Abstract
We compared B-cell phenotypes in Peyer's patches and solitary lymphoid follicles (organized gut-associated lymphoid tissue, GALT) with those in jejunal or ileal lamina propria. In situ, immunostaining showed that small B cells of naive [surface immunoglobulin D-positive (sIgD+) CD27–] and memory (sIgD± CD27+) phenotypes occurred almost exclusively in GALT, whereas the lamina propria contained only scattered sIgA+ CD27+ memory cells. In contrast, B-cell blasts and plasma cells negative for CD20 and often also for CD19 but with strong expression of CD38, CD27 and cytoplasmic IgA (cIgA), dominated in the lamina propria but were scarce in GALT. By flow cytometry, the proportion of dispersed CD19+ B lymphocytes varied from 4 to 42% among jejunal mucosal samples; between 5 and 50% of these were sIgD+, suggesting a variable contamination with GALT cells. B-cell blasts and plasma cells, identified by their large size and strong expression of CD38, were regularly found (25–35% of the total mononuclear cell population). Distinction between B-cell blasts and mature plasma cells was made by the presence or absence of human leucocyte antigen (HLA) class II molecules, CD45RA, CD19 and surface immunoglobulin. No CD19+ B cells outside GALT expressed CD5, but a very small portion of the lamina propria B-cell blasts were positive for CD28. Dispersed sIgA+ lamina propria cells expressed low levels of CD40, proliferated on CD40 ligation and constitutively secreted IgA in vitro. We concluded that the lamina propria B-cell compartment consists mainly of B-cell blasts and plasma cells but also has scattered, small sIgA+ cells that can proliferate in response to CD40 ligation and may therefore function as local memory cells for recall antigens.
Introduction
B cells exert a major protective immune defence function in the gut, principally by constituting the basis for the secretory IgA (SIgA) and SIgM system.1 Lamina propria plasma cells produce mainly dimeric IgA and represent the progeny of B cells primed in Peyer's patches, solitary lymphoid aggregates or appendix (collectively called gut-associated lymphoid tissue, GALT). Memory/effector B cells exit from GALT through draining lymph, and home from the blood circulation to the lamina propria via postcapillary venules.1 It is assumed that Peyer's patches largely give rise to the small intestinal B-cell population2,3 while the appendix primes B cells for the large intestine.4 The contribution to intestinal IgA-producing cells from peritoneal cavity B lymphocytes is well documented in mice5 but this has not been thoroughly examined in humans.
Differentiation of naive B cells to memory/effector cells after antigen encounter is manifested as changes in phenotype and morphology. Such cellular alterations have been observed in human tonsils and rat spleen.6,7 Briefly, naive B cells usually switch their surface (s) immunoglobulin phenotype from sIgD+ IgM+ to sIgD– IgM+, and may then proceed to another isotype (usually sIgG or sIgA) after secondary stimulation. The surface immunoglobulin is retained when the cell enters a memory pathway but is gradually lost during terminal plasma cell differentiation together with other B-cell markers such as CD20, CD45RA, HLA class II molecules and CD19, whereas CD38 is strongly up-regulated.8 The CD40 ligand (CD40L, CD154) seems to be a critical factor in the decision between these differentiation pathways because the presence of CD40L favours the memory state while differentiation into plasma cells is inhibited.9 Recently, CD27 was reported to distinguish naive from memory B cells in a more valid way than the presence or absence of sIgD; thus, memory cells with high levels of somatic mutation in their immunoglobulin variable genes usually expressed CD27 while cells with these genes in germline configuration were negative.10
Phenotypic alterations associated with B-cell responses in human Peyer's patches have not been studied in detail, with the exception of immunoglobulin isotype patterns.11–14 Thus, only a little information exists about the distribution of B-cell subsets in human GALT versus lamina propria.1,11,15 Intriguingly, both animal experiments and human studies have suggested that B-cell responses can take place in lamina propria apparently independent of GALT structures, but the nature of the B cells involved is unknown.16,17 In this context, it is of considerable interest that a major fraction of human intestinal IgA-producing cells has been reported to express CD5,18 a marker which is present on a fraction of tonsillar and peripheral sIgD+ cells,19 associated with B cells from the peritoneal cavity,20 or involved in autoimmunity.19 However, it remains controversial whether B1 cells (often CD5+) contribute to immune responses in the human intestine.21
The present study was undertaken to expand our knowledge on B cells, mainly those expressing IgA, in the human small intestine. A detailed immunohistochemical in situ study was supplemented by flow cytometric data obtained from dispersed mucosal cells, in addition to short-term in vitro experiments. Markers known to be induced or lost when B cells differentiate to plasma cells were used to identify the stage at which primed B cells enter the lamina propria, assuming that the phenotypes detected represent ‘snap-shots’ of intestinal B-cell homing.
Materials and methods
Immunohistochemistry
Biopsy specimens of macroscopically normal jejunum (n = 6) and ileum (n = 6) were used (median age 42 years, range 5–72 years). All samples were obtained for diagnostic purposes and parallel formalin-fixed material was examined to ensure normal histology. Specimens selected for immunohistochemistry were, within 15 min of sampling, embedded in OCT compound (Tissue-Tek, Miles Laboratories, Elkhart, IN), snap-frozen in liquid nitrogen and stored at −70° until cryosectioning at 4–6 µm. Sections were then fixed for 10 min in acetone at room temperature, wrapped in aluminium foil, and stored at −20°.
Multi-colour immunostaining was performed on tissue sections and cytospins (see later) in three steps with mixtures of pretitrated primary antibodies (Table 1) for 1 hr at room temperature, secondary reagents (biotinylated, Cy3- or fluorescein isothiocyanate (FITC) -conjugated goat anti-mouse IgG1 or IgG2a from Southern Biotechnology, Birmingham, AL, and rabbit anti-human cytokeratin from the authors' laboratory) for 1·5 hr, and tertiary reagents (streptavidin-Texas Red from BRL, Gaithersburg, MD and/or 7-amino-4-ethylcoumarin-3-acetic acid (AMCA)-conjugated goat anti-rabbit IgG, Vector Laboratories, Burlingame, CA) for 30 min. Controls were irrelevant isotype-matched primary antibodies and FITC-conjugated goat IgG used at concentrations comparable to those of the specific antibodies (Table 1). The specimens were examined in a Leitz DMRXE microscope (Leica, Wetzlar, Germany) equipped with a vertical illuminator and filter blocks for observation of red, green, blue and combined red/green emissions. Pictures were obtained in a Nikon EcLipse 800 fluorescence microscope equipped with a 3518 CCD video camera and captured by foto-station software (FotoWare A/S, Høvik, Norway).
Table 1.
Primary antibodies used for immunohistochemistry and flow cytometry
| Designation/ clone | Specificity (human) | Isotype/ label | Working concentration (µg/ml or dilution) | Source |
|---|---|---|---|---|
| BMA030 | CD3 | IgG2a | Purified Ig: 2·5,* 5·0 | Behringwerke, Marburg, Germany |
| SK7 | CD3 | IgG1 | Purified Ig: 2·5* | Becton-Dickinson |
| UCHT2 | CD5 | IgG1 | Purified Ig: 1/0·5* | PharMingen, San Diego, CA |
| L17F12 | CD5 | IgG2a | Purified Ig: 2·5,* 5·0 | Becton-Dickinson |
| HIB19 | CD19 | IgG1-CyChrome | Purified Ig: 5·0* | PharMingen |
| HD37 | CD19 | IgG1 | Supernatant: 1/20,* 1/10 | Dako, Glostrup, Denmark |
| L26 | CD20 | IgG2a | Supernatant: 1/40 | Dako |
| 3A12 | CD27 | IgG2a | Ascitic fluid: 1/1000,* 1/500 | R.A.W. van Lier, Amsterdam, NL |
| 15E8 | CD28 | IgG1 | Ascitic fluid: 1/1000,* 1/500 | R.A.W. van Lier |
| HB-7 | CD38 | IgG1 | Purified Ig: 0·13,* 0·25 | Becton Dickinson |
| 33071A | CD40 | IgG1 | Purified Ig: 2·5,* 5·0 | PharMingen |
| L48 | CD45RA | IgG1 | Purified Ig: 2·5,* 5·0 | Bectin-Dickinson |
| UCHL-1 | CD45RO | IgG2a | Supernatant: 1/40,* 1/10 | P.C.L. Beverly, London, UK |
| FN-1 | CDw78 | IgG1 | Supernatant: 1/80,* 1/20 | S. Funderud, Oslo, Norway |
| L243 | HLA-DR | IgG2a | Purified Ig: 1/80,* 1/40 | Becton-Dickinson |
| B7/21 | HLA-DP | IgG1 | Purified Ig: 1/20* | Becton-Dickinson |
| SK10 | HLA-DQ | IgG1 | Purified Ig: 1/80* | Becton-Dickinson |
| IgD26 | δ-chain | IgG1 | Supernatant:1/20,* 1/10 | D.Y. Mason, Oxford, UK |
| 6E2C1 | α-chain | IgG1 | Ascitic fluid: 1/1000 | T. Lea, Oslo, Norway |
| SK11 | l-selectin | IgG2a | Purified Ig: 2·5,*,5·0 | Becton-Dickinson |
| 4G8 | l-selectin | IgG1 | Purified Ig: 1·0 | R & D Systems, Abingdon, UK |
| X39 | KLH | IgG2a | Purified Ig: 2·5,* 5·0 | Becton-Dickinson |
| X40 | KLH | IgG1 | Purified Ig: 2·5,* 5·0 | Becton-Dickinson |
| MOPC-21 | None | IgG1-Tricolor | Purified Ig: 5·0* | Caltag Lab., San Fransisco, CA |
| Anti-IgD | δ-chain | Goat IgG-FITC | Purified Ig: 1/50,* 1/200 | Sigma |
| Anti-IgA | α-chain | Goat IgG-FITC | Purified Ig: 1/200* | Sigma |
| Anti-IgM | µ-chain | Goat IgG-FITC | Purified Ig: 1/50* | Sigma |
| R-505 | Cytokeratin | Rabbit Ig | Antiserum: 1/100 | Authors' laboratory |
Concentrations used for flow cytometry.
Flow cytometry
Cells were dispersed from the intestinal mucosa of nine jejunal resection specimens obtained from organ donors (median age 35 years, range 10–56 years) as previously described.15 In brief, the samples were rinsed in phosphate-buffered saline (PBS) and either used immediately or incubated on ice overnight with RPMI-1640 (Gibco, Paisley, UK) containing 5% (v/v) fetal calf serum (FCS) and Fungizone (0·25 µg/ml). After removal of the epithelium, mononuclear cells were obtained from the remaining mucosa by digestion of the tissue with a mixture of collagenase and dispase.
Paired immunostaining was performed in V-bottomed microtitre plates as follows: 0·05 × 106−0·25 × 106 cells in 50 µl medium (RPMI containing 2% FCS and 0·1% w/v NaN3) were incubated with mixtures (50 µl) of pretitrated primary antibody reagents (Table 1) for 15 min (4°, rocking shelf, in the dark), washed twice and then similarly incubated with mixtures of secondary reagents [phycoerythrin (PE) -conjugated goat anti-mouse IgG1 and FITC-conjugated goat anti-mouse IgG2a from Southern Biotechnology, Birmingham, AL]. In two experiments, three-colour staining was performed with addition of Cy-Chrome-conjugated anti-CD19 as a fourth incubation step after blocking with 10% mouse serum to examine the expression of markers only on B cells.
The cells were analysed within 1–2 hr in a Becton Dickinson FACScan with the lysys ii software analysis program. The instrument was calibrated with CaliBRITE™ beads (Becton Dickinson), compensation was set by eye, and 10 × 103 cells were acquired in list mode based on gating in the forward/side scatter dot plot.
Functional studies
To test proliferative responses and immunoglobulin production by isolated sIgA+ cells, dispersed mucosal samples (n = 6) were subjected to positive selection by magnetic beads or sorting on a FACS Vantage cell sorter (Becton Dickinson) as described below:
For positive bead selection, cells were incubated with mAb to IgA (clone 6E2C1, Table 1) in a small volume (10 × 106 cells in 0·5 ml) at 4° for 20 min on a rock and roller, then washed twice in PBS with 2% FCS (PBS/2%). Sheep anti-mouse IgG1-coated beads (Dynal, Oslo, Norway) were then added at a 1 : 1 bead-to-cell ratio, incubated as above, and bead-coated cells were removed with a magnet. The positively selected cells were grown in RPMI/20% at 37° and medium was exchanged every 2 hr. Before every exchange, bead-free cells were collected from the supernatant by magnet separation until the initial suspension was virtually free of cells, generally after three rounds of incubation in RPMI/20%. The purity of isolated cells was tested by flow cytometry and by immunostaining of cytospins. Then, 0·05 × 106 isolated sIgA+ cells were incubated (triplicates) in 96-well plates (Falcon, Becton Dickinson) together with different combinations of anti-light-chain monoclonal antibodies (mAbs) (anti-λ and anti-κ from Dako, Glostrup, Denmark, both used at 10 µg/ml), irradiated (70 Gy) CD40L-transfected L cells (a kind gift from Drs P. Garrone and J. Banchereau, Schering-Plough, France) at a 1 : 4 L cells-to-B-cells ratio, Pansorbin (0·005% v/v; from Calbiochem-Novabiochem Corp., La Jolla, CA) or no stimulus, in RPMI/10% for 5 days. Transforming growth factor-β (TGF-β; 0·6 ng/ml; from Pepro Tech EC Ltd, London, UK) and interleukin-10 (IL-10; 250 ng/ml; Pepro Tech) were added to all wells. Alternatively, fluorescence-activated cell sorter (FACS) sorted small sIgA+ cells (R1; see later) were similarly used. In three experiments, parallel wells from bead-selected cells were prepared with addition of recombinant human IL-2 (Amersham, Life Sciences, Buckinghamshire, UK), IL-4, IL-6, interferon-γ (IFN-γ; all from R & D Systems, Abingdon, UK), all at 10 ng/ml, or no added cytokines, to examine whether the IgA production could be influenced by such factors. Supernatants were harvested from wells with proliferative stimuli prior to pulsing individual wells with 1 µCi/well [3H]TdR for the last 16 hr of culture, then proliferation was tested by harvesting wells through Printer Filtermat A (Wallac, Turku, Finland) and counted in a 1205 Betaplate™ counter (Pharmacia/LKB, Piscataway, NL). Results were presented as the mean of three wells for every experimental condition. Production of IgA, IgM, and IgG in supernatants was examined by enzyme-linked immunosorbent assay (ELISA).
Results
Immunohistochemical observations
The results from immunohistochemical examinations are summarized in Table 2, and the distribution of various phenotypic markers is visualized in Fig. 1.
Table 2.
Expression of various markers on B cells in different human small intestinal mucosal compartments (Peyer′ spatches, PP; lamina propria, LP) evaluated by immunohistochemistry
| Marker | PP M-cell area | PP Dome area | PP Mantle zone | PP Marginal zone | PP Germinal centre | LP* |
|---|---|---|---|---|---|---|
| CD5 | −† | −† | −† | −† | −† | −† |
| CD19 | + | + | + | + | + | + |
| CD20 | + | − | + | + | + | −‡ |
| CD27 | −/+ | + | − | + | −/+ | + |
| CD28 | −† | −† | − | −† | − | − |
| CD38 | −/+ | + | − | − | −/+ | + |
| CD45RA | + | + | + | + | + | − |
| CD45RO | −§ | − | − | − | −† | −† |
| CDw78 | + | − | + | + | −/+ | − |
| HLA-DR | + | − | + | + | −/+ | − |
| IgD | −/+ | −§ | + | − | −/+ | −§ |
| IgM | +/− | + | + | −/+ | + | + |
| IgA | +/− | + | − | +/− | −/+ | + |
Most or all cells positive
most or all cells negative
more cells positive than negative
more cells negative than positive.
By immunohistochemistry, reproducible distinction between R1 and R2 cells in LP could not be performed
was positive on T cells
was positive on some cells
was positive on extremely few B cells.
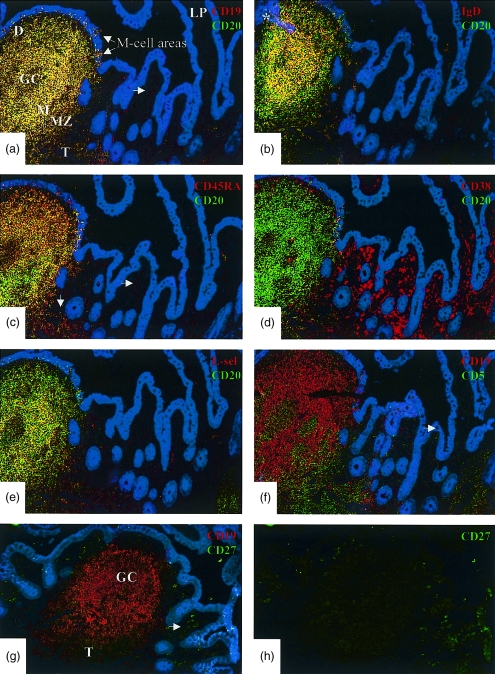
Figure 1.
Distribution of B-cell phenotypes in a Peyer's patch (PP) compared with adjacent lamina propria (LP) demonstrated by immunofluorescence staining. Photomicrographs depicting the periphery of the same Peyer's patch with adjacent villous lamina propria in serial sections (a–f) of a normal ileal biopsy specimen (original magnification × 250). Colour code of markers is indicated in each panel. Cytokeratin is stained blue to visualize the epithelium. Yellow indicates coexpression of markers. The topographic hallmarks are labelled in (a): D, dome; GC, germinal centre; M, mantle zone; MZ, marginal zone; and T, T-cell area. Note that the GC is only partially represented in these sections. Two M-cell areas in the follicle-associated epithelium above the dome are also shown. (a) Yellow cells expressing both CD19 and CD20 are present only in the follicle, dome area and M-cell pockets; occasional weakly stained red cells corresponding to B-cell blasts and plasma cells are seen in adjacent LP. (b) Yellow cells expressing CD20 and sIgD are present only in the follicle (section through mantle zone); green cells outside the follicle represent memory B cells in the marginal zone. Note that very few yellow (sIgD+ CD20+ naive) or green (IgD– CD20+ memory) cells are seen in the lamina propria. (The asterisk indicates area where the epithelium is accidentally overlaid on the follicle.) (c) Virtually all CD20+ cells coexpress CD45RA (yellow); occasional single CD45RA+ cells corresponding to the CD19+ cells in (a) are present in LP (horizontal arrow), and red cells at lower left represent naive T cells in PP (vertical arrow). (d) Co-staining for CD38 and CD20 shows that these markers are almost reciprocally expressed, in that most intrafollicular B cells bear no or low levels of CD38, whereas it is strongly expressed on large lamina propria cells representing B-cell blasts and plasma cells, as well as on a few germinal centre cells. (e) l-selectin is coexpressed on a fraction of CD20+ cells in the follicle as well as in M-cell pockets; red cells represent mainly naive T cells in PP (lower left). Note that very few l-selectin+ cells (CD20+ B cells or CD20– T cells) are present in LP. (f) Co-staining for CD19 and CD5 demonstrates lack of overlap between these markers, indicating that no intestinal B cells (i.e. only T cells) express CD5 as detected by this method. An intraepithelial CD5+ T cell is indicated (arrow). (g) Co-staining for CD19 and CD27 to visualize the distribution from PP to LP in the same biopsy specimen (note that this section as well as (h) were cut at a deeper level than those illustrated above). (h) Green separation of the same field. In the germinal centre and mantle zone, CD27 is only expressed by very few cells and therefore most of the follicle appears red when double-exposed. The follicle periphery, corresponding to the marginal zone, as well as the T-cell area (T), contain many CD27+ cells. By contrast, strongly CD27+ cells are present in adjacent LP, mostly representing plasma cells; CD19 expression on these cells is faint and they therefore appear brightly green.
GALT
B cells corresponding to the mantle and marginal zones of lymphoid follicles, as well as many cells in M-cell pockets and T-cell areas expressed CD19, CD20, CD45RA, HLA-DR and sIgD (or sIgA); in addition, many cells expressed l-selectin, as previously reported (Fig. 1a,b,c,e).11,15 In the germinal centres, B cells were positive for CD19 and CD20, the latter marker being considerably weaker than on mantle and marginal zone cells. Occasional rare CD19+ germinal centre cells appeared to coexpress CD45RO that was otherwise found only on T cells. No B cells positive for CD5 were detected (Fig. 1f).
The sIgD+ cells were also examined for coexpression of CD27; and occasional small such cells at the outer edges of germinal centres were found to be positive. CD27 was also found on sIgD– cells within the follicles, mainly corresponding to the marginal zones, as well as on T cells in the interfollicular areas (Fig. 1g,h). CDw78 was found only on HLA-DR+ B cells. CD38 was negative or weakly expressed by mantle and marginal zone lymphocytes and moderately positive on most germinal centre cells, although some at the latter reacted strongly (Fig. 1d). Plasma cells with cytoplasmic (c) IgA or cIgM, present along follicle peripheries and in the dome areas, also expressed high levels of CD38.
Small intestinal lamina propria
In jejunal specimens with normal morphology, as well as in the ileum outside GALT structures, very few CD20+ cells (< 1 per villus) were seen (Fig. 1a–e). They were small and usually expressed IgA as a small rim at the membrane (presumably sIgA); extremely few cells were sIgD+ (< 1 in every fifth villus; Fig. 1b). These rare B lymphocytes were also positive for CD19, CD45RA, CDw78 and HLA-DR but only weakly reactive for CD38 and negative for the T-cell memory marker CD45RO, as well as for CD28 and CD5 (Fig. 1f). Interestingly, all sIgA+ and most of the extremely rare lamina propria sIgD+ cells coexpressed CD27. Combined CD19 and CD20 immunostaining showed that cells with weak CD19 expression (corresponding to plasma cells; see later) were negative for CD20 (Fig. 1a).
Organized GALT structures (B-cell follicles with domes and T-cell areas) were not seen in any of the jejunal specimens, whereas solitary follicles or Peyer's patches (containing at least five aggregated follicles) were seen in all ileal samples. Virtually no cells expressing l-selectin were found in the lamina propria and naive T cells (CD45RA+) were almost absent as previously reported.22
Lamina propria cells with high CD38 levels (CD38hi; Fig. 1d) were large and expressed cytoplasmic and apparently sometimes surface immunoglobulin (usually IgA), high levels of CD27 (∼100%, Fig. 1g,h), often CD19 (> 50%; Fig. 1a), and sometimes also CD45RA (Fig. 1c). They were negative for CD20 (Fig. 1d), CDw78 and HLA-DR. IgD-producing plasma cells were extremely rare (< 1 cell in every fifth villus). Within the epithelium, no CD19+ cells were observed except in M-cell areas of GALT structures.
Flow-cytometric examination of dispersed lamina propria lymphoid cells
To obtain reliable quantitative data also for small lymphoid subsets that were difficult to evaluate by immunohistochemistry, flow cytometry was performed on dispersed cells from jejunal mucosa of organ donors. The data are summarized in Table 3.
Table 3.
Summary of flow-cytometric data obtained with dispersed mucosal cells, presented as percentage (median and range) of CD19+ cells in R1 (small cells) and of CD38hi cells in R2 (large cells)
| Marker | R1 | R2 |
|---|---|---|
| CD5 | 1·5 (0–3)* | 0·5 (0–1)* |
| CD19 | 12 (4–42)† | 50 (25–72) |
| CD27 | 70 (47–98) | 98 (98–100) |
| CD20 | 26 (7–48) | 0·5 (0–2) |
| CD40 | 75 (12–90) | 5 (0–20) |
| CD28 | 0·5 (0·5–1)* | 0·5 (0·5–1) |
| CD45RA | 95 (95–100) | 10 (5–35) |
| CD45RO | 1·5 (1–10)* | 2 (2–6)* |
| CDw78 | 79 (75–91) | 1·5 (1–3) |
| HLA-DR | 79 (75–92) | 1·5 (1–3) |
| HLA-DP | 80 (75–95) | 1·5 (1–3) |
| HLA-DQ | 48 (45–50) | 0·5 (0–0·5) |
| IgD | 10 (5–52) | 1 (0·5–1·5) |
| IgA | 73 (30–90) | 55 (46–56) |
| IgM | 8 (4–12) | 14 (6–20) |
Present on most T cells
based on total number of cells in R1.
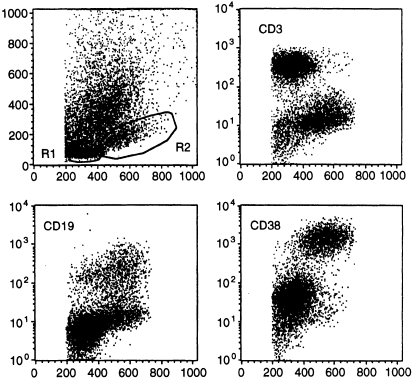
Based on gating in the forward/side-scatter dot plot, dispersed lamina propria cells were as previously reported15 separated into lymphocytes (small, R1) and blasts or plasma cells (large, R2) by their size and level of CD38 expression (Fig. 2). This distinction was arbitrary, because in the forward-scatter CD38 dot plot, some cells falling within the R1 gate always expressed high levels of CD38 (Fig. 2). Such CD38hi R1 cells could represent T or B cells. The fractions of B and T cells in R1 and R2 were nevertheless reproducibly different because T cells (CD3+) predominated (58–95%) in R1 whereas relatively few (< 10%) were present in R2 (Fig. 2). Conversely, while B cells (CD19+ or CD38hi) could be either very few or quite numerous in R1, they always predominated in R2 (Fig. 2, Table 3).
Figure 2.
Flow-cytometric definition of R1 and R2 cells in small intestinal mucosal samples. Dispersed mononuclear mucosal cells were obtained from organ donors and subjected to immunofluorescence staining for the respective markers by an indirect method as decribed in the Materials and Methods. Upper left panel shows gates (R1 and R2 cells) that were set on the basis of surface marker expression (y-axis; CD3, CD19, CD38) in relation to cell size (FSC, x-axis) on ungated populations. The cells falling outside R1 and R2 represent epithelial cells and dead cells. R1 cells (small) mainly consist of T cells (CD3+, upper right panel) with few cells expressing CD19 (lower right panel). R2 cells (large) mainly consist of B-cell blasts and plasma cells (CD38hi) with very few T cells (right panels); however, many large cells express CD19 (lower left). Some relatively small cells show rather strong CD38 expression and thus the distinction of R1 from R2 cells is not exact (lower right).
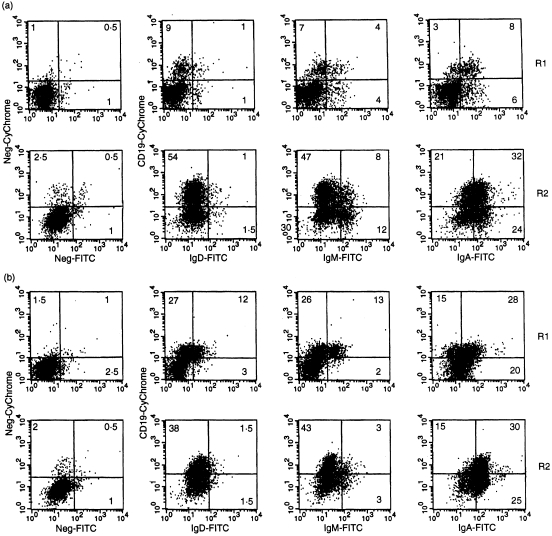
B cells (CD19+) in R1 usually expressed CD20, CD40, CD45RA, CDw78, HLA-DR, -DP and -DQ, as well as surface immunoglobulin, usually IgA (Table 3, Figs 3 and 4). Expression of CD80 and CD86 by these cells was quite low (I. N. Farstad, unpublished observations). In samples with few R1 B cells, < 5% expressed sIgD (< 1% of the total cell population) whereas in B-cell rich samples, up to 50% were sIgD+ (Fig. 3a,b). A very small fraction of the R1 sIgD+ cells (< 5%) expressed CD27, but in Peyer's patch samples up to 10% of the sIgD+ cells expressed this marker (I. N. Farstad, unpublished observations). However, occasional R1 cells were negative for most of these markers and then corresponded to the small CD38hi fraction as described above. In samples with many R1 B cells, including sIgD+ cells, a small fraction of B cells expressed CD45RO (Table 3, Fig. 4), a marker reported to be transiently present during terminal B-cell differentiation.23 Also l-selectin+ B cells and naive T cells could be found in such B-cell-rich samples (data not shown). Because immunohistochemistry revealed only very rare sIgD+, l-selectin+ and CD45RA+ T cells in the lamina propria outside GALT structures (Fig. 1b,c,e), this flow-cytometric finding most probably reflected that B-cell-rich samples were in fact contaminated by cells derived from solitary lymphoid follicles.
Figure 3.
Flow-cytometric analysis of R1 and R2 B-cell isotypes in small intestinal mucosal samples. Numbers in quadrants represent percentage positive cells. Left panels represent controls obtained with irrelevant primary antibodies conjugated to Tricolor (containing Cy5 and therefore similar to Cy-Chrome; y-axis) or FITC (x-axis). (a) Profiles of a sample with few B cells (10% CD19+ cells in R1): the main proportion of R1 CD19+ cells express sIgA while very few bear sIgD or sIgM. R2 cells are mainly sIgA+ and most cells in this sample also express CD19. In both R1 and R2 distinct fractions of cells without CD19 express surface immunoglobulin; this probably indicates that some of the immunoglobulin secreted from mature plasma cells is transiently membrane-bound [this also applies to (b)]. (b) Profiles of a sample with many B cells (42% CD19+ cells in R1): although sIgA+ cells dominate among the CD19+ cells, significant fractions of sIgD+ and sIgM+ cells are also present. R2 cells are mainly sIgA+ but fewer of these cells express CD19 than in (a).
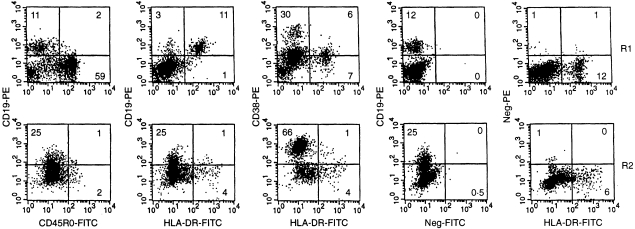
Figure 4.
Flow-cytometric examination of CD45RO, HLA-DR and CD38 on R1 and R2 B cells in B-cell-poor small intestinal mucosal sample. Numbers in quadrants represent percentage positive cells. Dispersed mononuclear cells were examined by flow cytometry and small (R1) versus large (R2) cells compared for the markers indicated. The fourth and fifth panels in the rows represent controls obtained with relevant versus irrelevant primary antibodies followed by similar secondary antibody conjugates. In the first panel row, very few R1 or R2 CD19+ cells express CD45RO, indicating that most cells expressing this marker are T cells. Conversely, most CD19+ cells in R1 express HLA-DR while very few R2 CD19+ cells are positive (second panel row). Few CD19+ HLA-DR– cells in R1 might reflect that distinction of R1 from R2 cells is not exact when based on size and CD38 (see Fig. 2), and these cells could in fact represent relatively small B-cell blasts. In the third panel row, CD38+ R1 cells are negative for HLA-DR while HLA-DR+ cells are negative or weakly positive for CD38. The latter cells represent CD19+ cells while the former represent a mixture of relatively small T- and B-cell blasts. In R2, HLA-DR is mainly present on very few CD38hi cells; the single HLA-DR+ cells in the lower right quadrant represent macrophages (see Results section).
R2 cells were mainly negative for CD20 but positive for CD27 (Table 3). A small fraction expressed CD45RA, CDw78, HLA-DR and HLA-DP, whereas most R2 cells were sIg+ and/or CD19+ (Table 3, Fig. 3a,b). CDw78 and HLA-DP were always present on HLA-DR+ cells, both with a linear relationship to HLA-DR, whereas HLA-DQ was mostly negative (Table 3). The HLA-DR+ cells in R2 negative for CD19 and CD38 (Fig. 4), represented macrophages.15
Extremely few B cells, and only < 20% of the sIgD+ ones present in the B-cell-rich samples, expressed CD5; the level was much lower than that on T cells which were always CD5+ (Table 3). A very small fraction both of R1 and R2 B cells expressed CD28, a molecule reported to identify B cells entering plasmacytoid differentiation.8 Their B-cell phenotype was confirmed with triple stainings for CD28, CD19 and HLA-DR; 0·5–1% of the CD19+ cells reproducibly expressed CD28, with or without concurrent HLA-DR (data not shown). As for CD5, however, CD28 was most abundant on T cells (Table 3).
Proliferation and immunoglobulin production by isolated sIgA+ cells
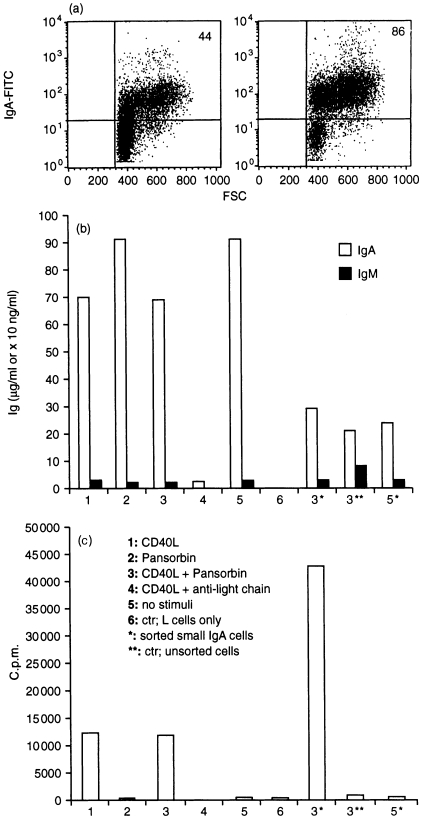
The proliferative and immunoglobulin-producing capacity of dispersed mucosal sIgA+ cells was examined in vitro. The purity of isolated cells was 70–90% as determined by flow-cytometric analysis with a goat anti-human IgA–FITC conjugate (Cat. no. 2050-02, Southern Biotechnology, 1 µg/ml; Fig. 5a), and in cytospins prepared from the isolated cells containing less than 2% CD3+ cells and less than 0·5% sIgD+ cells (data not shown). Bead selection for sIgA+ cells resulted in enrichment also of large (R2) cells (Fig. 5a), in accordance with the flow-cytometric data showing that many R2 cells expressed surface immunoglobulin (Fig. 3a,b). However, all samples investigated incorporated thymidine as a sign of proliferation in response to CD40 ligation. Addition of the polyclonal B-cell activator Pansorbin, which consists of formalinized particles of Staphylococcus aureus, strain Cowan I, had little effect on this CD40-induced proliferation (one representative experiment is shown in Fig. 5c). Samples in which many sIgD+ cells (> 3% in R1) were present in the starting population (i.e. before isolation of sIgA+ cells), tended to give larger counts per minute (c.p.m.) values, possibly reflecting a higher proliferative potential of contaminating GALT sIgA+ cells. Pansorbin alone, or a combination of surface immunoglobulin cross-linking and CD40 ligation, had minimal effect on proliferation; the latter protocol induced cell death as deemed by morphology in cytospins, probably explaining why immunoglobulin production was also minimal (Fig. 5b,c). Similar results were also obtained when the anti-light-chain antibodies were immobilized overnight (data not shown). Addition of Pansorbin alone failed to induce increased immunoglobulin production compared with unstimulated B cells (Fig. 5b).
Figure 5.
Examination of isolated sIgA+ cells. (a) Enrichment of sIgA+ cells from dispersed starting population (left) and after bead selection for sIgA+ cells (right) shown by flow cytometry as described in the Materials and Methods. Numbers in upper right corners indicate percentage positive cells (ungated). (b) Immunoglobulin production (µg/ml) by bead-selected and sorted versus unsorted sIgA+ cells as described in the Materials and Methods, subjected to different stimuli indicated by numbers. Note that the scale for sorted* and unsorted** cells is different (× 10 ng/ml) from that for bead-selected cells (µg/ml). (c) Proliferation (c.p.m. values) by bead-selected and sorted versus unsorted sIgA+ cells subjected to different stimuli indicated by numbers.
As expected, sorted sIgA+ cells within R1 (excluding B-cell blasts and plasma cells, as explained above) showed higher proliferation but much less immunoglobulin production than bead-separated cells (Fig. 5b,c). Sorting resulted in significantly lower numbers of cells available for culture; therefore, CD40L and Pansorbin treatment was compared only with unstimulated B cells and with results obtained for unfractionated (unsorted) lamina propria cells (Fig. 5b,c, asterisks).
Based on ELISA, the purity of sIgA+ cells versus other B-cell isotypes was supported by the disproportionately higher production of IgA than IgM (or IgG) in all samples (IgA and IgM produced by bead-selected, sorted and unsorted cells is shown in Fig. 5b). Addition of CD40L always resulted in lower levels of immunoglobulin being produced (Fig. 5b). Wells containing IL-2, IL-4, IL-6, IFN-γ, TGF-β, IL-10, or medium only, gave approximately similar IgA concentrations as wells containing both TGF-β and IL-10 (data not shown).
Discussion
It has been known since 1965 that IgA-producing plasma cells predominate in normal human intestinal lamina propria,24 and since 1979 that T cells outnumber sIgA+ cells in dispersed mucosal cell populations from the gut.25,26 However, the detailed composition of B lymphocytes, B-cell blasts and plasma cells within the intestinal lamina propria as opposed to organized GALT structures has not been reported. Such data may also throw light on the maturational stage at which primed B cells enter the lamina propria from peripheral blood.27
By combining results obtained with immunohistochemistry on mucosal biopsy specimens and data based on flow cytometry of dispersed mucosal mononuclear cells, we found that small (R1) B lymphocytes, the naive sIgD+ CD27– ones in particular, constitute only minor fractions of the total lamina propria mononuclear cell population (< 10% and < 1%, respectively). In contrast, B-cell blasts and plasma cells (R2) amounted to ∼30%. Importantly, however, the fraction of R1 B lymphocytes varied considerably among samples, the B-cell-rich ones containing substantial numbers of sIgD+ naive B cells (Fig. 3b) in addition to CD45RA-naive T cells and l-selectin+ naive B and T cells (data not shown). By immunohistochemistry, we could document that the latter subsets were virtually absent in the lamina propria outside GALT structures where they were well represented (Fig. 1). Taken together, our data suggested that cell populations presumed to be derived from the lamina propria, could be heavily contaminated with GALT cells, probably originating from solitary B-cell follicles known to occur scattered in both the jejunal and ileal mucosa.28 Thus, it is of outmost importance so as to avoid confusion in this field, that the sampling problems existing in the gut be borne in mind when studies aiming at characterization of dispersed lamina propria cells are performed.
Our data supported previous reports that CD45RO, a marker usually identifying memory T cells, may be expressed during terminal B-cell differentiation to plasma cells;23 we found that up to 2% of CD19+ or CD38hi cells were positive for CD45RO in B-cell rich samples. In situ, coexpression of CD19 and CD45RO was found only in the germinal centres; therefore, the presence of CD19+ CD45RO+ cells in B-cell rich samples suggested that they were contaminated with GALT cells.
We identified a consistent fraction (5–10%) of singly distributed small B lymphocytes bearing sIgA, CD20, CD27, CD45RA and HLA class II molecules, which we assumed represented memory B cells from the lamina propria. This subset expressed low levels of CD40 (Table 3) and was shown to proliferate in response to CD40 ligation but not in response to the polyclonal B-cell activator Pansorbin (Fig. 5c). Combined surface immunoglobulin cross-linking and CD40 ligation induced cell death despite the addition of TGF-β and IL-10 to the cultures; the explanation might be lack of T cells producing other important growth factors or lack of essential stromal elements. Nevertheless, the data raised the possibility that such lamina propria memory sIgA+ cells are capable of mounting a topical immune response to recall antigens at the effector site. This would be in keeping with previous reports that B-cell immune responses can be enhanced and even elicited29 outside Peyer's patches,16,17 although those experiments did not preclude a possible contribution from solitary lymphoid follicles. The bead-separated sIgA+ mucosal cells, which represented a mixture of memory cells and blasts, produced high levels of IgA in vitro (Fig. 5b). This production was virtually unaffected by the addition of Pansorbin, but less IgA was found in wells containing CD40L-transfected L cells, probably because cells expressing CD40 were driven into proliferation. As our cultures contained variable proportions of memory B cells and blasts, the latter expressing little CD40 and being responsive to increased immunoglobulin production by IL-10 stimulation, the relative influence of CD40L, Pansorbin, and cytokines on immunoglobulin production could not be addressed. Sorted memory cells stimulated with Pansorbin tended to produce more immunoglobulin than unstimulated cells, despite the presence of CD40L (Fig. 5b), but further experiments will be needed to clarify this issue.
R2 cells consisted of CD38hi CD20– cells, with or without CD19 and surface immunoglobulin; very few of them had retained CD45RA and HLA class II molecules. Because this B-cell phenotype has been identified also in human intestinal lymph,30 presumably on its way to the intestinal lamina propria, we believe that CD45RA and class II molecules are down-regulated at intestinal effector sites. Indeed, immunohistochemistry revealed CD45RA to be weakly expressed on some CD38hi or CD19+ lamina propria plasma cells (Fig. 1c) while class II molecules were hard to identify with this method. Among the class II molecules investigated, HLA-DP was shown by flow cytometry to be down-regulated along with HLA-DR, while HLA-DQ was virtually negative on these cells (Table 3). CD28, a molecule usually associated with T cells, has been reported to be expressed also by plasma cells8 and circulating immunoglobulin-producing cells generated by oral immunization.31 We observed CD28 only on a very small fraction of R2 cells, and on even fewer R1 cells, by flow cytometry, and could not detect it by immunohistochemistry. This discrepancy might reflect that CD28 is expressed only very transiently during plasma cell differentiation in human intestinal mucosa. By contrast, CD27 was present on virtually all cytoplasmic immunoglobulin-positive or sIgD– B cells in the lamina propria as judged by immunohistochemistry; therefore, it seemed to be a good marker both for memory B cells, B-cell blasts and plasma cells in the human small intestine. The rarely detectable sIgD+ CD27+ cells might be on their way to terminal differentiation.
CD5 was not present on lamina propria B cells and we found this marker expressed only at low levels on few GALT sIgD+ cells by employing two distinct mAbs to CD5 (Table 1). Conversely, Peters et al.18 reported that a substantial fraction of human intestinal IgA-producing cells in fact expressed CD5. Also circulating antibody-forming cells generated after oral immunization were reported to express CD5, and especially those producing anti-tetanus toxoid.31 CD5+ B cells constitute a relatively larger fraction in young individuals,19 but we found no CD5-expressing B cells in the lamina propria of individuals down to the age of 5 years. This discrepancy could not be due to methodological problems because compared with other reports,19,32 we observed the expected proportions of CD5+ tonsillar B cells (Farstad et al. unpublished data). Incubation of dispersed mucosal or tonsillar cells overnight with 20% normal human serum, as was done in the study of Peters et al.18 had no effect on CD5 expression either by mucosal sIgA+ cells or by tonsillar sIgD+ cells (data not shown). In mice, many intestinal B cells (B1 cells) represent the progeny of B cells derived from the peritoneum,5 and the human fetal omentum as well as lavage fluid from the adult human peritoneal cavity contain a substantial proportion of CD5+ B lymphocytes.33,34 Therefore, although our data suggested that CD5+ effector B cells do not occur in the human small intestine, this finding did not preclude the existence of B1 B cells as a separate lineage at this tissue site.
CDw78 was shown to be present only on HLA-DR+ B cells in the human small intestine. By flow cytometry, CDw78 showed a linear relationship to HLA-DR both on R1 CD19+ and on a fraction of R2 CD38hi cells, as did HLA-DP. These findings agreed with recently published data showing that CDw78 is associated with HLA-DR and -DP35 and may act as a co-stimulatory molecule on B cells.36
In conclusion, we have shown that dispersed lymphoid cells sampled to represent the small intestinal lamina propria, often were heavily contaminated with cells derived from organized GALT structures. Furthermore, we have identified a small population of scattered sIgA+ CD20+ CD27+ CD40+ CD38lo CD45RA+ HLA class II+ lamina propria cells that probably serve as local memory cells for recall antigens and not only as effector cells destined for terminal plasma cell differentiation. In contrast, CD20–CD27+ CD40–CD38hi B cells, which amounted to approximately one-third of the lamina propria mononuclear cells, constituted blasts that probably had recently arrived in the lamina propria as they expressed CD19, CD28, CD45RA, HLA class II as well as surface immunoglobulin (usually IgA), in addition to terminally differentiated plasma cells that mostly lacked these markers.
Acknowledgments
This work was supported by the Research Council of Norway, The Norwegian Cancer Society and Anders Jahres Fund. The skilful assistance provided by the technical staff at LIIPAT is greatfully acknowledged. Dr S. Funderud is thanked for kindly providing the FN-1 mAb.
References
- 1.Brandtzaeg P, Farstad IN, Johansen F-E, Morton HC, Norderhaug IN, Tamanaka T. The B-cell system of human mucosae and exocrine glands. Immunol Rev. 1999;171:45–87. doi: 10.1111/j.1600-065X.1999.tb01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craig SW, Cebra JJ. Peyer's patches: an enriched source of precursors for IgA-producing immunocytes in the rabbit. J Exp Med. 1971;134:188–200. doi: 10.1084/jem.134.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunn-Walters DK, Isaacson PG, Spencer J. Sequence analysis of human IgVH genes indicates that ileal lamina propria plasma cells are derived from Peyer's patches. Eur J Immunol. 1997;27:463–7. doi: 10.1002/eji.1830270217. [DOI] [PubMed] [Google Scholar]
- 4.Mizoguchi A, Mizoguchi E, Chiba C, Bhan AK. Role of appendix in the development of inflammatory bowel disease in TCR-α mutant mice. J Exp Med. 1996;184:707–15. doi: 10.1084/jem.184.2.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kroese FG, de Waard F, Bos NA. B-1 cells and their reactivity with the murine intestinal microflora. Semin Immuno. 1199;6:11–18. doi: 10.1006/smim.1996.0003. [DOI] [PubMed] [Google Scholar]
- 6.MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–39. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 7.Liu YJ, Zhang J, Lane PJ, Chan EY, MacLennan IC. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur J Immunol. 1991;21:2951–62. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- 8.Banchereau J, Rousset F. Human B lymphocytes: phenotype, proliferation and differentiation. Annu Rev Immunol. 1992;52:125–262. doi: 10.1016/s0065-2776(08)60876-7. [DOI] [PubMed] [Google Scholar]
- 9.Arpin C, Dechanet J, Van Kooten C, Merville P, Grouard G, Briere F, Liu YJ. Generation of memory B cells and plasma cells in vitro. Science. 1995;268:720–2. doi: 10.1126/science.7537388. [DOI] [PubMed] [Google Scholar]
- 10.Klein U, Rajewsky K, Kuppers P. Human immunoglobulin (Ig) M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679–89. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spencer J, Finn T, Isaacson PG. Human Peyer's patches: an immunohistochemical study. Gut. 1986;27:405–10. doi: 10.1136/gut.27.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spencer J, Finn T, Pulford KA, Mason DY, Isaacson PG. The human gut contains a novel population of B lymphocytes which resemble marginal zone cells. Clin Exp Immunol. 1985;62:607–12. [PMC free article] [PubMed] [Google Scholar]
- 13.Bjerke K, Brandtzaeg P. Immunoglobulin- and J chain-producing cells associated with lymphoid follicles in the human appendix, colon and ileum, including Peyer's patches. Clin Exp Immunol. 1986;64:432–41. [PMC free article] [PubMed] [Google Scholar]
- 14.Bjerke K, Brandtzaeg P. Terminally differentiated human intestinal B cells. IgA and IgG subclass-producing immunocytes in the distal ileum, including Peyer's patches, compared with lymph nodes and palatine tonsils. Scand J Immunol. 1990;32:61–7. doi: 10.1111/j.1365-3083.1990.tb02894.x. [DOI] [PubMed] [Google Scholar]
- 15.Farstad IN, Halstensen TS, Lazarovits AI, Norstein J, Fausa O, Brandtzaeg P. Human intestinal B-cell blasts and plasma cells express the mucosal homing receptor integrin α4β7. Scand J Immunol. 1995;42:662–72. doi: 10.1111/j.1365-3083.1995.tb03709.x. [DOI] [PubMed] [Google Scholar]
- 16.Husband AJ. Kinetics of extravasation and redistribution of IgA-specific antibody-containing cells in the intestine. J Immunol. 1982;128:1355–9. [PubMed] [Google Scholar]
- 17.Ogra PL, Karzon DT. The role of immunoglobulins in the mechanism of mucosal immunity to virus infection. Pediatr Clin North Am. 1970;17:385–400. doi: 10.1016/s0031-3955(16)32417-8. [DOI] [PubMed] [Google Scholar]
- 18.Peters MG, Secrist H, Anders KR, Nash GS, Rich SR, MacDermott RP. Normal human intestinal B lymphocytes. Increased activation compared with peripheral blood. J Clin Invest. 1989;83:1827–33. doi: 10.1172/JCI114088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kasaian MT, Casali P. Autoimmunity-prone B-1 (CD5 B) cells, natural antibodies and self recognition. Autoimmunity. 1993;5:315–29. doi: 10.3109/08916939309115755. [DOI] [PubMed] [Google Scholar]
- 20.Beagley KW, Murray AM, McGhee JR, Eldridge JH. Peritoneal cavity CD5 (Bla) B cells: cytokine induced IgA secretion and homing to intestinal lamina propria in SCID mice. Immunol Cell Biol. 1995;73:425–32. doi: 10.1038/icb.1995.66. [DOI] [PubMed] [Google Scholar]
- 21.Lycke N. Expanding our knowledge in mucosal immunology. Immunologist. 1999;7:35–8. [Google Scholar]
- 22.Halstensen TS, Scott H, Brandtzaeg P. Human CD8+ intraepithelial T lymphocytes are mainly CD45RA–RB+ and show increased co-expression of CD45RO in celiac disease. Eur J Immunol. 1990;20:1825–30. doi: 10.1002/eji.1830200829. [DOI] [PubMed] [Google Scholar]
- 23.Jensen GS, Poppema S, Mant MJ, Pilarski LM. Transition in CD45 isoform expression during differentiation of normal and abnormal B cells. Int Immunol. 1989;1:229–36. doi: 10.1093/intimm/1.3.229. [DOI] [PubMed] [Google Scholar]
- 24.Crabbé PA, Carbonara AO, Heremans JF. The normal human intestinal mucosa as a major source of plasma cells containing A-immunoglobulin. Lab Invest. 1965;14:235–48. [PubMed] [Google Scholar]
- 25.Bookman MA, Bull DM. Characteristics of isolated intestinal mucosal lymphoid cells in inflammatory bowel disease. Gastroenterology. 1979;77:503–10. [PubMed] [Google Scholar]
- 26.Goodacre R, Davidson R, Singal D, Bienenstock J. Morphologic and functional characteristics of human intestinal lymphoid cells isolated by a mechanical technique. Gastroenterology. 1979;76:300–8. [PubMed] [Google Scholar]
- 27.Parrott DMV. The gut as a lymphoid organ. Clin Gastroenterol. 1976;5:211–27. [PubMed] [Google Scholar]
- 28.Moghaddami M, Cummins A, Mayrhofer G. Lymphocyte-filled villi: comparison with other lymphoid aggregations in the mucosa of the human small intestine. Gastroenterology. 1998;115:1414–25. doi: 10.1016/s0016-5085(98)70020-4. [DOI] [PubMed] [Google Scholar]
- 29.Hamilton SR, Keren DF, Yardley JH, Brown G. No impairment of local intestinal immune responses to keyhole limpet haemocyanin in the absence of Peyer's patches. Immunology. 1981;42:431–5. [PMC free article] [PubMed] [Google Scholar]
- 30.Farstad IN, Norstein J, Brandtzaeg P. Phenotypes of B and T cells in human intestinal and mesenteric lymph. Gastroenterology. 1997;112:163–73. doi: 10.1016/s0016-5085(97)70231-2. [DOI] [PubMed] [Google Scholar]
- 31.Quiding-Jarbrink M, Lakew M, Nordstrom I, Banchereau J, Butcher EC, Holmgren J, Czerkinsky C. Human circulating specific antibody-forming cells after systemic and mucosal immunizations: differential homing commitments and cell surface differentiation markers. Eur J Immunol. 1995;25:322–7. doi: 10.1002/eji.1830250203. [DOI] [PubMed] [Google Scholar]
- 32.Holder MJ, Abbot SD, Milner AE, Gregory CD, Casamayor M, Johnson GD, MacLennan ICM, Gordon J. IL-2 expands and maintains IgM plasmablasts from a CD5+ subset contained within the germinal centre cell-enriched (surface IgD–/CD39– buoyant) fraction of human tonsil. Int Immunol. 1993;5:1059–66. doi: 10.1093/intimm/5.9.1059. [DOI] [PubMed] [Google Scholar]
- 33.Solvason N, Kearney JF. The human fetal omentum: a site of B cell generation. J Exp Med. 1992;175:397–404. doi: 10.1084/jem.175.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donze HH, Lue C, Julian BA, Kutteh WH, Kantele A, Mestecky J. Human peritoneal B-1 cells and the influence of continuous ambulatory peritoneal dialysis on peritoneal and peripheral blood mononuclear cell (PBMC) composition and immunoglobulin levels. Clin Exp Immunol. 1997;109:356–61. doi: 10.1046/j.1365-2249.1997.4541352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drbal K, Angelisova P, Rasmussen AM, Hilgert I, Funderud S, Horejsi V. The nature of the subset of MHC class II molecules carrying the CDw78 epitopes. Int Immunol. 1999;11:491–8. doi: 10.1093/intimm/11.4.491. [DOI] [PubMed] [Google Scholar]
- 36.Rasmussen AM, Horejsi V, Levy FO, et al. CDw78 – a determinant on a major histocompatibility complex class II subpopulation that can be induced to associate with the cytoskeleton. Eur J Immunol. 1997;27:3206–13. doi: 10.1002/eji.1830271218. [DOI] [PubMed] [Google Scholar]