Abstract
Mouse bone marrow-derived myeloid dendritic cells (DC) propagated in granulocyte–macrophage colony-stimulating factor and transforming growth factor-β1 (TGF-β1) (so-called ‘TGF-β DC’) are phenotypically immature, and prolong allograft survival. Interleukin-10 (IL-10) has been shown to inhibit the maturation of DC by down-regulating surface major histocompatibility complex (MHC) class II, co-stimulatory and adhesion molecule expression. Genetic engineering of TGF-β DC to overexpress IL-10 might enhance their tolerogenic potential. In this study, adenoviral (Ad) vectors encoding the mouse IL-10 gene were transduced into B10 (H2b) TGF-β DC. Transduction with Ad-IL-10 at a multiplicity of infection (MOI) of 50–100 resulted in a modest reduction in the incidence of DC expressing surface MHC class II, CD40, CD80 and CD86. Paradoxically, Ad-IL-10 transduction enhanced the allostimulatory activity of DC in mixed leucocyte reactions and cytotoxic T lymphocyte assays, and increased their natural killer cell stimulatory activity. Systemic injection of normal C3H recipients with Ad-IL-10-transduced B10-DC 7 days before organ transplantation, exacerbated heart graft rejection and augmented circulating anti-donor alloantibody titres. Contrasting effects were observed in relation to tumour growth. All mice preimmunized with Ad-IL-10-transduced, tumour antigen (B16F10)-pulsed DC developed palpable tumours, associated with significant inhibition of splenic anti-tumour cytotoxic T lymphocyte generation. Animals pretreated with control Ad-LacZ-transduced, B16F10-pulsed DC however, remained tumour free. These findings are consistent with the multifunctional immunomodulatory properties of mammalian IL-10.
INTRODUCTION
The antigen-presenting function of dendritic cells (DC) is associated with surface expression of major histocompatibility complex (MHC) class II and co-stimulatory molecules, that include CD40, CD80 (B7-1) and CD86 (B7-2).1,2 In contrast to mature DC, which are potent stimulators of T-cell activation and proliferation, immature DC deficient in surface co-stimulatory molecules exhibit tolerogenic potential, both in vitro3 and in vivo.4–7 Thus, mouse bone marrow (BM)-derived DC propagated in granulocyte–macrophage colony-stimulating factor (GM-CSF) and transforming growth factor-β1 (TGF-β1) (so-called TGF-β DC), that are phenotypically immature (MHC class II+, CD40lo CD80lo CD86lo) can induce antigen-specific T-cell hyporesponsiveness in vitro, and prolong the survival of vascularized cardiac allografts in a donor-specific manner.8,9 Although immature donor DC exhibit tolerogenic potential, they fail to induce indefinite graft survival.4,5,8 This may be due to the up-regulation of surface co-stimulatory molecules on the DC following their injection and interaction with host T cells.5,9 Conceivably, strategies that prevent or suppress the maturation of DC could enhance their in vivo tolerogenicity, with potential therapeutic implications.
Interleukin-10 (IL-10) was initially identified as a T helper type 2 (Th2) cell product that inhibited the synthesis of cytokines, particularly IL-2 and interferon-γ (IFN-γ), by Th1 lymphocytes.10,11 It suppresses both T-cell proliferative and cytolytic responses.12,13 These inhibitory effects appear to be mediated by impairment of the stimulatory function of antigen-presenting cells (APC),14,15 via down-regulation of MHC class II and co-stimulatory molecule expression.16–19 Moreover, IL-10 decreases the production of pro-inflammatory cytokines, including tumour necrosis factor-α (TNF-α), IL-1, IL-6, IL-8 and IL-12 by APC.20,21 Thus, IL-10 has been regarded as an immunosuppressive cytokine. We hypothesized that overexpression of mouse IL-10 in TGF-β DC, using a genetic engineering approach, might enhance their tolerogenicity. In this study, myeloid DC propagated from mouse BM in the presence of GM-CSF and TGF-β1 were transduced with adenoviral (Ad) vectors expressing cDNA encoding mouse IL-10. IL-10-transduced cells exhibited modest down-regulation of MHC class II, CD40, CD80 and CD86 expression, but enhanced capacity for allogeneic T-cell activation and induction of natural killer (NK) cell activity. These Ad-IL-10-transduced TGF-β DC exacerbated cardiac allograft rejection. By contrast, Ad-IL-10-transduced tumour antigen-pulsed DC showed diminished capacity to protect against growth of the B16F10 melanoma. The results are consistent with the complex immunomodulatory properties of mammalian IL-10. They are also in keeping with our contention15 that the immunosuppressive action of IL-10 appears to be more effective against indirect (tumour) than against direct (allo-) antigen recognition.
MATERIALS AND METHODS
Mice
Ten- to 12-week-old male mice of the C57BL/10/J (B10; H2b), C3H/HeJ (H2k) and BALB/c (H2d) strains were obtained from The Jackson Laboratory, Bar Harbor, ME. They were maintained in a specific pathogen-free facility of the University of Pittsburgh Medical Center. Six- to 8-week-old, female C57BL/6 (B6; H2b) mice purchased from Charles River Laboratories (Montreal, Canada) were maintained at McMaster University. The mice were provided with Purina rodent chow, and tap water ad libitum.
Propagation of TGF-β DC
The culture and selection procedures used to generate and purify DC were similar to those reported initially by Inaba et al.22 and were modified as described.23,24 Briefly, freshly isolated BM cells obtained from femurs of B10 or B6 mice were cultured in 24-well plates (2 × 106/well) in 2 ml of RPMI-1640 (Life Technologies, Gaithersburg, MD) supplemented with antibiotics and 10% v/v fetal calf serum (FCS) (Nalgene, Miami, FL) (referred to subsequently as complete medium), 4 ng/ml recombinant mouse GM-CSF, and 0·2 ng/ml recombinant human TGF-β1 (R and D Systems, Minneapolis, MN). The DC were purified by centrifugation over metrizamide as described,25 and referred to subsequently as TGF-β DC.8
Construction of recombinant Ad vectors expressing mammalian (mouse) IL-10
A 1050-base pair (bp) fragment of mouse IL-10 cDNA was excised from pCDSRα (obtained from the DNAX Research Institute, Palo Alto, CA) by bam HI and Hin dIII digestion. The shuttle plasmid pACCMV, containing 0–17 map units of human type 5 Ad genome with a human cytomegalovirus (CMV) promoter (760 bp), multicloning sites and simian virus-40 splicing junction/polyA signal (430 bp) inserted in the E1 region of the viral genome, was double-digested with bam HI and Hin dIII. The IL-10 fragment was then subcloned into the bam HI–Hin dIII site in pACCMV using T4 ligase to obtain the recombinant plasmid pACCMV–IL-10. This plasmid was cotransfected into 293 cells, along with a plasmid pAdBHG10 containing most of the rightward sequences (3·7–100 map units) of the human type 5 Ad genome with a partial deletion in the E3 region. The recombinant, replication-deficient Ad was rescued by homologous recombination. Ad-LacZ was used as control. High titres of recombinant Ad were amplified, purified, titrated and stored as described previously.26
Ad vector-mediated gene transfer
TGF-β DC cultured for 5 days as described above, were transduced with Ad-LacZ for 2 hr at 50 or 100 multiplicities of infection (MOI). Two days later, LacZ expression was quantified by X-gal staining27 to determine transduction efficiency. The efficiency of IL-10 gene transduction was determined on glass slides by immunocytochemical staining for the gene product, using anti-mouse IL-10 monoclonal antibody (mAb; clone JES5–16E3; PharMingen, San Diego, CA), followed by biotinylated polyclonal anti-rat immunoglobulin (1 : 100; PharMingen) and avidin–biotin complex–alkaline phosphatase (ABC–AP; Vector, Burlingame, CA). Production of IL-10 by the transduced DC was determined by enzyme-linked immunosorbent assay (ELISA) measurement of mouse IL-10 in the supernatants of 106 DC cultured for 24-hr periods from 0, 24 and 48 hr after gene transfer.
Flow cytometric analysis
Cells (5 × 105/tube) were stained for MHC class II expression using biotin-conjugated mouse anti-mouse IAb mAb (IgG2a, PharMingen), with fluorescein isothiocyanate (FITC) streptavidin as the secondary reagent. For identification of co-stimulatory molecules, primary hamster or rat mAbs, including anti-mouse CD40, CD80, or CD86 (PharMingen) followed by FITC-conjugated goat anti-hamster IgG, or goat anti-rat IgG2a were used as described.23 FITC-conjugated, isotype-matched irrelevant mAbs were used as negative controls. After staining, the cells were fixed in 1% paraformaldehyde in saline, and then analysed using an EPICS ELITE flow cytometer (Coulter Corporation, Hialeah, FL). Five thousand events were acquired for each sample.
Mixed leucocyte reactions (MLR)
One-way MLR cultures were performed in triplicate in 96-well, round-bottom microculture plates (Corning, Corning, NY). Nylon wool-eluted spleen T cells (45 min, 37°) were used as responders (2 × 105/well). Graded doses of γ-irradiated (20 Gy; X-ray source) DC were used as stimulators. Cultures were maintained in RPMI-1640 complete medium, for 72 hr in 5% CO2 in air. [3H]TdR (1 µCi/well) was added for the final 18 hr, and incorporation of [3H]TdR into DNA was assessed by liquid scintillation counting. Results were expressed as mean counts per minute (c.p.m.) ± 1SD.
Cytotoxic T lymphocyte (CTL) assays
For allogeneic CTL assay, freshly isolated spleen cells from normal C3H mice were cultured with γ-irradiated (20 Gy) B10-DC (ratio 10 : 1) for 5–7 days, then used as effectors. EL4 (H2b) lymphoma cells [TIB39; American Type Culture Collection (ATCC) Rockville, MD], were used as donor-specific targets. The P815 (H2d) mouse mastocytoma cell line (TIB64; ATCC), and the R1.1 (H2k) lymphoma cell line (TIB42; ATCC), were used as third party (specificity) and syngeneic controls, respectively. For quantification of CTL against tumour antigen, spleen cells harvested from mice 14 days after immunization with B16F10-DC or B16F10/Ad-IL-10-DC were cultured with B16F10 cells (ratio of 30–50 : 1) for 5 days, then used as effectors. B16F10 cells were used as targets and were labelled (4 × 106) with 100 µCi Na251CrO4 (New England Nuclear, Boston, MA). They were washed and plated at a concentration of 5 × 103 cells/well in 96-well, round-bottomed culture plates (Corning). Serial, two-fold dilutions of effector cells were added to give effector : target (E : T) ratios of 100 : 1, 50 : 1, 25 : 1 and 12·5 : 1 in a total volume of 200 µl/well. The percentage of specific 51Cr release was determined after incubating the plates for 4 hr at 37° in RPMI-1640 complete medium in 10% CO2 in air. An aliquot (100 µl) of supernatant was recovered from each well after centrifugation at 500 g for 1 min. Maximum 51Cr release was determined by osmotic lysis of the target cells. The per cent specific cytotoxicity was calculated using the following formula: % cytotoxicity = 100 × [(experimental c.p.m.) − (spontaneous c.p.m.)] / [(maximum c.p.m.) − (spontaneous c.p.m.)]. The results are expressed as means ± 1SD of percentage-specific 51Cr release in triplicate cultures.
Quantification of NK cell activity
Lysis of YAC-1 tumour cells (ATCC) detected by a 4-hr 51Cr-release assay was used as an indicator of NK cell activity. Calculation of the per cent cytotoxicity was similar to that for CTL activity, as described above.
Heterotopic heart transplantation
Surgical procedures were performed under inhalation anaesthesia using methoxyflurane (Pitman-Moore, Atlanta, GA). Vascularized heterotopic cardiac transplantation to an abdominal site was performed as described previously.28 The heart graft was monitored daily by abdominal palpation. Total cessation of cardiac muscle contraction was defined as rejection, which was confirmed by histological examination.
Tumour cell lines
B16F10 (H2b), a subclone of the spontaneously occurring murine melanoma B16, and EL4 (H2b), a T-lymphoma cell line, were both obtained from the ATCC, and cultured in modified Eagle's medium (MEM; Gibco-BRL, Gaithersburg, MD), supplemented with 10% v/v FCS, 2 mm l-glutamine, 100 units/ml penicillin, and 100 µg/ml streptomycin.
Preparation of tumour cell extract and pulsing of DC with tumour antigen
B16F10 tumour cell extract was prepared as described previously.29 B6 BM-derived TGF-β DC, propagated for 4 days as described above, were incubated with tumour extract at 37° for 18 hr in complete MEM at a ratio of 106 DC to the extract from 5 × 106 B16F10 tumour cells.
DC immunization and monitoring of tumour growth
B6 mice were injected intraperitoneally (i.p.) with 106 DC pulsed with tumour antigen (B16F10-DC), or with B16F10-DC transduced with Ad-LacZ (B16 F10/Ad-LacZ-DC) or Ad-IL-10 (B16F10/Ad-IL-10-DC). After 14 days, each animal was challenged with 104 B16F10 tumour cells by subcutaneous injection in the right hind flank. The injection site was monitored daily for tumour growth, and the incidence of tumour-free animals was recorded.
Complement-dependent cytotoxic antibody activity
Serum samples from organ transplant recipients were incubated at 56° for 30 min to inactivate complement. Thereafter, they were diluted serially in round-bottomed, 96-well microtitre plates (Corning) in Hanks' balanced salt solution (HBSS) containing 0·1% w/v bovine serum albumin (Sigma). Splenic target cells (5 × 105) from B10 (donor; H2b), BALB/c (third-party; H2d), or C3H mice (syngeneic; H2k) were added to each well, and the plates were incubated for 1 hr at room temperature. The cells were then washed twice in HBSS, and 100 μl of baby rabbit complement (Cedarlane, Hornby, Ontario) was added to each well. The plates were maintained for 30 min at 37° in 5% CO2 in air, washed twice with HBSS and the cells were incubated for an additional 3 hr in 100 μl HBSS supplemented with 10% FCS and 20 μl 3-[4,5-dimethylthiazol-2-yl]-3,5-diphenylformazan (MTT; Sigma). At the end of the incubation period, the cells were washed twice, and 150 μl dimethyl sulphoxide (Sigma) was added to each well. MTT formazan was dissolved by shaking the plate vigorously, followed by centrifugation at 1430 g for 1 min. Optical density was measured at 550 nm, using a kinetic microplate reader (Molecular Devices, Menlo Park, CA).
Statistical analyses
The significance of difference between means was determined using Student's t-test. A P value < 0·05 was considered significant.
RESULTS
Efficiency of Ad-IL-10 transduction of TGF-β DC
Ad vector transduction efficiency was consistently > 80% at 50 or 100 MOI, as determined by X-gal staining following transduction with Ad-LacZ (data not shown). This was similar to the transduction rate achieved with Ad-LacZ in mature mouse BM-derived DC.27 The expression of IL-10 protein in DC was confirmed by immunocytochemical staining of cytocentrifuge preparations with anti-mouse IL-10 mAb, which showed strong expression of the IL-10 gene product in the DC transduced with Ad-IL-10 (Fig. 1), and by quantification of IL-10 production by transduced Ad-IL-10 DC using ELISA. While 1 × 106 DC transduced with Ad-IL-10 at 100 MOI then cultured in 1 ml complete medium for 24 hr, from 0, 24, or 48 hr after gene transfer produced 10·2 ± 1·2, 15·7 ± 2·4 and 12·8 ± 1·9 ng of IL-10, respectively, the Ad-LacZ-transduced DC produced only trace amounts of IL-10 (0·29 ± 0·2, 0·18 ± 0·1 and 0·22 ± 0·4 ng, respectively). This confirmed sustained production of the transgene product (mouse IL-10) for at least 72 hr after gene transfer.
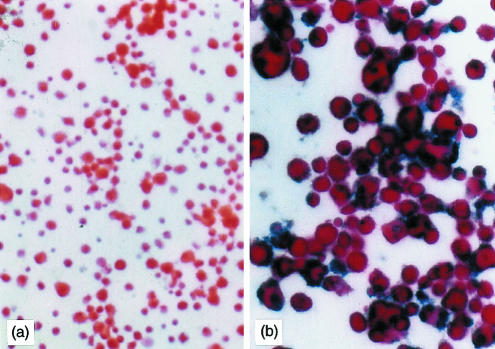
Figure 1.
Expression of IL-10 in TGF-β DC transduced with Ad-IL-10. B10 bone marrow-derived DC were propagated in the presence of GM-CSF and TGF-β1 for 5 days, and then transduced with (a) Ad-LacZ or (b) Ad-IL-10 at 50 MOI as described in the Materials and Methods. An avidin–biotin complex–alkaline phosphatase immunocytochemical staining procedure was used to identify intracellular IL-10, as seen in (b); counterstained with fast red. Magnification: × 70 (a); × 140 (b).
Phenotypic changes in TGF-β DC transduced with Ad-IL-10 vectors
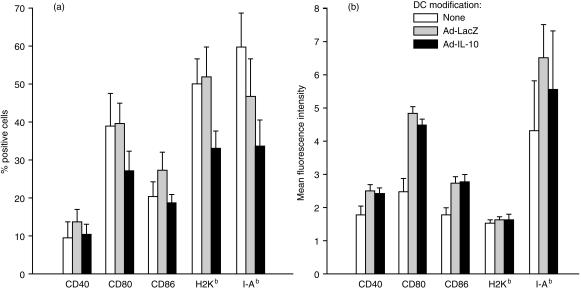
DC propagated from B10 mouse BM in GM-CSF+ TGF-β1 for 5 days were phenotypically immature. As reported previously,8 they expressed high surface levels of the mouse DC-restricted antigens (CD11c and DEC205) (data not shown), moderate MHC-class II (IAb), but low CD40, CD80 and CD86. The influence of Ad-LacZ or Ad-IL-10 transduction on the expression of cell key surface antigens was ascertained by flow cytometric analysis of the incidence of positive cells and the mean fluorescence intensity (MFI). Ad-LacZ gene transduction increased the MFI of CD40+, CD80+ and CD86+ cells (Fig. 2a). Transduction with Ad-IL-10 did not reverse the effect of Ad on MFI but reduced the incidence of CD80, CD86 and MHC class I/II positive cells compared with control gene transduction (Fig. 2). These effects of gene transfer were comparatively modest.
Figure 2.
Flow cytometric analysis of TGF-β DC propagated from B10 (H2b) bone marrow for 5 days and then transduced with Ad-LacZ or Ad-IL-10 (50 MOI), as described in the Materials and Methods. Data indicate the incidence of cells expressing molecules of interest on the cell surface (a), and the mean fluorescence intensity of these positive cells (b). The data are means ± 1SD of three separate experiments.
Overexpression of IL-10 enhances DC allostimulatory activity in MLR
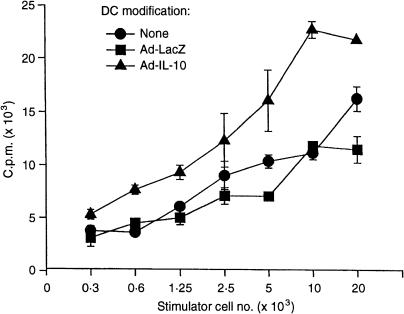
The allostimulatory activity of control and Ad-IL-10-transduced B10-DC was tested in vitro in one-way MLR, using C3H splenic T cells as responders. Non-transduced and Ad-LacZ-transduced DC were used as controls. Although transduction with the Ad vector encoding the reporter gene LacZ (at 50 MOI) marginally increased expression of surface co-stimulatory molecules on the DC (as described above), it did not enhance their allostimulatory activity, as shown in Fig. 3. However, allogeneic T-cell proliferation was enhanced significantly by stimulation with Ad-IL-10-transduced DC (Fig. 3), even though transduction with Ad-IL-10 reversed the minor increase in expression of co-stimulatory molecules induced by Ad-LacZ (Fig. 2). Thus, in contrast to the well-documented capacity of exogenous IL-10 protein to reduce DC allostimulatory activity,30,31 increased endogenous production of IL-10 by DC as the result of gene transfer enhanced their allostimulatory function. It is also apparent that (minor) changes in co-stimulatory molecule expression by DC following Ad-mediated gene transfer are not correlated with the allostimulatory function of the cells.
Figure 3.
Ad-IL-10 transduction enhances the allostimulatory function of TGF-β DC. Unmodified, bone marrow-derived TGF-β DC (B10, H2b) or DC transduced with either Ad-LacZ or Ad-IL-10 (50 MOI), were tested as stimulators of naïve, allogeneic (C3H, H2k) T cells in 3-day MLR. Data are mean c.p.m. ± 1SD of triplicate cultures, and are representative of three separate experiments.
DC transduced with Ad-IL-10 stimulate strong alloantigen-specific CTL and NK cell activities in allogeneic spleen cell populations
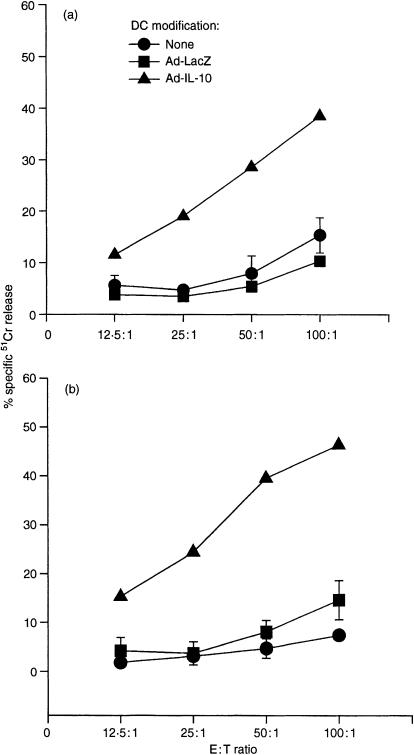
To determine the CTL activity of T cells stimulated by Ad-IL-10-transduced DC, C3H spleen T cells were cultured with γ-irradiated (20 Gy) B10 Ad-IL-10-transduced DC at a ratio of 10 : 1 for 5–7 days, then used as effectors in a 4-hr 51Cr-release assay. Compared with non-transduced and Ad-LacZ-transduced DC, which exhibited similar low levels of activity, Ad-IL-10-transduced DC elicited significantly enhanced CTL activity against allospecific (EL4) targets (Fig. 4a). No specific CTL activity was generated against syngeneic or third-party targets (data not shown). NK cell activity was determined by a 4-hr 51Cr-release assay, using YAC-1 tumour cells as targets. No significant NK-cell activity was detected in spleen cells stimulated with Ad-LacZ-transduced DC, whereas spleen cells cultured with Ad-IL-10-transduced DC exhibited NK cell activity that was related to the E : T ratio tested (Fig. 4b). These results indicate that transduction of DC with Ad-IL-10 not only enhances their capacity to induce alloantigen-specific CTL activity, but also augments their ability to induce non-specific (non-MHC-restricted) NK cell activity.
Figure 4.
TGF-β DC transduced with Ad-IL-10 (50 MOI) show markedly increased capacity to induce the generation of (a) CTL and (b) NK cell activity. Bone marrow-derived TGF-β DC (B10; H2b) were transduced with either Ad-LacZ or Ad-IL-10. (a) The DC were used as stimulators of CTL induction in 6-day MLR, with naive C3H (H2k) T cells as responders. Non-transduced DC were used as controls. Cytotoxicity against EL4 targets (H2b) was determined at various effector : target cell ratios in a 4-hr 51Cr-release assay. (b) The DC were used as inducers of NK cell activity in 6-day MLR, with naive C3H (H2k) spleen cells as responders. Non-transduced DC were used as controls. Cytotoxicity against YAC-1 targets was determined at various effector : target cell ratios, in a 4-hr 51Cr-release assay. Data are means ± 1SD of triplicate cultures, and are representative of three separate experiments.
Ad-IL-10-transduced DC exacerbate cardiac allograft rejection
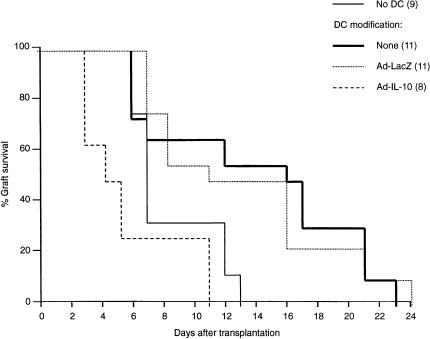
Next, the in vivo allostimulatory activity of Ad-IL-10-transduced DC was determined by the capacity of Ad-transduced TGF-β DC to modulate the survival of B10 vascularized heart grafts in C3H recipients as described.8 Seven days before B10 cardiac transplantation 2 × 106 B10 BM-derived TGF-β DC transduced with Ad-IL-10 or Ad-LacZ (control) were administered intravenously to C3H recipients (we had demonstrated previously5 that administration of donor immature DC 7 days before cardiac transplantation achieved maximal prolongation of graft survival). Compared with Ad-LacZ-transduced DC, which significantly prolonged graft survival in a similar fashion to unmodified TGF-β DC [median survival time (MST) 16 days, compared with 7 days in unmodified recipients; P < 0·01], Ad-IL-10-transduced DC exacerbated rejection (MST 5 days; P < 0·01) (Fig. 5). Histological examination of the rejected grafts, revealed no irrefutable evidence of humoral rejection in animals treated with Ad-IL-10-transduced DC. Taken together, the data show that over-expression of IL-10 in immature donor DC by Ad-mediated gene transfer enhances their allostimulatory activity, both in vitro and in vivo.
Figure 5.
Influence of Ad-IL-10 transduction on the capacity of TGF-β DC to prolong allogeneic vascularized organ graft survival. Bone marrow-derived TGF-β DC (B10; H2b) were transduced with either Ad-LacZ or Ad-IL-10 then injected (2 × 106) intravenously, 7 days before C3H (H2k) recipients received B10 cardiac allografts. Non-transduced DC were used as controls. Graft survival in the Ad-IL-10 DC group was reduced significantly compared with either the non-transduced DC or Ad-LacZ-transduced DC groups (P < 0·01). Numbers of mice per group are shown in parentheses.
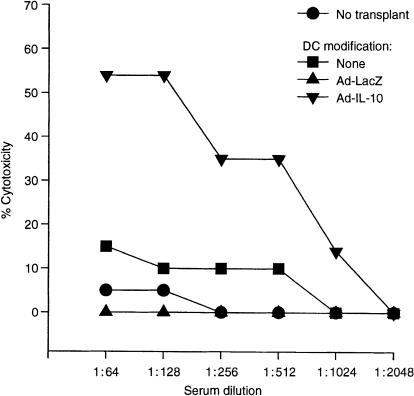
Ad-IL-10-transduced DC augment cytotoxic alloantibody production in cardiac allograft recipients
We examined circulating, anti-donor, complement-dependent cytotoxic antibody levels in sera of the variously treated cardiac allograft recipients. As shown in Fig. 6, pretreatment (day 7) with donor-derived TGF-β DC transduced to overexpress IL-10 augmented anti-donor alloantibody titres, determined 4 days after cardiac transplantation. DC treatment without heart transplantation generated low titres of cytotoxic alloantibody (target cell killing < 10%) and there was no significant difference between groups of mice given Ad-LacZ-transduced DC compared with control DC (data not shown).
Figure 6.
Serum complement-dependent lymphocytotoxic antibody titrrs against B10 (H2b) targets in normal C3H (H2k) mice (no transplant), or C3H recipients of B10 heart allografts, pretreated (day 7) with unmodified B10 TGF-b DC, Ad-LacZ-transduced DC (Ad-LacZ), or Ad-IL-10-transduced DC (Ad-IL-10). The sera were obtained 4 days after cardiac transplantation. The results (means ± 1SD) are representative of two separate experiments, with three animals per group at each time point.
IL-10 gene transduction inhibits the anti-tumour effect of tumour antigen-pulsed DC
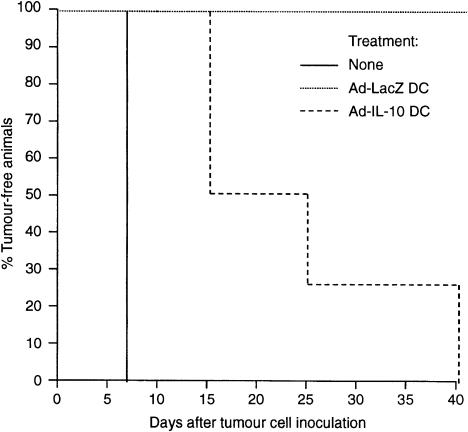
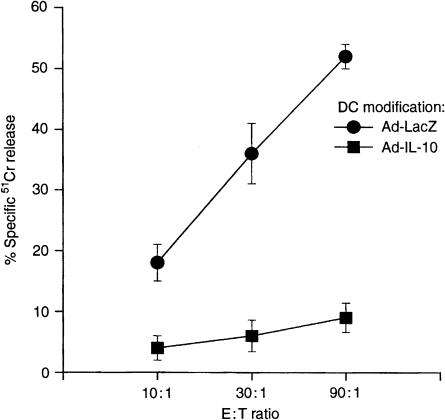
To determine the influence of Ad-IL-10 transduction on the anti-tumour activity of DC, B6 mice were injected i.p. with 106 DC pulsed with tumour antigen (B16F10-DC), or with antigen-pulsed DC transduced with Ad-LacZ or Ad-IL-10. After 14 days, each animal was challenged by subcutaneous injection of 104 B16F10 cells in the right hind flank. Tumour appearance and the incidence of tumour-free mice were monitored daily. Without DC treatment, all mice developed tumours within 7 days (Fig. 7). In contrast, all animals pretreated with non-transduced or Ad-LacZ-transduced B16F10-DC remained tumour-free by day 40 after tumour cell inoculation (Fig. 7). However, mice immunized with Ad-IL-10-transduced B16F10-DC each developed tumours by day 40 (50% by day 15). As shown in Fig. 8, the generation of CTL in spleens of animals immunized with Ad-IL-10-transduced B16F10-DC was significantly inhibited compared with control Ad-LacZtransduced B16F10-DC recipients, which exhibited marked anti-tumour CTL activity.
Figure 7.
Influence of Ad-IL-10 transduction on the anti-tumour activity of tumour antigen-pulsed DC. B6 mice were either untreated (no DC; n = 8) or injected i.p. with 106 TGF-β DC, that had been pulsed with Bl6F10 tumour antigen, and transduced with Ad-LacZ (n = 8), or Ad-IL-10 (n = 8), 14 days before challenge with 104 B16F10 tumour cells by subcutaneous injection. Non-transduced DC pulsed with tumour antigen gave similar results to those shown for Ad-LacZ DC (data not shown). Data are percentages of tumour-free animals in each group.
Figure 8.
Influence of Ad-IL-10 transduction of tumour antigen-pulsed TGF-β DC on the tumour-specific cytotoxic activity induced by these cells in spleens of immunized mice. Effector cells were harvested from mice 14 days after immunization with tumour antigen-pulsed DC transduced with either Ad-LacZ or Ad-IL-10. They were cultured with tumour target cells for 5 days. Cytotoxicity against tumour antigens was determined in a 4-hr 51Cr-release assay. Data are means ± 1SD of triplicate cultures, and are representative of three separate experiments.
DISCUSSION
The present studies demonstrate that overproduction of endogenous IL-10 by immature DC induced by Ad-mediated gene transfer exerts complex effects on DC biology. Transduction of the IL-10 gene only slightly inhibited surface expression of MHC class II, as well as CD40, CD80 and CD86 on immature TGF-β DC. Paradoxically, IL-10 gene transfer significantly enhanced the allostimulatory activity of the cells, both in vitro and in vivo, as demonstrated by elevated donor-specific MLR and CTL responses (Figs 3 and 4a), and exacerbation of cardiac allograft rejection (Fig. 5). These findings disagree with the effect of addition of exogenous murine IL-10 or viral IL-10 protein on the allostimulatory function of DC. Thus, exogenous murine IL-10 or viral IL-10 has been reported to inhibit both T-cell proliferative and cytotoxic responses12,13 by suppressing the function of APC.16–19,31 This apparent contradiction may reflect the complex and multifunctional roles of IL-10. Thus, DC endocytic and chemotactic migratory capacities can be increased by IL-10.31 On the other hand, its effects on T-cell responses have been shown to be influenced by timing of exposure to the cytokine and dosage. In contrast to low-dose IL-10, high-dose post-transplant administration of exogenous IL-10 can exacerbate mouse cardiac allograft rejection.15,33 We observed that Ad-IL-10 gene transduction achieved comparatively high production of IL-10 by DC, that was sustained over several days in vitro. Thus, interactions between DC and allogeneic T cells in the presence of high local levels of IL-10 may favour enhanced T-cell responses. Indeed, IL-10 is known to promote proliferation of CTL precursors,20 and systemic administration of IL-10 induces specific, and long-lasting anti-tumour responses.34 Moreover, IL-10 has been reported to act as a co-stimulator of B-cell growth and differentiation, and to enhance immunoglobulin production.20 Indeed, in the present study, exacerbation of allograft rejection was accompanied by augmentation of circulating anti-donor alloantibody titres. The augmentation of allostimulatory activity of IL-10-transduced DC may also reflect the alteration of DC status by infection with Ad vector.35 Moreover, whereas IL-10 can suppress alloimmune responses by inhibition of the maturation of immature DC, fully mature DC lose sensitivity to IL-10.36
On the basis of our investigation, transduction with Ad-LacZ (at least at 50 MOI) appears not to promote the phenotypic maturation of immature TGF-β DC, as shown by the incidence of cells expressing MHC class II and co-stimulatory molecules (Fig. 2). Others have reported that recombinant Ad does not perturb human DC maturation,37 or in contrast, that recombinant Ad enhances the immunostimulatory function of human DC by increasing their co-stimulatory and antigen-presenting functions, without triggering IL-12 secretion.38 In the latter study, much higher MOI were used to infect DC than were employed in the present investigation.
The present studies demonstrate differential effects of Ad-IL-10-transduced immature DC on anti-allograft and anti-(syngeneic) tumour responses. Pre-transplant administration of Ad-IL-10 DC exacerbated mouse cardiac allograft rejection (Fig. 5), whereas pretreatment of mice with tumour antigen-pulsed Ad-IL-10 DC reduced the anti-tumour effect observed with tumour antigen-pulsed Ad-LacZ-transduced DC (Fig. 6). Although we found no convincing histological evidence that the rapid rejection of cardiac allografts in the Ad-IL-10-transduced DC treated group was humorally mediated, circulating cytotoxic alloantibody titres were elevated in this group. Thus, an antibody-mediated effect cannot be excluded as a factor (in addition to enhanced anti-donor T-cell responses) to explain the exacerbated allograft rejection. Since it has been reported that tumour cells treated with IL-10, or transfected with the IL-10 gene, show reduced expression of MHC class I, and low sensitivity to specific CTL-mediated lysis,39,40 such down-regulation of MHC class I expression by IL-10 could conceivably contribute to defects in antigen recognition and impaired resistance to tumour growth. Taken together, these findings with IL-10-transduced myeloid DC reflect the complex immunomodulatory effects previously described for the native protein.20
Acknowledgments
This work was supported by National Institutes of Health Grants DK 29961 and AI41011. We are grateful to Schering-Plough, Kenilworth, NJ, for providing cytokines and to Ms Shelly L. Conklin for secretarial support.
Abbreviations
- ABC-AP
avidin–biotin complex–alkaline phosphatase
- Ad
adenoviral
- Ag
antigen
- APC
antigen-presenting cell
- B6
C57BL/6
- B10
C57BL/10 J
- BM
bone marrow
- C3H
C3H/HeJ
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- FCS
fetal calf serum
- hpf
high power field
- IL
interleukin
- MFI
mean fluorescence intensity
- MHC
major histocompatibility complex
- MLR
mixed leucocyte reaction
- MOI
multiplicity of infection
- MST
median survival time
- NK
natural killer
- Th
T helper
- TGF-β
transforming growth factor-β
REFERENCES
- 1.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 2.Austyn JM. The initiation of immune responses and allograft rejection. In: Rose ML, Yacoub MH, editors. Immunology of Heart and Lung Transplantation. Boston: Little, Brown; 1993. pp. 22–41. [Google Scholar]
- 3.Lu L, Mccaslin D, Starzl TE, Thomson AW. Mouse bone marrow-derived dendritic cell progenitors (NLDC 145+, MHC class II+, B7-1dim, B7-2–) induce alloantigen-specific hyporesponsiveness in murine T lymphocytes. Transplantation. 1995;60:1539–45. doi: 10.1097/00007890-199560120-00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rastellini C, Lu L, Ricordi C, Starzl TE, Rao AS, Thomson AW. GM-CSF stimulated hepatic dendritic cell progenitors prolong pancreatic islet allograft survival. Transplantation. 1995;60:1366–70. [PMC free article] [PubMed] [Google Scholar]
- 5.Fu F, Li Y, Qian S, Lu L, Chambers F, Starzl TE, Fung JJ, Thomson AW. Costimulatory molecule-deficient dendritic cell progenitors (MHC class II+, B7-1dim, B7-2–) prolong cardiac allograft survival in non-immunosuppressed recipients. Transplantation. 1996;62:659–65. doi: 10.1097/00007890-199609150-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayamizu K, Huie P, Sibley RK, Strober S. Monocyte-derived dendritic cell precursors facilitate tolerance to heart allografts after total lymphoid irradiation. Transplantation. 1998;66:1285–91. doi: 10.1097/00007890-199811270-00004. [DOI] [PubMed] [Google Scholar]
- 7.Suri RM, Niimi M, Wood KJ, Austyn JM. Immature dendritic cell pretreatment induces cardiac allograft prolongation. J Leukocyte Biol. 1998;32(Suppl. 2):32. (Abstract) B16. [Google Scholar]
- 8.Lu L, Li W, Fu F, Chambers FG, Qian S, Fung JJ, Thomson AW. Blockade of the CD40–CD40L pathway potentiates the capacity of donor-derived dendritic cell progenitors to induce long-term cardiac allograft survival. Transplantation. 1997;64:1808–15. doi: 10.1097/00007890-199712270-00031. [DOI] [PubMed] [Google Scholar]
- 9.Lu L, Li W, Zhong CP, Qian S, Fung JJ, Thomson AW, Starzl TE. Increased apoptosis of immunoreactive host cells and augmented donor leukocyte chimerism, not sustained inhibition of B7 molecule expression, are associated with prolonged cardiac allograft survival in mice preconditioned with immature donor dendritic cells plus anti-CD40L mAb. Transplantation. 1999;68:747–57. doi: 10.1097/00007890-199909270-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse helper T cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–95. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore KW, Vieira P, Fiorentino DF, Trounstine ML, Khan TA, Mosmann TR. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein–Barr virus gene BCRFI. Science. 1990;248:1230–4. doi: 10.1126/science.2161559. [DOI] [PubMed] [Google Scholar]
- 12.Bejarano MT, De Waal Malefyt R, Abrams JS, et al. Interleukin-10 inhibits allogeneic proliferative and cytotoxic T cell responses generated in primary mixed lymphocyte cultures. Int Immunol. 1992;4:1389–97. doi: 10.1093/intimm/4.12.1389. [DOI] [PubMed] [Google Scholar]
- 13.de Waal Malefyt R, Yssel H, De Vries JE. Direct effects of IL-10 on subsets of human CD4+ T-cell clones and resting T cells: Specific inhibition of IL-2 production and proliferation. J Immunol. 1993;150:4754–65. [PubMed] [Google Scholar]
- 14.Fiorentino DF, Zlotnik A, Vieira P, et al. IL-10 acts on the antigen presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–51. [PubMed] [Google Scholar]
- 15.Li W, Fu F, Lu L, Narula SK, Fung JJ, Thomson AW, Qian S. Differential effects of exogenous interleukin-10 on cardiac allograft survival: inhibition of rejection by recipient pretreatment reflects impaired host accessory cell function. Transplantation. 1999;68:1402–8. doi: 10.1097/00007890-199911150-00029. [DOI] [PubMed] [Google Scholar]
- 16.de Waal Malefyt R, Haanen J, Spits H, et al. Interleukin-10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–24. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding L, Shevach EM. IL-10 inhibits mitogen-induced T cell proliferation by selectively inhibiting macrophage costimulatory function. J Immunol. 1992;148:3133–9. [PubMed] [Google Scholar]
- 18.Beissert S, Hosoi J, Grabbe S, Asahina A, Granstein RD. IL-10 inhibits tumor antigen presentation by epidermal antigen-presenting cells. J Immunol. 1995;154:1280–6. [PubMed] [Google Scholar]
- 19.Steinbrink K, Wölfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–80. [PubMed] [Google Scholar]
- 20.de Waal Malefyt R, Moore KW. Interleukin-10. In: Thomson AW, editor. The Cytokine Handbook. 3. San Diego: Academic Press; 1998. pp. 333–64. [Google Scholar]
- 21.D'andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon-γ-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–8. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inaba K, Inaba M, Romani N, Aya H, DeGuchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu L, Woo J, Rao AS, et al. Propagation of dendritic cell progenitors from normal mouse liver using GM-CSF and their maturational development in the presence of type-1 collagen. J Exp Med. 1994;179:1823–34. doi: 10.1084/jem.179.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu L, Rudert WA, Qian S, et al. Growth of donor-derived dendritic cells from the bone marrow of liver allograft recipients in response to granulocyte/macrophage colony-stimulating factor. J Exp Med. 1995;182:379–87. doi: 10.1084/jem.182.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu L, Bonham CA, Chambers FG, Watkins SC, Hoffman RA, Simmons RL, Thomson AW. Induction of nitric oxide synthase in mouse dendritic cells by interferon γ, endotoxin and interaction with allogeneic T cells: nitric oxide production is associated with dendritic cell apoptosis. J Immunol. 1996;157:3577–86. [PubMed] [Google Scholar]
- 26.Xing Z, Ohkawara Y, Jordana M, Graham FL, Gauldie J. Adenoviral vector-mediated interleukin-10 expression in vivo: intramuscular gene transfer inhibits cytokine responses in endotoxemia. Gene Ther. 1997;4:140–9. doi: 10.1038/sj.gt.3300371. [DOI] [PubMed] [Google Scholar]
- 27.Lu L, Gambotto A, Lee W-C, Qian S, Bonham CA, Robbins PD, Thomson AW. Adenoviral delivery of CTLA4Ig into myeloid dendritic cells promotes their in vitro tolerogenicity and survival in allogeneic recipients. Gene Ther. 1999;6:554–63. doi: 10.1038/sj.gt.3300862. [DOI] [PubMed] [Google Scholar]
- 28.Ono K, Lindsey ES. Improved technique of heart transplantation in rats. J Thorac Cardiovasc Surg. 1969;7:225–9. [PubMed] [Google Scholar]
- 29.Yang S, Darrow TL, Vervaert CE, Seigler HF. Immunotherapeutic potential of tumor antigen-pulsed and unpulsed dendritic cells generated from murine bone marrow. Cell Immunol. 1997;179:84–95. doi: 10.1006/cimm.1997.1151. [DOI] [PubMed] [Google Scholar]
- 30.Enk AH, Angeloni VL, Udey MC, Katz SI. Inhibition of Langerhans cell antigen-presenting function by IL-10. A role for IL-10 in induction of tolerance. J Immunol. 1993;151:2390–8. [PubMed] [Google Scholar]
- 31.Takayama T, Nishioka Y, Lu L, Lotze MT, Tahara H, Thomson AW. Retroviral delivery of viral IL-10 into myeloid dendritic cells markedly inhibits their allostimulatory activity and promotes the induction of T cell hyporesponsiveness. Transplantation. 1998;66:1567–74. doi: 10.1097/00007890-199812270-00001. [DOI] [PubMed] [Google Scholar]
- 32.Sato K, Nagayama H, Tadokoro K, Juji T, Takahashi TA. Extracellular signal-regulated kinase, stress-activated protein kinase/c-Jun N-terminal kinase, and p38mapk are involved in IL-10-mediated selective repression of TNF-alpha-induced activation and maturation of human peripheral blood monocyte-derived dendritic cells. J Immunol. 1999;162:3865–72. [PubMed] [Google Scholar]
- 33.Qian S, Li W, Li Y, Fu F, Lu L, Fung JJ, Thomson AW. Systemic administration of cellular interleukin-10 can exacerbate cardiac allograft rejection in mice. Transplantation. 1996;62:1709–14. doi: 10.1097/00007890-199612270-00002. [DOI] [PubMed] [Google Scholar]
- 34.Berman RM, Suzuki T, Tahara H, Robbins P, Narula SK, Lotze MT. Systemic administration of cellular interleukin-10 induces an effective, specific, and long-lived immune response against established tumors in mice. J Immunol. 1996;157:231–8. [PubMed] [Google Scholar]
- 35.Tillman BW, De Gruijl TD, Luykx-De Bakker SA, Scheper RJ, Pinedo HM, Curiel TJ, Gerritsen WR, Curiel DT. Maturation of dendritic cells accompanies high-efficiency gene transfer by a CD40-targeted adenoviral vector. J Immunol. 1999;162:6378–83. [PubMed] [Google Scholar]
- 36.Kalinski P, Schuitemaker JH, Hilkens CM, Kapsenberg ML. Prostaglandin E2 induces the final maturation of IL-12-deficient CD1a+CD83+ dendritic cells: the levels of IL-12 are determined during the final dendritic cell maturation and are resistant to further modulation. J Immunol. 1998;161:2804–9. [PubMed] [Google Scholar]
- 37.Zhong L, Granelli-Piperno A, Cho Y, Steinman RM. Recombinant adenovirus is an efficient and non-perturbing genetic vector for human dendritic cells. Eur J Immunol. 1999;29:964–72. doi: 10.1002/(SICI)1521-4141(199903)29:03<964::AID-IMMU964>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 38.Rea D, Schagen FHE, Hoeben RC, Mehtali M, Havenga MJE, Toes REM, Melief CJM, Offringa R. Adenoviruses activate human dendritic cells without polarization toward a T-helper type 1-inducing subset. J Virol. 1999;73:10245–53. doi: 10.1128/jvi.73.12.10245-10253.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuda M, Salazar F, Petersson M, et al. Interleukin-10 pre-treatment protects target cells from tumor- and allo-specific cytotoxic T-cells and down-regulates HLA class I expression. J Exp Med. 1994;180:2371–6. doi: 10.1084/jem.180.6.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petersson M, Charo J, Salazar-Onfray F, et al. Constitutive IL-10 production accounts for the high NK sensitivity, low MHC class I expression, and poor transporter associated with antigen processing (TAP) -1/2 function in prototype NK target YAC-1. J Immunol. 1998;161:2099–105. [PubMed] [Google Scholar]