Abstract
Oral administration of antigen has been shown to be effective for both positive and negative modulation of immune responses. In the present study we characterized changes in the reactivity of the immune system after oral immunization with allogeneic spleen cells. Mice were orally immunized for 10 consecutive days with fresh allogeneic spleen cells, and the phenotype, proliferative response, cytotoxic activity and cytokine production profile of recipient spleen cells were assessed 1 or 7 days after the last immunization dose. Although no significant changes in the proportion of CD4+, CD8+ or CD25+ cells were observed in the spleen of orally immunized mice, significant activation of alloreactivity in spleen cells was found. Cells from orally immunized mice exhibited enhanced proliferation and cytotoxic activity after stimulation with specific allogeneic cells in vitro, and produced considerably higher concentrations of interferon-γ (IFN-γ) and significantly less interleukin (IL)-4 than did cells from control mice. The production of IL-2 was essentially unchanged and that of IL-10 was only slightly increased. The systemic allosensitization induced by oral immunization was demonstrated in vivo by increased resistance to the growth of allogeneic tumours induced by subcutaneous inoculation of high doses of tumour cells. In addition, orthotopic corneal allografts in orally immunized recipients were rejected more rapidly (in a second-set manner) than in control, untreated recipients. These data show that oral immunization with allogeneic cells modulates individual components of the immune response and that specific transplantation immunity, rather than tolerance, is induced in the treated recipients.
Introduction
Oral administration of antigen has been shown to be an effective way to modulate immune responses. This approach has recently attracted attention with the perspective of using mucosal immunization to treat autoimmune diseases1 or to suppress transplantation reactions.2,3 However, the effects of oral immunization are still far from uniform. In some cases, inhibition of the immune response after oral immunization was observed and this phenomenon has been called oral tolerance.4 Other studies have shown, in contrast, that oral immunization stimulates the immune system, and oral administration of antigen has been suggested as a route for vaccination.5 Application of various immunological models has revealed that many different factors determine the final outcome of mucosal immunization. The main variables are type, dose and form of antigen, genotype, immune status and age of the recipient, and the frequency and timing of immunization.6,7
Contradictory results have been obtained also at the level of cytokine production after oral immunization. Some authors described a typical T helper (Th)2/Th3 type of cytokine response characterized by increased production of interleukin (IL)-4, IL-10 and transforming growth factor-β (TGF-β).8,9 In other models, however, enhanced production of interferon-γ (IFN-γ) and decreased secretion of IL-4 were observed after oral immunization.10,11 It appears that distinct cytokine production profiles may be found in cells isolated from mucosal and systemic lymphoid organs.12
In a transplantation model, Sayegh et al.2 showed that oral administration of allogeneic spleen cells down-regulated the immune response to histocompatibility antigens and prevented sensitization of skin allografts in mice. In their extensive studies, Niederkorn and co-workers3,9,13 demonstrated that oral immunization with allogeneic spleen or corneal cells enhanced the survival of orthotopic corneal allografts in mice. When cells used for immunization were conjugated with the cholera toxin B subunit (CTB), tolerance induction was even more effective and resulted in the inhibition of cell-mediated cytotoxicity (CMC), delayed-type hypersensitivity (DTH) and mixed lymphocyte culture (MLC) reactivity. These changes were associated with decreased secretion of IL-2 and IFN-γ and enhanced production of IL-10 and TGF-β.9 Therefore, oral administration of allogeneic cells was suggested to be effective for down-regulating sensitization to transplantation antigens and for the improved survival of corneal allografts.
Here we describe a study showing that oral administration of allogeneic cells induces specific transplantation immunity, rather than tolerance, in the treated animals. Oral immunization with allogeneic spleen cells specifically activated the immune system, as revealed by enhanced MLC reactivity and CMC and by changes in production of cytokines in vitro. These changes correlated with specific sensitization in vivo, as demonstrated by resistance to the growth of allogeneic tumours induced by high doses of tumour cells and by a second-set type rejection of orthotopic corneal allografts in orally immunized mice.
Materials and methods
Animals
Mice of inbred strains BALB/c (H2d), C57BL/10Sn (B10, H2b), A/Ph (H2a), CBA/J (H2k) and DBA/1 (H2q), were from the breeding colony of the Institute of Molecular Genetics, Prague. The mice, of both sexes, were used at 8–12 weeks of age. Rats of the inbred strain, AVN, were obtained from the Institute of Physiology, Academy of Sciences, Prague.
Oral immunization
Suspensions of spleen cells from B10 mice were prepared in phosphate-buffered saline (PBS), and the indicated numbers of cells (1·9–60 × 106 cells/dose/day) were administered directly, in a volume of 0·2 ml, into the stomach using a gavage tube. Ten daily immunization doses were applied in total, and the mice were tested 1 or 7 days after the last immunization. Each experiment was repeated at least three times. Control groups of mice included animals that were either untreated or were treated with 0·2 ml per oral dose of PBS as frequently as the active treatment in the experimental animals. Each experiment contained a comparable proportion of untreated and PBS-treated mice. There were no differences in the tested parameters between untreated and PBS-treated animals.
CTB (Sigma Chemicals, St. Louis, MO) was conjugated to cells by incubating 30 × 106 spleen cells/ml with 100 µg/ml of CTB in PBS for 2 hr at 37° with frequent shaking. The cell suspension was washed three times in PBS and the cells were used for oral immunization.
MLC reaction
Reactive spleen cells (0·75 × 105/ml) were incubated in 0·2 ml of RPMI-1640 medium (Sigma Chemicals) containing 10% heat-inactivated fetal calf serum (FCS) (Sigma), antibiotics (100 U/ml of penicillin, 100 µg/ml of streptomycin), 10 mm HEPES buffer and 5 × 10−5m 2-mercaptoethanol (complete RPMI-1640 medium) in 96-well tissue culture plates (Nunclon, Roskilde, Denmark) with 1 × 105 irradiated (3000 rads) stimulatory cells. Cell proliferation was determined by adding 0·5 µCi of [3H]thymidine to each well for the last 6 hr of the 96-hr incubation period.
Cytotoxicity assay
Spleen cells (3 × 106 cells/ml) from control BALB/c mice, or from BALB/c mice immunized orally with B10 cells, were incubated in tissue culture flasks (Costar, Cambridge, MA) containing 5 ml of complete RPMI-1640 medium and irradiated (3000 rads) B10 spleen or CBA/J cells (1 × 106 cells/ml). After 96 hr of incubation, the cells were harvested, adjusted to appropriate concentrations and tested for cytotoxicity against 51Cr-labelled B10 or CBA/J concanavalin A (Con A)-induced blasts.14 The per cent specific cytotoxicity was calculated according to the following formula:
Cytokine production and determination
Spleen cells (1·5 × 106/ml) from control or orally immunized mice were incubated in 1 ml of complete RPMI-1640 in 24-well tissue culture plates (Flow Laboratories, McLean, MI), alone or with 1·5 × 106 irradiated (3000 rads) stimulatory spleen cells. Cell culture supernatants were harvested at various time-points (48–96 hr) and stored at −20° until assayed. The presence of IL-2, IL-4, IL-10 and IFN-γ in the supernatants was measured by enzyme-linked immunosorbent assay (ELISA)15 using sets of cytokine-specific capture and detection monoclonal antibodies (mAbs) purchased from PharMingen (San Diego, CA) and exactly according to the manufacturer's instructions. For quantification of cytokine levels, standards for IL-2, IL-4, IL-10 and IFN-γ (all purchased from Genzyme, Cambridge, MA) were included in all ELISA determinations.
Allogeneic tumour growth assay
Cells of the fibrosarcoma, MC11, originally induced by methylcholanthrene in B10 male mice,16 were grown in vitro in tissue cultures. MC11 cells (12 × 106 in 0·2 ml of PBS) were injected subcutaneously in a dorsal part of the recipient's body. The size of the tumours was measured every other day.
RNA preparation and reverse transcription–polymerase chain reaction (RT–PCR)
Spleen cells from control BALB/c mice, or from BALB/c mice immunized orally with B10 cells, were stimulated for 24 hr in vitro with irradiated (3000 rads) B10 cells. Total RNA was isolated from the cells using TRIzol Reagent (Life Technologies, Grand Island, NY). Two micrograms of total RNA was reverse transcribed into cDNA in a 20-µl reaction mixture, as described previously.17 Two microlitres of the cDNA preparation was amplified in a PCR cycler (MJ Research, Watertown, MA), in a reaction mixture containing 5′ and 3′ primers (Stratagene, La Jolla, CA). After PCR, the products were electrophoresed on an ethidium bromide-stained agarose gel.
Orthotopic corneal grafting
Corneas were grafted orthotopically, using the technique described by She et al.18 and modified as described previously.19 In brief, corneal grafts (2 mm in diameter) were transferred from B10 donors to anaesthetized BALB/c recipients and were secured in the graft bed using 10 interrupted sutures of Ethilon 11–0 (Ethicon, Edinburgh, Midlothian, UK). The sutures were removed 7 days after grafting. The survival of the grafts was evaluated using the following criteria: density of infiltration, opacity, oedema and intensity of neovascularization.
Flow cytometry
The proportions of CD4+, CD8+ and CD25+ cells in spleens of normal and orally immunized BALB/c mice were determined with flow cytometry (FACSTAR; Becton-Dickinson, Mountain View, CA) using specific mAb obtained from PharMingen.
Statistical analysis
Statistical significance of difference between control and experimental groups was calculated using the Student's t-test.
Results
Phenotype of spleen cells from orally immunized mice
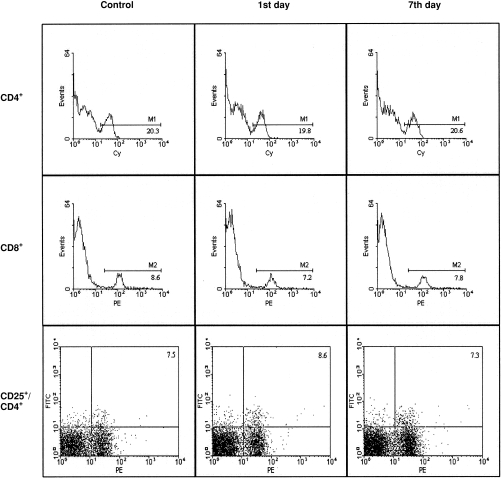
Flow cytometry analysis of major T-cell subpopulations among spleen cells from normal BALB/c mice and BALB/c mice immunized orally with B10 spleen cells revealed that oral immunization had no significant effect on the proportion of CD4+ and CD8+ cells or activated CD25+/CD4+ cells (Fig. 1).
Figure 1.
Percentages of CD4+, CD8+ and CD25+/CD4+ cells in spleens of control and orally immunized BALB/c mice. BALB/c mice were immunized for 10 consecutive days with 30 × 106 spleen cells from B10 donors, and the percentages of CD4+, CD8+ and CD25+/CD4+ cells were determined (by flow cytometry) in spleens of treated mice 1 (1st day) or 7 (7th day) days after the last immunization dose. One representative figure of five similar ones is shown. Cy, cy-chrome; FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Proliferative response and cytotoxicity
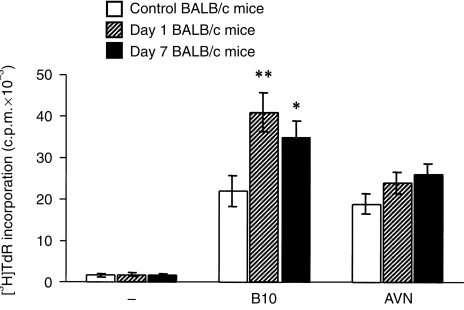
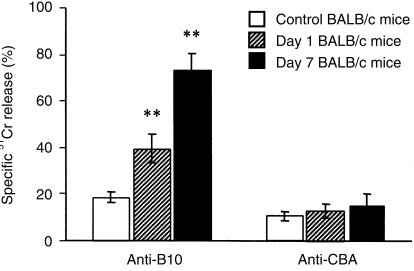
Compared with cells from control mice, spleen cells from BALB/c mice orally immunized with B10 cells and tested 1 and 7 days after the last immunization dose exhibited a significantly higher proliferative response in MLC when stimulated with allogeneic cells used for the immunization (Fig. 2). This sensitization was antigen specific, as no significant differences in the reactivity were observed if the cells from immunized mice were stimulated with irradiated rat cells (Fig. 2). After in vitro sensitization with irradiated cells of the donor haplotype, spleen cells from orally immunized mice displayed significantly higher cytotoxicity against donor-derived blast cells than cells from control mice (Fig. 3).
Figure 2.
Mixed lymphocyte culture (MLC) reactivity of spleen cells from control and orally immunized mice. Spleen cells from control BALB/c mice, or BALB/c mice 1 or 7 days after the last immunization with 30 × 106 spleen cells from B10 mice, were cultured unstimulated (–) or were stimulated with irradiated B10 or AVN spleen cells. Each bar represents the mean value (± SD) from six individual mice. Values with asterisks are significantly (*P < 0·05, **P < 0·01) different from the value for control mice. c.p.m., counts per minute.
Figure 3.
Cell-mediated cytotoxicity (CMC) of spleen cells from control and orally immunized mice. Spleen cells from control BALB/c mice, or BALB/c mice 1 or 7 days after the last oral immunization with B10 spleen cells, were stimulated for 5 days in vitro with irradiated B10 or CBA/J spleen cells, and their cytotoxicity against 51Cr-labelled concanavalin A (Con A) blasts of B10 or CBA/J haplotype, respectively, was determined at a ratio of 10 : 1 (effector to target cells). Each bar represents the mean value (± SD) from three individual mice. Values with asterisks differ significantly (**P < 0·01) from the value for control mice.
Production of cytokines
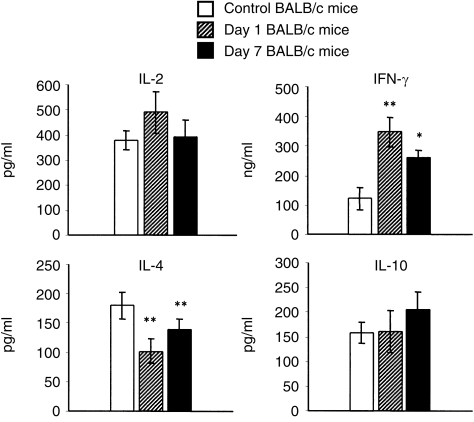
Spleen cells from control BALB/c mice or BALB/c mice orally immunized with B10 cells were cultured with irradiated B10 spleen cells and the concentrations of IL-2, IL-4, IL-10 and IFN-γ in cell culture supernatants were determined by ELISA. Preliminary experiments showed that the optimal level of IL-2 was achieved after 48 hr, IFN-γ after 72 hr, and IL-4 and IL-10 after 96-hr of incubation (data not shown). The results summarized in Fig. 4 show that oral immunization modulated production of some cytokines: the production of IFN-γ was significantly increased, while the production of IL-4 was decreased in the immunized mice. The production of IL-2 was not significantly changed by oral immunization and the production of IL-10 was slightly enhanced.
Figure 4.
Production of T helper 1 (Th1) and T helper 2 (Th2) cytokines by spleen cells from control and orally immunized mice. Spleen cells from control BALB/c mice, or BALB/c mice 1 or 7 days after the last oral immunization with B10 spleen cells, were stimulated in vitro with irradiated B10 cells and the production of interleukin (IL)-2, IL-4, IL-10 and interferon-γ (IFN-γ) was determined by enzyme-linked immunosorbent assay (ELISA). Each bar represents the mean value (± SD) from six individual mice. Values with asterisks differ significantly (*P < 0·05, **P < 0·01) from the value for control mice.
To confirm that the enhanced levels of IFN-γ found after oral immunization were caused by enhanced gene expression, we analysed expression of the gene for IFN-γ. Spleen cells from control and orally immunized mice were stimulated in vitro with irradiated allogeneic cells and the concentration of mRNA transcript for IFN-γ was determined by RT–PCR. Figure 5 shows that the expression of IFN-γ mRNA was apparently enhanced only in cells from orally immunized mice.
Figure 5.
Expression of mRNA for interferon-γ (IFN-γ) in spleen cells from control BALB/c mice (lanes 1 and 2) or BALB/c mice, 1 (lanes 3 and 4) or 7 (lanes 5 and 6) days after the last oral immunization dose with B10 spleen cells. Spleen cells from tested mice were stimulated for 24 hr in vitro with irradiated B10 cells. Total RNA was isolated, reverse transcribed, and amplified using the polymerase chain reaction (PCR) with 5′ and 3′ primers for IFN-γ and β-actin. PCR products were electrophoresed; the ethidium bromide-stained agarose gel is shown.
To verify that the observed sensitization after oral administration of allogeneic cells was a property only of the particular strain combination of mice used, we tested production of IFN-γ in three other inbred strains of mice immunized orally with allogeneic cells. As demonstrated in Fig. 6, spleen cells from orally immunized A/Ph, CBA/J and DBA/1 mice produced a significantly higher level of IFN-γ than spleen cells from control mice.
Figure 6.
Production of interferon-γ (IFN-γ) by spleen cells from orally immunized A/Ph, CBA/J and DBA/1 mice. The mice were immunized for 10 consecutive days with 30 × 106 B10 spleen cells and the production of IFN-γ by spleen cells from control untreated mice or orally immunized mice 1 or 7 days after the last immunization dose was evaluated. Each bar represents the mean value (± SD) from three individual mice. Values with asterisks represent reactivities that are statistically different (*P < 0·05, **P < 0·01) from control mice.
Effect of CTB on oral immunization
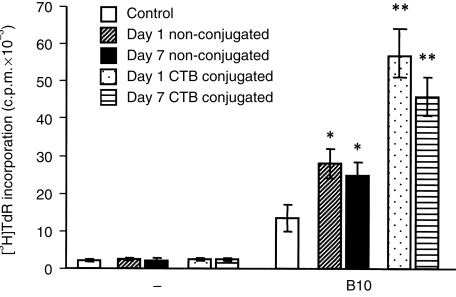
CTB has been shown to modulate immunity to orally administered antigens. To test the effect of CTB in our model, spleen cells were conjugated with CTB and administered orally. As demonstrated in Fig. 7, spleen cells from mice immunized orally with CTB-conjugated allogeneic cells proliferated after stimulation with specific allogeneic cells in vitro to a significantly greater extent than spleen cells from control untreated mice, and even more than the cells from mice immunized orally with allogeneic non-conjugated cells.
Figure 7.
Mixed lymphocyte culture (MLC) reactivity of spleen cells from mice orally immunized with cholera toxin subunit B (CTB)-conjugated or non-conjugated allogeneic cells. Spleen cells were from control BALB/c mice, BALB/c mice 1 or 7 days after the last immunization dose with non-conjugated B10 cells or from BALB/c mice 1 or 7 days after the last dose of CTB-conjugated B10 cells. For immunization, 30 × 106 non-conjugated or CTB-conjugated cells were administered orally for 10 consecutive days. Spleen cells from each individual mice were cultured unstimulated (–) or were stimulated with irradiated B10 cells. Each bar represents the mean value (± SD) from three individual mice. Values with asterisks represent significant proliferation (*P < 0·05, **P < 0·01) from values obtained for control mice. c.p.m., counts per minute.
Effects of oral immunization on the growth of allogeneic tumours
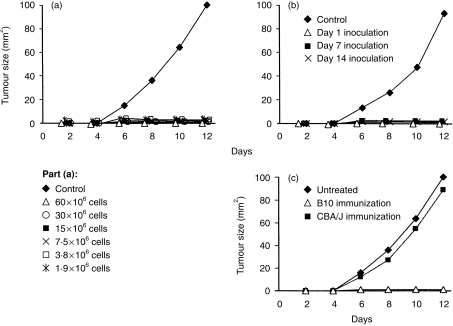
Allogeneic tumour cells generally do not grow in normal mice unless a sufficiently high dose of tumour cells is used. In our model of methylcholantherene-induced sarcoma MC11 in B10 mice, fewer than 103 tumour cells induce progressively growing tumours in syngeneic B10 cells. However, owing to allotransplantation immunity, greater than 10 × 106 tumour cells are required to induce tumours in allogeneic BALB/c mice. It was found that 12 × 106 MC11 sarcoma cells induced progressively growing tumours in 100% of untreated recipients. If the recipient BALB/c mice were immunized orally with B10 cells before tumour cell inoculation, the tumour growth was completely inhibited. Inhibition of the tumour growth was achieved with a wide range of allogeneic cells used for oral immunization (Fig. 8a), and suppression of tumour growth was demonstrated 1, 7 and 14 days after the last immunization dose (Fig. 8b). The specificity of oral immunization is demonstrated in Fig. 8(c). The MC11 sarcomas grew progressively in untreated recipients or in recipients orally immunized with unrelated CBA/J spleen cells, but did not grow in mice immunized with B10 cells.
Figure 8.
Growth of allogeneic tumours in control and orally immunized mice. (a) Control BALB/c mice, or BALB/c mice 7 days after the last oral immunization with 60 × 106, 30 × 106, 15 × 106, 7·5 × 106, 3·8 × 106 or 1·9 × 10 spleen cells from B10 mice, were inoculated subcutaneously with 12 × 106 cells of MC11 sarcoma of B10 origin and the growth of the tumours was determined. (b) Growth of MC11 sarcomas in control BALB/c mice, and in BALB/c mice orally immunized with 30 × 106 B10 cells and inoculated with tumour cells 1, 7 or 14 days after the last immunization dose. (c) Growth of MC11 sarcomas in untreated BALB/c mice, and in BALB/c mice inoculated with tumour cells 7 days after the last oral immunization with 30 × 106 B10 or CBA/J spleen cells. Each group consisted of five to seven mice.
Survival of corneal allografts in orally immunized mice
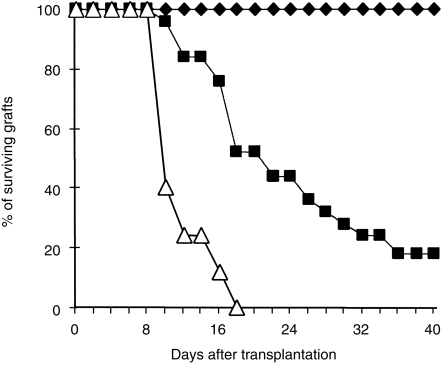
The majority of orthotopic corneal allografts from B10 donors in untreated BALB/c recipients are rejected, with a mean survival time of 20 days. In the recipients immunized orally with B10 spleen cells, corneal allografts were rejected more rapidly and none of the allograft survived beyond day 18 after transplantation (Fig. 9).
Figure 9.
Survival of orthotopic corneal allografts in control and orally immunized mice. Survival of syngeneic corneal grafts (✦) (10 mice), B10 corneal allografts in normal BALB/c recipients (▪) (15 mice) and in BALB/c recipients grafted 7 days after the last immunization dose with 30 × 106 B10 spleen cells (▵) (15 mice).
Discussion
Administration of antigens through mucosal surfaces has been shown to be effective for modulating the immune response. Oral immunization has been demonstrated as an approach for vaccination but, in contrast, oral administration of antigen has also been suggested to be useful for inducing tolerance. The final immune response after oral immunization is still difficult to predict because many factors, some of them so far not well recognized, can influence the outcome of oral immunization.6,7
Here we showed that oral immunization with allogeneic spleen cells for 10 consecutive days modulates the production of cytokines in the spleen of treated animals and induces systemic transplantation immunity, rather than tolerance. The allospecific sensitization was demonstrated in vitro by enhanced MLC reactivity and increased CMC, and in vivo by increased resistance to the growth of allogeneic tumours and by a second-set type of rejection of corneal allografts. The ability of orally administered allogeneic cells to induce immunity was demonstrated through a wide range of cell concentrations (from 1·9 to 60 × 106 per dose) and was proved 1, 7 and 14 days after the last immunization dose.
Although it is generally accepted that oral administration of soluble antigens preferentially induces tolerance, while administration of particulate antigens stimulates immunity, our results are somewhat different from findings described in other transplantation systems. It has been shown that oral immunization with allogeneic cells diminishes allotransplantation immunity in vivo2,3,20,21 and induces hyporeactivity in vitro.9,21 The latter finding was supported by observations of weaker MLC reactivity and decreased production of Th1 cytokines in cells from orally immunized animals. We found that the proliferation of spleen cells was enhanced from orally immunized mice in MLC and the cells produced significantly enhanced levels of the Th1 cytokine, IFN-γ, and decreased amounts of IL-4. The production of IL-2 and IL-10 were non-significantly modulated in immunized mice. Thus, there was no apperent shift to a Th1 or Th2 type of immune response after oral immunization with allogeneic cells. Allosensitization in vivo was demonstrated by increased resistance to the growth of allogeneic tumours induced by high doses of allogeneic tumour cells. In our model, at least 10 × 106 allogeneic tumour cells were required to induce progressively growing tumours in normal animals. In animals with neonatally induced transplantation tolerance, fewer than 1 × 106 tumour cells are sufficient to induce progressively growing sarcomas (V. Holáň, unpublished). Here we showed that inoculation of 12 × 106 tumour cells did not induce tumours in orally treated animals, while the same dose of tumour cells induced tumours in all untreated recipients. The induction of allotransplantation immunity was shown to be donor specific, as oral immunization with unrelated third-party spleen cells did not induce resistance to the tumour growth. In addition, we showed that corneal allografts in orally immunized mice were rejected more rapidly (in a second-set manner) in orally immunized recipients than in control mice. These findings differ from those of Niederkorn and co-workers,3,13 who showed that oral immunization with allogeneic cells promotes corneal allograft survival in mice. The tolerogenic potential of orally administered allogeneic cells was increased when they were coupled to CTB.3 In our model, coupling of the cells used for oral immunization to CTB further increased their immunogenicity rather than supported tolerance induction. Spleen cells from mice immunized orally with CTB-conjugated allogeneic cells responded in MLC with a stronger proliferative response than cells from control mice and even with a stronger response than cells from mice immunized orally with non-conjugated cells. Production of IFN-γ by spleen cells from mice immunized with CTB-conjugated cells was higher than production of IFN-γ by spleen cells from mice immunized with non-conjugated allogeneic cells (data not shown). Altogether, our results show that oral immunization with allogeneic cells induces specific transplantation immunity, rather than tolerance, in the treated animals. It is difficult to find reasons for the discrepancies between our results and those of some other groups. One possibility may be that distinct results could be caused by genetic differences among the mouse strains used, as different strains may have a different genetic predisposition for the development of Th1 or Th2 responses. Niederkorn et al. used, in their extensive studies on oral tolerance, high-responder CB6F1 mice, which all rejected orthotopic corneal allografts, in most cases within 20 days after grafting.3,13 On the contrary, 20–40% of untreated BALB/c mice have been demonstrated to accept B10 corneal allografts permanently.22,23 In this study, we showed that if A/Ph, CBA/J or DBA/1 strains of mice were used instead of BALB/c mice, oral immunization with allogeneic spleen cells generally stimulated specific transplantation immunity rather than induced tolerance. Another factor that can influence the outcome of oral immunization is the type of cells used for immunization. Niederkorn and co-workers regularly used, for tolerization studies, corneal epithelial and endothelial cells, but they also tested allogeneic adherent spleen cells and obtained hyporeactivity to corneal allografts.13 In other models of oral immunization, spleen cells were also used.2,21 In this work, spleen cells were used for oral immunization, but we also compared thymus cells or adherent spleen cells that were prepared as described by Ma et al.13 In all cases, sensitization rather than the induction of hyporeactivity was observed (unpublished results). Other authors used different time schedules for oral immunization and distinct test systems. However, when we mimicked these immunization/tolerization schedules, transplantation immunity was still preferentially induced. Genetically determined differences and different immunological reactivities of individual strains of mice thus appear to be the main factors responsible for the different results obtained.
Of particular interest were changes in cytokine production after oral immunization. In orally immunized mice, we found a significant increase in the production of IFN-γ and a decrease in the secretion of IL-4. These observations agree with the findings of some other models of mucosal tolerance10,11,24,25 but contrast with others.26,27 The preferential stimulation of IFN-γ production after mucosal administration of antigen was observed in various models.10,11,28,29 IFN-γ supports the Th1 immune response, which promotes a reduction in the number of IL-4-producing Th2 cells.30 In contrast, IL-4 determines development of a Th2-type response,31 which is generally associated with a state of transplantation tolerance.32,33 Increased production of IFN-γ and lower levels of IL-4 observed after oral immunization can support the development of transplantation immunity and might explain the absence of tolerance in our system. We tested cytokine production using spleen cells and it may be argued that in other lymphoid organs another type of cytokine response might occur. It has been shown that distinct cytokine production profiles can be found in mucosal and systemic lymphoid organs.12 For example, using Peyer's patch (PP) cells from mice orally immunized with allogeneic corneal cells, Niederkorn and co-workers found a diminished Th1-type cytokine response and increased production of IL-10.9 However, when immunoreactivity was tested of PP cells from mice orally immunized with allogeneic spleen cells, the results were comparable with those described here for spleen cells.34 PP cells from orally immunized mice produced considerably more IFN-γ and significantly less IL-4 than PP cells from control mice. The production of IL-2 and IL-10 by PP cells was increased in orally immunized mice.34 Thus, no apparent shift to a Th1- or Th2-type cytokine response in spleen or PP cells was found after oral immunization.
Altogether, our results show that oral immunization with fresh allogeneic cells in mice may induce systemic transplantation immunity rather than tolerance. This has to be borne in mind when considering oral tolerogenic approaches for induction of transplantation tolerance.
Acknowledgments
We wish to thank Professor J. V. Forrester, University of Aberdeen, for useful advice and suggestions during this work and for reading the manuscript. This work was supported by grant nos 310/99/0360, 310/97/1261 and 310/99/D044 from the Grant Agency of the Czech Republic, grant no. 3964-3 from the Ministry of Health of the Czech Republic, project nos VS 97099, MSM 113100003 and J13/98 111100005 from the Ministry of Education of the Czech Republic, and NATO Linkage grant CRG.LG 972853.
Glossary
Abbreviations
- CMC
cell-mediated cytotoxicity
- CTB
cholera toxin subunit B
- DTH
delayed-type hypersensitivity
- IFN
interferon
- IL
interleukin
- MLC
mixed lymphocyte culture
- PP
Peyer's patches
- RT–PCR
reverse transcription–polymerase chain reaction
- TGF
transforming growth factor
References
- 1.Weiner HL, Friedman A, Miller A, et al. Oral tolerance: immunological mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Annu Rev Immunol. 1994;12:809–37. doi: 10.1146/annurev.iy.12.040194.004113. [DOI] [PubMed] [Google Scholar]
- 2.Sayegh MH, Zhang ZJ, Hancock WW, Kvok CA, Carpenter CB, Weiner HL. Down-regulation of the immune response to histocompatibility antigens and prevention of sensitization of skin allografts by orally administered alloantigen. Transplantation. 1992;53:163–6. doi: 10.1097/00007890-199201000-00033. [DOI] [PubMed] [Google Scholar]
- 3.He Y-G, Mellon J, Niederkorn JY. The effect of oral immunization on corneal allograft survival. Transplantation. 1996;91:920–6. doi: 10.1097/00007890-199603270-00014. [DOI] [PubMed] [Google Scholar]
- 4.Garside P, Mowat AM. Mechanisms of oral tolerance. Crit Rev Immunol. 1997;17:119–37. doi: 10.1615/critrevimmunol.v17.i2.10. [DOI] [PubMed] [Google Scholar]
- 5.Yang DM, Fairweather N, Button LL, McMaster WR, Kahl LP, Liew FY. Oral Salmonella typhimurium AroA– vaccine expressing a major leishmanial surface protein gp63 preferentially induces T helper 1 cells and protective immunity against leishmaniasis. J Immunol. 1990;145:2281–5. [PubMed] [Google Scholar]
- 6.Peng H-J, Turner MW, Strobel S. The kinetics of oral hyposensitization to a protein antigen are determined by immune status and the timing, dose and frequency of antigen administration. Immunology. 1989;67:425–30. [PMC free article] [PubMed] [Google Scholar]
- 7.Faria AMC, Garcia G, Rios MJC, Michalaros CL, Vaz NM. Decrease in susceptibility to oral tolerance induction and occurrence of oral immunization to ovalbumin in 20-week-old mice: the effect of interval between oral exposures and rate of antigen intake in the oral immunization. Immunology. 1993;78:147–51. [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman A, Weiner HL. Induction of anergy or active suppression following oral tolerance is determined by antigen dose. Proc Natl Acad Sci USA. 1994;91:6688–92. doi: 10.1073/pnas.91.14.6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma D, Li X-Y, Mellon J, Niederkorn JY. Immunological phenotype of host orally immunized with corneal alloantigens. Invest Opthalmol Vis Sci. 1998;39:744–53. [PubMed] [Google Scholar]
- 10.Marth T, Strober W, Kelsall BI. High dose oral tolerance in ovalbumin TCR– transgenic mice: systemic neutralization of IL-12 augments TGF-β secretion and T cell apoptosis. J Immunol. 1996;157:2348–57. [PubMed] [Google Scholar]
- 11.Chen Y, Inobe J-I, Weiner HL. Inductive events in oral tolerance in the TCR transgenic adoptive transfer mode. Cell Immunol. 1997;178:62–8. doi: 10.1006/cimm.1997.1119. 10.1006/cimm.1997.1119. [DOI] [PubMed] [Google Scholar]
- 12.Tonkonogy SL, Swain SL. Distinct lymphokine production by CD4+ T cells isolated from mucosal and systemic lymphoid organs. Immunology. 1993;80:574–80. [PMC free article] [PubMed] [Google Scholar]
- 13.Ma D, Mellon J, Niederkorn JY. Oral immunization as a strategy for enhancing corneal allograft survival. Br J Ophthalmol. 1997;81:778–84. doi: 10.1136/bjo.81.9.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holáň V, Mitchison NA. Haplotype-specific suppressor T cells mediating linked suppression of immune responses elicited by third-party H-2 alloantigens. Eur J Immunol. 1993;13:652–7. doi: 10.1002/eji.1830130809. [DOI] [PubMed] [Google Scholar]
- 15.Holáň V, Sedláč ková K, Rů ž ič ková M. Production of high levels of Th1 and Th2 cytokines in mice with acquired transplantation tolerance. Cell Immunol. 1996;174:7–12. doi: 10.1006/cimm.1996.0287. 10.1006/cimm.1996.0287. [DOI] [PubMed] [Google Scholar]
- 16.Bubenı´k J, Indrová M, Ně meč ková Š, Malkovský M, von Broen B, Pálek V, Andrlı´ková J. Solubilized tumour-associated antigens of methylcholanthrene-induced sarcomas. Comparative studies by in vitro sensitization of lymph-node cells, macrophage electrophoretic mobility assay and transplantation tests. Int J Cancer. 1987;21:348–55. doi: 10.1002/ijc.2910210316. [DOI] [PubMed] [Google Scholar]
- 17.Holáň V, Kuffová L, Zajı´cová A, Krulová M, Filipec M, Holler P, Janč árek A. Urocanic acid enhances IL-10 production in activated CD4+ T cells. J Immunol. 1998;161:3237–41. [PubMed] [Google Scholar]
- 18.She S-C, Steahly LP, Moticka MJ. A method for performing full-thickness, orthotopic, penetrating keratoplasty in the mouse. Opthalmic Surg. 1990;21:781–5. [PubMed] [Google Scholar]
- 19.Holáň V, Hašková Z, Filipec M. Transplantation immunity and tolerance in the eye Rejection and acceptance of orthotopic corneal allografts in mice. Transplantation. 1996;62:1050–4. doi: 10.1097/00007890-199610270-00003. [DOI] [PubMed] [Google Scholar]
- 20.Gorczynski RM, Chen Z, Zeng H, Fu XM. A role of persisting antigen, antigen presentation, and ICAM-1 in increased renal graft survival after oral or portal vein donor-specific immunization. Transplantation. 1998;66:339–49. doi: 10.1097/00007890-199808150-00011. [DOI] [PubMed] [Google Scholar]
- 21.Ishido N, Matsuoka J, Matsuno T, Nakagawa K, Tanaka N. Induction of donor-specific hyporesponsiveness and prolongation of cardiac allograft survival by jejunal administration of donor splenocytes. Transplantation. 1999;68:1377–82. doi: 10.1097/00007890-199911150-00026. [DOI] [PubMed] [Google Scholar]
- 22.Sonoda Y, Streilein JW. Orthotopic corneal transplantation in mice. Evidence that the immunogenetics rules of rejection do not apply. Transplantation. 1992;54:694–704. doi: 10.1097/00007890-199210000-00026. [DOI] [PubMed] [Google Scholar]
- 23.Hašková Z, Usiu A, Pepose JS, Ferguson TA, Stuart PM. CD4+ T cells are critical for corneal, but not skin, allograft rejection. Transplantation. 2000;69:483–7. doi: 10.1097/00007890-200002270-00004. [DOI] [PubMed] [Google Scholar]
- 24.McMenamin C, Pimm C, McKersey M, Holt PG. Regulation of IgE responses to inhaled antigen in mice by antigen-specific γδ T cells. Science. 1994;265:1869–71. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- 25.Marth T, Strober W, Kelsall BL. High dose oral tolerance in ovalbumin TCR-transgenic mice. Systemic neutralization of IL-12 augments TGF-β secretion and T cell apoptosis. J Immunol. 1996;157:2348–57. [PubMed] [Google Scholar]
- 26.Chen Y, Inobe J, Weiner HL. Induction of oral tolerance to myelin basic protein in CD8-depleted mice. J Immunol. 1995;155:910–6. [PubMed] [Google Scholar]
- 27.Hancock WW, Polanski M, Yhang J, Blogg A, Weiner HL. Suppression of insulitis in non-obese diabetic (NOD) mice by oral insulin administration associated with selective expression of interleukin-4 and -10, transforming growth factor-β, and prostaglandin-E. Am J Pathol. 1995;147:1193–9. [PMC free article] [PubMed] [Google Scholar]
- 28.McMenamin C, Holt PG. The natural immune response to inhaled soluble protein antigens involves major histocompatibility complex (MHC) class I-restricted CD8+ T cell-mediated but MHC class II-restricted CD4+ T cell-dependent immune deviation resulting in selective suppression of immunoglobulin E production. J Exp Med. 1993;178:889–99. doi: 10.1084/jem.178.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.George A. Generation of gamma interferon response in murine Peyer's patches following oral immunization. Infect Immun. 1996;64:4606–11. doi: 10.1128/iai.64.11.4606-4611.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Street NE, Mosmann TR. Functional diversity of T lymphocytes due to secretion of different cytokine patterns. FASEB J. 1991;5:171–7. doi: 10.1096/fasebj.5.2.1825981. [DOI] [PubMed] [Google Scholar]
- 31.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:625–73. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 32.Chen A, Field E. Enhanced type 2 and diminished type 1 cytokines in neonatal tolerance. Transplantation. 1995;59:933–41. doi: 10.1097/00007890-199504150-00002. [DOI] [PubMed] [Google Scholar]
- 33.Onodera K, Hancock WW, Graser E, Volk HD, Lehmann M, Chandraker A, Sayegh MH, Kupiec-Weglinski JW. Th2-type cytokines in the ‘infectious’ tolerance pathway. Transplant Proc. 1997;29:1290–1. doi: 10.1016/s0041-1345(96)00522-2. 10.1016/S0041-1345(96)00522-2. [DOI] [PubMed] [Google Scholar]
- 34.Frič J, Krulová M, Zajı´cová A, Holáč V. Phenotype, immunological reactivity and cytokine production profile of Peyer's patch cells from mice immunized orally with allogeneic cells. Folia Biol (Praha) 2000;46:119–25. [PubMed] [Google Scholar]