Abstract
Ficolins are a group of multimeric proteins that contain collagen-like and fibrinogen-like (FBG) sequences. Three types of ficolins have been characterized: H-, L- and M-ficolins. Both H- and L-ficolins have demonstrated lectin activities. In the present study, the FBG domain of M-ficolin was expressed and shown to bind to N-acetyl-d-glucosamine. M-ficolin mRNA was expressed in monocytes but not in the more differentiated macrophages and dendritic cells. By flow cytometry, surface biotinylation and immunoprecipitation, we showed that M-ficolin was associated with the surface of promonocytic U937 cells. M-ficolin transiently expressed in COS-7 cells was also clearly detected on the cell surface by immunoprecipitation. By flow cytometry, M-ficolin was detected on peripheral blood monocytes but not on lymphocytes or granulocytes. Immobilized rabbit anti-M-ficolin F(ab′)2 mediated U937 cell adhesion, and the antibody also inhibited phagocytosis of Escherichia coli K-12 by U937 cells. Therefore, M-ficolin might act as a phagocytic receptor or adaptor on circulating monocytes for micro-organism recognition and may potentially mediate monocyte adhesion.
INTRODUCTION
Like the collectins and the complement protein C1q, ficolins represent a distinct group of multimeric proteins composed of apparently identical polypeptides, each containing a short N-terminal segment followed by a middle collagen-like sequence and then by a C-terminal globular, fibrinogen-like (FBG) domain.1 These polypeptides form triple helices in the collagen-like region and are further oligomerized into ‘bundle-of-tulips’ structures.1–3 Three types of ficolin have been characterized in humans: H-, L- and M-ficolin, which have distinct tissues of origin and distribution. L-ficolin is synthesized in the liver and found in the blood circulation.4 Adult plasma contains, on average, a level of L-ficolin that is threefold higher than found in cord blood, implying a protective role of this lectin.5 L-ficolin indeed binds to sugar structures via its FBG domains5 and, on binding to carbohydrates on bacteria, promotes clearance by phagocytosis.4 Recently, Matsushita et al.6 reported that, like the collectin mannan-binding lectin (MBL), L-ficolin formed a complex with the MBL-associated proteases (MASPs) and binding of this complex to Salmonella typhimurium activated the complement system. H-ficolin was initially identified as a serum autoantigen, the Hakata Antigen, recognized by antibodies in patients suffering from systemic lupus erythematosus and other autoimmune diseases.3 It is synthesized in the liver by hepatocytes and bile duct epithelial cells and is secreted into both blood circulation and bile.7 It is also synthesized by ciliated bronchial and Type II alveolar epithelial cells and is secreted into bronchus and the alveolar space.7 H-ficolin is a lectin that binds to carbohydrate structures found on bacteria and may therefore play an important role in both systemic and mucosal immune defence systems.
M-ficolin is synthesized in peripheral blood monocytes.8 However, its expression is down-regulated during monocyte differentiation and its mRNA is not detectable in mature macrophages.8 By serial analysis of gene expression, M-ficolin mRNA has been found to be abundant in peripheral blood monocytes, accounting for 0·44% of the total mRNA in the cells.9 However, M-ficolin mRNA is not detectable in monocyte-derived dendritic cells and was detected only at a very low level in monocyte-derived macrophages in vitro.9,10 M-ficolin expression is clearly a marker of circulating monocytes although its function in monocytes has yet to be characterized. Ficolins also have other binding properties. M-ficolin was initially identified as a membrane-associated protein in pig uterus, based on its affinity for transforming growth factor-β1 (TGF-β1).11 L-ficolin has been shown to bind to corticosteroids,12 and both L- and M-ficolin have also been reported to be elastin-binding proteins.13,14 The physiological implications of such interactions remain to be investigated.
In the present study, the FBG domain of M-ficolin has been expressed and demonstrated to bind to N-acetyl-d-glucosamine (GlcN Ac), a sugar residue common on the surface of micro-organisms. In contrast to the post-translational secretion of both L- and H-ficolins into the blood circulation and/or other secretions, M-ficolin is detected on the surface of monocytes that mediate U937 cell adhesion and phagocytosis of Escherichia coli K-12.
MATERIALS AND METHODS
GlcN Ac–Sepharose was prepared as described by Fornstedt & Porath.15 The premyeloid HL60 cells, the promonocytic U937 cells and the monocytic THP-1 cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD) and cultured in RPMI-1640 supplemented with 10% fetal calf serum (FCS) at 37° in the presence of 5% CO2. COS-7 cells were cultured in Dulbecco's modified Eagle's minimal essential medium (DMEM) containing 10% FCS (complete DMEM).
Expression constructs
cDNA encoding the M-ficolin FBG domain was amplified, using the M-ficolin cDNA (U1) clone as a template,16 with a forward (5′-CTGGAATTCCAGTCGTGTGCGACAGGC-3′) and a reverse (5′-ATTCTCGAGCTAGGCGGGCCGCACCTT-3′) primer. The DNA fragment was subcloned into the EcoRI/XhoI site of the pGEX-4T.1 vector (pGEX/FBG) (Amersham Pharmacia Biotech, Uppsala, Sweden). The complete open reading frame of clone U1 (nucleotide 1–978) was amplified similarly with a forward (5′-CCGGAATTCAGTCAAAGGCCAGAGAGC-3′) and a reverse (5′-ATTCTCGAGCGGCGGGCCGCACCTTCAT-3′) primer and cloned into the EcoRI/XhoI site of the pcDNA3.1/myc-His vector (pcDNA3/FC) (Invitrogen, Groningen, the Netherlands). The polymerase chain reaction (PCR) was carried out for 30 cycles of 94° for 30 seconds, 53° for 30 seconds and 72° for 1 min. Both expression constructs were verified by sequencing from both directions.
Expression and purification of recombinant FBG
Preparation of the glutathione-S-transferase (GST)–FBG fusion protein was carried out essentially as described by Smith & Johnson.17 E. coli BL21 was transformed with the expression construct pGEX/FBG and cultured overnight in L-broth containing ampicillin (0·1 mg/ml). The overnight culture was diluted 50-fold in the same medium and cultured for ≈ 4 hr, to an optical density (OD) at 600 nm of 0·5, before addition of isopropyl B-D-thiogalactopyranoside (IPTG) to a final concentration of 0·1 mm. Induction with IPTG was carried out for 5 hr at 37° and the bacteria were harvested by centrifugation. The bacteria were resuspended in STE buffer (10 mm Tris, 150 mm NaCl and 1 mm EDTA, pH 8·0) and frozen at −80°. To purify the GST–FBG fusion protein, 5 mm dithiothreitol (DTT) was added to the bacterial lysate, which was subsequently solubilized with 1·4% Sarkosyl as described by Frangioni & Neel.18 Cell debris was removed by centrifugation (10 000 g) for 10 min at 4°. The clarified bacterial lysate was made 4% with respect to Triton-X-100 and, upon dilution with an equal volume of PBS, incubated for 30 min at room temperature. The solubilized fraction was then bound to glutathione (GSH)–Sepharose.
Binding of FBG to GlcNAc
GST–FBG bound to GSH–Sepharose (1 ml) was resuspended in 1 volume of phosphate-buffered saline (PBS) containing thrombin (50 U/ml) and incubated at room temperature for 16 hr. The supernatant was recovered after a brief centrifugation and, upon addition of 1 mm CaCl2, applied to a GlcNAc–Sepharose column (5-ml bed volume). The column was washed five times, each with two bed volumes of PBS containing 1 mm CaCl2, and bound proteins were eluted batchwise with 0·1 m GlcNAc in the wash buffer. The eluate was examined by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and silver staining.
Preparation of anti-M-ficolin antibody
Two New Zealand White rabbits were each immunized with 0·5 mg of FBG purified on the GlcNAc–Sepharose column and boosted three times at 3-week intervals, each time with 0·2 mg of purified FBG. Serum was collected 2 weeks after the last booster. Immunoglobulin G (IgG) was isolated from the antiserum (10 ml) by three cycles of Na2SO4 (16%) precipitation. The IgG fraction was dialysed against 0·07 m phosphate buffer (pH 6·3) and further purified by passing through a Q-Sepharose column (2 × 30 cm) pre-equilibrated with 0·07 m phosphate buffer (pH 6·3). To prepare the F(ab′)2 fragment, purified non-immune or anti-FBG IgG (50 mg) in 0·1 m sodium acetate (pH 4·5) was digested overnight with pepsin (1 mg) at 37°. The digestion was stopped by addition of 0·3 ml of 2 m Tris buffer (pH 8·0) and clarified by centrifugation for 10 min at 2000 g. The supernatant was applied to a Sephadex G-200 column (1·6 × 100 cm) and eluted with TBS (10 mm Tris and 150 mm NaCl, pH 7·3). Fractions of 2·5 ml were collected and those containing the F(ab′)2, as judged by SDS–PAGE, were pooled.
Transfection
COS-7 cells were trypsinized and transferred to six-well tissue culture plates, at a density of 2 × 105 cells/well, and cultured overnight. Cells in each well were transfected with the pcDNA3/FC expression plasmid (2 µg) using the SuperFect reagent (Qiagen, Hilden, Germany) and the transfected cells were washed with DMEM and cultured for a further 48 hr in complete DMEM.
Cell-surface biotinylation
The transfected COS-7 cells (1 × 107) were washed twice in Hanks' balanced salt solution (HBSS; 0·4 mm KH2PO4, 0·3 mm Na2HPO4, 137 mm NaCl, 5·4 mm KCl, 42 mm NaHCO3, 5·6 mm d-glucose, pH 7·4) and then incubated for 30 min at room temperature with Sulfo-N-hydroxysuccinimide (NHS)-biotin (0·1 mg/ml). HL60, U937 and THP-1 cells were washed twice in HBSS and resuspended to a density of 1 × 108 cells/ml in HBSS. The cells were then gently mixed with 0·1 volume of sulfo-NHS-biotin (10 mg/ml) for 30 min at 4°. Unbound biotin was quenched by addition of an equal volume of 0·1 m glycine for 1 hr at 4°. Cells were harvested by centrifugation (400 g) for 5 min at 4° and washed twice with HBSS.
Immunoprecipitation
Surface-biotinylated cells were lysed by incubation for 1 hr at 4° in a lysis buffer containing 50 mm Tris, 100 mm NaCl, 0·5% (v/v) Nonidet P-40 (NP-40) and protease inhibitors (1 mm phenyl methyl sulphonyl fluoride [PMSF], 2 µg/ml of leupeptin and 10 µg/ml of aprotonin). The lysate was clarified by centrifugation (20 000 g) for 10 min at 4°. The supernatant was then precleared by incubation for 1 hr at 4° with protein A–Sepharose (40 µl). The precleared supernatant was incubated for 3 hr at 4° either with non-immune or with the anti-FBG rabbit serum (5 µl) and then for 1 hr with protein A–Sepharose. The resins were collected by brief centrifugation and washed sequentially, each for 20 min, in wash buffer 1 (50 mm Tris, 150 mm NaCl and 0·5% (v/v) NP-40, pH 7·5), wash buffer 2 (50 mm Tris, 0·5 m NaCl and 0·1% (v/v) NP-40, pH 7·5), and wash buffer 3 (50 mm Tris, 50 mm NaCl and 0·1% (v/v) NP-40, pH 7·5). The resins were boiled in SDS–PAGE sample buffer for 5 min and the eluted proteins were separated on a 12·5% (w/v) gel. The separated proteins were electroblotted onto nitrocellulose. The blots were blocked in a buffer containing 10 mm Tris, 150 mm NaCl and 1% (w/v) bovine serum albumin (BSA) (pH 7·4) and developed with streptavidin–alkaline phosphatase and the substrates nitroblue tetrazolium (NBT) and 5-bromo-4-chloro-3-indolyl phosphate (BCIP).
Flow cytometry
Peripheral blood leucocytes were isolated by centrifugation over a Ficoll–Hypaque gradient and, after washing, resuspended to 1 × 107 cells/ml in HBSS. U937 cells were washed similarly and resuspended to 1 × 107 cells/ml in HBSS. Surface proteins were cross-linked for 1 hr at 4° with sulpho-ethyleneglycobis(succinimidylsuccinate) (sulpho-EGS) and, after washing three times in HBSS, the cells were incubated for 10 min at 4° in HBSS containing 2% goat serum and 0·1% NaN3 (w/v). The cells (1 × 106) were resuspended in HBSS and incubated for 40 min on ice with either non-immune or anti-FBG IgG (40 µg). The cells were washed in HBSS containing 5% FCS and 0·1% NaN3 (w/v) and then incubated for 45 min on ice with fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG. Cells were washed in HBSS, fixed in 1% paraformaldehyde and analysed on a Coulter Elite Epics ESP flow cytometer (Beckman Coulter, Inc., Fullerton, CA).
U937 adhesion to F(ab′)2 coated wells
Microtitre plates (96-well) were coated with the F(ab′)2 fragment of either non-immune or anti-FBG rabbit IgG at different concentrations and then blocked for 30 min at 37° with BSA (1 mg/ml) in PBS. U937 cells were resuspended to a concentration of 1 × 106/ml in RPMI-1640 containing BSA (1 mg/ml) and 0·1 ml was dispensed to each well. The plates were incubated for 1 hr at 37°. Non-adhered cells were removed by washing with a buffer (PBS containing 1 mm CaCl2, 1·5 mm MgCl2 and 0·5 mg/ml BSA) and adherent cells were fixed with formalin solution (10% in PBS) for 10 min at room temperature.19 The plates were rinsed with PBS, and adherent cells were stained with 0·1% crystal violet in 20% methanol for 10 min at room temperature. Unbound dye was removed by washing with PBS, and methanol (0·2 ml/well) was added to each well to elute bound crystal violet. The eluate was subsequently measured at an absorbance (A) of 600 nm using an SLT microtitre plate reader (SLT Labinstruments, Grodig, Austria).
Phagocytosis
Freshly harvested U937 cells were resuspended at a concentration of 1 × 106/ml in RPMI-1640 containing 20 mm HEPES (pH 7·2) and 1% (w/v) BSA. To each 1 × 106 cells (in 1 ml), the F(ab′)2 fragment of either non-immune or anti-human FBG rabbit IgG (100 µg) was added and the cells were incubated for 1 hr at 4° with gentle mixing. As an additional control, the cells were incubated in the absence of antibody. FITC-labelled E. coli K-12 particles (Molecular Probes, Eurgene, OR), were added to the cells at a particle : cell ratio of 50 : 1, and mixed at 37°. Aliquots of the cells were retrieved and washed in PBS by centrifugation at 400 g. E. coli particles that were not ingested were quenched with equal volumes of Trypan blue (2 mg/ml) in 20 mm sodium acetate (pH 4·4) and 150 mm NaCl. Cells were then fixed with 2% paraformaldehyde and uptake of E. coli particles was measured by flow cytometry. Each experiment was carried out in triplicate.
RESULTS
Expression and demonstration of M-ficolin FBG domain as a lectin domain
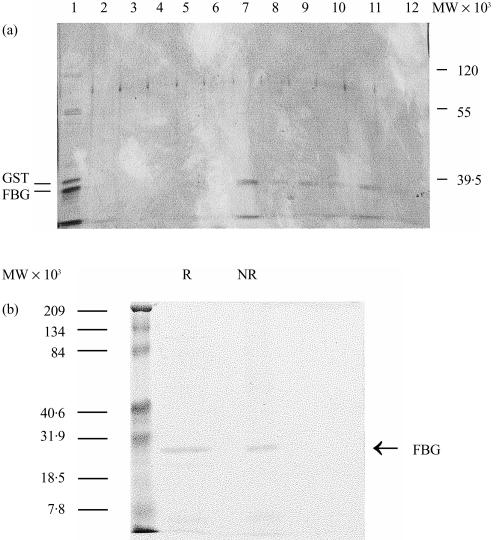
In a previous study, the FBG domain of L-ficolin was prepared by collagenase digestion of purified L-ficolin and shown to bear the lectin activity of the protein.5 In the present study, the FBG domain of M-ficolin was expressed as a GST fusion protein (GST–FBG) in E. coli. The fusion protein, found exclusively in the insoluble fraction, was solublized in 1·4% (v/v) Sarkosyl and the solublized GST–FBG was bound to GSH–Sepharose. The GST–FBG fusion protein bound to the resin was digested with thrombin. The released FBG domain was shown to bind to GlcNAc–Sepharose and could be eluted with GlcNAc, thus demonstrating specific binding of the FBG domain to GlcNAc (Fig. 1a). However, a significant fraction of the FBG domain applied to the GlcNAc-Sepharose column did not bind to the column, implying a relatively low affinity of the FBG domain for GlcNAc (Fig. 1a). No oligomeric species of the FBG domain was detected by SDS–PAGE under non-reducing conditions (Fig. 1b), and even after cross-linking (data not shown), indicating that it was expressed as a monomer. This is consistent with its relatively low affinity for GlcNAc.
Figure 1.
Chracterization of M-ficolin fibrinogen-like (FBG) domain. (a) The FBG domain of M-ficolin was expressed as a glutathione-S-transferase (GST) fusion protein. The fusion protein bound to GSH–Sepharose was digested in gel with thrombin and the FBG domain released into the supernatant was collected and applied to an N-acetyl-d-glucosamine (GlcNAc)–Sepharose column. Unbound proteins, shown in lane 1, included predominantly the 24 000-molecular weight (MW) FBG domain and some contaminating GST (the 26 000-MW band). The resin was washed five times each with two bed volumes of phosphate-buffered saline (PBS) and then eluted with 0·1 m GlcNAc in the same buffer. The unbound proteins (lane 1), the washings (lanes 2–6) and the GlcNAc eluate (lanes 7–12) were analysed by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) on a 12·5% (w/v) gel and visualized by silver-staining. (b) The purified FBG was examined by SDS–PAGE on a 12·5% (w/v) gel in the presence (R) or absence (NR) of 2-mercaptoethanol and the gel was stained with Coomassie Brilliant blue. The molecular weight standards are indicated on the left.
Expression of M-ficolin on the surface of monocytes
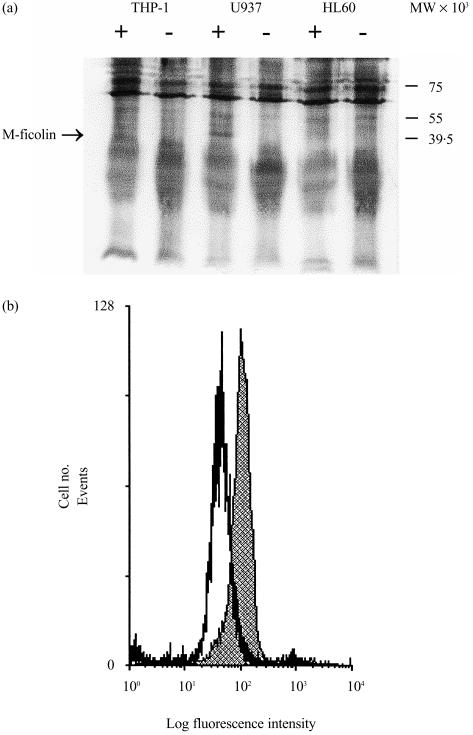
M-ficolin is predominantly synthesized in monocytes.8 As porcine M-ficolin was initially identified as a membrane-associated protein in the uterus,11 we considered the possibility that M-ficolin is associated with monocyte surface structures. Three monocytic cell lines, HL60, U937 and THP-1, were surface biotinylated and then M-ficolin was immunoprecipitated from the cell lysate using a rabbit polyclonal antibody against the recombinant FBG domain. Proteins precipitated with non-immune rabbit serum from the different cell lines were used as negative controls. Precipitated proteins were probed with streptavidin–alkaline phosphatase to identify cell surface proteins only. As shown in Fig. 2(a), a 40 000-molecular weight (MW) band was detected in the U937 cell lysate. It was precipitated with the anti-FBG but not with the non-immune rabbit serum. The putative M-ficolin band was not precipitated from HL60 and THP-1 cell lysates with either anti-FBG or non-immune rabbit serum (Fig. 2a). This is consistent with the lack of M-ficolin mRNA in HL60 and THP-1 cells, as judged by reverse transcription–polymerase chain reaction (RT–PCR)8 (data not shown). U937 cells were also analysed for surface M-ficolin expression by flow cytometry using the anti-FBG antibodies. As shown in Fig. 2(b), a significant amount of M-ficolin was detected on U937 cells.
Figure 2.
Detection of M-ficolin on monocytoid cells. (a) Detection of M-ficolin on HL60, U937 and THP-1 cells by immunoprecipitation of biotinylated surface proteins. Surface-biotinylated cells were lysed using 0·5% (v/v) Nonidet P-40 (NP-40) and the supernatant precleared with Protein A–Sepharose. The supernatant was then incubated with either non-immune (−) or rabbit anti-fibrinogen-like (FBG) (+) rabbit serum and then with Protein A–Sepharose. The resin was harvested by centrifugation and, upon washing, eluted with sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) sample buffer. The eluted proteins were separated by SDS–PAGE on a 12·5% (w/v) gel and electroblotted onto nitrocellulose membranes. Biotinylated cell surface proteins were detected with alkaline phosphatase-conjugated streptavidin and visualized using nitroblue tetrazolium (NBT) and 5-bromo-4-chloro-3-indolyl phosphate (BCIP). (b) Detection of M-ficolin on U937 cells by flow cytometry. U937 cells were washed and briefly surface cross-linked with sulpho-ethyleneglycobis(succinimidylsuccinate) (sulpho-EGS). The cells were blocked and then incubated with either non-immune or anti-FBG rabbit serum. After washing, the cells were further incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit F(ab′)2. After washing, the cells were fixed and analysed by flow cytometry on a Coulter Elite Epics ESP flow cytometer. The shaded profile denotes fluorescence detected on U937 cells with the anti-FBG antiserum while the open profile represents signals detected with non-immune rabbit serum.
M-ficolin is associated with the surface of transfected COS-7 cells
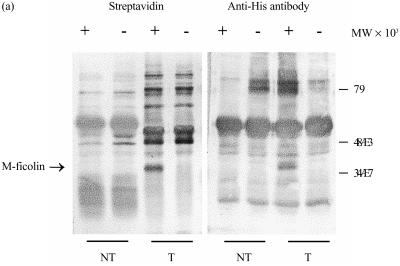
M-ficolin apparently lacks typical membrane-anchorage sequences, and the molecular mechanism by which it is associated with the U937 cell surface is not clear. In order to examine whether membrane association is an intrinsic property of M-ficolin or is defined by the specific surface features or molecules on U937 cells, M-ficolin was transiently expressed in an apparently irrelevant cell type, COS-7 cells. An expression construct was made in the pcDNA 3.1 vector to express M-ficolin with a C-terminal His tag. COS-7 cells transfected with the expression vector were surface biotinylated, and M-ficolin was immunoprecipitated from the cell lysate using the anti-FBG antibody or, as a control, with non-immune rabbit serum. As shown in Fig. 3, the 40 000-MW M-ficolin polypeptide immunoprecipitated from the transfected COS-7 cells with the anti-FBG antibody was a biotinylated protein. The identity of the 40 000-MW band as M-ficolin was confirmed by Western blotting using an anti-His monoclonal antibody (mAb). The biotinylated 40 000-MW band was not detected in the precipitate from transfected COS-7 cells using non-immune rabbit serum or in the precipitate from non-transfected COS-7 cells using anti-FBG serum, further confirming the identity of the polypeptide as M-ficolin (Fig. 3). Therefore, association with the cell surface is probably an intrinsic property of M-ficolin.
Figure 3.
Immunoprecipitation of biotinylated surface proteins of transfected COS-7 cells. Non-transfected COS-7 cells (NT), or those transfected with the His-tagged M-ficolin expression construct pcDNA3/FC (T), were surface biotinylated. The cells were lysed in 0·5% (v/v) Nonidet P-40 (NP-40) and the supernatants were, upon preclearance with Protein A–Sepharose, first incubated with either non-immune (−) or anti-FBG (+) rabbit serum and then with Protein A–Sepharose. Resin-bound proteins were, after washing, eluted with sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) sample buffer and separated on a 12·5% (v/v) gel. The separated proteins were electroblotted and probed with either alkaline phosphatase-conjugated streptavidin or a mouse anti-His monoclonal antibody. The anti-His antibody was further probed with alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G (IgG). The blots were developed with nitroblue tetrazolium (NBT) and 5-bromo-4-chloro-3-indolyl phosphate (BCIP).
Expression of M-ficolin on peripheral blood monocytes
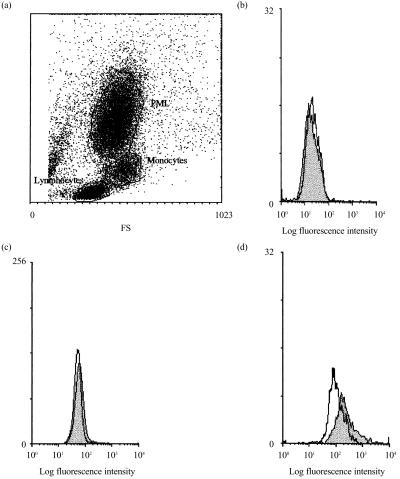
M-ficolin mRNA is predominantly expressed in circulating monocytes.16 In this study, the expression of M-ficolin on monocyte surface was also investigated. Isolated monocytes were briefly surface cross-linked with sulpho-EGS to prevent possible elution of M-ficolin from the cell surface during the subsequent detection of M-ficolin using the polyclonal rabbit anti-FBG antibody. Stained leucocytes were gated into three populations based on forward and side scattering, i.e. lymphocytes, monocytes and granulocytes (Fig. 4a), and each population was analysed for staining using the anti-FBG antibody. No significant antibody staining was detected on lymphocytes and granulocytes. This is consistent with the lack of M-ficolin mRNA in lymphoblastoid Molt-4 cells7 and granulocytes (data not shown). However, a low, but significant, level of M-ficolin was detected on monocytes (Fig. 4d).
Figure 4.
Immunofluorescence detection of M-ficolin on the surface of leucocytes by flow cytometry. Leucocytes were separated from whole blood (20 ml) using Ficoll–Hypaque gradient and, after washing, briefly cross-linked with sulpho-ethyleneglycobis(succinimidylsuccinate) (sulpho-EGS). The cells were stained as in Fig. 2(b). (a) A dot-plot of the leucocytes as defined by forward (FS) and side (SS) scattering. The lymphocyte, granulocyte and monocyte populations, as gated in (a), were individually analysed for fluorescence signals as shown in (b), (c) and (d), respectively. Filled profiles are fluorescence signals detected with the anti-FBG antiserum and the open profiles represent signals detected with non-immune rabbit serum. PML, polymorphonuclear leucocytes.
M-ficolin mediates phagocytosis of E. coli by U937 cells
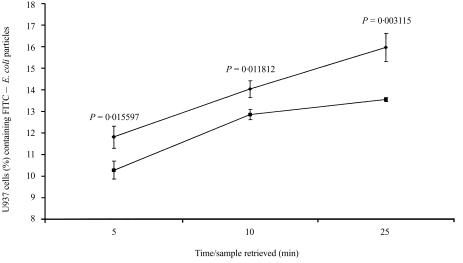
It was attempted to evaluate the function of U937 cell surface-associated M-ficolin. As the FBG domain of M-ficolin binds to GlcNAc, a sugar residue commonly found on Gram-negative bacteria such as E. coli, it is possible that the surface M-ficolin mediates phagocytosis of E. coli. FITC-labelled E. coli K-12 particles were presented to U937 cells. U937 cells were preincubated with the F(ab′)2 fragments of either non-immune or anti-FBG rabbit IgG before incubation with the E. coli particles. E. coli particles that were not bound, or bound but not ingested, by U937 cells were quenched with Trypan blue and the uptake of E. coli particles was measured by flow cytometry. As shown in Fig. 5, compared with non-immune F(ab′)2, the anti-FBG F(ab′)2 significantly inhibited E. coli uptake by U937 cells. Therefore, U937 cell-surface M-ficolin probably functions as a phagocytic receptor or adaptor for Gram-negative bacteria.
Figure 5.
Uptake of Escherichia coli K-12 by U937 cells. U937 cells were washed and resuspended to a density of 1 × 106/ml in RPMI-1640 containing 1% (w/v) bovine serum albumin (BSA) and 20 mm HEPES (pH 7·4). One millilitre of the cell suspension was transferred to each of three tubes that were subsequently incubated for 1 hr at 4° with the F(ab′)2 fragments of non-immune or anti-FBG rabbit IgG or an equivalent volume of phosphate-buffered saline (PBS). The cells were then warmed to 37° and mixed with fluorescein isothiocyanate (FITC)-conjugated E. coli K-12 particles in a cell : particle ratio of 1 : 50. The samples were incubated at 37° and samples were removed from the tubes at 5, 10 and 25 min. Extracellular E. coli particles were quenched with equal volumes of Trypan blue and the cells were analysed by flow cytometry to determine the percentage of U937 cells that had ingested the FITC-labelled E. coli K-12 particles. The values at each time-point represent the mean of three independent experiments with the indicated standard deviation. •, U937 cells preincubated with the F(ab′)2 fragment of non-immune rabbit immunoglobulin G (IgG); ▪, U937 cells preblocked with the anti-FBG antibody. The P-values were obtained by two-tailed t-test and are indicated for each time-point.
M-ficolin mediates U937 cell adhesion
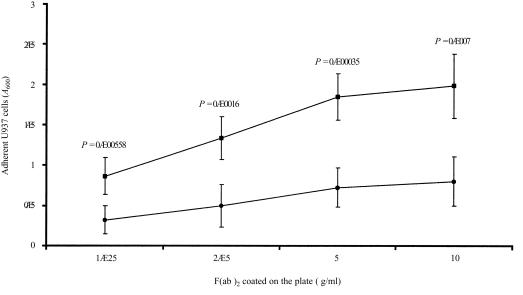
Monocytes circulate only transiently in the bloodstream before migration into extravascular tissues where they differentiate into macrophages.20 Monocyte emigration involves adhesion to endothelial cells and transmigration through the endothelial junctions and the basement membrane.21 The contribution of M-ficolin to monocyte adhesion was investigated using U937 cells. As shown in Fig. 6, U937 cells adhered to immobilized F(ab′)2 of the anti-FBG antibody in a concentration-dependent manner, and at each concentration of F(ab′)2 coating, the anti-FBG antibody-mediated U937 cell adhesion was significantly more effective than that of the non-immune IgG. Therefore, the FBG domain is involved in adhesion as the antibody was raised against the FBG domain.
Figure 6.
Adhesion of U937 cells to the F(ab′)2 fragments of rabbit anti-FBG immunoglobulin G (IgG). Microtitre plates (96-well) were coated with the F(ab′)2 of non-immune or anti-FBG rabbit IgG at the concentrations indicated on the graph. U937 cells were washed and incubated with the plates for 1 hr at 37°. Non-adherent cells were removed by washing and adherent cells were stained with crystal violet. Bound dye was, after washing, eluted with methanol and the absorbance (A) at 600 nm was measured using a microtitre plate reader. The value at each substrate concentration point represents the mean (± SD) of five independent experiments, and U937 cell adhesion on non-immune (•) and anti-FBG (▪) IgG F(ab′)2 at each concentration point were compared by the two-tailed t-test
DISCUSSION
Monocytes and macrophages express a rich spectrum of surface receptors for the recognition of pathogens.20 Some receptors, e.g. the mannose receptor, directly bind to specific arrays/patterns of molecules on pathogens.22 Others, such as Fc, complement and collectin receptors, only recognize pathogens that have been opsonized by various humoral molecules, e.g. antibodies, complement fragments and collectins. The collectins bind to carbohydrate structures on pathogens and directly promote phagocytosis through the collectin receptor(s).23–25 Ficolins are a family of proteins that resemble collectins in structure,1 and both H- and L-ficolins have also been shown to bind to carbohydrate structures found on Gram-negative bacteria.4,5,7 Binding of L-ficolin to S. typhimurium directly opsonizes the bacteria for enhanced phagocytosis by polymorphonuclear leucocytes (PMNs)4 and this is probably mediated by an L-ficolin receptor(s) on PMNs. The finding that L-ficolin formed a functional complex with the MASPs that bound to S. typhimurium and subsequently activated the complement system, implied that L-ficolin could mediate killing and clearance of pathogens more effectively through complement activation and complement receptor-mediated phagocytosis.6 Here we have shown that M-ficolin also has lectin activity and, like L-ficolin,5 it binds to GlcNAc via the FBG domain. As GlcNAc is a common carbohydrate moiety on Gram-negative bacteria, M-ficolin may also recognize these pathogens.
M-ficolin was initially identified from the membrane fraction of pig uterus.11 We considered the possibility that human M-ficolin, which is predominantly expressed in monocytes and also in the monocytoid U937 cells, is also expressed on the surface of these cells. By immunoprecipitation and flow cytometry, significant M-ficolin has been detected on U937 cells. By flow cytometry, it has also been detected on monocytes, but not on lymphocytes and granulocytes. Association with the surface of monocytes or U937 cells is apparently an intrinsic property of M-ficolin instead of that of monocytes or U937 cells because, upon transient expression in the unrelated COS-7 cells, it was also clearly detected on the cell surface. The mechanism by which M-ficolin is associated with the cell surface was demonstrated in the adhesion study described above. M-ficolin was shown to mediate U937 cell adhesion to the immobilized F(ab′)2 fragment of the anti-FBG antibody and therefore M-ficolin was probably linked to the cell cytoskeleton either directly or indirectly through its cytoskeleton-linked receptor(s). M-ficolin-mediated U937 cell adhesion may therefore be a novel pathway of monocyte emigration into extravascular tissues.
The other putative function of monocyte surface M-ficolin was implicated by its affinity for GlcNAc, a common sugar structure on Gram-negative bacteria and other pathogens. We showed that the F(ab′)2 fragment of the anti-FBG antibody significantly inhibited U937 cell uptake of E. coli K-12 and therefore M-ficolin may contribute to pathogen recognition and killing by monocytes. The three ficolins have similar structures and recognize similar carbohydrate moieties on microbes, implying certain degrees of functional overlap or redundancy among these proteins. However, the distinct cells of origin and tissues of distribution of the three ficolins, i.e. L-ficolin in the blood circulation, M-ficolin on monocyte surface, and H-ficolin in both blood circulation and mucosal tissues, can expose the three ficolins to distinct microbe populations and immune effector systems.
Owing to the lack of a mAb, it is difficult to evaluate the extent of M-ficolin association with monocyte and U937 cell surfaces. However, this has been demonstrated by multiple and independent approaches and in several types of cells, i.e. circulating monocytes, U937 and COS-7 cells. The adhesion of U937 cells to the anti-FBG F(ab′)2 fragment and the inhibitory effects of the anti-FBG antibody to U937 cell phagocytosis of E. coli K-12 also indirectly indicated the presence of M-ficolin on U937 cells. Detailed investigation of the functional properties of monocyte surface M-ficolin awaits the development of reagents with greater specificity, such as mAbs.
Acknowledgments
The authors thank Ng Bee Ling for assistance with flow cytometry and both Yue Siew Chin and Susan Ong for help in image processing. This project was jointly supported by the National Medical Research Council research grant RP6600016. C. Teh was also supported by a National University of Singapore PhD scholarship.
Abbreviations
- EGS
ethyleneglycobis(succinimidylsuccinate)
- HBSS
Hanks' balanced salt solution
- NBT
nitroblue tetrazolium
- BCIP
5-bromo-4-chloro-3-indolyl phosphate
REFERENCES
- 1.Lu J, Le Y. Ficolins and the fibrinogen-like domain. Immunobiology. 1998;199:190–9. doi: 10.1016/S0171-2985(98)80026-0. [DOI] [PubMed] [Google Scholar]
- 2.Ohashi T, Erickson HP. Two oligomeric forms of plasma ficolin have differential lectin activity. J Biol Chem. 1997;272:14220–6. doi: 10.1074/jbc.272.22.14220. [DOI] [PubMed] [Google Scholar]
- 3.Sugimoto R, Yae Y, Akaiwa M, et al. Cloning and characterization of the Hakata antigen, a member of the ficolin/opsonin p35 lectin family. J Biol Chem. 1998;273:20721–7. doi: 10.1074/jbc.273.33.20721. [DOI] [PubMed] [Google Scholar]
- 4.Matsushita M, Endo Y, Taira S, et al. A novel human serum lectin with collagen- and fibrinogen-like domains that functions as an opsonin. J Biol Chem. 1996;271:2448–54. doi: 10.1074/jbc.271.5.2448. [DOI] [PubMed] [Google Scholar]
- 5.Le Y, Lee SH, Kon OL, Lu J. Human L-ficolin: plasma levels, sugar specificity, and assignment of its lectin activity to the fibrinogen-like (FBG) domain. FEBS Lett. 1998;425:367–70. doi: 10.1016/s0014-5793(98)00267-1. [DOI] [PubMed] [Google Scholar]
- 6.Matsushita M, Endo Y, Fujita T. Complement-activating complex of ficolin and mannose-binding lectin-associated serine protease. J Immunol. 2000;164:2281–4. doi: 10.4049/jimmunol.164.5.2281. [DOI] [PubMed] [Google Scholar]
- 7.Akaiwa M, Yae Y, Sugimoto R, Suzuki SO, Iwaki T, Izuhara K, Hamasaki N. Hakata antigen, a new member of the ficolin/opsonin p35 family, is a novel human lectin secreted into bronchus/alveolus and bile. J Histochem Cytochem. 1999;47:777–86. doi: 10.1177/002215549904700607. [DOI] [PubMed] [Google Scholar]
- 8.Lu J, Le Y, Kon OL, Chan J, Lee SH. Biosynthesis of human ficolin, an Escherichia coli-binding protein, by monocytes: comparison with the synthesis of two macrophage-specific proteins, C1q and the mannose receptor. Immunology. 1996;89:289–94. doi: 10.1046/j.1365-2567.1996.d01-732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashimoto S, Suzuki T, Dong HY, Yamazaki N, Matsushima K. Serial analysis of gene expression in human monocytes and macrophages. Blood. 1999;94:837–44. [PubMed] [Google Scholar]
- 10.Hashimoto S, Suzuki T, Dong HY, Nagai S, Yamazaki N, Matsushima K. Serial analysis of gene expression in human monocyte-derived dendritic cells. Blood. 1999;94:845–52. [PubMed] [Google Scholar]
- 11.Ichijo H, Hellman U, Wernstedt C, Gonez LJ, Claesson-Welsh L, Heldin CH, Miyazono K. Molecular cloning and characterization of ficolin, a multimeric protein with fibrinogen- and collagen-like domains. J Biol Chem. 1993;268:14505–13. [PubMed] [Google Scholar]
- 12.Edgar PF. Hucolin, a new corticosteroid-binding protein from human plasma with structural similarities to ficolins, transforming growth factor-β1-binding proteins. FEBS Lett. 1995;375:159–61. doi: 10.1016/0014-5793(95)01205-s. [DOI] [PubMed] [Google Scholar]
- 13.Harumiya S, Omori A, Sugiura T, Fukumoto Y, Tachikawa H, Fujimoto D. EBP-37, a new elastin-binding protein in human plasma: structural similarity to ficolins, transforming growth factor-β1-binding proteins. J Biochem. 1995;117:1029–35. doi: 10.1093/oxfordjournals.jbchem.a124802. [DOI] [PubMed] [Google Scholar]
- 14.Harumiya S, Takeda K, Sugiura T, Fukumoto Y, Tachikawa H, Miyazono K, Fujimoto D, Ichijo H. Characterization of ficolins as novel elastin-binding proteins and molecular cloning of human ficolin-1. J Biochem. 1996;120:745–51. doi: 10.1093/oxfordjournals.jbchem.a021474. [DOI] [PubMed] [Google Scholar]
- 15.Fornstedt N, Porath J. Characterization studies on a new lectin found in seeds of Vicia ervilia. FEBS Lett. 1975;57:187–91. doi: 10.1016/0014-5793(75)80713-7. [DOI] [PubMed] [Google Scholar]
- 16.Lu J, Tay PN, Kon OL, Reid KBM. Human ficolin: cDNA cloning, demonstration of peripheral blood leucocytes as the major site of synthesis and assignment of the gene to chromosome 9. Biochem J. 1996;313:473–8. doi: 10.1042/bj3130473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith DB, Johnson KS. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1998;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 18.Frangioni JV, Neel BG. Solubilization and purification of enzymatically active glutathione S-transferase (pGEX) fusion proteins. Anal Biochem. 1993;210:179–97. doi: 10.1006/abio.1993.1170. [DOI] [PubMed] [Google Scholar]
- 19.Wilkins JA, Stupack D, Stewart S, Shen C. β1 integrin-mediated lymphocyte adherence to extracellular matrix is enhanced by phorbol ester treatment. Eur J Immunol. 1991;21:517–22. doi: 10.1002/eji.1830210239. [DOI] [PubMed] [Google Scholar]
- 20.Gordon S. The macrophages. Bioessays. 1995;17:977–86. doi: 10.1002/bies.950171111. [DOI] [PubMed] [Google Scholar]
- 21.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 22.Stahl PD, Ezekowitz RA. The mannose receptor is a pattern recognition receptor involved in host defense. Curr Opin Immunol. 1998;10:50–5. doi: 10.1016/s0952-7915(98)80031-9. [DOI] [PubMed] [Google Scholar]
- 23.Holmskov U, Malhotra R, Sim RB, Jensenius JC. Collectins: collagenous C-type lectins of the innate immune defense system. Immunol Today. 1994;15:67–74. doi: 10.1016/0167-5699(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 24.Hoppe HJ, Reid KBM. Collectins – soluble proteins containing collagenous regions and lectin domains – and their roles in innate immunity. Protein Sci. 1994;3:1143–58. doi: 10.1002/pro.5560030801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu J. Collectins: collectors of microorganisms for the innate immune system. Bioessays. 1997;19:509–18. doi: 10.1002/bies.950190610. [DOI] [PubMed] [Google Scholar]