Abstract
We have previously shown that tolerance can be induced against acute experimental autoimmune encephalomyelitis (EAE) in Lewis rats by bone marrow-derived dendritic cells (DC) that have been pulsed in vitro with encephalitogenic myelin basic protein peptide 68–86 (MBP 68–86), and injected subcutaneously into healthy rats prior to immunization with MBP 68–86 plus complete Freund's adjuvant. To elucidate better the properties of tolerogenic DC, we here compared plastic-adherent DC with floating, non-adherent DC, which were cultured for 7 days in the presence of granulocyte–macrophage colony-stimulating factor plus interleukin-4 (IL-4). Adherent DC expressed high levels of IL-10 mRNA and protein, and low levels of IL-12 mRNA and showed high expression of CD54 compared with floating DC. Proliferation, nitrite concentration and capacity for antigen presentation were lower in adherent DC than in floating DC. There were no differences between adherent and floating DC regarding expression of CD11c, OX62, major histocompatibility complex class II, CD80, or CD86. Most importantly, we observed that adherent DC induced tolerance to EAE in vivo when injected subcutaneously into Lewis rats prior to immunization, while floating DC did not. Adherent DC-mediated tolerance to EAE was associated with augmented proliferation, nitric oxide production and frequency of apoptotic cells as well as with up-regulation of transforming growth factor-β (TGF-β) -expressing cells in T-cell areas of lymph nodes. Tolerance induction by adherent DC seems to be related to a nitric oxide–apoptosis pathway and to up-regulation of TGF-β-expressing cells.
Introduction
There is now growing evidence that the regulation of immunity or tolerance takes place primarily at the level of dendritic cells (DC). DC constitute a complex system of cells which, under different conditions, can present antigen, inducing such contrasting states as immunity and tolerance.1,2 The heterogeneity of DC can be studied by a variety of methods: flow sorting of fluorescence labelled DC; different cytokine culture conditions; and adherent versus floating states of DC. According to the method used, DC with different phenotypes and functions can be obtained. Distinct DC subsets exhibit different characteristics, and mediate polarization of immune responses towards T helper type 1 (Th1) or Th2 responses. For example, a lymphoid-related subset induces high levels of the Th1 cytokines interferon-γ (IFN-γ) and interleukin-2 (IL-2), but little or no Th2 cytokines. In contrast, a myeloid-related subset induces large amounts of Th2 cytokines IL-4 and IL-10, in addition to IFN-γ and IL-2.3 CD8α+ DC drive the development of Th1-type immune responses, whereas conventional CD8α– DC induce the differentiation of Th2-type responses.4 It is postulated that mature DC may be best suited to achieve in vivo anti-tumour and anti-infection effects because of their capacity to efficiently present antigen to naÝve T cells. Immature DC, on the other hand, may induce tolerance rather than immunity.5
In the present study, we compared adherent and floating DC derived from healthy Lewis rat bone marrow regarding phenotypes, properties and functions. Adherent DC expressed high levels of IL-10 mRNA and protein, and low levels of IL-12 mRNA, and showed high expression of CD54 compared to floating DC. In vivo, adherent DC that had been pulsed in vitro with encephalitogenic myelin basic protein peptide 68–86 (MBP 68–86) induced tolerance to experimental autoimmune encephalomyelitis (EAE) when injected subcutaneously (s.c.) to Lewis rats prior to immunization with MBP 68–86 and complete Freund's adjuvant (CFA). The results suggest that the mechanism of DC-induced tolerance might result from a nitric oxide (NO)-mediated apoptosis pathway and/or up-regulation of transforming growth factor-β (TGF-β) -expressing cells.
Materials and methods
Animals and antigen preparation
Male Lewis rats, body weight 150–180 g, were purchased from Zentralinstitut fur Versuchstierzucht, Hannover, Germany. Guinea-pig MBP 68–86 (YGSLPQKSQRSQDENPV) were produced in an automatic Tecan-Syro Synthesizer (Multisytech, Bochum, Germany).
Preparation of bone marrow-derived DC
Bone marrow cells were flushed from femurs and tibias of healthy Lewis rats and passed through a wire mesh to remove small pieces of bone and debris. Subsequently, the red blood cells were osmotically lysed. After washing, cells were suspended in serum-free Dulbecco's modified Eagle's minimal essential medium (DMEM; Gibco, Paisley, UK) supplemented with 1% MEM amino acids (Gibco), 2 mm glutamine (Flow Laboratories, Irvine, UK), 50 IU/ml penicillin, 50 µg/ml streptomycin (Gibco) and 10 mm HEPES (Sigma, St Louis, MO), and placed into 75-cm2 Falcon culture flasks (Nunc, Copenhagen, Denmark) for 2 hr at 37° in 5% CO2. Floating cells were removed by aspirating medium. Adherent cells were washed five times with phosphate-buffered saline (PBS) and cultured for 7 days in medium containing 10% fetal calf serum (Gibco), 10 ng/ml of recombinant rat granulocyte–macrophage colony-stimulating factor (rrGM-CSF; R & D Systems, Minneapolis, MN) and 10 ng/ml of rrIL-4. Fresh medium was changed after 3–4 days. Floating, non-adherent DC were collected by shaking and aspirating the medium, followed by harvesting adherent DC with a cell scrap.
Pulsing of DC with encephalitogenic MBP 68–86 in vitro
Adherent and floating DC (1 × 106/ml) were incubated with MBP 68–86 at a concentration of 50 µg/0·5 ml at 37° for 4 hr. Cells were then washed three times to remove excess antigen, and resuspended at 1 × 106/ml in serum-free medium. DC were injected s.c. into healthy Lewis rats in a volume of 1 ml.
Induction of EAE and evaluation of clinical signs
First, we observed whether injection of DC prevented induction of clinical EAE. After 4 weeks, these DC-injected rats were immunized in both hind footpads with 200 µl of inoculum containing 10 µg of MBP 68–86, 2 mg Mycobacterium tuberculosis (strain H37RA; Difco, Detroit, MI), 100 µl saline and 100 µl Freund's incomplete adjuvant (Difco). Clinical scores were graded according to the following criteria: (0) asymptomatic, (1) loss of distal half of tail tonicity, (2) loss of entire tail tonicity, (3) hindlimb paresis, (4) hindlimb paralysis, and (5) tetraplegia. Clinical observations were made in a blinded fashion by at least two investigators.
Flow cytometry
Cell surface molecules and intracellular cytokines were analysed by flow cytometry (Becton Dickinson, Mountain View, CA). Selected monoclonal antibodies (mAbs) to rat molecules [CD11c, OX62, CD80, CD86, major histocompatibility complex (MHC) class II, CD54] and appropriate isotype controls were used. Anti-rat CD11c mAb and isotype controls were obtained from Serotec (Oxford, UK). Anti-rat OX62, phycoerythrin (PE)-conjugated anti-rat MHC class II mAb (OX6), PE-conjugated anti-rat B7-1 mAb (CD80), anti-rat B7-2 mAb (CD86) and anti-rat CD54 mAb were from PharMingen (San Diego, CA). DC were incubated with unlabelled anti-rat CD11c, OX62, CD86, CD54 and isotype control, then followed by fluorescein isothiocyanate (FITC)-conjugated anti-mouse secondary antibodies. Cell suspensions were fixed with 4% paraformaldehyde and then permeabilized with 0·15% saponin. Cells were stained with PE-conjugated anti-rat IL-10 mAb (PharMingen).
Preparation of mononuclear cells and T cells
To prepare mononuclear cells (MNC) and T cells, Lewis rats were killed on day 14 post-immunization (p.i.). Spleen and lymph nodes were removed. Suspensions of MNC were prepared by pressing the tissues through a wire mesh to obtain a single-cell suspension. For preparation of T cells, MNC suspensions from lymph nodes were washed and incubated in culture dishes for 2 hr at 37° in 5% CO2. The non-adherent fraction was collected and passed through a 20-ml nylon wool column. Purified T cells were enriched by depletion of nylon wool-adherent cells.
Proliferation and antigen presentation in vitro
Proliferative responses were examined by [3H]thymidine incorporation. For DC proliferation, adherent and floating DC (4 × 103 cells/200 µl) were added in 96-well, round-bottom microtitre plates (Nunc), respectively. For antigen-induced T-cell proliferation, MNC suspensions (4 × 105 cells/200 µl) were incubated in the presence or absence of MBP 68–86 (10 µg/ml). For antigen presentation assays, adherent and floating DC (4 × 103) were cultured with purified lymph node T cells (2 × 105) in the presence of MBP 68–86 (10 µg/ml). After 60 hr, all cells were pulsed with [3H]thymidine (10 µl, 100 µCi/ml; Amersham, Little Chalfont, UK). After 12 hr, cells were harvested onto glass fibre filters and measured in a β-scintillation counter. Results were expressed as counts per minute (c.p.m.) in triplicate.
Assay of IFN-γ-secreting cells
Enzyme-linked immunospot (ELISPOT) assays were used to detect single cells secreting IFN-γ. Nitrocellulose-bottom microtitre plates (Millititre-HAM plates; Millipore Co., Bedford, UK) were coated with 100 µl aliquots of mouse anti-rat IFN-γ mAb (DB1; Innogenetics, Ghent, Belgium) at 15 µg/ml. MNC (4 × 105/200 µl) were added to individual wells in the presence or absence of MBP 68–86 (10 µg/ml). After 48 hr of culture and washing, plates were incubated with 100 µl polyclonal rabbit anti-rat IFN-γ (1 : 400; Innogenetics), biotinylated anti-rabbit IgG (1 : 500; Dakopatts, Copenhagen, Denmark) and then avidin–biotin peroxidase complex (ABC) (1 : 200; Vector, Burlingame, CA). Red-brown immunospots corresponding to individual cells that had secreted IFN-γ were counted in a dissection microscope.
Measurement of nitrite
Because secreted NO quickly reacts with oxygen-yielding nitrite, the level of nitrite as a reflection of NO production in culture supernatants was measured using modified Griess reagent (Sigma). DC (4 × 103/200 µl) were cultured in the absence of MBP 68–86. MNC (4 × 105/200 µl) were cultured in the presence or absence of MBP 68–86 (10 µg/ml). After 48 hr, 100 µl of supernatants from cultured DC and MNC was mixed with 100 µl of Griess reagent. After 10 min reaction at room temperature, absorbance at 540 nm was measured using an automatic plate reader. Nitrite concentration was determined by comparison with a sodium nitrite standard curve in culture medium.
Immunohistochemistry
Spleen and lymph nodes from the killed animals were snap-frozen in liquid nitrogen. Cryostat sections (8 µm thick) were fixed in acetone. Endoperoxidase was inactivated in H2O2. Non-specific binding was blocked with 1% blocking reagent (Boehringer Mannheim, Mannheim, Germany). Sections were incubated with polyclonal rabbit anti-rat TGF-β1 antibodies (Santa Cruz Biotechnology Inc., Santa Cruz, CA), followed by biotin-conjugated anti-rabbit antibodies and the ABC (Vector) reactive system. Omission of the primary antibodies served as negative controls. The numbers of TGF-β1 positive cells were calculated per 100 mm2 of tissue sections.
Assay for apoptosis
Lymph nodes from the killed animals were immediately snap-frozen in liquid nitrogen. Cryostat sections (8 µm) were fixed in acetone for 10 min. Sections were stained with FITC-dUTP (Boehringer Mannheim) according to the manufacturer's instructions. Permeabilization was done with 0·1% Triton X-100 in 0·1% sodium citrate for 2 min on ice, 50 µl of TdT-mediated biotin–dUTP nick-end labelling (TUNEL) reaction mixture was added to tissue samples and incubated in a humidified chamber for 60 min at 37° in the dark. The numbers of apoptotic cells were calculated per 100 mm2 of tissue sections.
In situ hybridization
In situ hybridization (ISH) was used to detect IL-12 and IL-10 mRNA as previously described.6 A mixture of four different 48-base long synthetic oligonucleotide probes was used in order to increase the sensitivity of the method. The oligonucleotide sequences were obtained from GenBank using the MacVector System. Rat probes for IL-12 p40 (GenBank accession no. M86771) were complementary to bases 147–194 and 595–642, and for IL-12 p35 (acc. no. M86672) to bases 190–238 and 706–753. The mouse probes of IL-10 (acc. no. M378977) were complementary to bases 79–126, 134–181, 184–231 and 402–449. Control slides were hybridized with the same total amount of a sense probe with nucleotide sequence for exon 4 of rat IFN-γ. A constant ratio of the guanine/cytosine content of approximately 60% was employed. Synthetic oligonucleotide probes (Scandinavian Gene synthesis AB, Koping, Sweden) were labelled with [35S]deoxyadenosine 5′-thiotriphosphate (New England Nuclear, Cambridge, MA) with terminal dexynucleotidyl transferase (Amersham). Cells were hybridized for 18 hr at 42° with 106 labelled probe per 100 µl hybridization mixture. After emulsion autoradiography, development and fixation, the coded slides were examined by dark-field microscopy at × 10 magnification for positive cells containing > 15 grains per cell in a star-like distribution. The intracellular distribution of grains was always checked by light microscopy at × 20 or × 40 magnification. There were no difficulties in differentiating between positive and negative cells. The results are expressed as number of labelled cells per 105 DC. The control probe used in parallel with the cytokine probes produced a uniformly weak background signal without revealing any positive cells.
Statistics
Differences between three groups were evaluated by anova. Differences between groups were determined by Student's t-test. P < 0·05 was considered significant.
Results
Phenotype and cytokine expression of adherent and floating DC
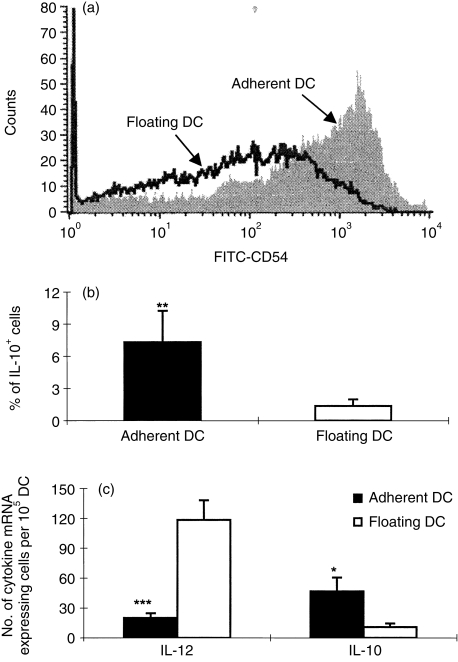
After 7 days of culture in the presence of GM-CSF + IL-4, adherent DC and floating DC were obtained at mean levels of 54% (± 5·3) and 46% (± 5·3), respectively. Expression of surface molecules was assessed on adherent and floating DC. Adherent DC expressed CD11c (55·5 ± 9·2%), OX62 (66·5 ± 5·0%), MHC class II (62·5 ± 12·0%), CD80 (36 ± 7·1%) and CD86 (42·5 ± 27·6%). Floating DC expressed CD11c (49·5 ± 5·0%), OX62 (55·5 ± 5·0%), MHC class II (48·3 ± 13·6%), CD80 (25·0 ± 7·4%) and CD86 (29·7 ± 19·1%). Overall, adherent DC seem to express slightly higher levels of these molecules, but there were no statistically significant differences. Adherent DC showed higher levels of IL-10 mRNA expression (P < 0·05), lower levels of IL-12 mRNA expression (P < 0·001) and higher levels of CD54 expression (P < 0·005) compared to floating DC. Similarly, higher levels of IL-10 protein expression were displayed by adherent DC compared to floating DC (P < 0·01)(Fig. 1).
Figure 1.
Phenotype and cytokine profiles of adherent and floating DC. DC from rat bone marrow were cultured for 7 days in the presence of GM-CSF and IL-4. Adherent DC and floating DC were isolated. (a) Adherent DC and floating DC were analysed by flow cytometry for CD54 surface marker. (b) DC were stained with PE-conjugated anti-rat IL-10 and then examined by flow cytometry. (c) DC were examined for IL-10 and IL-12 mRNA expression by in situ hybridization as described in the Materials and methods. Data are representative of three independent experiments; *P < 0·05; **P < 0·01; ***P < 0·001.
Comparison of proliferative response, NO production and antigen presentation
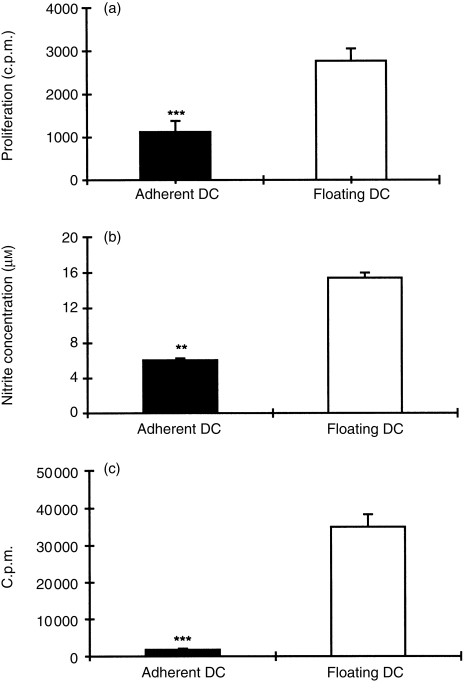
To evaluate the characteristics of adherent and floating DC, we compared proliferative response, NO production and capacity of antigen presentation. Figure 2(a) shows that proliferation of adherent DC was lower than that of floating DC (P < 0·001). Adherent DC also exhibited low levels of nitrite concentration compared to floating DC (P < 0·01) (Fig. 2b). Figure 2(c) indicates that capacity of antigen presentation was lower in adherent DC than floating DC (P < 0·001).
Figure 2.
Proliferative response (a), NO production (b) and antigen presentation (c) of adherent DC and floating DC. Proliferation was assessed at 72 hr by the uptake of [3H]thymidine. Nitrite was measured at 72 hr by Griess reagent. For antigen presentation assays, T cells (1 × 106/ml; 200 µl/well) were incubated with DC (T cells : DC ratio 50 : 1) in the presence of specific antigen MBP 68–86 peptide (10 µg/ml) for 72 hr. Data (mean ± SD) are representative of three independent experiments; **P < 0·01; ***P < 0·001.
Adherent DC in vitro pulsed with MBP 68–86 induce tolerance to EAE
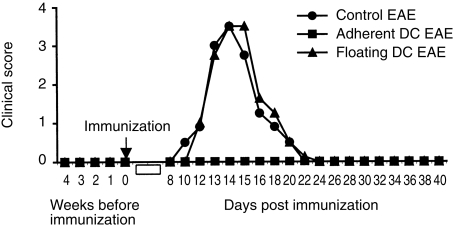
Healthy rats were injected s.c. with MBP 68–86-pulsed adherent and floating DC as described in the Materials and methods. Control EAE rats received injection of PBS alone. To evaluate whether injection of MBP 68–86-pulsed DC could induce clinical EAE, we first observed these rats for 4 weeks. No clinical signs occurred, suggesting that MBP 68–86-pulsed DC did not induce clinical EAE. Four weeks after DC injection, these rats were immunized with MBP 68–86 and CFA. Tolerance to EAE was observed in rats injected with adherent DC, whereas those injected with floating DC or PBS did not exhibit any protection from EAE (Fig. 3).
Figure 3.
Tolerance induction by adherent DC. Lewis rats were s.c. injected with MBP 68–86-pulsed adherent DC (n = 6) and floating DC (n = 6) from bone marrow at 1 × 106 DC/rat, respectively. After 4 weeks, these DC-treated rats and control EAE rats injected with PBS (n = 6) were immunized with MBP 68–86 and CFA. The graph shows mean clinical scores of EAE. These data are compiled from two independent experiments.
Adherent DC-induced tolerance is associated with augmented proliferation, IFN-γ and NO production
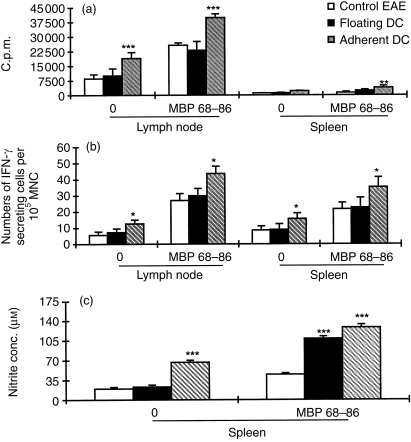
To understand the mechanisms by which adherent DC induced tolerance to EAE in vivo, proliferative responses, IFN-γ and NO production by spleen and lymph node MNC were measured on day 14 p.i. Figure 4(a) shows that spontaneous and MBP 68–86-induced proliferative responses of lymph node (P < 0·001) and spleen MNC (P < 0·01) obtained from rats injected with adherent DC were higher than in rats injected with floating DC or in PBS-injected control rats.
Figure 4.
Proliferative response (a), IFN-γ secretion (b) and NO production (c) of lymph node and spleen MNC from rats treated with adherent DC, floating DC or rats receiving PBS (control EAE) on day 14 p.i. with encephalitogenic MBP 68–86 and CFA (n = 4). These data (mean ± SD) represent one of two to three independent experiments with essentially identical results; *P < 0·05; **P < 0·01; ***P < 0·001.
Spontaneous and MBP 68–86-induced IFN-γ-secreting cells were also elevated among lymph node and spleen MNC from rats treated with adherent DC compared to rats treated with floating DC or PBS-treated control rats (P < 0·05 for both comparisons) (Fig. 4b).
Spleen MNC from rats treated with adherent DC spontaneously produced high levels of nitrite concentration compared to rats treated with floating DC or PBS-treated control rats (P < 0·001 for both comparisons). After stimulation with MBP 68–86, spleen MNC both from rats treated with adherent and with floating DC produced higher levels of nitrite concentration than rats treated with PBS (P < 0·001) (Fig. 4c).
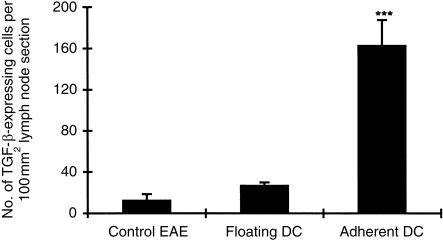
Adherent DC up-regulate TGF-β-expressing cells in lymph nodes
Rats injected with adherent DC showed dramatic up-regulation of TGF-β-expressing cells in lymph nodes compared to rats receiving floating DC or PBS injection (P < 0·001 for both comparisons). In the latter, only a few TGF-β-expressing cells were seen among lymph node cells (Fig. 5).
Figure 5.
Numbers of TGF-β-expressing cells detected by immunohistochemistry in lymph node sections obtained on day 14 p.i. with encephalitogenic MBP 68–86 and CFA from rats pretreated with adherent DC, floating DC, or rats receiving PBS (control EAE). Numbers of cells were counted in five random fields at magnification × 200. Results are expressed as mean ± SD per 100 mm2 of tissue area from each experimental group (four rats); ***P < 0·001.
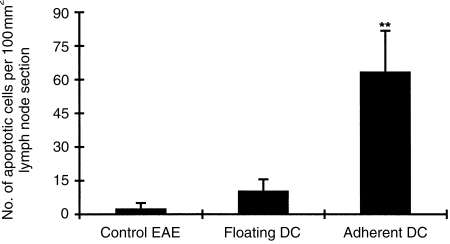
Adherent DC enhance apoptosis among lymph node cells
Previous studies have demonstrated that CD4+ T-cell apoptosis contributes to the recovery from EAE.7 To determine whether adherent DC can induce cell apoptosis, the frequency of apoptotic cells was measured in lymph nodes from rats of the three groups. Figure 6 shows that lymph nodes from rats injected with adherent DC contained higher levels of apoptotic cells compared to rats receiving floating DC or PBS (P < 0·01 for both comparisons).
Figure 6.
Numbers of apoptotic cells in lymph node sections on day 14 p.i. from rats treated with adherent DC, floating DC, or PBS (control EAE) were detected by TUNEL as described in the Materials and methods. Results are expressed as the mean ± SD per 100 mm2 of tissue area from each experimental group (four rats); **P < 0·01.
Discussion
DC constitute a complex system of cells which, under different culture conditions and expressing different phenotype, can induce immunity or tolerance. DC plasticity is also reflected in their differentiation.8 Two DC subsets, the classical myeloid-related DC and a novel lymphoid-related DC, have been identified. Recent evidence suggests that each of these subsets may have different roles in the generation of peripheral tolerance or immunity and of type of T-cell response.3,4,9,10 We compared here the phenotypes, properties and functions of adherent versus floating bone marrow DC in vitro as well as in vivo. Adherent and floating DC were found to express similar levels of CD11c, OX62, MHC class II, B7-1 and B7-2. However, in accordance with previous observations,11 adherent DC expressed low levels of IL-12 and high levels of IL-10 in vitro when compared to floating DC. Functional heterogeneity between bone marrow-derived adherent and floating DC could have important implications for the development of DC-based immunotherapies.
We speculate that high IL-10 and low IL-12 expression by adherent DC could play a critical role for in vivo tolerance induction. It has been reported that IL-10 is required for induction of T-cell tolerance, and converts stimulatory DC into tolerogenic antigen-presenting cells.12,13 Studies have also shown that retroviral delivery of viral IL-10 into myeloid DC markedly limits their allostimulatory activity and promotes the induction of T-cell hyporesponsiveness.14 Gao et al.15 found that tolerogenic DC express high levels of IL-10 and have a skewed balance between IL-10 and IL-12 production. IL-12 appears to be the most crucial DC product, driving the development of naÝve precursors into Th1 cells.16 Recently, Kalinski et al.17 suggested that DC can be divided into three types of subsets based on IL-12-producing capacity: the type-1 DC subset, which produces high levels of IL-12 and induces Th1-cell differentiation in naÝve Th cells; the type-2 DC subset, producing low levels of IL-12 and high levels of co-stimulatory molecules, resulting in Th2-cell responses; and the type-3 DC subset treated with IL-10, expressing low levels of IL-12 and co-stimulatory molecules, giving rise to tolerogenic DC. In the present study, the high levels of IL-10 produced by adherent DC may in turn inhibit IL-12 expression, suggesting that adherent DC may exhibit tolerogenic properties.
Apart from distinct cytokine profiles of adherent DC (IL-10high, IL-12low) and floating DC (IL-10low, IL-12high), adherent DC show lower NO production, proliferation and antigen presentation capacity compared to floating DC. Because adherent DC expressed high levels of IL-10 compared to floating DC, it is possible that adherent DC inhibit antigen presentation, proliferation and NO production. Recently, Steinman et al.18 proposed that immature DC phagocytose tissue cells to tolerance or regulate self-reactive T cells.
Most importantly, in agreement with our hypothesis, adherent DC successfully induced tolerance to EAE in Lewis rats compared to floating DC. However, the properties of tolerogenic DC are not clear. In the present study, an opposite skewed balance between IL-10 and IL-12 was observed for adherent and floating DC, which constitutes a possible mechanism by which adherent DC expressing IL-10 exhibit a tolerogenic property. In the present study, we observed that adherent DC expressed higher levels of CD54 compared to floating DC. DC are specialized to mediate several physiological components of immunogenicity, such as the acquisition of antigen in tissues, the migration to lymphoid organs, and the identification and activation of antigen-specific T cells. High levels of CD54 by DC probably contribute to these functions. The function of CD54 of adherent DC in immunological tolerance is just beginning to be studied.
TGF-β plays a dominant role in the generation of immune tolerance. Many studies have described induction of immune tolerance accompanied by up-regulation of TGF-β-secreting regulatory T cells.19–24 Nasal administration of acetylcholine receptor (AChR) in a Lewis rat model experimental autoimmune myasthenia gravis has indicated a role of TGF-β-producing cells. The effects of in vitro suppression of AChR-primed lymphocyte proliferation could be blocked by anti-TGF-β mAb.25 In vivo, oral antigen-induced tolerance has revealed that the protective effect can be reversed by administration of anti-TGF-β mAb.24,26,27 In the present study, there was an increase of TGF-β-expressing cells in rats injected with adherent DC compared to rats receiving floating DC or PBS, suggesting that TGF-β-expressing cells also play a major role in the adherent DC-induced tolerance to EAE.
Both antigen-pulsed adherent and floating DC can in vivo prime T cells without subsequent immunization, reflected by augmented antigen-specific proliferation in rats receiving antigen-pulsed adherent and floating DC compared to healthy control rats (data not shown). However, we found that adherent DC-induced tolerance in Lewis rats was accompanied by enhanced spontaneous and specific antigen-induced proliferative responses, IFN-γ and NO production. Frequencies of apoptotic cells in sections of lymph nodes were augmented in adherent DC-treated rats. A frequently proposed cascade for the development of organ-specific autoimmune disease involves the induction and expansion of Th1 cells, which secrete IFN-γ and thereby activate macrophages and other effector cells to produce tissue-damaging molecules such as NO. However, several parts of this concept have been repeatedly challenged, particularly in the murine EAE model. IL-12 protects from Th1-mediated experimental autoimmune uveitis (EAU).28 It has been demonstrated that both IFN-γ and NO are two potential down-regulating molecules in EAE.29–31 IFN-γ suppressed EAE by inducing inducible NO synthase (iNOS) and subsequent NO production.32 IL-12 protects from Th1-mediated EAU by curtailing development of uveitogenic effector T cells. This phenomenon involves hyperinduction of IFN-γ, causing up-regulation of iNOS and production of NO, which protects, at least in part, by triggering apoptotic deletion of antigen-specific cells at a critical time-point during antigen priming28. A positive correlation between recovery from EAE and the levels of NO was observed.7,31 Interestingly, when recovered rats are treated with N-methyl-l-arginine acetate (l-NMA) alone (inhibitor of NO production), 100% of animals develop a relapse of EAE in Lewis rats,31 suggesting a central role for NO in the immunoregulation of EAE. Of course, the role of NO in the immune system comprises both effector (e.g. tissue destruction or apoptosis of autoreactive T cells) and regulatory (e.g. modulation of cytokine responses) functions, depending on concentration of NO and target cells in the local microenvironment. In view of the data reported here, we propose that reduction of inflammatory cells within the central nervous system might be caused by their elimination through apoptosis.
In summary, tolerogenic DC may constitute a separate differentiation pathway, as suggested by Suss and Shortman.33 IL-10high IL-12low DC population derived from adherent DC can contribute to induction of tolerance, possibly through up-regulation of TGF-β-expressing cells and/or induction of IFN-γ, NO and the apoptosis pathway. The present study also provides insight into the mechanisms of DC tolerance induction and should facilitate the design of an ideal vaccine aimed at applying immune tolerance in autoimmune diseases.
Acknowledgments
This work has been supported by grants from the Swedish MS Society (NHR), the Swedish Medical Research Council and Karolinska Institute Research Funds. Dr Jian-She Yang is a guest researcher from the First Affiliated Hospital, Shihezi Medical College, Shihezi, P.R.China
Glossary
Abbreviations
- DC
dendritic cells
- EAE
experimental autoimmune encephalomyelitis
- MBP
myelin basic protein
- MNC
mononuclear cell
- NO
nitric oxide
- p.i.
post-immunization
REFERENCES
- 1.Finkelman FD, Lees A, Birnbaum R, Gause WC, Morris SC. Dendritic cells can present antigen in vivo in a tolerogenic or immunogenic fashion. J Immunol. 1996;157:1406–14. [PubMed] [Google Scholar]
- 2.Kurts C, Kosaka H, Carbone FR, Miller JF, Heath WR. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8 (+) T cells. J Exp Med. 1997;186:239–45. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pulendran B, Smith JL, Caspary G, Brasel K, Pettit D, Maraskovsky E, Maliszewski CR. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci USA. 1999;96:1036–41. doi: 10.1073/pnas.96.3.1036. 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maldonado-Lopez R, De Smedt T, Michel P, et al. CD8 alpha+ and CD8alpha– subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–92. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Labeur MS, Roters B, Pers B, Mehling A, Luger TA, Schwarz T, Grabbe S. Generation of tumor immunity by bone marrow-derived dendritic cells correlates with dendritic cell maturation stage. J Immunol. 1999;162:168–75. [PubMed] [Google Scholar]
- 6.Link J, Soderstrom M, Olsson T, Hojeberg B, Ljungdahl A, Link H. Increased transforming growth factor-beta, interleukin-4, and interferon-gamma in multiple sclerosis. Ann Neurol. 1994;36:379–86. doi: 10.1002/ana.410360309. [DOI] [PubMed] [Google Scholar]
- 7.Xiao BG, Huang YM, Xu LY, Ishikawa M, Link H. Mechanisms of recovery from experimental allergic encephalomyelitis induced with myelin basic protein peptide 68–86 in Lewis rats: a role for dendritic cells in inducing apoptosis of CD4+T cells. J Neuroimmunol. 1999;97:25–36. doi: 10.1016/s0165-5728(99)00041-7. 10.1016/S0165-5728(99)00041-7. [DOI] [PubMed] [Google Scholar]
- 8.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 9.Salomon B, Cohen JL, Masurier C, Klatzmann D. Three populations of mouse lymph node dendritic cells with different origins and dynamics. J Immunol. 1998;160:708–17. [PubMed] [Google Scholar]
- 10.Smith AL, De St Groth BF. Antigen-pulsed CD8α dendritic cells generate an immune response after subcutaneous injection without homing to the draining lymph node. J Exp Med. 1999;189:593–8. doi: 10.1084/jem.189.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakraborty A, Li L, Chakraborty NG, Mukherji B. Stimulatory and inhibitory differentiation of human myeloid dendritic cells. Clin Immunol. 2000;94:88–98. doi: 10.1006/clim.1999.4826. 10.1006/clim.1999.4826. [DOI] [PubMed] [Google Scholar]
- 12.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–80. [PubMed] [Google Scholar]
- 13.Groux H, Bigler M, De Vries JE, Roncarolo MG. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J Exp Med. 1996;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takayama T, Nishioka Y, Lu L, Lotze MT, Tahara H, Thomson AW. Retroviral delivery of viral interleukin-10 into myeloid dendritic cells markedly inhibits their allostimulatory activity and promotes the induction of T-cell hyporesponsiveness. Transplantation. 1998;66:1567–74. doi: 10.1097/00007890-199812270-00001. [DOI] [PubMed] [Google Scholar]
- 15.Gao JX, Madreas J, Zeng W, Cameron MJ, Zhang Z, Wang JJ, Zjong R, Grant D. CD40-deficient dendritic cells producing interleukin-10, but not interleukin-12, induce T-cell hyporesponsiveness in vitro and prevent acute allograft rejection. Immunology. 1999;98:159–70. doi: 10.1046/j.1365-2567.1999.00863.x. 10.1046/j.1365-2567.1999.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–76. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 17.Kalinski P, Hilkens CMU, Wierenga EA, Kapsenberg ML. T cell priming by type 1 and type 2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–7. doi: 10.1016/s0167-5699(99)01547-9. 10.1016/S0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 18.Steinman RM, Turely S, Mellrman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–6. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller SD, Tan LJ, Kennedy MK, Dal Canto MC. Specific immunoregulation of the induction and effector stages of relapsing EAE via neuroantigen-specific tolerance induction. Ann N Y Acad Sci. 1991;636:79–94. doi: 10.1111/j.1749-6632.1991.tb33440.x. [DOI] [PubMed] [Google Scholar]
- 20.Miller SD, Tan LJ, Pope L, McRae BL, Karpus WJ. Antigen-specific tolerance as a therapy for experimental autoimmune encephalomyelitis. Int Rev Immunol. 1992;9:203–22. doi: 10.3109/08830189209061791. [DOI] [PubMed] [Google Scholar]
- 21.Bai XF, Shi FD, Xiao BG, Li HL, Van Der Meide PH, Link H. Nasal administration of myelin basic protein prevents relapsing experimental autoimmune encephalomyelitis in DA rats by activating regulatory cells expressing IL-4 and TGF-beta mRNA. J Neuroimmunol. 1997;80:65–75. doi: 10.1016/s0165-5728(97)00133-1. 10.1016/S0165-5728(97)00133-1. [DOI] [PubMed] [Google Scholar]
- 22.Xiao BG, Zhang GX, Shi FD, Ma CG, Link H. Decrease of LFA-1 is associated with upregulation of TGF-beta in CD4 (+) T cell clones derived from rats nasally tolerized against experimental autoimmune myasthenia gravis. Clin Immunol Immunopathol. 1998;89:196–204. doi: 10.1006/clin.1998.4537. 10.1006/clin.1998.4537. [DOI] [PubMed] [Google Scholar]
- 23.Ma CG, Zhang GX, Xiao BG, Link H. Cellular mRNA expression of interferon-gamma (IFN-gamma), IL-4 and transforming growth factor-beta (TGF-beta) in rats nasally tolerized against experimental autoimmune myasthenia gravis (EAMG) Clin Exp Immunol. 1996;104:509–16. doi: 10.1046/j.1365-2249.1996.50755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–40. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 25.Shi FD, Li HL, Wang HB, Bai XF, Van Der Meide PH, Link H, Ljunggren HG. Mechanisms of nasal tolerance induction in experimental autoimmune myasthenia gravis: identification of regulatory cells. J Immunol. 1999;162:5757–63. [PubMed] [Google Scholar]
- 26.Inobe J, Slavin AJ, Komagata Y, Chen Y, Liu L, Weiner HL. IL-4 is a differentiation factor for transforming growth factor-beta secreting Th3 cells and oral administration of IL-4 enhances oral tolerance in experimental allergic encephalomylitis. Eur J Immunol. 1998;28:2780–90. doi: 10.1002/(SICI)1521-4141(199809)28:09<2780::AID-IMMU2780>3.0.CO;2-J. 10.1002/(SICI)1521-4141(199809)28:09<2780::AID-IMMU2780>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 27.Miller A, Lider O, Roberts A, Sporn MB, Weiner HL. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor β after antigen-specific triggering. Proc Natl Acad Sci USA. 1992;89:421–5. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarrant TK, Silver PB, Wahlsten JL, Rizzo LV, Chan CC, Wiggert B, Caspi RR. IL-12 protects from a Th-1-mediated autoimmune disease, experimental autoimmune uveitis, through a mechanism involving interferon-γ, nitric oxide, and apoptosis. J Exp Med. 1999;189:219–30. doi: 10.1084/jem.189.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramashaw IA. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol. 1996;157:3223–7. [PubMed] [Google Scholar]
- 30.Cowden WB, Cullen FA, Staykova MA, Willenborg DO. Nitric oxide is a potential down-regulating molecule in autoimmune disease: inhibition of nitric oxide production renders PVG rats highly susceptible to EAE. J Neuroimmunol. 1998;88:1–8. doi: 10.1016/s0165-5728(98)00040-x. 10.1016/S0165-5728(98)00040-X. [DOI] [PubMed] [Google Scholar]
- 31.O'brien NC, Charlton B, Cowden WB, Willenborg DO. Nitric oxide plays a critical role in the recovery of Lewis rats from experimental autoimmune encephalomyelitis and the maintenance of resistance to reinduction. J Immunol. 1999;163:6841–7. [PubMed] [Google Scholar]
- 32.Willenborg DO, Fordham SA, Staykova MA, Ramshaw IA, Cowden WB. IFN-γ is critical to the control of murine autoimmune encephalomyelitis and regulates both in the periphery and in the target tissue: a possible role for nitric oxide. J Immunol. 1999;163:5278–86. [PubMed] [Google Scholar]
- 33.Suss G, Shortman K. A subset of dendritic cells kills CD4 T cells via Fas/Fas-ligand-induced apoptosis. J Exp Med. 1996;183:1789–96. doi: 10.1084/jem.183.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]