1995;182:1462–79. doi: 10.1084/jem.182.5.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silverman DJ, Santucci LA. Potential for free radical-induced lipid peroxidation as a cause of endothelial cell injury in rocky mountain spotted fever. Infect Immun. 1988;56:3110–5. doi: 10.1128/iai.56.12.3110-3115.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong JE, Santucci LA, Tian LA, Silverman DJ. Superoxide dismutase-dependent, catalase-sensitive peroxides in human endothelial cells infected by Rickettsia rickettsii. Infect Immun. 1998;66:1293–8. doi: 10.1128/iai.66.4.1293-1298.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jornot L, Junod AF. Variable glutathione levels and expression of antioxidant enzymes in human endothelial cells. Am J Physiol. 1993;264:482–9. doi: 10.1152/ajplung.1993.264.5.L482. [DOI] [PubMed] [Google Scholar]
- 35.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA. 1991;88:4651–5. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–9. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]