Abstract
Among peripheral T cells, the expression of CD4 and CD8 is almost mutually exclusive. However, here we show, using flow cytometric analysis, that ex vivo approximately 6% of rat T cells stained for both CD4 and CD8. These double positive cells were also detected by confocal microscopy. Only around 50% of double positive cells expressed the CD8β chain, the remaining cells expressed the CD8α chain alone. Double positive cells were blast-like with a phenotype, distinct from that of either CD4 or CD8 single positive cells, suggestive of an activated state. Previous reports of double positive T cells have also suggested that coexpression of CD4 and CD8 is linked to the activation state of the cell. There was an indication that priming animals with a hapten-carrier complex increased the ratio of CD8αα : αβ expressing double positive T cells, although we did not detect an increase in the frequency of double positive T cells following priming. We also show that the frequency of double positive cells was reduced following thymectomy and with age. In conclusion, these studies show that peripheral T cells expressing both CD4 and CD8 can be detected in the rat and that they are phenotypically distinct from CD4 and CD8 single positive T cells.
INTRODUCTION
On the majority of peripheral T cells, the expression of CD4 and CD8 is mutually exclusive and commonly CD4+ T cells modulate the function of other cells while CD8+ T cells are cytotoxic. However, it is becoming increasingly recognized that these distinctions are not so clear cut, with reports in the literature of cytotoxic CD4+ T cells,1 non-cytotoxic CD8+ T cells2 and T cells that express both CD4 and CD8.3–18 Double positive cells have been observed in vitro following activation of human,3,4 swine5,6 and rat7,8 T cells and also in vivo in healthy humans3,9,10 and swine.5,11 Their frequency is increased during conditions where the immune system is activated: bacterial infection in mice12 and humans,13 viral infection in humans,9 hapten-carrier primed rats,14 autoimmune disease9,15,16 and after transplantation in rats17 and humans.18 These reports indicate that the coexpression of CD4 and CD8 may be linked to the activation state of the T cell. Contrary to these findings, it has been suggested that murine and human double positive cells, obtained after in vitro activation, could be artefacts produced by the association of CD4 and CD8 single positive cells during flow cytometric analysis or the absorption of CD8 by CD4 single positive T cells.19 The origin of double positive T cells is also unclear. It has been suggested that double positive T cells are double positive thymocytes which have been released from the thymus prematurely.20 In humans, thymectomy totally eliminated double positive T cells,15 however, in rats, thymectomy had no effect on their number17 and, in swine, thymectomy actually resulted in an increase in double positive T cells.21
The purpose of this present study was to determine whether double positive T cells exist in the periphery of normal rats and, if so, to analyse the phenotype of these cells. The dependence of double positive T cells on the thymus and the possible link between T-cell activation and coexpression of CD4 and CD8 were also explored.
MATERIALS AND METHODS
Animals
PVG.RT1c rats were obtained from the specific pathogen-free breeding facilities of the Sir William Dunn School of Pathology (Oxford, UK). Animals were thymectomized (Tx) at 6 weeks of age. Age- and sex-matched sham Tx (ShTx) animals had their thymi exposed but not removed.
Monoclonal antibodies (mAbs)
The mouse mAb used in these studies were as follows: OX7 (anti-rat Thy-1),22 OX8 (anti-rat CD8α chain hinge region),23 OX21 (anti-human C3b inactivator),24 OX22 (anti-rat CD45RC),25 OX39 (anti-rat interleukin (IL)-2Rα chain).26 These were produced in the Medical Research Council (MRC) Cellular Immunology Unit. R73 (anti-rat T-cell receptor (TCR)αβ),27 341 (anti-rat CD8β chain),28 G28 (anti-rat CD8α chain immunoglobulin fold)28 and 10·78 (anti-rat NKR.P1)29 were kindly provided by Dr T. Hunig (University of Würzburg, Germany) and IA.29 (anti-rat intracellular adhesion molecule-1 (ICAM-1))30 was a gift from Dr M. Miyasaka (Osaka University, Japan). Purified 341 immunoglobulin (used in microscopy experiments), fluorescein isothiocyanate (FITC)-conjugated OX8 (OX8-FITC) and R73 (R73-FITC) were from Serotec (Kidlington, UK), multiple labelling grade indocarbocyanine (Cy3)-conjugated donkey anti-mouse (DAM–Cy3) was from Jackson ImmunoResearch Laboratories Inc. (West Grove, PA), streptavidin quantum red (SA-QR) was from Sigma (Sigma Chemical Co., St Louis, MO) and phycoerythrin (PE)-conjugated DAM (DAM–PE) was from Chemicon (Temecula, CA). FITC-conjugated and biotinylated W3/25 (anti-rat CD4, W3/25-FITC and W3/25-B, respectively)31 were prepared in this laboratory by Mr S. Simmonds, following standard techniques.
Antigens and priming
Dinitrophenol–bovine gamma globulin (DNP–BGG) was prepared, as previously described.32 Animals were immunized intraperitoneally (i.p.) with 0·5, 1 or 2 mg alum-precipitated DNP–bovine serum albumin (BSA) in 0·5 ml phosphate-buffered saline (PBS). Unprimed animals received 0·5 ml PBS intraperitoneally.
Confocal microscopy
The thoracic ducts of animals were cannulated, as previously described.33 Thoracic duct lymphocytes (TDL) were collected over 24 hr and filtered with lens tissue to generate a single cell suspension. Cells were incubated with either OX8, G28, 341 or OX21 followed by DAM–Cy3 and then W3/25–FITC. CD4+ cells were purified by incubating with anti-FITC coated magnetic microbeads (Miltenyi Biotec, Gladbach, Germany) and passing them down a magnetic-activated cell sorting (MACS) column (Miltenyi Biotec), according to the manufacturer's instructions. Purified, labelled cells were attached to poly-l-lysine coated glass coverslips, fixed with 2% paraformaldehyde in PBS and stored in 0·5% paraformaldehyde in PBS overnight at 4°. Coverslips were mounted in Vectashield (Vector Laboratories, Peterborough, UK) and sealed with nail polish. Images were obtained using a BioRad MRC-1000/1024 hybrid confocal laser scanning microscope (Hemel Hempstead, UK) (running under Comos 7·0a) equipped with an argon/krypton laser and coupled to a Nikon Diaphot 200 inverted microscope (60 × PlanApo oil-immersion objective; numerical aperture 1·4). Kalman-filtered images (N = 6–10) were collected with iris aperture (1·2 mm) and the minimum laser power that filled the whole grey scale in the low-scan/low-signal mode. For multiple labelling, sequential images were obtained and no ‘bleedthrough’ was detected between channels. The frequency of double positive cells was calculated after merging red and green pseudocoloured images. For each marker 1–1·5 × 103 cells were counted.
Flow cytometric analysis
One week after immunization with DNP–BSA (see Antigens and priming) mesenteric lymph nodes (LNs) were removed, pressed through wire mesh and filtered with lens tissue to generate a single cell suspension. Cells were incubated with either OX8, 341 or OX21 followed by DAM–PE. Cells were then incubated with R73–FITC and W3/25-B. Finally, SA-QR was added. Data was acquired on a FACScan (Becton Dickinson, Palo Alto, CA), after appropriate compensation using single-labelled cells and control mAb. Viable lymphocytes were analysed using Cellquest software (Becton Dickinson), after gating on forward- and side-scatter profiles.
TDL were used for the analysis of double positive cells from normal, Tx and ShTx animals. The labelling and analysis of TDL from Tx and ShTx animals was performed as described above. For the phenotypic analysis of TDL from normal animals, a panel of mAbs (see text) was developed with DAM–PE. This was followed by OX8–FITC and W3/25-B together with SA-QR.
RESULTS
Double positive cells are detected ex vivo
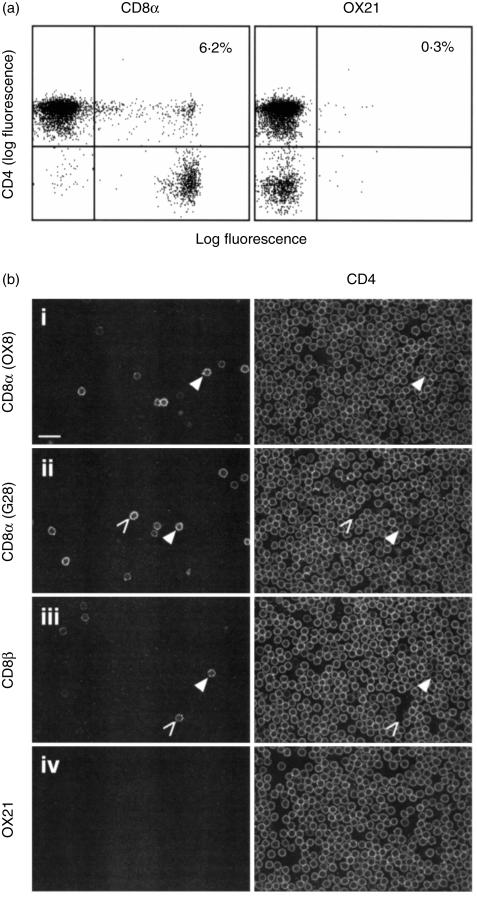
To determine whether double positive T cells exist in the periphery of normal rats, mesenteric LN cells were triple labelled for TCRαβ (R73), CD4 (W3/25) and CD8 (OX8) or a control mAb (OX21). TCRαβ+ cells were gated on and the expression of CD4 against CD8 or OX21 was analysed by flow cytometry (Fig. 1a). Only 0·3% of T cells stained with both W3/25 and OX21, but 6·2% stained with both W3/25 and OX8.
Figure 1.
Double positive cells are detected ex vivo. (a) Mesenteric LN cells were triple labelled for TCRαβ (R73), CD4 (W3/25) and CD8α chain (OX8) or a control mAb (OX21) and analysed by flow cytometry. R73+ cells were gated on and the expression of W3/25 against OX8 or OX21 was analysed. (b) TDL were double stained for CD4 (W3/25) and CD8α chain (OX8, row i, or G28, row ii), CD8β chain (341, row iii) or a control mAb (OX21, row iv) before purifying CD4+ cells on a MACS column, as described in Materials and methods. Equatorial optical sections were collected sequentially through labelled, fixed cells on a confocal microscope. W3/25 images were collected in the green channel and OX8, G28, 341 and OX21 images were collected in the red channel. Examples of cell staining for CD4 and CD8α or CD8β are indicated with closed arrowheads, and for CD8α or CD8β alone with open arrowheads. Bar = 20 µm. Data are representative of three independent experiments.
To exclude the possibility that double positive cells are artefacts produced by the association of CD4+ and CD8+ cells during flow cytometric analysis, confocal microscopy was used to examine single cells labelled for both CD4 and CD8. Figure 1(b) shows CD4+ cells, purified from TDL, double labelled for CD4 (W3/25) and CD8α chain (OX8, row i, or G28, row ii), CD8β chain (34l, row iii) or a control mAb (OX21, row iv). Cells were labelled with both OX8 and G28 anti-CD8α chain mAbs, as OX8 labels the hinge region, whilst G28 labels the immunoglobulin fold. In the merged images, examples of cell staining for CD4 and CD8α or CD8β are indicated with closed arrowheads, and for CD8α or CD8β alone with open arrowheads. Of W3/25+ cells, 2·6% stained with OX8 and 2·7% with G28, but only 1·1% stained with 341. No staining was seen with the control mAb, OX21. The concordance of the results with OX8 and G28 mAbs indicates that the CD8α chains on these cells express both OX8 and G28 epitopes, this was also observed by flow cytometric analysis (data not shown).
Double positive cells have a phenotype distinct from that of CD4 and CD8 single positive cells
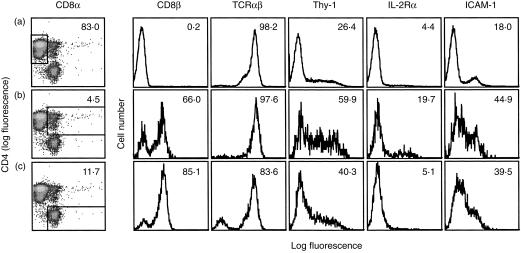
To determine whether double positive T cells have a phenotype distinct from that of CD4 and CD8 single positive T cells, TDL were triple labelled for CD4 (W3/25), CD8 (OX8) and one of a panel of various other cell surface markers (Thy-1, CD45RC, IL-2Rα, OX40, CD26, l-selectin, CD8β chain, TCRαβ, ICAM-1, leucocyte-function associated antigen-1 (LFA-1), very late antigen-4 (VLA-4) and NKR.P1). CD4 single positive (Fig. 2, row A), double positive (Fig. 2, row B) and CD8 single positive (Fig. 2, row C) cells were examined by flow cytometry, with gates set to define these three cell types. As expected, CD4+ CD8α− cells were negative for the CD8β chain, approximately 60% of the CD4+ CD8α+ cells expressed the CD8β chain (which correlates with the confocal data, shown in Fig. 1b, row iii) and 85% of the CD4− CD8α+ cells were positive for the CD8β chain. All CD4+ CD8α− and CD4+ CD8α+ cells expressed TCRαβ, but 17% of CD4− CD8α+ cells were negative for TCRαβ. This percentage was approximately the same as the percentage of CD4− CD8α+ cells that did not express the CD8β chain. Labelling cells for the natural killer (NK) cell marker NKR.P1, with the mAb 10.78 (data not shown), showed that the few CD4− CD8α+ cells which were positive (approximately 7%) were insufficient to account for the total percentage of CD8β− TCRαβ− cells. The remainder are likely to be TCRγδ cells. Of the other cell surface markers analysed, Thy-1, IL-2Rα and ICAM-1 were the most informative (Fig. 2). A higher frequency of cells stained for these three markers amongst the double positive subset compared with either the CD4 or CD8 single positive subsets. A small increase in the expression of OX40 was also observed amongst double positive cells compared to CD4 and CD8 single positive cells (6·8% positive versus 0·7%, data not shown) and NKR.P1 expression was increased on double positive and CD8 single positive cells compared to CD4 single positive cells (6·9% positive versus 0·4%, data not shown). No clear differences in labelling of the three cell subsets, defined by CD4 and CD8, was seen with CD45RC, CD26, l-selectin, LFA-1 and VLA-4 (the majority of cells were positive for these markers, data not shown).
Figure 2.
Double positive T cells have a distinct phenotype from CD4 and CD8 single positive T cells. TDL were triple labelled for CD8α chain (OX8), CD4 (W3/25) and CD8β chain (341), TCRαβ (R73), Thy-1 (OX7), IL-2Rα chain (OX39) or ICAM-1 (IA.29). CD4 single positive (row A), double positive (row B) or CD8 single positive (row C) cells were gated on and the expression of CD8β chain, TCRαβ, Thy-1, IL-2Rα chain and ICAM-1 on these three T-cell subsets was analysed by flow cytometry. Numbers shown in the top right represent either the percentage of cells within a defined subset (first column) or the percentage of cells within a subset that are stained with each marker (all other columns). Data are representative of five independent experiments.
To determine whether any of the three cell subsets described above contained blast-like cells, their forward and side scatter profiles were analysed (data not shown). Both forward and side scatter were greater for the double positive cells than for the CD4 or CD8 single positive cells; forward scatter 58 versus 48 and side scatter 14 versus 11.
The frequency of double positive cells does not increase following priming
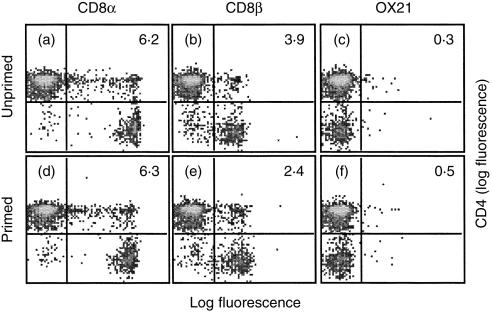
To examine the link between T-cell activation and coexpression of CD4 and CD8, the frequency of double positive T cells was determined in primed animals. One week after in vivo priming with alum-precipitated DNP–BSA, mesenteric LN cells were triple labelled for TCRαβ (R73), CD4 (W3/25) and CD8α (OX8), CD8β (341) or a control mAb (OX21) and analysed by flow cytometry. Figure 3 shows the expression of CD4 against CD8α chain (a and d), CD8β chain (b and e) or OX21 (c and f), after gating on TCRαβ+ cells, from unprimed and primed animals. Consistent with the confocal microscopy experiments shown in Fig. 1(b) (row iii) and flow cytometric analysis shown in Fig. 2(B), approximately twice as many cells stained with OX8 than with 341. However, there was no significant difference in the frequency of double positive T cells detected in unprimed and primed animals (6·2% versus 6·3%). Similar results were seen with spleen cells and at two weeks after priming (data not shown). Less than 0·5% positive cells were detected with the control mAb, OX21.
Figure 3.
The frequency of double positive T cells does not increase following priming. Animals were primed i.p. with 2 mg alum-precipitated DNP–BSA in 0·5 ml PBS and were left for 1 week, unprimed animals received PBS alone. Mesenteric LN cells from unprimed (a–c) and primed (d–f) animals were triple labelled for TCRαβ (R73), CD4 (W3/25) and CD8α chain (OX8), CD8β chain (341) or a control mAb (OX21). R73+ cells were gated on and the expression of W3/25 against OX8, 341 or OX21 was analysed by flow cytometry. Numbers shown in the top right quadrants represent the percentage of T cells that were double labelled. Data are representative of two independent experiments.
The ratio of CD8αα : αβ expressing double positive T cells increases following priming
Table 1 summarizes the data from the flow cytometric analysis of primed and unprimed animals, described above. Although priming did not increase the frequency of double positive T cells, the proportion of double positive T cells expressing CD8αβ decreased (also see Fig. 3b,e) and the proportion expressing CD8αα increased significantly in primed animals.
Table 1.
The ratio of CD8αα : αβ expressing double positive T cells increases with priming
| Amount of DNP–BSA used for priming (mg) | ||||
|---|---|---|---|---|
| 0 | 0·5 | 1 | 2 | |
| CD8 αα+ and αβ+ cells (OX8+) | 6·0 | 6·1 | 5·7 | 6·0 |
| CD8 αβ+ cells (341+) | 3·4 | 2·6* | 1·5* | 2·0* |
| CD8 αα+ cells | 2·6 | 3·6* | 4·3* | 4·0* |
Double positive cells are shown as a percentage of total T cells in unprimed and primed animals. Animals were immunized i.p. with 0·5, 1 or 2 mg alum precipitated DNP–BSA in 0·5 ml PBS. Unprimed animals received 0·5 ml PBS i.p. One week after immunization with DNP–BSA, mesenteric LN cells were stained for TCRαβ (R73), CD4 (W3/25) and CD8α chain (OX8), CD8β chain (341) or a control mAb (OX21). Labelled cells were analysed by flow cytometry. TCRαβ+ cells were gated on and the percentage of double positive T cells was calculated. The percentage of cells expressing CD8αα alone was calculated by subtracting the percentage of 341 labelled cells from the percentage of OX8 labelled cells. Values given are the means from two independent experiments.
Denotes values which are three SD or more different from the value for unprimed animals (n = 20 000).
The frequency of double positive T cells decreases after thymectomy and with age
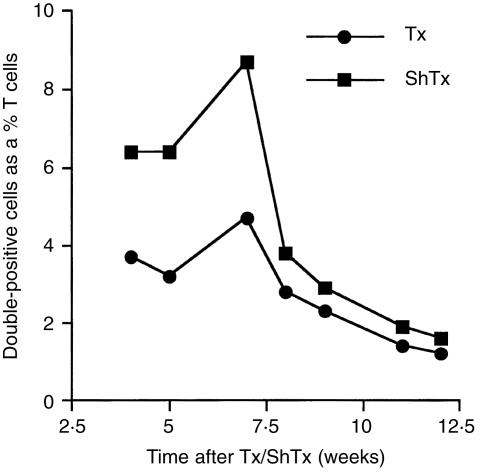
To assess the dependence of double positive T cells on the thymus and the effect of age on these cells, the frequency of double positive T cells in Tx and ShTx animals was compared at various time points after thymectomy. TDL from Tx and ShTx animals were triple labelled for TCRαβ (R73), CD4 (W3/25) and CD8 (OX8). TCRαβ+ cells were gated on and the expression of CD4 against CD8 was analysed by flow cytometry. Figure 4 shows that the frequency of double positive T cells in animals that had been Tx at 6 weeks of age was reduced for up to seven weeks after thymectomy, as compared to ShTx animals (3·7 versus 6·4% 4 weeks after thymectomy). Although, thymectomy did not eliminate this population. By 8 weeks after thymectomy the frequency of double positive T cells in ShTx and Tx animals was comparable, approximately 3% (although there were always slightly more in ShTx animals, see Fig. 4). From eight weeks onwards after thymectomy, there was a decrease in the frequency of double positive T cells with age in both Tx and ShTx animals, to approximately 1·4% in an 18-week-old animal (see Fig. 4).
Figure 4.
The frequency of double positive T cells is reduced after thymectomy and with age. TDL from Tx and ShTx animals were triple labelled for TCRαβ (R73), CD4 (W3/25) and CD8α chain (OX8). R73+ cells were gated on and the expression of W3/25 against OX8 was analysed by flow cytometry. Values in the graph represent double positive cells as a percentage of T cells.
DISCUSSION
These studies show that a peripheral double positive T-cell population exists in vivo in the rat. These cells are not an artefact of flow cytometric analysis, as they were also detected by confocal microscopy. As double positive T cells expressed a phenotype distinct from that of CD4 and CD8 single positive T cells (see below), it is unlikely that double positive T cells arose by CD4 single positive T cells adsorbing CD8 (or vice versa).
Rat double positive cells all expressed TCRαβ. They expressed levels of CD4 comparable to CD4 single positive T cells, but the level of CD8 expressed on double positive T cells varied whilst on CD8 single positive T cells it was homogeneous (this can be seen most clearly in Fig. 3). This observation may imply that in vivo double positive T cells are generated from CD4 single positive T cells that are induced to express varying levels of CD8, as described for human,4,34,35 swine6,21 and rat8 double positive T cells in vitro.
All rat double positive T cells expressed CD8α chain. Recently, a novel form of CD8α chain, not recognized by the G28 mAb, was reported on rat macrophages36 and mast cells.37 G28 mAb reactivity was examined in these present studies, to ascertain which form of the CD8α chain was expressed on double positive T cells. It would appear to be the same as that found on CD8 single positive T cells, as equivalent staining was seen with OX8 and G28 mAbs.
However, only around half of rat double positive T cells expressed the CD8β chain, so double positive T cells can be subdivided into cells that express the CD8αβ heterodimer (although not necessarily exclusive to some homodimer expression) and cells that express CD8α alone, presumably in the form of a homodimer.38 In these present studies, priming had reciprocal effects on CD8 αβ and αα expressing double positive T cells and it has been suggested that CD8 αβ and αα expressing T cells are functionally distinct.39 Functional differences between CD8 αβ and αα expressing double positive T cells could initially be assessed by comparing the phenotype of these two T-cell subsets. In the present study, this experiment was not done, but the phenotype of CD8 αβ and αα expressing double positive T cells combined was analysed.
Total double positive cells have a phenotype distinct from CD4 and CD8 single positive cells; they express increased levels of Thy-1, IL-2Rα, ICAM-1 and OX40. Thy-1 is a marker of recent thymic emigrants (RTE)40 and is up-regulated upon T-cell activation in the rat.26 However, RTE are TCRαβlo40 and double positive cells all expressed high levels of TCRαβ, suggesting that they are not RTE. ICAM-130,41 and OX4026 are up-regulated upon CD4+ T-cell activation and so the increased frequency of double positive cells expressing these markers is also indicative of an activated state. IL-2Rα is found on activated cells, but has also been implicated as a marker of cells with a regulatory capacity in both mice42,43 and rats (Dr L. Stephens, personal communication). However, on rat TDL, IL-2Rα is not a definitive marker for regulatory cells44 (Dr L. Stephens, personal communication). Taken together with the data on Thy-1, ICAM-1 and OX40 expression, it is likely that the increased frequency of IL-2Rα+ cells amongst the double positive population is suggestive of an activated phenotype rather than a regulatory one. In support of an activated phenotype, double positive cells were blast-like, as measured by forward and side scatter.
Despite indications from their phenotype, the frequency of double positive T cells was not increased in alum-precipitated DNP–BSA primed animals. Other studies have reported an increase in the frequency of double positive cells following in vivo priming of mice with Salmonella12 and of rats with alum-precipitated DNP–BGG, using Bordetella pertussis as an adjuvant.14 Perhaps a more potent activation stimulus than a hapten-carrier complex alone is required to observe a detectable increase in the frequency of double positive T cells. Animals primed with DNP–BGG make larger antibody responses than animals primed with DNP–BSA and including Bordetella pertussis as an adjuvant further increased the antigenicity of DNP–BGG (data not shown). This effect of pertussis toxin has previously been noted.45 Unfortunately, the frequency of double positive T cells in these animals was not examined.
Although priming did not increase the frequency of double positive T cells, it did alter the ratio of CD8αα : αβ expressing double positive T cells, leading to an increase in the proportion of cells expressing the α chain alone. Although it is difficult to draw conclusions from such small groups of animals, these findings correlate with in vitro observations in rat8,28 (E. Kenny, unpublished data) and human35 where, following T-cell activation, double positive T cells express the CD8α chain alone. This suggests that CD8αα and CD8αβ expressing double positive cells may be distinct T-cell subsets.
The frequency of double positive T cells in animals Tx at 6 weeks of age was reduced for up to 7 weeks after thymectomy, as compared to ShTx animals. These results correlate with in vitro observations where the frequency of T cells that can be induced to become double positive is decreased if the cells are purified from Tx donors (E. Kenny et al., manuscript in preparation). This suggests that double positive T cells are of thymic origin and that at least some have left the thymus not long ago. However, double positive cells have not left the thymus prematurely, nor have RTE mice as they are TCRαβhi.40 These observations are in contrast to those of Godden et al. who reported no effect of thymectomy on the number of rat double positive T cells.17 This discrepancy may be explained as they analysed double positive T cells participating in an alloresponse in cyclosporine-treated heart allografted animals, whilst in the present study, the global population of double positive T cells in untreated animals was analysed. Thymectomy did not eliminate rat double positive T cells, as described in humans;15 approximately half remained. This, and the fact that double positive T cells could still be detected in aged animals, suggests that not all double positive T cells have recently left the thymus (or indeed are necessarily of thymic origin).
By 8 weeks after thymectomy, the frequency of double positive T cells in Tx and ShTx animals was comparable, presumably due to the reduction in thymic output in older animals. From 8 weeks onwards after thymectomy, the frequency of double positive T cells varied inversely with age in both ShTx and Tx animals. These results correlate with in vitro observations where the frequency of T cells that can be induced to become double positive decreases when cells are purified from aged animals (E. Kenny et al. manuscript in preparation).
In summary, a population of double positive T cells exists in the periphery of normal rats. This population contains T cells that have recently left the thymus and activated cells, and it is comprised of two, possibly distinct, subsets: CD8 homodimer and CD8 heterodimer expressing cells.
Acknowledgments
We are very grateful to Mr Steve Simmonds and Mr Mike Puklavec for technical assistance. We would also like to thank Dr Leigh Stephens for reading the manuscript and for sharing unpublished data. This work was supported by grants from the Medical Research Council, the Wellcome Trust and the E. P. Abraham Fund. Emma Kenny has a studentship from the Medical Research Council and Ana Pombo is funded by The Royal Society.
Abbreviations
- B
biotinylated
- DAM
donkey anti-mouse
- LN
lymph node
- mAb
monoclonal antibody
- NMS
normal mouse serum
- NRS
normal rat serum
- RTE
recent thymic emigrant
- SA-QR
streptavidin quantum red
- ShTx
sham thymectomized
- TDL
thoracic duct lymphocytes
- Tx
thymectomized
REFERENCES
- 1.Hahn S, Gehri R, Erb P. Mechanism and biological significance of CD4-mediated cytotoxicit. Immunol Rev. 1995;146:57–79. doi: 10.1111/j.1600-065x.1995.tb00684.x. [DOI] [PubMed] [Google Scholar]
- 2.Le Gros G, Erard F. Non-cytotoxic, IL-4, IL-5, IL-10 producing CD8+ T cells: their activation and effector function. Curr Opin Immunol. 1994;6:453–7. doi: 10.1016/0952-7915(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 3.Blue ML, Daley JF, Levine H, Schlossman SF. Coexpression of T4 and T8 on peripheral blood T cells demonstrated by two-color fluorescence flow cytometr. J Immunol. 1985;134:2281–6. [PubMed] [Google Scholar]
- 4.Paliard X, Malefijt RW, de Vries JE, Spits H. Interleukin-4 mediates CD8 induction on human CD4+ T-cell clones. Nature. 1988;335:642–4. doi: 10.1038/335642a0. [DOI] [PubMed] [Google Scholar]
- 5.Saalmuller A, Reddehase MJ, Buhring HJ, Jonjic S, Koszinowski UH. Simultaneous expression of CD4 and CD8 antigens by a substantial proportion of resting porcine T lymphocytes. Eur J Immunol. 1987;17:1297–301. doi: 10.1002/eji.1830170912. [DOI] [PubMed] [Google Scholar]
- 6.Zuckermann FA, Husmann RJ. Functional and phenotypic analysis of porcine peripheral blood CD4/CD8 double-positive T cells. Immunology. 1996;87:500–12. [PMC free article] [PubMed] [Google Scholar]
- 7.Bevan DJ, Chisholm PM. Co-expression of CD4 and CD8 molecules and de novo expression of MHC class II antigens on activated rat T cells. Immunology. 1986;59:621–5. [PMC free article] [PubMed] [Google Scholar]
- 8.Ramirez F, McKnight AJ, Silva A, Mason D. Glucocorticoids induce the expression of CD8 alpha chains on concanavalin A-activated rat CD4+ T cells: induction is inhibited by rat recombinant interleukin 4. J Exp Med. 1992;176:1551–9. doi: 10.1084/jem.176.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortolani C, Forti E, Radin E, Cibin R, Cossarizza A. Cytofluorimetric identification of two populations of double positive (CD4+,CD8+) T lymphocytes in human peripheral bloo. Biochem Biophys Res Commun. 1993;191:601–9. doi: 10.1006/bbrc.1993.1260. [DOI] [PubMed] [Google Scholar]
- 10.Kay NE, Bone N, Hupke M, Dalmasso AP. Expansion of a lymphocyte population co-expressing T4 (CD4) and T8 (CD8) antigens in the peripheral blood of a normal adult male. Blood. 1990;75:2024–9. [PubMed] [Google Scholar]
- 11.Pescovitz MD, Sakopoulos AG, Gaddy JA, Husmann RJ, Zuckermann FA. Porcine peripheral blood CD4+/CD8+ dual expressing T-cells. Vet Immunol Immunopathol. 1994;43:53–62. doi: 10.1016/0165-2427(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 12.Tamauchi H, Sasahara T, Habu S. CD4+ CD8+ cells lacking self-Mls reactive T cells are induced in mesenteric lymph nodes of Salmonella enteritidis-infected mice. Immunol Lett. 1993;37:123–30. doi: 10.1016/0165-2478(93)90021-s. [DOI] [PubMed] [Google Scholar]
- 13.Ottenhoff TH, Elferink DG, Klatser PR, de Vries RR. Cloned suppressor T cells from a lepromatous leprosy patient suppress Mycobacterium leprae reactive helper T cells. Nature. 1986;322:462–4. doi: 10.1038/322462a0. [DOI] [PubMed] [Google Scholar]
- 14.Spickett GP, Mason DW. Demonstration of the stability of the membrane phenotype of T helper cells after priming and boosting with a hapten-carrier conjugate. Eur J Immunol. 1983;13:785–8. doi: 10.1002/eji.1830130916. [DOI] [PubMed] [Google Scholar]
- 15.Berrih S, Gaud C, Bach MA, Le Brigand H, Binet JP, Bach JF. Evaluation of T cell subsets in myasthenia gravis using anti-T cell monoclonal antibodies. Clin Exp Immunol. 1981;45:1–8. [PMC free article] [PubMed] [Google Scholar]
- 16.De Maria A, Malnati M, Moretta A, et al. CD3+4–8–WT31-(T cell receptor gamma+) cells and other unusual phenotypes are frequently detected among spontaneously interleukin 2- responsive T lymphocytes present in the joint fluid in juvenile rheumatoid arthritis. A clonal analysis. Eur J Immunol. 1987;17:1815–9. doi: 10.1002/eji.1830171221. [DOI] [PubMed] [Google Scholar]
- 17.Godden U, Herbert J, Stewart RD, RoSeries B. A novel cell type carrying both Th and Tc/s markers in the blood of cyclosporine-treated, allografted rats. Transplantation. 1985;39:624–8. doi: 10.1097/00007890-198506000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Burdick JF, Beschorner WE, Smith WJ, McGraw D, Bender WL, Williams GM, Solez K. Characteristics of early routine renal allograft biopsies. Transplantation. 1984;38:679–84. doi: 10.1097/00007890-198412000-00026. [DOI] [PubMed] [Google Scholar]
- 19.Kelly K, Pilarski L, Shortman K, Scollay R. CD4+ CD8+ cells are rare among in vitro activated mouse or human T lymphocytes. Cell Immunol. 1988;117:414–24. doi: 10.1016/0008-8749(88)90130-x. [DOI] [PubMed] [Google Scholar]
- 20.Bonomo A, Kehn PJ, Shevach EM. Premature escape of double-positive thymocytes to the periphery of young mice. Possible role in autoimmunit. J Immunol. 1994;152:1509–14. [PubMed] [Google Scholar]
- 21.Zuckermann FA, Gaskins HR. Distribution of porcine CD4/CD8 double-positive T lymphocytes in mucosa- associated lymphoid tissues. Immunology. 1996;87:493–9. [PMC free article] [PubMed] [Google Scholar]
- 22.Mason DW, Williams AF. The kinetics of antibody binding to membrane antigens in solution and at the cell surface. Biochem J. 1980;187:1–20. doi: 10.1042/bj1870001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brideau RJ, Carter PB, McMaster WR, Mason DW, Williams AF. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980;10:609–15. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- 24.Hsiung L, Barclay AN, Brandon MR, Sim E, Porter RR. Purification of human C3b inactivator by monoclonal-antibody affinity chromatography. Biochem J. 1982;203:293–8. doi: 10.1042/bj2030293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spickett GP, Brandon MR, Mason DW, Williams AF, Woollett GR. MRC OX-22, a monoclonal antibody that labels a new subset of T lymphocytes and reacts with the high molecular weight form of the leukocyte-common antigen. J Exp Med. 1983;158:795–810. doi: 10.1084/jem.158.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paterson DJ, Jefferies WA, Green JR, Brandon MR, Corthesy P, Puklavec M, Williams AF. Antigens of activated rat T lymphocytes including a molecule of 50 000 Mr detected only on CD4 positive T blasts. Mol Immunol. 1987;24:1281–90. doi: 10.1016/0161-5890(87)90122-2. [DOI] [PubMed] [Google Scholar]
- 27.Hunig T, Wallny HJ, Hartley JK, Lawetzky A, Tiefenthaler G. A monoclonal antibody to a constant determinant of the rat T cell antigen receptor that induces T cell activation. Differential reactivity with subsets of immature and mature T lymphocytes. J Exp Med. 1989;169:73–86. doi: 10.1084/jem.169.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torres-Nagel N, Kraus E, Brown MH, Tiefenthaler G, Mitnacht R, Williams AF, Hunig T. Differential thymus dependence of rat CD8 isoform expression. Eur J Immunol. 1992;22:2841–8. doi: 10.1002/eji.1830221113. [DOI] [PubMed] [Google Scholar]
- 29.Chambers WH, Vujanovic NL, DeLeo AB, Olszowy MW, Herberman RB, Hiserodt JC. Monoclonal antibody to a triggering structure expressed on rat natural killer cells and adherent lymphokine-activated killer cells. J Exp Med. 1989;169:1373–89. doi: 10.1084/jem.169.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamatani T, Miyasaka M. Identification of monoclonal antibodies reactive with the rat homolog of ICAM-1, and evidence for a differential involvement of ICAM-1 in the adherence of resting versus activated lymphocytes to high endothelial cells. Int Immunol. 1990;2:165–71. doi: 10.1093/intimm/2.2.165. [DOI] [PubMed] [Google Scholar]
- 31.Williams AF, Galfre G, Milstein C. Analysis of cell surfaces by xenogeneic myeloma-hybrid antibodies: differentiation antigens of rat lymphocytes. Cell. 1977;12:663–73. doi: 10.1016/0092-8674(77)90266-5. [DOI] [PubMed] [Google Scholar]
- 32.Little J, Eisen H. Preparation of immunogenic 2,4-dinitrophenyl and 2,4,6-trinitrophenyl proteins. Meth Immunol Immunochem. 1967;1:128–33. [Google Scholar]
- 33.Gowans JL. The recirculation of lymphocytes from blood to lymph in the rat. J Physiol. 1959;146:54–69. doi: 10.1113/jphysiol.1959.sp006177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blue ML, Daley JF, Levine H, Craig KA, Schlossman SF. Biosynthesis and surface expression of T8 by peripheral blood T4+ cells in vitro. J Immunol. 1986;137:1202–7. [PubMed] [Google Scholar]
- 35.Hori T, Paliard X, de Waal Malefijt R, Ranes M, Spits H. Comparative analysis of CD8 expressed on mature CD4+ CD8+ T cell clones cultured with IL-4 and that on CD8+ T cell clones: implication for functional significance of CD8 beta. Int Immunol. 1991;3:737–41. doi: 10.1093/intimm/3.7.737. [DOI] [PubMed] [Google Scholar]
- 36.Hirji N, Lin TJ, Befus AD. A novel CD8 molecule expressed by alveolar and peritoneal macrophages stimulates nitric oxide production. J Immunol. 1997;158:1833–40. [PubMed] [Google Scholar]
- 37.Lin TJ, Hirji N, Nohara O, Stenton GR, Gilchrist M, Befus AD. Mast cells express novel CD8 molecules that selectively modulate mediator secretion. J Immunol. 1998;161:6265–72. [PubMed] [Google Scholar]
- 38.Littman DR, Thomas Y, Maddon PJ, Chess L, Axel R. The isolation and sequence of the gene encoding T8: a molecule defining functional classes of T lymphocytes. Cell. 1985;40:237–46. doi: 10.1016/0092-8674(85)90138-2. [DOI] [PubMed] [Google Scholar]
- 39.Gelfanov V, Gelfanova V, Lai YG, Liao NS. Activated alpha beta-CD8+, but not alpha alpha-CD8+, TCR-alpha beta+ murine intestinal intraepithelial lymphocytes can mediate perforin- based cytotoxicity, whereas both subsets are active in Fas-based cytotoxicity. J Immunol. 1996;156:35–41. [PubMed] [Google Scholar]
- 40.Hosseinzadeh H, Goldschneider I. Recent thymic emigrants in the rat express a unique antigenic phenotype and undergo post-thymic maturation in peripheral lymphoid tissues. J Immunol. 1993;150:1670–9. [PubMed] [Google Scholar]
- 41.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–34. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 42.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 43.Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+ CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212–8. [PubMed] [Google Scholar]
- 44.Fowell D, Mason D. Evidence that the T cell repertoire of normal rats contains cells with the potential to cause diabetes. Characterization of the CD4+ T cell subset that inhibits this autoimmune potential. J Exp Med. 1993;177:627–36. doi: 10.1084/jem.177.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryan M, McCarthy L, Rappuoli R, Mahon BP, Mills KH. Pertussis toxin potentiates Th1 and Th2 responses to co-injected antigen: adjuvant action is associated with enhanced regulatory cytokine production and expression of the co-stimulatory molecules B7-1, B7-2 and CD28. Int Immunol. 1998;10:651–62. doi: 10.1093/intimm/10.5.651. [DOI] [PubMed] [Google Scholar]