Abstract
During eosinophil (EOS) accumulation at sites of allergic inflammation, an initial step is the binding of EOS to adhesion molecules expressed on vascular endothelial cells (EC). We have previously observed that adhesion of peripheral blood EOS to recombinant human vascular cell adhesion molecule-1 (rh-VCAM-1) stimulates the respiratory burst of EOS. Although the biological consequence of this activation remains to be elucidated, reactive oxygen species such as hydrogen peroxide (H2O2) may modify the adhesive property of EOS. In the present study, we examined whether H2O2 modifies the adhesive property of EOS. EOS were isolated from the peripheral blood of healthy subjects. Adhesion of the EOS to paraformaldehyde-fixed human umbilical vein EC (HUVEC), stimulated or not stimulated with tumour necrosis factor-α (TNF-α; 100 pm for 24 hr), was examined in the presence or absence of H2O2. H2O2 significantly enhanced adhesion of EOS to both resting and TNF-α-stimulated fixed HUVEC (P < 0·01, respectively). Such enhancing effects were inhibited by anti-β2 integrin antibody or anti-CD11b antibody, but not by anti-CD11a or anti-α4 integrin antibody. H2O2 also enhanced EOS adhesion to rh-intracellular cell adhesion molecule-1 (ICAM-1) but not to rh-VCAM-1. Finally, H2O2 enhanced the expression of both CD11b and CD18 on EOS. These results indicate that H2O2 directly augments the adhesive property of EOS through β2 integrin.
Introduction
Eosinophils (EOS) preferentially accumulate at sites of allergic inflammation and because of this probably play crucial roles in the pathophysiology of diseases, such as asthma, through the release of a variety of inflammatory mediators.1,2 For EOS to participate in allergic inflammation, it is necessary that they migrate from the peripheral circulation into the tissues. An initial step of this process is the interaction of circulating EOS with adhesion proteins expressed on the surface of vascular endothelial cells (EC). Accumulating evidences have established that EOS integrin adhesion molecules such as α4 integrins, including α4β1 (very late activation antigen-4 [VLA-4], CD49d/CD29), and β2 integrins, including αLβ2 (lymphocyte function-associated antigen-1 [LFA-1], CD11a/CD18) and αMβ2 (Mac-1, CD11b/CD18), play important roles in the adhesion to, and the transmigration through, EC.3,4 Vascular cell adhesion molecule (VCAM)-1, a counter ligand for α4β1, is a potent inducer of spontaneous adhesion of blood EOS in vitro.5,6 Administration of anti-α4β1 antibodies (Ab) in vivo inhibits the accumulation of EOS induced by soluble inflammatory mediators and the antigen-induced EOS infiltration into guinea-pig skin or mouse trachea.7,8 Monoclonal Ab (mAb) against intracellular cell adhesion molecule-1 (ICAM-1), a ligand for β2 integrins, also inhibited EOS airway infiltration in a primate model of asthma.9 Although recombinant human (rh)-ICAM-1 requires second signals, such as granulocyte–macrophage colony-stimulating factor (GM-CSF) or Mn2+, for inducing adhesion of blood EOS,10–12 it has been shown that the β2 integrin/ICAM-1 pathway is essentially involved in EOS transmigration across EC monolayers in vitro.13,14
We have previously reported that adhesion of EOS to rh-VCAM-1 activates a respiratory burst of EOS.6 This suggests that the interaction between EOS α4 integrin and endothelial VCAM-1 is an important step, not only for selective EOS recruitment into the tissue but also as an initial action in the activation of EOS effector functions. The biological consequences of the activation of such a specific effector function remains unknown; however, it is possible that the released oxygen species modify either the morphological or functional status of EC, such as the permeability, adhesion molecule expression and adhesiveness to leucocytes.15–17 Oxygen species also alter the functional status of leucocytes. For example, hydrogen peroxide (H2O2) promotes adhesion of neutrophils18 and monoblastoid U-937 cells19 via CD11b/CD18 molecules. Here we report that H2O2 enhances EOS adhesion to both resting and tumour necrosis factor-α (TNF-α)-stimulated EC through the enhanced expression and activation of β2 integrin of EOS.
Materials and methods
Reagents
Percoll was purchased from Pharmacia (Uppsala, Sweden). Hanks' balanced salt solution (HBSS), newborn calf serum (NCS), fetal calf serum (FCS), trypsin–EDTA, l-glutamine and penicillin–streptomycin were obtained from Life Technologies (Grand Island, NY). Anti-CD16 mAb-coated magnetic microbeads were obtained from Miltenyl Biotec (Auburn, CA). H2O2 was obtained from Wako Pure Medical Co. (Osaka, Japan). Human umbilical vein endothelial cells (HUVEC) and endothelial cell basal medium (EBM) medium were obtained from Clonetics (Palo Alto, CA). rh-TNF-α, rh-VCAM-1 and rh-ICAM-1 were obtained from R & D Systems (Minneapolis, MN). Other reagents were purchased from Sigma (St. Louis, MO) unless stated otherwise.
Separation of EOS
EOS were isolated from peripheral blood specimens, of normal volunteers, that contained less than 5% EOS in the total leucocytes. Subjects ranged in age from 26 to 39 years, and gender distribution was equal. Isolation of EOS was performed by negative immunomagnetic bead selection, as previously described.14 Briefly, heparinized blood was diluted with HBSS without Ca2+ and centrifuged for 20 min at 700 g over 1·090 g/ml Percoll. Plasma, the mononuclear cell band and Percoll were removed, and the red blood cells in the pellet were lysed by hypotonic shock. The resulting granulocytes were washed with HBSS (chilled to 4°) supplemented with 2% NCS (HBSS/NCS), then incubated with anti-CD16 Ab-coated magnetic beads for 40 min at 4°. The cells were filtered through a steel wool column in a magnetic field (Miltenyl Biotec) to remove neutrophils bound to magnetic beads. CD16-negative EOS (> 98% pure and > 99% viable) were collected, washed and resuspended in HBSS supplemented with 5% FCS (HBSS/FCS).
Preparation of HUVEC
HUVEC were cultured on type-IV collagen-coated tissue-culture flasks. When confluent, HUVEC were passaged into collagen-coated 96-well-tissue culture plates, and either medium alone or TNF-α (at 100 pm) was added and culture continued for 24 hr in 5% CO2 at 37°. Following culture, the incubated mixture was decanted and the HUVEC were washed three times with HBSS. HUVEC were fixed with 100 µl of 1% paraformaldehyde in phosphate-buffered saline (PBS) at ambient temperature for 15 min to block the generation of mediators. After washing these cells three times with HBSS, 200 µl of 1% glycine in HBSS was added and incubated at ambient temperature for 1 hr to quench the residual paraformaldehyde. Plates were then decanted and washed three times with HBSS before use.
EOS adhesion assay
EOS adhesion was assessed as residual EOS peroxidase (EPO) activity of adherent EOS, as previously described.6,10,14 Briefly, EOS (100 µl of 1 × 105 cells/ml in HBSS/FCS) were placed onto HUVEC monolayers or rh-adhesion molecule-coated plates, in the presence or absence of an activator, and incubated for 30 min at 37°. After five washes with HBSS (preincubated to 37°), 100 µl of HBSS/FCS was added to the reaction wells. As standards, 100 µl of serially diluted cell suspension (1 × 103, 3 × 103, 1 × 104, 3 × 104 and 1 × 105 cells/ml) was added to empty wells. EPO substrate (Tris buffer, pH 8·0, containing 1 mm o-phenylenediamine, 1 mm H2O2 and 0·1% Triton-X-100) was then added to all of the wells. After a 30-min incubation at room temperature, 50 µl of 4-m H2SO4 was added to stop the reaction, and the absorbance at 490 nm was measured. Percent EOS adhesion was calculated from the log dose–response curve. EOS viability after the incubation exceeded 98%, as assessed by using Trypan Blue dye exclusion.
Expression of CD11b and CD18 on EOS
The expression of CD11b and CD18 on EOS was examined by flow cytometric analysis. EOS were exposed to either 1 µm H2O2 or buffer (HBSS/5% FCS) alone for 30 min at 37°. The cells were washed three times and resuspended in PBS (chilled to 4°) supplemented with 2% bovine serum albumin (BSA) and 0·2% sodium azide (fluorescence-activated cell sorter [FACS] buffer). Cells (1 × 105/100 µl) were incubated with fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD18 (clone MHM23, immunoglobulin G1 [IgG1]; Dako A/S, Glostup, Denmark) and/or phycoerythrin (PE)-conjugated mouse anti-human C3bi (CD11b) (clone 2LPM19c, IgG1; Dako A/S) on ice for 30 min. PE- and FITC-conjugated mouse IgG1 (Dako A/S) were used as isotype-matched controls. Cells were then washed and resuspended in FACS buffer. Mean fluorescence was measured on at least 10 000 events using a fluorescence-activated cell sorter (FACScan; Becton-Dickinson, San Jose, CA, USA). Relative mean fluorescence was determined by subtraction of values for the IgG1 isotype-matched control.
Statistics
Data are presented as mean ± SEM. For statistical analysis, the Student's t-test was used for paired comparisons and analysis of variance (anova) for repeated measures; Scheffe constants were used for comparison of more than two variables to determine a significance. A P-value of less than 0·05 was considered significant.
Results
Effect of H2O2 on EOS adhesion
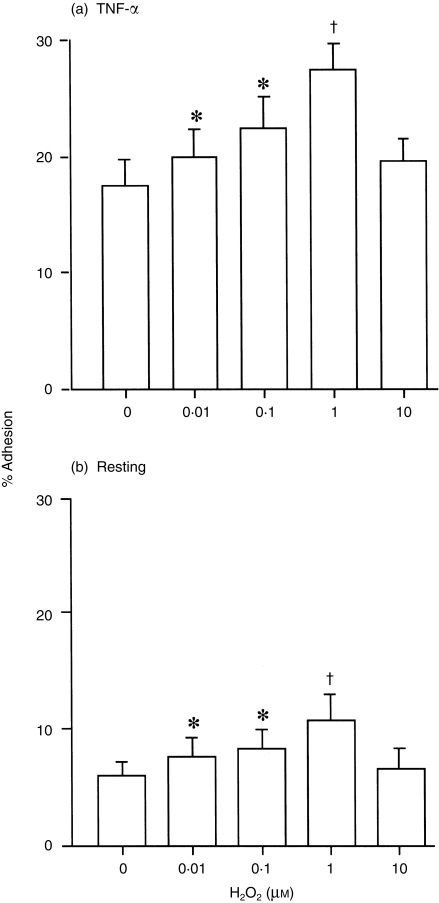
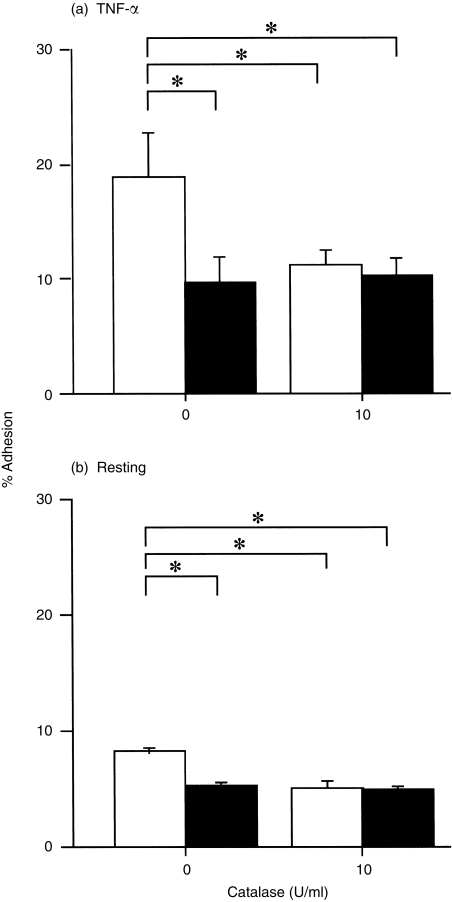
Initial experiments were conducted to determine whether H2O2 modifies adhesion of EOS to paraformaldehyde-fixed HUVEC. After 30 min of incubation, 0·01–1 µm H2O2 significantly enhanced adhesion of EOS to TNF-α-stimulated (100 pm, 24 hr) HUVEC (0·01 µm, P < 0·05; 0·1 µm, P < 0·05; 1 µm, P < 0·01; n = 8, respectively; Fig. 1a). Similarly, H2O2 promoted adhesion of EOS to resting HUVEC (0·01 µm, P < 0·05; 0·1 µm, P < 0·05; 1 µm, P < 0·01; n = 8, respectively; Fig. 1b). In both experiments the maximum effect of H2O2 was obtained at 1 µm (% adhesion: TNF-α-stimulated HUVEC, 27·4 ± 2·6%; resting HUVEC 10·8 ± 2·5%) and was comparable to the maximum effect of N-formyl-methionyl-leucyl-phenylalanine (FMLP, 1 µm: TNF-α-stimulated HUVEC, 27·0 ± 2·2%; resting HUVEC, 10·9 ± 3·3%, n = 5). Although 10 µm H2O2 did not augment EOS adhesion, the cell viability exceeded 98% under this experimental condition. Moreover, 1–10 µm H2O2 did not modify the pH of the assay buffer (data not shown). To confirm the specificity of the H2O2 effect, EOS were exposed to 1 µm H2O2 in the presence or absence of catalase (from Aspergillus nigar; Sigma), which rapidly degrades H2O2. The adhesion to both TNF-α-stimulated and resting HUVEC was then determined: EOS adhesion augmented by 1 µm H2O2 was blocked by 10 U/ml catalase (% inhibition of adhesion to TNF-α-stimulated HUVEC, 90·7 ± 3·4%, Fig. 2a; % inhibition of adhesion to resting HUVEC, 97·2 ± 2·6%, n = 5, respectively, Fig. 2b). H2O2 at 1 µm also augmented eosinophil adhesion to unfixed TNF-α-stimulated and resting HUVEC, and adhesion to both was blocked by 10 U/ml catalase (data not shown).
Figure 1.
Effect of H2O2 on eosinophil (EOS) adhesion to tumour necrosis factor-α (TNF-α)-stimulated (a) and resting (b) fixed, human umbilical vein endothelial cells (HUVEC). EOS were incubated in the presence or absence of H2O2 for 30 min at 37°. *P < 0·05, †P < 0·01 versus control (no H2O2), n = 8, respectively.
Figure 2.
Effect of 10 U/ml of catalase on eosinophil (EOS) adhesion to tumour necrosis factor-α (TNF-α)-stimulated (a) and resting (b) fixed, human umbilical vein endothelial cells (HUVEC). Open bars represent adhesion of EOS in the presence of 1 µm H2O2. Closed bars represent adhesion of EOS in the absence of H2O2. *P < 0·01, n = 5, respectively.
To examine whether H2O2 directly affects the adhesive property of EOS, EOS adhesion to 1% BSA-coated microassay plates was examined: 1 µm H2O2 augmented the adhesion (buffer control, 16·2 ± 2·5% versus H2O2, 25·1 ± 2·3%; P = 0·001, n = 6).
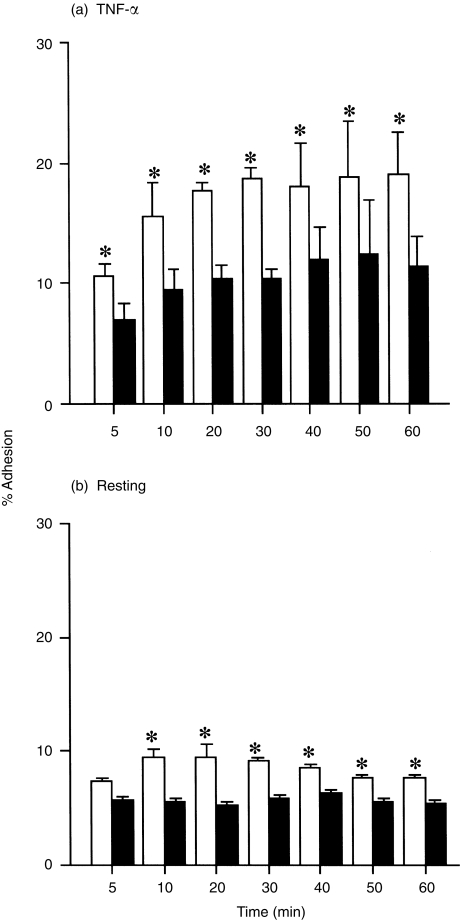
The reaction kinetics of the H2O2 effect on adhesion of EOS was examined using TNF-α-stimulated HUVEC. The H2O2 effect was observed at 5 min after addition of H2O2 and lasted for at least 60 min (P < 0·05 at each time-point examined, n = 4; Fig. 3a). The H2O2 effect on adhesion of EOS to the resting HUVEC was observed at 10 min after addition of H2O2 and lasted for 60 min (P < 0·05 at each time-point examined except 5 min, n = 4; Fig. 3b).
Figure 3.
Kinetics of the H2O2 effect on adhesion of eosinophils (EOS) to tumour necrosis factor-α (TNF-α)-stimulated-(a) (n = 4) and resting (b) (n = 4) human umbilical vein endothelial cells (HUVEC). Open bars represent adhesion of EOS in the presence of 1 µm H2O2. Closed bars represent adhesion of EOS in the absence of H2O2. *P < 0·05.
Effect of anti-integrin mAbs on the H2O2-induced adhesion of EOS
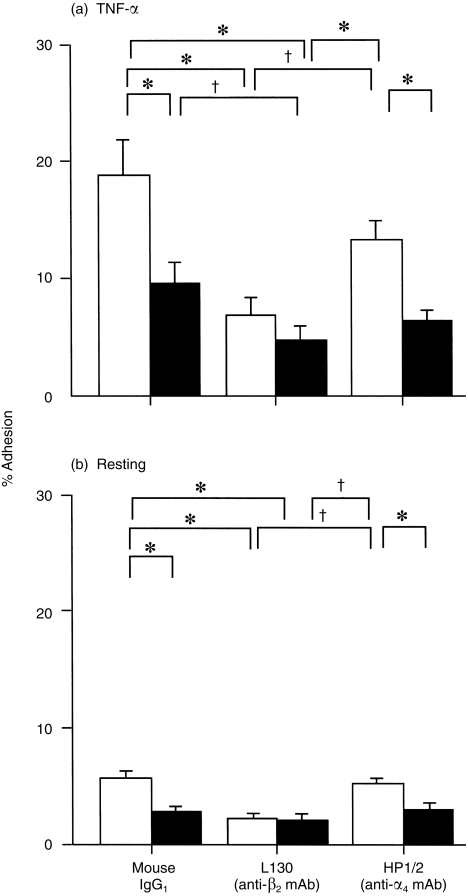
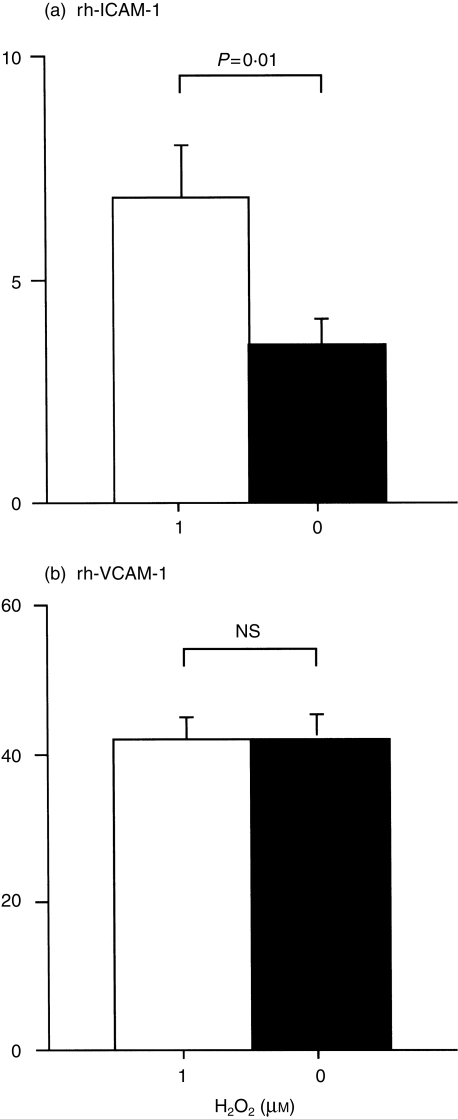
To identify the EOS integrin(s) involved in the H2O2 effect, EOS were pretreated with either anti-α4 integrin mAb (clone HP1/2, mouse IgG1, 3 µg/ml; Cosmo Bio., Tokyo, Japan), anti-β2 integrin mAb (clone L130, mouse IgG1, 3 µg/ml; Becton-Dickinson) or an isotype-matched control mouse IgG1 (3 µg/ml; Cappel, Aurora, Ohio, USA) at ambient temperature for 15 min, and then EOS adhesion was examined. H2O2 at 1 µm significantly enhanced the adhesion of EOS to TNF-α-stimulated HUVEC in the presence of mouse IgG1 or anti-α4 Ab (P < 0·01, n = 5, respectively, Fig. 4a). In contrast, the H2O2 effect was blocked by anti-β2 mAb (Fig. 4a). Similarly, H2O2 enhanced the EOS adhesion to resting HUVEC in the presence of mouse IgG1 or anti-α4 Ab (P < 0·01, n = 3, respectively), but the effect was blocked by anti-β2 Ab (Fig. 4b). To further confirm whether H2O2 enhanced the adhesion via β2 integrin, adhesion of EOS to immobilized rh-ICAM-1, a ligand for β2 integrin, or to rh-VCAM-1, a ligand for α4 integrin, was examined: 1 µm H2O2 enhanced adhesion of EOS only to rh-ICAM-1 (rh-ICAM-1, 3·5 ± 0·7% in medium alone versus 6·8 ± 1·3% in medium + H2O2, P = 0·01, Fig. 5a; rh-VCAM-1, 41·8 ± 3·6% in medium alone versus 41·6 ± 4·0% in medium + H2O2, P = NS, n = 5, Fig. 5b).
Figure 4.
Effects of anti-β2 integrin monoclonal antibody (mAb) (L130) and anti-α4 integrin mAb (HP1/2) on adhesion of eosinophils (EOS) to tumour necrosis factor-α (TNF-α)-stimulated (a) and resting (b) human umbilical vein endothelial cells (HUVEC). Mouse immunoglobulin G1 (IgG1) is an isotype-matched control. Open bars represent adhesion of EOS in the presence of 1 µm H2O2. Closed bars represent adhesion of EOS in the absence of H2O2. *P < 0·01, †P < 0·05, n = 5, respectively.
Figure 5.
Effect of 1 µm H2O2 on adhesion of eosinophils (EOS) to recombinant human intracellular cell adhesion molecule-1 (rh-ICAM-1) (a) and rh-vascular cell adhesion molecule-1 (VCAM-1) (b). Open bars represent adhesion in the presence of 1 µm H2O2. Closed bars represent adhesion in the absence of H2O2. n = 5, respectively.
To further identify EOS β2 integrin(s) involved in the H2O2 effect, EOS were pretreated with either anti-CD11a mAb (clone G25.2, mouse IgG2a, 5 µg/ml; Becton-Dickinson), anti-CD11b mAb (clone D12, mouse IgG2a, 5 µg/ml; Becton-Dickinson), or isotype-control mouse IgG2a (5 µg/ml; Cappel), and then EOS adhesion was examined in the presence or absence of 1 µm H2O2. The EOS adhesion augmented by H2O2 was partially inhibited by anti-CD11b antibody (% inhibition: adhesion to TNF-α-stimulated HUVEC, 60·3 ± 18·1%; adhesion to resting HUVEC, 68·6 ± 16·0%; P < 0·01, n = 5, respectively; results not shown). On the other hand, neither anti-CD11a antibody nor mouse IgG2a modified the augmented EOS adhesion to TNF-α-stimulated or resting HUVEC (data not shown). The effect of the combination of anti-CD11a and anti-CD11b did not differ from anti-CD11b antibody alone (data not shown).
Effect of H2O2 on EOS expression of CD11b and CD18
To examine whether H2O2 modifies the number of CD11b or CD18 molecules on EOS, the expression of those molecules was determined by flow cytometric analysis. Following stimulation with 1 µm H2O2, the expression of both CD11b and CD18 was enhanced (mean fluorescence index [MFI]: CD11b, 204·0 ± 26·3, control, versus 236·0 ± 24·0, H2O2, P < 0·05; CD18, 48·0 ± 8·3, control, versus 56·0 ± 6·3, H2O2, P < 0·01, n = 3, respectively; results not shown).
Role of H2O2 as an autocrine mediator for EOS adhesion
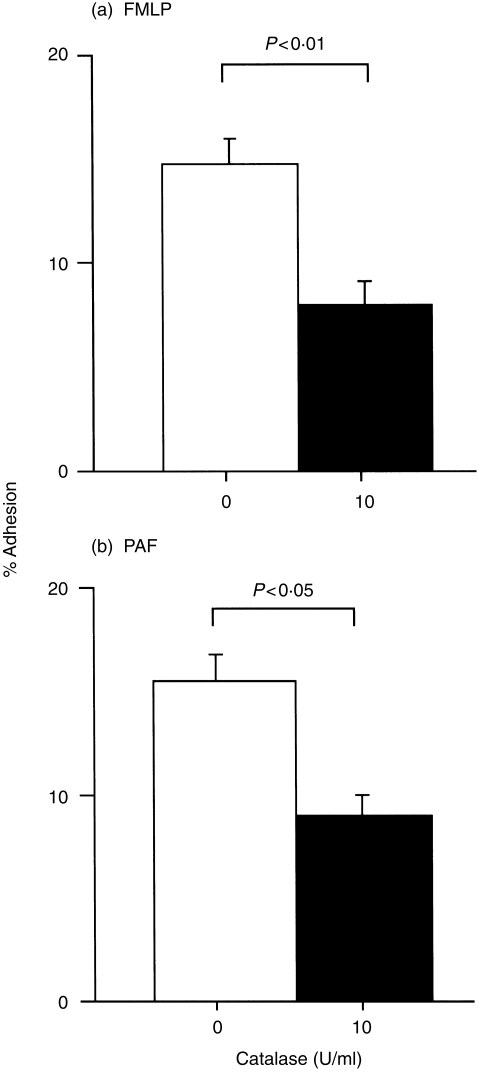
Because EOS are capable of generating radical oxygen species in response to a variety of inflammatory mediators, endogenous H2O2 may play roles in the development of stimulus-dependent adhesion of EOS in an autocrine mechanism. To examine this possibility, adhesion of EOS to paraformaldehyde-fixed resting HUVEC in response to GM-CSF, phorbol myristate acetate (PMA), FMLP, or platelet-activating factor (PAF), was examined in the presence or absense of 10 U/ml of catalase. Catalase did not modify spontaneous-, 100 pm GM-CSF-induced, or 1 ng/ml phorbol 12-myristate 13-acetate (PMA)-induced adhesion (control versus catalase: spontaneous, 5·2 ± 0·9% versus 5·1 ± 0·8%; GM-CSF, 12·2 ± 1·4% versus 12·5 ± 2·8%; PMA, 21·6 ± 3·7% versus 22·2 ± 4·2%; n = 5, P = NS, respectively; results not shown). In contrast, EOS adhesion induced by both FMLP and PAF (both at 1 µm) was partially inhibited by 10 U/ml of catalase (control versus catalase: FMLP, 14·8 ± 1·4% versus 8·0 ± 1·3%, P < 0·01, Fig. 6a; PAF, 15·4 ± 1·6% versus 9·0 ± 1·4%, P < 0·05, n = 5, Fig. 6b, respectively).
Figure 6.
Effect of 10 U/ml catalase on adhesion of eosinophils (EOS) induced by N-formyl-methionyl-leucyl-phenylalanine (FMLP) (a) and platelet-activating factor (PAF) (b) (both at 1 µm). Open bars represent adhesion of EOS in the presence of 1 µm H2O2. Closed bars represent adhesion of EOS in the absence of H2O2. n = 5, respectively.
Discussion
Our findings extend earlier observations concerning interaction between EOS and endothelial adhesion molecules,3,4,6,10 and suggest the potentially important biological consequence of the EOS respiratory burst during this process. First, nanomolar concentrations of H2O2 induced EOS adhesion to resting EC. Second, H2O2 also promoted adhesion of EOS to EC activated with a proinflammatory cytokine, TNF-α. The H2O2 effects appear to involve EOS β2 integrins, particularly CD11b/CD18. The counter ligands on HUVEC for the enhanced adhesion are postulated to be ICAMs, because CD11b/CD18 binds to ICAM-1, ICAM-2, and ICAM-3.3,4 Indeed, H2O2 augmented adhesion of EOS to rh-ICAM-1. Although EOS α4 integrin(s) could also interact with endothelial adhesion proteins such as VCAM-1, the anti-α4 mAb did not affect the H2O2 -induced EOS adhesion. Furthermore, H2O2 did not enhance adhesion of EOS to rh-VCAM-1, indicating that α4 integrin is not involved in the H2O2-induced adhesion. Finally, we have provided evidence that H2O2 directly enhances eosinophil expression of CD11b and CD18. Although both the number and functional state of integrins can be regulated by a variety of mediators3,4,20,21 our data suggest that the increased expressions of these molecules are, at least in part, involved in the enhancing effects for EOS adhesion. Intracellular signalling mechanisms associated with the H2O2 effect were not evaluated in this study, but should be clarified in future work.
At the sites of allergic inflammation, multiple inflammatory cells, including EOS and macrophages, may generate oxygen species.22,23 It has been reported that bronchoalveolar lavage (BAL) cells obtained following an antigen challenge generated 8–10 µm superoxide anion.23 EOS obtained from BAL following a segmental antigen challenge possess a greater ability to generate superoxide anion, as well as to induce adhesion and expression of CD11b, than that shown by EOS in peripheral circulation.24 H2O2 can be detected at micromolar concentrations in normal human sera.25 Although the precise concentration of oxygen species at the inflammatory sites remains unknown, these findings suggest that it is possible to achieve an H2O2 concentration similar to that used in this study at an allergic inflammation site.
As EOS are capable of generating radical oxygen species in response to a wide range of inflammatory mediators, EOS-derived H2O2 may play roles in the development of adhesive property of the cells. In this context, we observed that both FMLP- and PAF-induced EOS adhesion was partially inhibited by catalase while such an inhibitory effect was not observed when GM-CSF or PMA was the stimulant. These results suggest that EOS-derived H2O2 may be involved in the development of EOS adhesion as an autocrine/paracrine mediator in vivo when certain inflammatory mediators, such as FMLP or PAF, are involved.
Our observations agree with earlier work on other types of cells. Skogkund et al. reported that 30–300 µm H2O2 induced the adhesion of monoblastoid U-937 cells to plastic, and that this adhesion was inhibited by mAb against CD11b and CD18, but not by mAb against CD11a or CD11c.19 Fraticelli et al. reported that H2O2 caused the up-regulation of CD11b and CD18 on neutrophils concurrently with a shedding of l-selectin and increased the adherence of neutrophils to EC.18 These reports and our present observations suggest that H2O2 can induce a direct proadhesive effect on certain types of leucocytes, including EOS, via enhanced expression of CD11b/CD18.
Our observations raise possibilities as to the biological significance of the expression and function of EOS β2 integrin during interaction with H2O2. When activated, a variety of inflammatory cells, including EOS, are capable of generating radical oxygen species at sites of allergic inflammation, and therefore EOS will probably be exposed to H2O2. Meanwhile, H2O2 enhances the expression of ICAM-1 on EC and increases the ability of EC to potentiate leucocyte adhesion.15,16 Thus, H2O2 alters the adhesive property of both EOS and EC, and thereby augments the interaction between these two kinds of cells in a β2 integrin-dependent manner. The effect of H2O2 on EOS β2 integrin may also modify the subsequent transmigration of EOS across EC, because it has been well documented that the β2 integrin/ICAM-1 pathway is crucial in this process.13,14 Thus, H2O2 may modify the adhesion and transendothelial migration of EOS, and contribute to the phenotypic change of EOS in the airway. These effects would contribute to the eventual manifestations of inflammation in allergic diseases such as asthma.
Acknowledgments
The authors thank Ms Akemi Yokote for her excellent technical assistance.
Glossary
Abbreviations
- EC
endothelial cells
- EOS
eosinophils
- EPO
eosinophil peroxidase
- FMLP
N-formyl-methionyl-leucyl-phenylalanine
- H2O2
hydrogen peroxidase
- HUVEC
human umbilical vein endothelial cells
- ICAM-1
intracellular cell adhesion molecule-1
- PAF
platelet-activating factor
- VCAM-1
vascular cell adhesion molecule-1
References
- 1.Gleich GJ, Kita H, Adolphson CR. Eosinophils. In: Frank SM, Austen KF, Claman HN, Unanue ER, editors. Samter's Immunological Diseases. 5. Boston, MA: Little, Brown; 1994. p. 205. [Google Scholar]
- 2.Weller PF. Human eosinophils. J Allergy Clin Immunol. 1997;100:283. doi: 10.1016/s0091-6749(97)70237-9. [DOI] [PubMed] [Google Scholar]
- 3.Bochner BS. Cellular adhesion and its antagonism. J Allergy Clin Immunol. 1997;100:581. doi: 10.1016/s0091-6749(97)70158-1. [DOI] [PubMed] [Google Scholar]
- 4.Wardlaw AJ. Molecular basis for selective eosinophil trafficking in asthma: a multistep paradigm. J Allergy Clin Immunol. 1999;104:917. doi: 10.1016/s0091-6749(99)70069-2. [DOI] [PubMed] [Google Scholar]
- 5.Schleimer RP, Sterbinsky SA, Kaiser J, et al. IL-4 induces adherence of human eosinophils and basophils but not neutrophils to endothelium: association with expression of VCAM-1. J Immunol. 1992;148:1086. [PubMed] [Google Scholar]
- 6.Nagata M, Sedgwick JB, Bates ME, Kita H, Busse WW. Eosinophil adhesion to vascular cell adhesion molecule-1 activates superoxide anion generation. J Immunol. 1995;155:2194. [PubMed] [Google Scholar]
- 7.Weg VB, Williams TJ, Lobb RR, Nourshargh S. A monoclonal antibody recognizing very late activation antigen-4 inhibits eosinophil accumulation in vivo. J Exp Med. 1993;177:561. doi: 10.1084/jem.177.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakajima H, Sano H, Nishimura T, Yoshida S, Iwamoto I. Role of vascular cell adhesion molecule 1/very late activation antigen 4 and intracellular adhesion molecule 1/lymphocyte function-associated antigen 1 interactions in antigen-induced eosinophil and T cell recruitment into the tissue. J Exp Med. 1994;179:1145. doi: 10.1084/jem.179.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wegner CD, Gundel RH, Reilly P, Haynes N, Letts LG, Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) in the pathogenesis of asthma. Science. 1990;247:456. doi: 10.1126/science.1967851. [DOI] [PubMed] [Google Scholar]
- 10.Nagata M, Sedgwick JB, Kita H, Busse WW. Granulocyte–macrophage colony-stimulating factor augments ICAM-1 and VCAM-1 activation of eosinophil function. Am J Respir Cell Mol Biol. 1998;19:158. doi: 10.1165/ajrcmb.19.1.3001. [DOI] [PubMed] [Google Scholar]
- 11.Godding V, Stark JM, Sedgwick JB, Busse WW. Adhesion of activated eosinophils to respiratory epithelial cells is enhanced by tumor necrosis factor-α and interleukin-1β. Am J Respir Cell Mol Biol. 1995;13:555. doi: 10.1165/ajrcmb.13.5.7576691. [DOI] [PubMed] [Google Scholar]
- 12.Håkansson L. Measurement of neutrophils and eosinophils adhesion to E-selectin, VCAM-1, and ICAM-1 by the use of transfected fibroblast cell lines. J Immunol Methods. 1994;176:53. doi: 10.1016/0022-1759(94)90350-6. [DOI] [PubMed] [Google Scholar]
- 13.Ebisawa M, Bochner BS, Georas SN, Schleimer RP. Eosinophil transendothelial migration induced by cytokines. I. Role of endothelial and eosinophil adhesion molecules in IL-1β-induced transendothelial migration. J Immunol. 1992;149:4021. [PubMed] [Google Scholar]
- 14.Yamamoto H, Sedgwick JB, Busse WW. Differential regulation of eosinophil adhesion and transmigration by pulmonary micro-vascular endothelial cells. J Immunol. 1998;161:971. [PubMed] [Google Scholar]
- 15.Bradley JR, Johnson DR, Pober JS. Endothelial activation by hydrogen peroxide. Selective increases of intercellular adhesion molecule-1 and major histocompatibility complex class I. Am J Pathol. 1993;142:1598. [PMC free article] [PubMed] [Google Scholar]
- 16.Sellak H, Franzini E, Hakim J, Pasquire C. Reactive oxygen species rapidly increase endothelial ICAM-1 ability to bind neutrophils without detectable upregulation. Blood. 1994;83:2669. [PubMed] [Google Scholar]
- 17.Siflinger-Birnboim A, Lum H, Del Vecchio PJ, Malik AB. Involvement of Ca2+ in the H2O2-induced increase in endothelial permeability. Am J Physiol. 1996;270:L973. doi: 10.1152/ajplung.1996.270.6.L973. [DOI] [PubMed] [Google Scholar]
- 18.Fraticelli A, Serrano CV, Bochner BS, Capogrossi MC, Zweier JL. Hydrogen peroxide and superoxide modulate leukocyte adhesion molecule expression and leukocyte endothelial adhesion. Biochem Biophys Acta. 1996;1310:251. doi: 10.1016/0167-4889(95)00169-7. [DOI] [PubMed] [Google Scholar]
- 19.Skoglund G, Cotgreave I, Rincon J, Patarroyo M, Ingelman-Sutdberg M. H2O2 activates CD11b/CD18-dependent cell adhesion. Biochem Biophys Res Commun. 1988;157:443. doi: 10.1016/s0006-291x(88)80269-9. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto K, Sterbinsky SA, Bickel CA, Zhou DW, Kovach NL, Bochner BS. Regulation of α4 integrin-mediated adhesion of human eosinophils to fibronectin and vascular cell adhesion molecule-1 (VCAM-1) J Allergy Clin Immunol. 1997;99:648. doi: 10.1016/s0091-6749(97)70027-7. [DOI] [PubMed] [Google Scholar]
- 21.Weber C, Kitayama J, Springer TA. Differential regulation of β1 and β2 integrin avidity by chemoattractants in eosinophils. Proc Natl Acad Sci USA. 1996;93:10939. doi: 10.1073/pnas.93.20.10939. 10.1073/pnas.93.20.10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calhoun WJ, Reed HE, Moest DR, Stevens CA. Enhanced superoxide production by alveolar macrophages and air-space cells, airway inflammation, alveolar macrophage density change after segmental antigen bronchoprovocation in allergic subjects. Am Rev Respir Dis. 1992;145:317. doi: 10.1164/ajrccm/145.2_Pt_1.317. [DOI] [PubMed] [Google Scholar]
- 23.Sanders SP, Zweier JL, Harrison SJ, Trush MA, Rembish SJ, Liu MC. Spontaneous oxygen radical production at sites of antigen challenge in allergic subjects. Am J Respir Crit Care Med. 1995;151:1725. doi: 10.1164/ajrccm.151.6.7767513. [DOI] [PubMed] [Google Scholar]
- 24.Sedgwick JB, Calhoun WJ, Vrtis RF, Bates ME, McAllister PK, Busse WW. Comparison of airway and blood eosinophil function after in vivo antigen challenge. J Immunol. 1992;149:3710. [PubMed] [Google Scholar]
- 25.Varma SD, Devamanoharan PS. Hydrogen peroxide in human blood. Free Radic Res Commun. 1991;14:125. doi: 10.3109/10715769109094124. [DOI] [PubMed] [Google Scholar]