Abstract
CD22 is a B-cell-restricted transmembrane protein, which acts as a negative regulator of B-cell signalling. CD22 also has lectin-like adhesive properties. When expressed on transfected fibroblasts, it is capable of mediating adhesion to other cells via recognition of cell-surface glycoconjugates terminating in α2,6-linked sialic acids. In previous studies in the mouse, CD22 was implicated as a bone marrow homing receptor for recirculating immunoglobulin D+ (IgD+) B cells through recognition of sialylated ligands on marrow sinusoidal endothelium. As the adhesive function of CD22 can be masked when α2,6-linked sialic acids are co-expressed at the cell surface, the aim of the present study was to investigate whether recirculating B cells have unmasked forms of CD22 that could be involved in bone marrow homing. Using α2,6-sialyllactose coupled to biotinylated polyacrylamide as a probe for detection of unmasked CD22, we showed that ≈ 2–5% of IgD+ murine B cells in the spleen and mesenteric lymph nodes were able to bind this synthetic ligand. In the bone marrow, however, the fraction of IgD+ B cells with unmasked CD22 was increased by two- to fivefold. B cells from CD22-deficient mice were not stained with the polyacrylamide probe, confirming that staining of B cells in wild-type mice was caused by CD22 and not by other potential sialic acid-binding lectins. In conclusion, we have identified a new subset of mature B cells in the mouse with unmasked CD22. This subset of recirculating B cells may bind to CD22 ligands on bone marrow sinusoidal endothelium, leading to their selective homing and subsequent enrichment in this tissue.
Introduction
CD22 is a B-cell-specific member of the immunoglobulin superfamily with seven immunoglobulin-like domains. It is found on the B-cell surface from the pre-B-cell stage up to the stage of mature B cells, but is lost from the surface as B cells further differentiate into plasma cells. CD22 is also referred to as Siglec-2, as it is a member of the Siglec family1 of sialic acid (Sia)-binding immunoglobulin-like lectins that includes sialoadhesin (Siglec-1), CD33 (Siglec-3), myelin-associated glycoprotein (Siglec-4), Siglec-5, Siglec-6, Siglec-7, Siglec-8 and Siglec-9.1–10
The best understood function of CD22 is as a negative regulator of B-cell receptor (BCR) signalling (reviewed in ref. 11). After BCR stimulation, CD22 is tyrosine phosphorylated on its cytoplasmic tail, resulting in recruitment and activation of SRC homology 2 domain containing tyrosine phosphatase (SHP-1), a tyrosine phosphatase that acts as an inhibitor of several signalling pathways in lymphocytes.12–15 The biological functions of CD22 in vivo were investigated by several groups using CD22-deficient (CD22–/–) mice.16–19 Although B-cell development in these mice was largely unaltered, B cells were in a hyperactivated state owing to an increased and prolonged Ca2+ signal after BCR stimulation. CD22-deficient B cells showed an increased rate of apoptosis in vitro and a shorter lifespan in vivo. However, the immune status of CD22–/– mice and responses to antigenic challenges were largely normal. Although studies with the CD22–/– mice revealed an important role for CD22 in modulating the strength of the BCR signal and thereby controlling the signalling threshold, they did not uncover the biological function of the lectin-like domain of CD22.16–19
The lectin and cell-binding properties of human and mouse CD22 have been extensively investigated by using either soluble chimeric forms of CD22 containing the extracellular immunoglobulin-like domains fused to the Fc region of human IgG (CD22-Fc) or by transfecting full-length CD22 into monkey COS cells or Chinese hamster ovary (CHO) cells. These recombinant forms of CD22 mediated Sia-dependent binding to various cell types, including erythrocytes, B cells, T cells and activated endothelial cells.3,4,20–23 Specificity studies showed that CD22 has an absolute requirement for α2,6-linked Sia, preferably on multiantennary N-linked oligosaccharide chains.3,24,25 The presence of this target motif is dependent on expression of the α2,6 sialyltransferase, ST6GalI, which transfers Sia to the non-reducing termini of N-glycans.26,27 The gene encoding ST6GalI contains several distinct promoters and is highly regulated in different cell types, especially B lymphocytes, hepatocytes and endothelial cells.23,28,29
While CD22 can mediate Sia-dependent binding to cells as a purified protein or on transfected fibroblasts, binding to target cells is lost when the receptor is expressed on cells bearing α2,6-linked Sia.23,30,31 Owing to the low expression of ST6GalI, COS cells and CHO cells do not normally display N-glycans with α2,6-linked Sia and this explains why CD22 is able to mediate cell–cell adhesion when expressed ectopically on these cells. Indeed, the co-expression of CD22 and ST6GalI in CHO and COS cells results in the abrogation of any CD22-dependent binding.23,30 In comparison, B cells show a regulated expression pattern of the ST6GalI enzyme, and usually display high levels of α2,6-linked Sia on the cell surface.23,27,31–33 The issue of whether CD22 on B cells can mediate interactions with exogenously added ligands was addressed recently by analysing the binding of human peripheral blood B cells to a synthetic probe consisting of α2,6-sialyllactose coupled to polyacrylamide. It was found that the Sia-binding activity of CD22 was undetectable in resting B cells but was markedly increased on a subset of B cells after activation.31 Although the molecular basis for unmasking CD22 on activated B cells was not determined, possibilities include the activation-induced down-regulation of ST6GalI or increased activity of a sialidase. Both would be predicted to result in reduced surface levels of α2,6-linked Sia and an increased Sia-dependent binding activity of CD22.
To date, the best evidence that CD22 can mediate Sia-dependent adhesion to other cells in vivo comes from studies of CD22–/– mice and the identification of CD22 ligands on bone marrow endothelial cells. First, it was shown that CD22–/– mice had a strongly reduced population of mature recirculating B cells in the bone marrow.16 These are mostly long-lived, resting, immunoglobulin D+ (IgD+) B cells that have undergone the last step of their maturation in the periphery and then circulated back to various organs, among them the bone marrow. Second, using a soluble recombinant form of murine CD22 containing the first three domains of CD22 fused to the Fc region of human immunoglobulin G1 (IgG1) (CD22-Fc), sialylated ligands for CD22 were shown to be expressed on sinusoidal endothelium of the murine bone marrow, but not on endothelial cells in other tissues examined.34 Injected CD22-Fc was able to bind bone marrow endothelium in vivo and this treatment inhibited accumulation of B cells in the bone marrow by ≈ 50%, whereas there was no effect on numbers of B cells in the spleen. CD22–/– mice were also shown to have a lower number of immunoglobulin M (IgM)-secreting plasma cells in the bone marrow compared with wild-type mice. Taken together, these results suggest that CD22 functions as a bone marrow-specific homing receptor for recirculating B cells that contain precursors for IgM-secreting plasma cells.34 However, for this hypothesis to be correct, it would be expected that the recirculating B cells that home to the bone marrow would be able to bind sialylated ligands on endothelium and hence have unmasked forms of CD22 at the cell surface. Here we demonstrate that a minor subset of mature B cells is able to bind CD22 ligands and that this subset is significantly enriched in the bone marrow as compared to the lymph node and spleen.
Materials and methods
Animals
Female BALB/C and C57BL/6 mice were obtained from Charles River, Kent, UK and used at 6–9 weeks of age. Age-matched female CD22–/– mice on a C57BL/6 background16 were also used in one experiment.
Reagents
Unless indicated otherwise, all reagents were purchased from Sigma (Poole, Dorset, UK). Biotinylated polyacrylamide (PAA) glycoconjugates carrying either NeuAcα2,3Galβ1,4Glc (α2,3-PAA) or NeuAcα2,6Galβ1,4Glc (α2,6-PAA) were purchased from Syntesome (Munich, Germany). These conjugates have a molecular mass of ≈ 30 kDa and on a molar basis contain 20% of saccharide and 5% biotin. Rat monoclonal antibody (mAb) RA3.6B2 anti-B220-phycoerythrin (PE), rat mAb 11-26c.2a anti-mouse IgD and mouse mAb CY34 anti-CD22-PE were purchased from Pharmingen (San Diego, CA). Streptavidin conjugated to fluorescein isothiocyanate (FITC) and Vectorshield mountant were from Vector Laboratories (Peterborough, Cambridgeshire, UK). Arthobacter ureafaciens sialidase was purchased from ICN (Basingstoke, Hampshire, UK).
Cell preparation
COS cells were transiently transfected by electroporation with cDNAs encoding either full-length mouse CD223 or mouse sialoadhesin2 or were sham-transfected with phosphate-buffered saline (PBS) in place of DNA. After 4 days, cells were lifted with PBS containing 5 mm EDTA and 0·2% bovine serum albumin (BSA), and resuspended at 5 × 106 cells/ml. Bone marrow, lymph node and spleens were dissected from killed mice. The tissues were mechanically disrupted, filtered through a 0·1-mm cell strainer (Fred Baker, Cheshire, UK) and resuspended as a single cell suspension at 5 × 107 cells/ml.
Labelling and analysis of cells
All incubations were performed at 4°. Aliquots (100 µl) of the transfected COS cells and murine cell populations were distributed amongst wells of 96-well plates. The cells were then incubated with 10 µg/ml of either α2,6-PAA or α2,3-PAA for 1 hr, washed and then incubated in saturating concentrations of streptavidin-FITC for 1 hr. For double-labelling experiments, cells were incubated with anti-CD22-PE, anti-B220-PE or anti-IgD for 1 hr at 4°. For IgD staining, cells were then incubated with anti-rat tetramethylrhodamine isothiocyanate (TRITC) conjugate. Cells were fixed in 2% formaldehyde and analysed using a Zeiss Axioplan fluorescent microscope equipped with a × 100 objective. Approximately 2000 cells were routinely scored in duplicate for both single- and double-labelling experiments. Significance was assessed using the two-tailed Student's t-test. For confocal microscopy, cells were stained and fixed as described above and then analysed using a Zeiss 410 laser-scanning confocal microscope equipped with a × 64 objective.
Results
Detection of unmasked CD22 on B cells with α2,6-PAA
To analyse the Sia-dependent binding activity of CD22 on the surface of murine B cells, we used α2,6-PAA as a probe, as described previously for human B cells.31 The sensitivity and specificity of this synthetic probe was first tested in binding assays with monkey COS cells transiently transfected with full-length murine CD22. COS cells were chosen because they have been used extensively in the past for studying the adhesive properties of CD22 and do not express α2,6-linked Sia that masks the binding site on CD22.3,21,22,35 Under the conditions used, ≈ 20% of COS cells expressed surface CD22, as assessed by fluorescence microscopy (Table 1) and a similar percentage of cells was brightly stained when parallel binding assays were performed with α2,6-PAA (Table 1). This shows that the α2,6-PAA probe was able to efficiently detect unmasked forms of CD22 expressed at the cell surface. Binding of this reagent was specific, because only background staining was observed with α2,3-PAA, a similar synthetic glycoconjugate that does not bind CD22, but does bind other Siglecs, such as sialoadhesin36 (Table 1).
Table 1.
Detection of unmasked forms of CD22 on transfected COS cells and lymphoid cell suspensions
| Cell population | % Of cells positive for CD22 | % Of cells positive for α2,6-PAA | % Of cells positive for α2,3-PAA |
|---|---|---|---|
| CD22-transfected COS cells | 20·0 ± 6·2 | 21·0 ± 6·4 | 2·6 ± 0·7 |
| Sham-transfected COS cells | 0·9 ± 0·1 | 1·4 ± 0·1 | 1·0 ± 0·2 |
| Bone marrow | 4·7 ± 1·8 | 0·9 ± 0·1 | 0·3 ± 0·2 |
| Lymph node | 12·2 ± 2·5 | 1·0 ± 0·1 | 0·1 ± 0·1 |
| Spleen | 21·0 ± 7·0 | 1·0 ± 0·1 | 0·4 ± 0·2 |
The indicated cell populations were incubated with NeuAcα2,6Galβ1,4Glc coupled to biotinylated polyacrylamide (α2,6-PAA) or NeuAcα2,3Galβ1,4Glc coupled to biotinylated polyacrylamide (α2,3-PAA) and binding revealed with streptavidin-coupled fluorescein isothiocyanate (FITC). Separate samples of cells were stained for surface CD22 expression using anti-CD22 monoclonal antibody (mAb) followed by anti-mouse-FITC antibody. The percentage of cells staining positively for each marker when analysed by fluorescence microscopy is shown. CD22 bound specifically to α2,6-PAA but did not bind α2,3-PAA. In other experiments, COS cells transfected with mouse sialoadhesin cDNA were found to bind specifically to the α2,3-PAA probe (results not shown). Data represent mean values ± SD from four to 18 experiments.
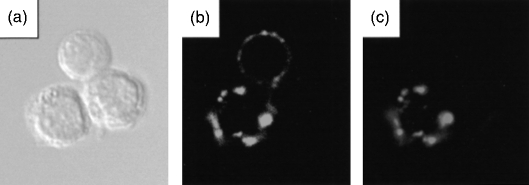
We next analysed binding of PAA conjugates to cell suspensions prepared from the bone marrow, spleen and mesenteric lymph nodes of BALB/c mice (Table 1; Fig. 1). Irrespective of the tissue, we found that the α2,6-PAA conjugate bound to ≈ 1% of the isolated cells, whereas the α2,3-PAA conjugate bound to only ≈ 0·1–0·4% of the cells (Table 1). Staining with α2,6-PAA was usually observed as punctate blebs at the cell surface (Fig. 1c) and was considerably weaker than the staining observed with transfected COS cells (results not shown). The cells labelled with α2,6-PAA had the morphological appearance of small lymphocytes (Fig. 1a). In contrast, the small proportion of bone marrow, spleen and lymph node cells labelled with α2,3-PAA had a granular appearance, suggesting that they were myeloid cells (data not shown).
Figure 1.
Double labelling of B cells and their analysis using confocal microscopy. Cell suspensions from mouse mesenteric lymph node were stained with NeuAcα2,6Galβ1,4Glc coupled to biotinylated polyacrylamide (α2,6-PAA) followed by streptavidin-coupled fluorescein isothiocyanate (FITC), and then labelled with rat anti-mouse immunoglobulin D (IgD) followed by anti-rat-TRITC. (a) Phase contrast image of three lymph node cells. (b) Staining for IgD shows that two of the three cells are IgD+ B cells. (c) Staining with α2,6-PAA reveals that one of the three cells is a IgD+ B cell that is able to bind α2,6-PAA.
Identification and characterization of B-cell subsets that bind α2,6-PAA
To determine whether the cells that stained with α2,6-PAA corresponded to the CD22+ IgD+ population of recirculating B cells, a series of double-staining experiments was performed, in which cell suspensions were simultaneously labelled with α2,6-PAA and anti-CD22, anti-IgD (Fig. 1) or anti-B220, a pan B-cell marker (Table 2). The results of these experiments clearly showed that, irrespective of the tissue, 80–90% of the cells labelled with α2,6-PAA were B cells, as defined with anti-B220. The identity of the remaining 10–20% of labelled cells was not investigated. A similar percentage of the α2,6-PAA-labelled cells was also stained with anti-IgD and anti-CD22, thereby confirming that the majority of labelled cells were, indeed, CD22+ IgD+ recirculating B cells.
Table 2.
Double labelling of bone marrow, lymph node and spleen cells with NeuAcα2,6Galβ1,4Glc coupled to biotinylated polyacrylamide (α2,6-PAA) and B-cell-specific antibodies
| Cell population | mAb | % Of cells positive for mAb | % Of cells positive for α2,6-PAA, also positive for mAb | % Of cells positive for mAb, also positive for α2,6-PAA |
|---|---|---|---|---|
| Bone marrow | B220 | 11·3 ± 2·4 | 82·5 ± 8·3 | 6·0 ± 0·5* |
| IgD | 5·6 ± 2·0 | 74·0 ± 8·8 | 10·0 ± 1·3† | |
| CD22 | 4·7 ± 1·8 | 76·0 ± 8·4 | 14·9 ± 5·0‡ | |
| Lymph Node | B220 | 21·7 ± 5·0 | 88·3 ± 8·1 | 4·0 ± 1·0 |
| IgD | 15·5 ± 5·5 | 84·0 ± 4·7 | 5·4 ± 1·0§ | |
| CD22 | 12·2 ± 2·5 | 76·0 ± 2·8 | 6·6 ± 1·9 | |
| Spleen | B220 | 35·0 ± 9·0 | 86·0 ± 10·8 | 3·0 ± 0·7 |
| IgD | 27·8 ± 6·2 | 72·4 ± 10·3 | 2·1 ± 0·1 | |
| CD22 | 21·0 ± 7·0 | 93·3 ± 11·5 | 4·8 ± 1·0 |
Cells were stained with α2,6-PAA, as described in the legend to Table 1, and then labelled using monoclonal antibodies (mAbs) to B220 (pan B cell), CD22 or immunoglobulin D (IgD) (long-lived recirculating B cells). Regardless of the tissue of origin, the majority of α2,6-PAA-labelled cells were also labelled with the B-cell markers. However, the bone marrow was enriched ≈ two- to fivefold in B cells that bind α2,6-PAA compared with lymph node and spleen (last column). Data represent mean values ± SD from three to five experiments.
Significantly different from corresponding values with lymph node (P < 0·05) and spleen (P < 0·001).
Significantly different from corresponding values with lymph node (P < 0·05) and spleen (P < 0·001).
Significantly different from corresponding values with spleen (P < 0·05).
Significantly different from corresponding values with spleen (P < 0·001).
When the fraction of IgD- or CD22-positive cell populations that bound α2,6-PAA were compared, interesting differences were found (last column in Table 2). In the spleen and lymph nodes, only ≈ 2% and 5% of the IgD+ B cells were labelled with α2,6-PAA, respectively, while in the bone marrow, ≈ 10% of IgD+ cells bound α2,6-PAA (Table 2). Thus, we observed a two- to fivefold enrichment of IgD+ B cells bearing an unmasked form of CD22 in the bone marrow, as compared to the periphery. A similar enrichment of α2,6-PAA-binding cells in the bone marrow was observed following double staining with anti-CD22 mAb. (Table 2). In addition, lymph node cells had a higher proportion of B cells that bound the α2,6-PAA probe compared with the spleen (Table 2). Finally, it should be noted that the immunofluorescence microscopy appeared to detect the CD22high B cells (e.g. in the bone marrow, 4·7% of cells were CD22 positive), which probably correspond to the IgD+ mature B cells (5·6%), but did not detect the preB and immature B-cell populations that express CD22 weakly.16
Binding of α2,6-PAA to B cells requires the presence of CD22
The double-labelling experiments showed that the majority of cells labelled with α2,6-PAA also expressed CD22 (Table 2). Although this supports the notion that binding of the probe is CD22 dependent, it is possible that other Sia-binding lectins exist on this subset of B cells, which could account for the staining observed. In order to directly assess the importance of CD22 in binding of α2,6-PAA to B cells, a double-labelling experiment was carried out in which bone marrow and spleen cells from CD22–/– mice on a C57Bl/6 background were compared with cells from age-matched, wild-type C57B1/6 mice (Table 3). As in the previous experiments, the percentage of α2,6-PAA-positive cells observed in wild-type cells was ≈ 0·8% in the bone marrow and ≈ 1% in the spleen, and the majority of those were B cells (Table 3). A similar enrichment of IgD+ α2,6-PAA+ cells was also seen in the bone marrow, although the absolute numbers were lower than those shown in Table 2 (6·4% in the bone marrow compared with 1·4% in the spleen; Table 3). In contrast, α2,6-PAA staining in CD22–/– mice was reduced to 0·1–0·2% in bone marrow and spleen and none of the α2,6-PAA+ cells were B cells (Table 3). In conclusion, the α2,6-PAA reagent specifically interacted with B cells in a CD22-dependent manner and there appeared to be no other α2,6-Sia-binding receptors present on B cells that could be detected under the conditions used.
Table 3.
B cells from CD22-deficient (CD22–/–) mice do not bind NeuAcα2,6Galβ1,4Glc coupled to biotinylated polyacrylamide (α2,6-PAA)
| CD22 genotype | Cell population | mAb | % Of cells positive for mAb | % Of cells positive for α2,6-PAA | % Of cells positive for α2,6-PAA, also positive for mAb | % Of cells positive for mAb, also positive for α2,6-PAA |
|---|---|---|---|---|---|---|
| +/+ | Bone marrow | B220 | 11·2 | 0·8 | 88·8 | 6·5 |
| IgD | 6·3 | 0·7 | 60·0 | 6·4 | ||
| CD22 | 4·7 | 0·9 | 66·6 | 12·1 | ||
| –/– | Bone marrow | B220 | 6·0 | 0·0 | 0·0 | 0·0 |
| IgD | 2·9 | 0·2 | 0·0 | 0·0 | ||
| CD22 | 0·1 | 0·0 | 0·0 | 0·0 | ||
| +/+ | Spleen | B220 | 43·9 | 1·0 | 71·4 | 1·6 |
| IgD | 43·9 | 0·8 | 75·0 | 1·4 | ||
| CD22 | 29·3 | 1·1 | 100·0 | 3·8 | ||
| –/– | Spleen | B220 | 31·2 | 0·1 | 0·0 | 0·0 |
| IgD | 37·0 | 0·1 | 0·0 | 0·0 | ||
| CD22 | 0·3 | 0·2 | 0·0 | 0·0 |
Bone marrow and spleen cells from wild-type (+/+) or CD22-deficient (–/–) mice were double labelled for α2,6-PAA and B-cell markers as described in the legends to Tables 1 and 2. Unlike B cells from wild-type mice, B cells from CD22–/– mice were not labelled with α2,6-PAA. Data show mean counts from duplicate slides in a single experiment.
Discussion
The data presented here demonstrate the existence of a new subset of murine B cells that bind specifically to α2,6-PAA. Significantly, this is a minor fraction of the mature, recirculating B cells in the lymph node and spleen that is enriched two- to fivefold in the bone marrow. Under the conditions used, binding of α2,6-PAA to the B-cell subset seemed to be strictly dependent on expression of CD22 because there was no staining of B cells in CD22–/– mice. These findings, taken together with our previous finding that CD22 ligands are present on bone marrow endothelium,34 support the notion that CD22 plays a direct role in the homing of B cells to the bone marrow. According to the model depicted in Fig. 2, the minor fraction of B cells with unmasked CD22 (visualized in the present study with the α2,6-PAA probe) would be able to bind sialylated ligands on the bone marrow endothelium. This binding would lead to transmigration across the sinusoidal endothelium and the accumulation of this subset of cells in the bone marrow. If correct, this sequence of events would be analogous to l-selectin-dependent homing of lymphocytes to peripheral lymph nodes. In this case, l-selectin on lymphocytes binds sialylated, fucosylated and sulphated oligosaccharide ligands attached to mucin-like molecules on high endothelial venules.37 The initial carbohydrate-dependent tethering mediated by l-selectin on lymphocytes is thought to lead to firm adhesion, mediated by integrins, and subsequent diapedesis involving tissue-specific chemokines.38 Further work is required to determine the roles of CD22, selectins, integrins and chemokines in the homing of B cells to the bone marrow.
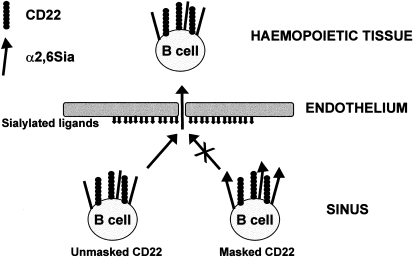
Figure 2.
Schematic diagram depicting the potential role of CD22 in mediating homing of B-cell subsets to the bone marrow. Mature recirculating immunoglobulin D+ (IgD+) B cells enter the bone marrow sinus via the bloodstream. Unmasked CD22 on subsets of these cells (as demonstrated in the present study) may engage sialylated ligands expressed on bone marrow sinusoidal endothelial cells.34 This could lead to transmigration into the haemopoietic spaces and subsequent enrichment of B cells with unmasked CD22 in the bone marrow. In contrast, B cells expressing CD22, whose binding site is masked by endogenous α2,6-linked sialic acid (α2,6 Sia) (the majority in peripheral organs), do not interact with sialylated ligands on bone marrow endothelium and hence remain in the circulation.
The probe used to detect unmasked forms of murine CD22 in the present study contained N-acetyl neuraminic acid linked to lactose (NeuAcα2,6Galβ1,4Glc-PAA) and was the same probe used previously to detect unmasked CD22 on activated human B cells.31 In that study, binding of α2,6-PAA was detected by flow cytometry, in contrast to fluorescence microscopy used here. To date we have not been able to detect the unmasked population of murine B cells using flow cytometry, whereas activated human B cells can be readily detected under the same labelling conditions (L. Nitschke, unpublished). One explanation for this difference might be related to earlier findings that murine CD22 prefers the N-glycolyl form of Sia over the N-acetyl form, whereas human CD22 binds both forms equally.39–41 Therefore, it is probable that murine B cells carrying unmasked CD22 would have been more easily detected and given brighter labelling if the assays had been carried out with the N-glycolyl form of α2,6-PAA (NeuGcα2,6Galβ1,4Glc-PAA), a reagent that is not currently available. Despite this potential limitation, it is important to point out that COS cells transfected with mouse CD22 were brightly labelled with the N-acetyl form of the α2,6-PAA probe, showing that unmasked mouse CD22 can be readily detected under the conditions used in the present study.
Previous experiments with CHO cells and COS cells have established that co-expression of CD22 with ST6Gal results in CD22 masking.23,42 Therefore, the simplest explanation for why only a subset of B cells can bind the α2,6-PAA probe is that the majority of B cells have masked forms of CD22 (owing to high levels of α2,6-linked Sia) and only a small proportion of mature recirculating B cells have unmasked CD22 (owing to lower levels of α2,6-linked Sia). This could result from lower expression of enzymes, such as ST6GalI, or expression of a sialidase that removes the cis-interacting Sia. Further work is needed to explore these possibilities. For human B cells, it was recently shown that after activation with anti-IgM and anti-CD40, a fraction of cells carried unmasked forms of CD22.31 Thus, it is tempting to speculate that the novel subset of murine B cells with an unmasked form of CD22, described here, represents a recently activated population of mature B cells. If this is the case, α2,6-PAA+ B cells should express activation markers, a theory that may be investigated by flow cytometry in the future.
Acknowledgments
This work was supported by the Wellcome Trust and the Human Frontiers Science Program.
Glossary
Abbreviations
- BCR
B-cell receptor
- CD22-Fc
extracellular domains of CD22 fused to the Fc region of human IgG1
- CD22–/– mice
CD22-deficient mice
- CHO
Chinese hamster ovary
- mAb
monoclonal antibody
- α2,3-PAA
NeuAcα2,3Galβ1,4Glc coupled to biotinylated polyacrylamide
- α2,6-PAA
NeuAcα2,6Galβ1,4Glc coupled to biotinylated polyacrylamide
- Sia
sialic acid
References
- 1.Crocker PR, Clark EA, Filbin M, et al. Siglecs: a family of sialic-acid binding lectins [letter] Glycobiology. 1998;8:v. doi: 10.1093/oxfordjournals.glycob.a018832. [DOI] [PubMed] [Google Scholar]
- 2.Crocker PR, Mucklow S, Bouckson V, et al. Sialoadhesin, a macrophage sialic acid binding receptor for haemopoietic cells with 17 immunoglobulin-like domains. EMBO J. 1994;13:4490. doi: 10.1002/j.1460-2075.1994.tb06771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelm S, Pelz A, Schauer R, et al. Sialoadhesin, myelin-associated glycoprotein and CD22 define a new family of sialic acid-dependent adhesion molecules of the immunoglobulin superfamily. Curr Biol. 1994;4:965. doi: 10.1016/s0960-9822(00)00220-7. [DOI] [PubMed] [Google Scholar]
- 4.Freeman SD, Kelm S, Barber EK, Crocker PR. Characterization of CD33 as a new member of the sialoadhesin family of cellular interaction molecules. Blood. 1995;85:2005. [PubMed] [Google Scholar]
- 5.Cornish AL, Freeman S, Forbes G, et al. Characterization of siglec-5, a novel glycoprotein expressed on myeloid cells related to CD33. Blood. 1998;92:2123. [PubMed] [Google Scholar]
- 6.Nicoll G, Ni J, Liu D, Klenerman P, Munday J, Dubock S, Mattei MG, Crocker PR. Identification and characterization of a novel siglec, siglec-7, expressed by human natural killer cells and monocytes. J Biol Chem. 1999;274:34089. doi: 10.1074/jbc.274.48.34089. [DOI] [PubMed] [Google Scholar]
- 7.Patel N, Brinkman-Van der Linden EC, Altmann SW, et al. OB-BP1/Siglec-6. A leptin- and sialic acid-binding protein of the immunoglobulin superfamily. J Biol Chem. 1999;274:22729. doi: 10.1074/jbc.274.32.22729. [DOI] [PubMed] [Google Scholar]
- 8.Floyd H, Ni J, Cornish AL, Zeng Z, Liu D, Carter KC, Steel J, Crocker PR. Siglec-8. A novel eosinophil-specific member of the immunoglobulin superfamily. J Biol Chem. 2000;275:861. doi: 10.1074/jbc.275.2.861. [DOI] [PubMed] [Google Scholar]
- 9.Zhang JQ, Nicoll G, Jones C, Crocker PR. Siglec-9. A novel sialic acid binding member of the immunoglobulin superfamily expressed broadly on human blood leukocytes. J Biol Chem. 2000;275:22121–6. doi: 10.1074/jbc.M002788200. [DOI] [PubMed] [Google Scholar]
- 10.Angata T, Varki A. Cloning, characterization and phylogenetic analysis of Siglec-9, a new member of the CD33-related group of Siglecs. Evidence for co-evolution with sialic acid synthesis pathways. J Biol Chem. 2000;275:22127–35. doi: 10.1074/jbc.M002775200. [DOI] [PubMed] [Google Scholar]
- 11.Cyster JG, Goodnow CC. Tuning antigen receptor signaling by CD22: integrating cues from antigens and the microenvironment. Immunity. 1997;6:509. doi: 10.1016/s1074-7613(00)80339-8. [DOI] [PubMed] [Google Scholar]
- 12.Schulte RJ, Campbell MA, Fischer WH, Sefton BM. Tyrosine phosphorylation of CD22 during B cell activation. Science. 1992;258:1001. doi: 10.1126/science.1279802. [DOI] [PubMed] [Google Scholar]
- 13.Peaker CJ, Neuberger MS. Association of CD22 with the B cell antigen receptor. Eur J Immunol. 1993;23:1358. doi: 10.1002/eji.1830230626. [DOI] [PubMed] [Google Scholar]
- 14.Leprince C, Draves KE, Geahlen RL, Ledbetter JA, Clark EA. CD22 associates with the human surface IgM–B-cell antigen receptor complex. Proc Natl Acad Sci USA. 1993;90:3236. doi: 10.1073/pnas.90.8.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doody GM, Justement LB, Delibrias CC, Matthews RJ, Lin J, Thomas ML, Fearon DT. A role in B cell activation for CD22 and the protein tyrosine phosphatase SHP. Science. 1995;269:242. doi: 10.1126/science.7618087. [DOI] [PubMed] [Google Scholar]
- 16.Nitschke L, Carsetti R, Ocker B, Kohler G, Lamers MC. CD22 is a negative regulator of B-cell receptor signalling. Curr Biol. 1997;7:133. doi: 10.1016/s0960-9822(06)00057-1. [DOI] [PubMed] [Google Scholar]
- 17.O'keefe TL, Williams GT, Davies SL, Neuberger MS. Hyperresponsive B cells in CD22-deficient mice. Science. 1996;274:798. doi: 10.1126/science.274.5288.798. 10.1126/science.274.5288.798. [DOI] [PubMed] [Google Scholar]
- 18.Otipoby KL, Andersson KB, Draves KE, et al. CD22 regulates thymus-independent responses and the lifespan of B cells. Nature. 1996;384:634. doi: 10.1038/384634a0. [DOI] [PubMed] [Google Scholar]
- 19.Sato S, Miller AS, Inaoki M, Bock CB, Jansen PJ, Tang ML, Tedder TF. CD22 is both a positive and a negative regulator of B lymphocyte antigen receptor signal transduction: altered signaling in CD22-deficient mice. Immunity. 1996;5:551. doi: 10.1016/s1074-7613(00)80270-8. [DOI] [PubMed] [Google Scholar]
- 20.Law CL, Aruffo A, Chandran KA, Doty RT, Clark EA. Ig domains 1 and 2 of murine CD22 constitute the ligand-binding domain and bind multiple sialylated ligands expressed on B and T cells. J Immunol. 1995;155:3368. [PubMed] [Google Scholar]
- 21.Stamenkovic I, Seed B. The B-cell antigen CD22 mediates monocyte and erythrocyte adhesion. Nature. 1990;345:74. doi: 10.1038/345074a0. [DOI] [PubMed] [Google Scholar]
- 22.Engel P, Nojima Y, Rothstein D, Zhou LJ, Wilson GL, Kehrl JH, Tedder TF. The same epitope on CD22 of B lymphocytes mediates the adhesion of erythrocytes, T and B lymphocytes, neutrophils, and monocytes. J Immunol. 1993;150:4719. [PubMed] [Google Scholar]
- 23.Hanasaki K, Varki A, Powell LD. CD22-mediated cell adhesion to cytokine-activated human endothelial cells. Positive and negative regulation by alpha 2–6-sialylation of cellular glycoproteins. J Biol Chem. 1995;270:7533. doi: 10.1074/jbc.270.13.7533. [DOI] [PubMed] [Google Scholar]
- 24.Powell LD, Sgroi D, Sjoberg ER, Stamenkovic I, Varki A. Natural ligands of the B cell adhesion molecule CD22 beta carry N-linked oligosaccharides with alpha-2,6-linked sialic acids that are required for recognition. J Biol Chem. 1993;268:7019. [PubMed] [Google Scholar]
- 25.Powell LD, Varki A. The oligosaccharide binding specificities of CD22 beta, a sialic acid-specific lectin of B cells. J Biol Chem. 1994;269:10628. [PubMed] [Google Scholar]
- 26.Weinstein J, Lee EU, McEntee K, Lai PH, Paulson JC. Primary structure of beta-galactoside alpha 2,6-sialyltransferase. Conversion of membrane-bound enzyme to soluble forms by cleavage of the NH2-terminal signal anchor. J Biol Chem. 1987;262:17735. [PubMed] [Google Scholar]
- 27.Hennet T, Chui D, Paulson JC, Marth JD. Immune regulation by the ST6Gal sialyltransferase. Proc Natl Acad Sci USA. 1998;95:4504. doi: 10.1073/pnas.95.8.4504. 10.1073/pnas.95.8.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lo NW, Lau JT. Transcription of the beta-galactoside alpha 2,6-sialyltransferase gene in B lymphocytes is directed by a separate and distinct promoter. Glycobiology. 1996;6:271. doi: 10.1093/glycob/6.3.271. [DOI] [PubMed] [Google Scholar]
- 29.Wuensch SA, Huang RY, Ewing J, Liang X, Lau JTY. Murine B cell differentiation is accompanied by programmed expression of multiple novel b-galactoside a2,6-sialyltransferase mRNA forms. Glycobiology. 2000;10:67. doi: 10.1093/glycob/10.1.67. 10.1093/glycob/10.1.67. [DOI] [PubMed] [Google Scholar]
- 30.Sgroi D, Koretzky GA, Stamenkovic I. Regulation of CD45 engagement by the B-cell receptor CD22. Proc Natl Acad Sci USA. 1995;92:4026. doi: 10.1073/pnas.92.9.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Razi N, Varki A. Masking and unmasking of the sialic acid-binding lectin activity of CD22 (Siglec-2) on B lymphocytes. Proc Natl Acad Sci USA. 1998;95:7469. doi: 10.1073/pnas.95.13.7469. 10.1073/pnas.95.13.7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Erikstein BK, Funderud S, Beiske K, Aas-Eng A, Lange Davies C, Blomhoff HK, Smeland EB. Cell cycle-dependent regulation of CDw75 (beta-galactoside alpha-2,6-sialyltransferase) on human B lymphocytes. Eur J Immunol. 1992;22:1149. doi: 10.1002/eji.1830220507. [DOI] [PubMed] [Google Scholar]
- 33.Hanasaki K, Varki A, Stamenkovic I, Bevilacqua MP. Cytokine-induced beta-galactoside alpha-2,6-sialyltransferase in human endothelial cells mediates alpha 2,6-sialylation of adhesion molecules and CD22 ligands. J Biol Chem. 1994;269:10637. [PubMed] [Google Scholar]
- 34.Nitschke L, Floyd H, Ferguson DJ, Crocker PR. Identification of CD22 ligands on bone marrow sinusoidal endothelium implicated in CD22-dependent homing of recirculating B cells. J Exp Med. 1999;189:1513. doi: 10.1084/jem.189.9.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson GL, Fox CH, Fauci AS, Kehrl JH. cDNA cloning of the B cell membrane protein CD22: a mediator of B–B cell interactions. J Exp Med. 1991;173:137. doi: 10.1084/jem.173.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crocker PR, Freeman S, Gordon S, Kelm S. Sialoadhesin binds preferentially to cells of the granulocytic lineage. J Clin Invest. 1995;95:635. doi: 10.1172/JCI117708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sassetti C, Tangemann K, Singer MS, Kershaw DB, Rosen SD. Identification of podocalyxin-like protein as a high endothelial venule ligand for l-selectin: parallels to CD34. J Exp Med. 1998;187:1965. doi: 10.1084/jem.187.12.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campbell JJ, Butcher EC. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr Opin Immunol. 2000;12:336. doi: 10.1016/s0952-7915(00)00096-0. 10.1016/S0952-7915(00)00096-0. [DOI] [PubMed] [Google Scholar]
- 39.Kelm S, Schauer R, Manuguerra JC, Gross HJ, Crocker PR. Modifications of cell surface sialic acids modulate cell adhesion mediated by sialoadhesin and CD22. Glycoconj J. 1994;11:576. doi: 10.1007/BF00731309. [DOI] [PubMed] [Google Scholar]
- 40.van der Merwe PA, Crocker PR, Vinson M, Barclay AN, Schauer R, Kelm S. Localization of the putative sialic acid-binding site on the immunoglobulin superfamily cell-surface molecule CD22. J Biol Chem. 1996;271:9273. [PubMed] [Google Scholar]
- 41.Brinkman-Van der Linden EC, Sjoberg ER, Juneja LR, Crocker PR, Varki N, Varki A. Loss of N-glycolylneuraminic acid in human evolution. Implications for sialic acid recognition by siglecs. J Biol Chem. 2000;275:8633. doi: 10.1074/jbc.275.12.8633. [DOI] [PubMed] [Google Scholar]
- 42.Sgroi D, Varki A, Braesch-Andersen S, Stamenkovic I. CD22, a B cell-specific immunoglobulin superfamily member, is a sialic acid-binding lectin. J Biol Chem. 1993;268:7011. [PubMed] [Google Scholar]