Abstract
Although γδ cells are commonly hypothesized to provide a ‘first line of defence’, γδ-cell-deficient mice are generally only marginally more susceptible to pathogens. Because γδ cells are enriched within epithelia, it is important to resolve whether immunoprotective capacity towards epithelial-tropic pathogens is absent from the γδ-cell compartment, or whether such activity is present but simply redundant with that of αβ T cells. In this work, following infection of the intestinal epithelium of αβ T-cell-deficient mice with the coccidian parasite, Eimeria vermiformis, γδ cells were shown to support the rapid activation of other lymphoid cells and to confer a transferable antipathogen effect that could be eradicated by neutralization of interferon-γ. However, unlike αβ T cells, these effects of γδ cells showed no evidence of functional immunological memory. These results are directly relevant to coccidiosis, an economically significant disease of livestock, and should have general relevance to infections involving αβ T-cell deficiencies, e.g. cryptosporidiosis in patients with acquired immune deficiency syndrome (AIDS).
Introduction
The subdivision of the T-lymphocyte compartment into subsets bearing either an αβ or a γδ T-cell receptor (TCR) appears to have been conserved for at least 450 million years.1 Although many studies have shown that both cell types are capable of producing various cytokines and mediating cytolysis,2–5 the question of how each cell type contributes to particular immune responses remains unresolved.1,6,7 This is particularly true for natural infections of epithelial cells, for while TCR-γδ+ cells are often rare in the systemic circulation (1–2% of peripheral T cells) they are commonly highly represented among intraepithelial lymphocytes (IELs).8–11 In that context, they have been proposed to mount a ‘first line of defence’12 in response to recognition of stress-induced major histocompatibility complex (MHC) ‘sentinel antigens’ expressed by infected epithelial cells.12,13
As an example of a natural epithelial-tropic infection, we have studied coccidiosis caused by Eimeria vermiformis (phylum: Apicomplexa), an intracellular protozoan pathogen, largely (if not completely) restricted to the intestinal epithelium where γδ cells are enriched. Different species of Eimerian parasitize a broad range of vertebrate hosts in which the immune response is measured primarily by each of two criteria: attenuation of primary infection; and the development of immunity to secondary challenge. Following primary infection, the immune system reduces the number of oocysts released into the faeces and the time-period over which oocyst release occurs (patent period). Nevertheless, E. vermiformis is intrinsically self-limiting, and an immunodeficient mouse will become cleared of the pathogen within ≈ 3 weeks. This aspect allows clean segregation of the responses to primary inocula and to subsequent secondary inocula, even in immunocompromised hosts. In immunocompetent mice, immunity to secondary infection is extremely efficient, and essentially no infectious oocysts are released into the faeces, even after administration of inocula 1000 times greater than given in the primary challenge (ref. 14; A. L. Smith & A. C. Hayday, unpublished).
Thymus-dependent responses are largely responsible for the host defences that follow either primary or secondary challenge.15,16 In attempts to clarify such responses, it was recently shown that both primary and secondary infections are exacerbated in αβ T-cell-deficient mice,17 just as such mice are defective in specific immunity towards most pathogens so far utilized.18–22 By contrast, γδ-cell-deficient mice were not obviously defective in either primary or secondary responses.17
Despite these results, three observations indicate that γδ cells are somehow involved in the response to Eimeria. First, there are increases in γδ+ IELs during infection either of mice23 or chickens.24 Second, γδ cells promote germinal centre formation in αβ T-cell-deficient mice repeatedly infected with E. vermiformis.25 Third, Eimeria-infected γδ-cell-deficient mice show an enhanced αβ T-cell-dependent immunopathology,17 reflecting an immunoregulatory role for γδ cells that has by now been implicated in numerous immune responses to infectious agents or to self.17,26–30
However, as is the case for αβ T cells, immunoregulation may be neither the only nor the major role of γδ cells. Indeed, treatment of mice with anti-TCR-γδ antibodies exacerbates the early phases of certain bacterial, viral and protozoal infections,29,31–34 with similar, more extensive results being obtained from TCR-δ–/– mice.18,19,35–37 For these reasons, the potential immunoprotective effects of γδ cells towards an epithelial cell pathogen, delivered via the natural route, warranted further clarification. In particular, we wanted to know whether immunoprotective capacity was absent from the γδ-cell pool, or whether any such activity that existed was simply redundant with the αβ T-cell compartment.
Materials and methods
Animals
TCR-β–/– and TCR-(β × δ) mice were crossed onto a C57.BL/6 background (> 10 generations) and bred and maintained in specific pathogen-free (SPF) isolators, in an accredited facility. C57.BL/6 and some TCR-β–/– mice were originally purchased from Jackson Laboratory (Bar Harbor, ME). Mice were given an invariant diet (Hamster chow 3500, Agway, Waverly, NY) and water ad libitum. Animals were 6–10 weeks of age at primary infection and secondary infections were initiated 4–6 weeks thererafter.
Parasites and oocyst enumeration
E. vermiformis (a kind gift of Prof. K. S. Todd, University of Illinois, Urbana–Champaign, IL) were maintained by passage in vivo, with oocysts purified and sporulated as described previously.38 Sporulation was scored microscopically and mice were given 102 or 103 sporulated oocysts in 100 µl of water by oral gavage. At the beginning of patency (7 days postinfection [p.i.]), mice were individually caged and maintained over autoclaved sand from which the faeces were collected at 24-hr periods, until no oocysts could be detected. Oocysts were released from disaggregated faeces by salt flotation and counted in girded McMaster chambers.
Preparation of lymphocytes
Spleens and mesenteric lymph nodes were removed from freshly killed mice using sterile technique, and lymphocyte suspensions were prepared by gently disrupting diced organs through a steel mesh into sterile phosphate-buffered saline (PBS), pH 7·2, containing 2% fetal calf serum (FCS), and maintained on ice. Erythrocytes were removed from spleen preparations by ‘flash lysis’ according to a standard technique.39 Small intestinal intraepithelial lymphocytes (IEL) were prepared as described previously.23 Lymphocytes were washed, resuspended and counted microscopically. Viability always exceeded 90%, according to Trypan Blue exclusion.
Adoptive transfer
For adoptive transfer, cells were resuspended in PBS (pH 7·2) containing 2% FCS, and injected intraperitoneally into recipient mice. Controls received PBS (pH 7·2), containing 2% FCS, with no cells. Recipient mice were challenged with E. vermiformis 24–48 hr after adoptive transfer, as described by Rose et al.38,40
Flow cytometry
Cells were washed and resuspended at 5 × 106/ml in staining buffer at 4° (PBS pH 7·2, 2% FCS, 0·01% sodium azide). Antibodies used for fluorescence-activated cell sorter (FACS) analysis were anti-CD3, anti-γδ, anti-αIEL, anti-CD8α and anti-CD8β (all purchased from Pharmingen Inc., San Diego, CA). Briefly, 5 × 105 cells in 100 µl of staining buffer were stained on ice for 30 min, washed and fixed in 1% paraformaldahyde before analysis on a FACScan (Becton-Dickinson, Palo Alto, CA) using cellquest software.
Depletion of interferon-γ (IFN-γ) in vivo
Fifty micrograms of rat anti-IFN-γ (XMG1.2; Endogen, Woburn, MA) was injected intravenously in 100 µl of sterile PBS (pH 7·2) via the orbital plexus 3 hr prior to initiation of infection. Control animals received 50 µg of rat immunoglobulin G (IgG). This concentration of XMG 1.2 has previously been used for in vivo depletion of IFN-γ.41
Results
Infection of T-cell-deficient strains
To determine if γδ cells conferred a reproducible, protective effect against Eimeria, which was independent of that provided by αβ T cells, infections of C57.BL/6.TCR-β–/– mice (that contain γδ cells) were compared with those of C57.BL/6.TCR-(β×δ)–/– mice (that contain no T cells). C57.BL/6 mice were included as controls. Immunoprotection was assessed by measuring the scale and duration of oocyst output following primary and secondary infections (Table 1). To accommodate inevitable variations in the reproductive efficiency of parasites harvested from passage in vivo, and to enable the appropriate comparisons to be made, all secondary infections were accompanied with parallel primary infections of mice of the same strain (Table 1; right-hand columns Exp. 1, groups D, E and F; Exp. 3, group C). All individual experiments were internally controlled, and direct comparisons were never made between one experiment and another.
Table 1.
Primary and secondary infection of T-cell receptor (TCR)-β–/– and TCR-(β × δ)–/– mice with Eimeria vermiformis
| Primary infection* | Secondary infection* | |||||||
|---|---|---|---|---|---|---|---|---|
| Exp. no. | Group | Strain of mouse: (β–/– lacks αβ T cells; β × δ–/– lacks αβ and γδ T cells) | No. of mice (deaths) | Oocyst output (× 106)† | Patent period (in days)† | No. of mice (deaths) | Oocyst output (× 106)† | Patent period (in days)† |
| 1 | A | TCR-β–/– | 6 (0) | 58·6 ± 6·3 | 14·0 ± 0·6 | 5 (0) | 41·2 ± 5·9 | 13·3 ± 0·3 |
| B | TCR-(β × δ)–/– | 6 (0) | 86·3 ± 10·8 | 13·4 ± 0·2 | 5 (1) | 74·5 ± 10·7 | 13·8 ± 0·3 | |
| C | TCR-(β × δ)+/– | 6 (0) | 9·5 ± 2·3 | 6·5 ± 0·2 | 6 (0) | 0·0 ± 0·0 | 0·0 ± 0·0 | |
| Primary infection controls‡ | ||||||||
| D | TCR-β–/– | 6 (0) | 38·9 ± 3·7 | 13·0 ± 0·4 | ||||
| E | TCR-(β × δ)–/– | 7 (2) | 81·8 ± 9·4 | 13·8 ± 0·4 | ||||
| F | C57.BL/6 | 5 (0) | 9·2 ± 2·7 | 7·4 ± 0·2 | ||||
| 2 | A | TCR-β–/– | 4 (0) | 44·7 ± 10·7 | 14·4 ± 0·4 | Not done | ||
| B | TCR-(β × δ)–/– | 4 (0) | 78·0 ± 8·1 | 13·8 ± 1·5 | Not done | |||
| C | C57.BL/6 | 4 (0) | 8·8 ± 0·6 | 7·3 ± 1·5 | Not done | |||
| 3 | A | TCR-(β × δ)–/– | 7 (1) | 134·0 ± 13·1 | 16·5 ± 0·6 | 6 (0) | 117·7 ± 19·2 | 14·2 ± 0·4 |
| B | C57.BL/6 | 5 (0) | 9·2 ± 1·1 | 8·0 ± 0·3 | Not done | |||
| C | TCR-(β × δ)–/– | Primary infection control | 5 (1) | 63·0 ± 24·4 | 13·8 ± 0·3 | |||
Secondary infections with 103 sporulated oocysts were initiated 4–7 weeks postprimary infection. Data is representative of two further experiments that are not presented.
Data from the oocyst output and the patent period are expressed as mean ± SE. Within experiments, different font types indicate significant differences (P < 0·05): italic denotes significantly greater susceptibility than C57.BL/6 mice; bold italic denotes significantly greater susceptibility than αβ T-cell-deficient mice.
Primary infection controls were naive mice infected in parallel with the secondary infections shown in the right-hand columns.
As anticipated from previous analyses of αβ Τ-cell-deficient mice,17 oocyst production during primary infection of T-cell-deficient TCR-β–/– or TCR-(β × δ)–/– mice greatly exceeded that of immunologically intact mice [either (β × δ)+/– heterozygotes or C57.BL/6]. Likewise, while immunologically intact mice exhibited complete immunity to reinfection (Table 1; Exp. 1 group C), all αβ T-cell-deficient mice remained fully susceptible to reinfection (Table 1; Exp. 1, groups A and B; Exp. 3, group A). However, TCR-γδ-intact, TCR-β–/– mice were significantly less susceptible to infection than completely T-cell-deficient TCR-(β × δ)–/– mice (Table 1, Exp. 1, groups A and B, D and E; Exp. 2, groups A and B; P < 0·05). Increased susceptibility of mice with no T cells was further illustrated by deaths (≈ 10% of mice) that occurred uniquely in TCR-(β × δ)–/– mice, between 8 and 9 days p.i., corresponding to peak parasite numbers in the intestine (Table 1). Indeed, TCR-(β × δ)–/– mice showed the highest susceptibility to infection of any of numerous mutant mouse strains tested (A. L. Smith & A. C. Hayday, unpublished). Compared with controls, the duration of oocyst output (patent period) was greater in TCR-β–/– and TCR-(β × δ)–/– mice, but was not reproducibly different between the two mutant strains.
Although these data indicate that γδ cells can contribute some anti-eimerian function, the magnitude of oocyst yield from infected TCR-β–/– mice did not differ significantly between primary and secondary infections, suggesting that γδ cells in mice receiving a primary infection mediated a protective effect as efficiently as γδ cells from pre-exposed mice. This issue is explored further below. Likewise, the highly susceptible TCR-(β × δ)–/– mice were equally susceptible to primary and secondary infection.
Cellularity of TCR-β–/– mesenteric lymph nodes (MLN) during infection
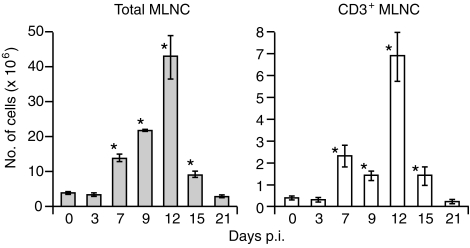
Mesenteric lymph node (MLN) cells of intact animals show marked expansion during infection with E. vermiformis.23,42 To determine whether this activation of lymphoid cells can be provoked by responding γδ cells, the numbers and phenotype of MLN cells were compared during infections of TCR-β–/– and TCR-(β × δ)–/– mice. There was an average of 3·52 ± 0·44 × 106 (mean ± SE) MLN cells in uninfected TCR-β–/– mice. This number increased progressively more than 10-fold to 42·53 ± 6·41 × 106 at 12 days p.i., before returning to resting levels by 21 days p.i. (Fig. 1). During the course of infection, CD3+ TCR-γδ+ lymphocytes composed 13·7 ± 1·1% of MLN cells, and remained largely negative for both CD4 and CD8. The remaining lymphocytes were predominantly CD3– B220+ B cells (data not shown). In contrast, there was no reproducible increase in the cellularity of the MLNs in TCR-(β × δ)–/– mice (uninfected mice 3·48 ± 0·67 × 106; mice 12 days p.i. 3·03 ± 0·27 × 106). Therefore, the MLN cell expansion in infected TCR-β–/– mice, which is mostly B cells, is dependent on direct and/or indirect effects of γδ cells.
Figure 1.
Cellularity of mesenteric lymph nodes (MLNs) from T-cell receptor (TCR)-β–/– mice infected with Eimeria vermiformis. MLN cells were harvested, at the time-points indicated on the figure, from TCR-β–/– mice infected with 1000 sporulated oocysts. CD3+ cells were enumerated using flow cytometry with fluorescein isothiocyanate (FITC)-conjugated anti-CD3. Results are expressed as mean ± SE (n = 3 at each time-point). *Significant difference versus uninfected mice analysed in parallel (P < 0·05). p.i., postinfection.
Adoptive transfer of MLN cells from naive and infected TCR-β–/– mice
To confirm that the difference in susceptibilities of TCR-β–/– and TCR-(β × δ)–/– mice were attributable to the differences in their lymphoid populations, 5 × 107 MLN cells from TCR-β–/– mice were adoptively transferred to T-cell-deficient TCR-(β × δ)–/– recipients. Control mice received PBS, pH 7·2. This regimen, applied to conventional strains, has previously been used to demonstrate transferable immunological protection afforded by CD4+ T cells.38,40 In those studies, MLN cells were more effective than spleen cells.40
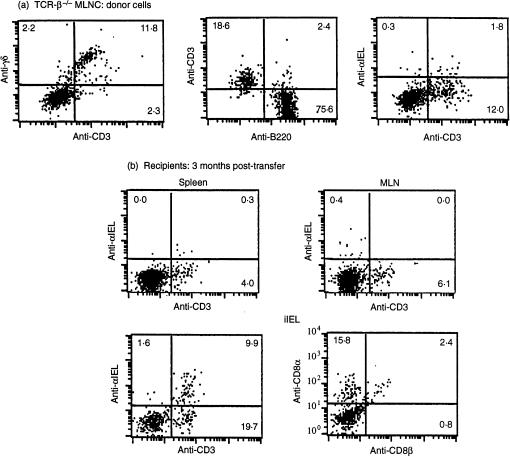
Successful transfer and repopulation of recipients with γδ cells was determined by flow cytometry. Because unmanipulated TCR-(β × δ)–/– mice harbour no CD3+ cells, the requirement for a congenic marker to determine T-cell transfer is obviated. Substantial numbers of CD3+ TCR-γδ+ cells were detected in TCR-(β × δ)–/– recipients within 1 day of transfer, and T cells became established long term among splenic, MLN and intestinal IEL populations; representative plots are shown in Fig. 2. Interestingly, while γδ cells in the spleen and MLN remained CD4– CD8–, a significant proportion of γδ+ IEL acquired the CD8αα homodimer (Fig. 2b), which is commonly expressed by γδ IELs of normal43,44 and TCR-β–/– mice (our unpublished data; ref. 45). Furthermore, > 30% of TCR-γδ+ IEL (but neither splenic nor MLN cells) displayed the integrin αIELβ,7 which is ordinarily expressed by ≈ 90% of IEL on which it promotes association with E-cadherin on the gut epithelium.46–49 Nevertheless, the fact that not all gut-associated γδ cells expressed αIELβ7 is apparently consistent with the finding that γδ cells are less dependent on αIELβ7 than are CD8+ αβ T cells.49 In summary, γδ+ MLN cell inocula reconstituted diverse gut-associated lymphoid compartments of T-cell-deficient mice.
Figure 2.
Reconstitution of T-cell receptor (TCR)-(β × δ)–/– mice with mesenteric lymph node (MLN) cells from TCR-β–/– mice: phenotypic analysis. (a) TCR-β–/– MLN cells; donor cells. (b) Recipients, 3 months post-transfer. Representative flow cytometry plots from at least four individual mice are presented. Antibodies used were phycoerythrin (PE) -conjugated anti-γδ, -anti-CD3, -anti-B220, -anti-αIEL, and -anti-CD8-α, and fluorescein isothiocyanate-conjugated anti-CD3 and -anti-CD8β. iIEL, intestinal intraepithelial lymphocytes.
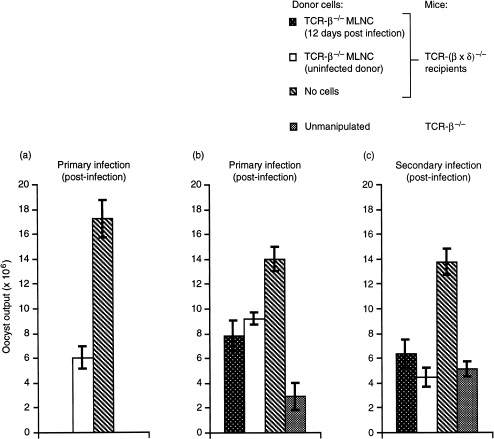
To test the immunoprotective capacity of transferred MLN cells, all mice were infected with 100 sporulated oocysts. A reduction from 1000 oocysts was made to reduce the lethality in infected TCR-(β × δ)–/– mice (see Table 1). The data in Fig. 3(a) show clearly that, compared with PBS-treated controls, TCR-β–/– MLN cells could reduce oocyst output in recipient TCR-(β × δ)–/– mice by 30–60% (P < 0·05), similar to the differences between unmanipulated TCR-β–/– mice and TCR-(β × δ)–/– mice, confirming that those differences are attributable to differences in the lymphoid compartments of the two strains.
Figure 3.
Adoptive transfer of T-cell receptor (TCR)-γδ T cells. Comparison of the efficacy of naive and responding (12 days postinfection) TCR-β–/– mesenteric lymph nodes (MLNs) as donor cells. (a) TCR-(β × δ)–/– mice received cells from naive TCR-β–/– mice (open bar); (b) and (c) TCR-(β × δ)–/– mice received cells from naive TCR-β–/– mice (open bars) or from infected mice (stippled bars), and were challenged once (panel b), rested, and then rechallenged (panel c). Results are expressed as mean ± SE from groups of four to 10 individually housed animals. Oocyst counts from adoptive-transfer recipients were comparable and statistically significantly different from counts from TCR-(β × δ)–/– mice that received phosphate-buffered saline (PBS) but no cells (P < 0·05). Fifty million TCR-β–/– MLN cells were adoptively transferred into TCR-(β × δ)–/– mice (both strains on a C57.BL/6 background). Primary infection was initiated with 100 sporulated oocysts 24–48 hr post-transfer, and secondary infection was initiated (with 100 oocysts) 4 weeks later.
Adoptive transfer analyses of immunological memory
MLN cells displayed measurable increases in number during the 2 weeks of the primary immune response to E. vermiformis infection (Fig. 1). To test whether this reflected enhanced immune responsiveness to the pathogen, further transfer experiments were set up in which the immunoprotective efficacies of TCR-β–/– MLN cells from naive mice and from mice 12 days p.i., respectively, were compared. Both MLN cell preparations conferred significant and comparable degrees of protection (35·8–44·3%) to recipient TCR-(β × δ)–/– animals (Fig. 3b). Thus, the active immunoprotective elements of the inoculum were not obviously enhanced by prior exposure to the pathogen.
To test this further, the adoptive-transfer recipients were rested and rechallenged. The resistance of recipients receiving either inoculum was again comparable. Although it was slightly better than it had been to primary challenge (55·8–67·9%) (Fig. 3c), it was noticeably different to the 99% protection of TCR-(β × δ)–/– recipients afforded by adoptive transfer of TCR-αβ+ 12-day p.i. MLN cell inocula (data not shown; ref. 17). Indeed, the resistance to infection in the rechallenged recipients of TCR-β–/– MLN cells was comparable with that of TCR-β–/– mice of the same cohort from which the transfer inocula were derived (Fig. 3c). The comparability of MLN-cell inocula from naïve or previously infected mice is consistent with the observation that γδ cell-bearing TCR-β–/– mice show no enhanced resistance following repeated infection with higher doses of parasite (Table 1).
An IFN-γ-dependent mechanism reduces susceptibility of TCR-β–/– mice to that of TCR-(β × δ)–/– mice
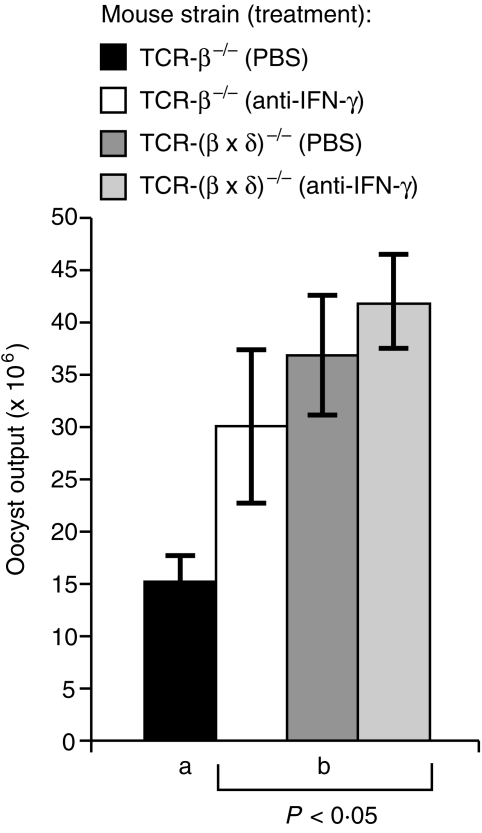
IFN-γ plays an important role in the T-cell-intact animal in limiting infection with E. vermiformis (refs 42,50; A. L. Smith & A. Hayday, submitted). Moreover, γδ+ cells have been reported to produce IFN-γ under various circumstances.1,2,4,5,51 Therefore, an antibody-depletion strategy was applied to test whether the relative resistance of TCR-β–/– mice, as compared with TCR-(β × δ)–/– mice, involved IFN-γ. TCR-β–/– mice were infected with 250 oocysts of a particularly effective inoculum. Comparison of oocyst output from anti-IFN-γ-treated and rat IgG-treated C57.BL/6 mice revealed a significant (P < 0·05) increase in oocyst production, similar to that reported previously,50 which confirmed the efficacy of the antibody treatment (data not shown). Strikingly, anti-IFN-γ-treatment increased the susceptibility of TCR-β–/– mice to the level seen in TCR-(β × δ)–/– mice, but did not significantly increase the susceptibility of TCR-(β × δ)–/– mice (Fig. 4). Therefore, IFN-γ is an important component of the immunoprotective mechanism present in TCR-β–/– mice.
Figure 4.
Exacerbation of infection in T-cell receptor (TCR)-β–/– mice by anti-interferon-γ (IFN-γ) treatment. Results are expressed as mean ± SE from groups of five or six individually housed animals; the bar annotated ‘a’ was significantly different (P < 0·05) from the bars annotated with ‘b’. Mice were injected intravenously with 50 µg of rat anti-IFN-γ or rat immunoglobulin G (IgG) as indicated, and infected with sporulated oocysts 3 hr later.
Discussion
The present study used complementary genetic and cellular approaches to test the capacity of γδ cells to support an immunoprotective function against E. vermiformis, a natural pathogen of the murine small intestinal epithelium and an example of the coccidian parasites that plague birds, reptiles and mammals. The results of both approaches are consistent: TCR-β–/– mice that lack αβ T cells but contain γδ cells are more resistant to infection than are TCR-β × δ–/– mice that lack γδ cells; and lymph node cells from γδ-intact mice transfer protection to γδ-deficient mice. The importance of γδ cells is further highlighted by the striking, infection-driven stimulation (direct or indirect) of other lymphoid cells, particularly B cells, that is seen in infected TCR-β–/– mice but not in γδ-deficient TCR-(β × δ)–/– mice.
At the same time, no significant, functional immunological memory is evident in the γδ-driven activities: TCR-β–/– mice do not exhibit an enhanced response during secondary infection and adoptive transfers of γδ cells are essentially equally efficacious whether drawn from naive or previously challenged mice. Consistent with this, the patent period in γδ-intact TCR-β–/– mice was the same as in T-cell-deficient, TCR-(β × δ)–/– mice, indicating that although the γδ-cell-driven activities in TCR-β–/– mice consistently reduce parasite growth, they are unable to resolve infection, a function that we have previously attributed to αβ Τ cells.17 In these several respects, the actions of γδ cells seem to comply with the characteristics of the innate response, rather than the adaptive αβ-T-cell response.
The experiments presented do not define whether the immunoprotective effects of γδ cells are direct effects, or indirect effects driven via the activation of other effector leucocytes. Indeed, despite a plethora of studies, the same is true for the more potent immunoprotective effects of CD4+ αβ+ T helper 1 (Th1) cells toward Eimeria. In that case, IFN-γ production has been strongly implicated (refs 40,50; A. L. Smith & A. C. Hayday, submitted), as it is in the immunological response of TCR-β–/– mice described here. IFN-γ is known to be produced by at least some subsets and clones of γδ cells.1,2,4,5,51 Furthermore, IFN-γ depletion failed to significantly increase the susceptibility of γδ-deficient TCR-(β × δ)–/– mice, a result that is consistent with the previous finding that natural killer (NK) cells and other non-T lymphocytes are not major effectors of IFN-γ-mediated resistance to E. vermiformis.52 Likewise, B cells have previously been shown to make only minor contributions to the anti-Eimeria response in either chickens or mice (refs 14,16,38; A. L. Smith & A. C. Hayday, submitted), and so it is unlikely that the promotion of IgG production is a primary role for IFN-γ. Taken together, these data encourage the view that γδ cells might act directly against infected enterocytes, consistent with previous reports that IFN-γ production by IELs can directly induce villus epithelial damage.53 While it is also possible that γδ cells regulate epithelial recovery via fibroblast growth factor VII (FGFVII) production,3 the dramatic results of IFN-γ depletion indicate that any effects of FGFVII must be insufficient on their own to account for the immunoprotective contributions of γδ cells.
The adoptive transfer approaches that are shown here to complement the genetic analysis of γδ-cell function, may in future be combined with the use of mice that have genetic disruptions in specific γδ-cell subsets.54 Such studies will reveal whether the same cells are responsible for the three bioactivities of γδ cells now evident in the Eimeria system: namely, immunoprotective effects (Table 1; Figs 3, 4); lymphoid stimulation (Fig. 1; ref. 25); and the suppression of immunopathology.17 Possibly, γδ+ IELs are responsible for some phenotypes and systemic γδ T cells for others. This issue is unresolved by the studies presented here because we show that MLN inocula can partially reconstitute the IEL compartment. The definition of relevant subsets of γδ cells will also cast light on the nature of γδ-cell stimulation in this system. It has recently been shown that murine and human γδ cells respond to MHC class I-related antigens expressed by activated cells, including human enterocytes,1,13 but there has as yet been no implication of these molecules in the response to infection.
The capacity of γδ cells to support some level of protection against the parasite, and at the same time to induce lymphocyte responses, apparently without the development of memory, could reasonably describe the functions of macrophages, NK cells, or other members of the innate immune compartment. This comparison is further provoked by the lack of diversity of the γδ-TCR, characteristic of epithelium-associated γδ cell repertoires,1,7 and the capacity of the γδ-TCR to engage autologous ‘stress-antigens’ seemingly unmodified by foreign antigen.1 Other links between γδ and NK cells are evident in the report that stimulation of bronchoalveolar lavage cells with anti-CD3 and recombinant interleukin-2 (rIL-2) induces γδ T cells to express NK1.1 and to lyse YAC1 cells, the prototypic NK-cell target.55 Furthermore, independent studies have attributed the mild immunoprotective effects of γδ cells towards Listeria to a collaboration between γδ cells and NK cells.56,57
On the other hand, the classification of γδ cells within the adaptive response seems clear. For example, the TCR-δ-chain complementarity-determining region-3 (CDR3) shows greater diversity than observed for any αβ T cells.1,25,58 Given this, and given the generally accepted view that the activation of lymphocytes with specific receptors is followed by the transition of those lymphocytes into a memory state, it may be that γδ cells engage peripheral autologous ‘activation/stress’ antigens early in their development, and become activated to a ‘constitutive memory’ state.1,13
It might be argued that the capacity of γδ cells to support lymphoid activation (Fig. 1) and immunoprotection (Figs 3, 4) is largely irrelevant because it is only revealed when mice are deficient in αβ T cells. (αβ T-cell-intact, γδ-cell-deficient mice are no more susceptible than wild-type animals to either primary or secondary infection.17) Nonetheless, there are important instances of defective αβ T-cell function, particularly the CD4+ compartment in patients with acquired immune deficiency syndrome (AIDS). Likewise, there may be particular times or anatomical sites in normal, healthy animals when αβ T-cell function is compromised and hence γδ cell-driven immunoprotection would be particularly significant. This will be investigated.
Acknowledgments
The work was supported by NIH grants AI 27855 and AI38942 (to A. C. Hayday, by the Wellcome Trust (to A. C. Hayday), and by the BBSRC (to A. L. Smith). Laboratory facilities were provided by the Dunhill Medical Trust. We wish to thank Jasmine Sia for technical support and our colleagues in the laboratory for valuable input.
References
- 1.Hayday AC. γδ cells – a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 2.Ishikawa H, Li Y, Abeliovich A, Yamamoto S, Kaufmann SHE, Tonegawa S. Cytotoxic and interferon γ-producing activities of γδ T cells in the mouse intestinal epithelium are strain dependent. Proc Natl Acad Sci USA. 1993;90:8204–8. doi: 10.1073/pnas.90.17.8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boismenu R, Havran WL. Modulation of epithelial cell growth by intraepithelial gamma delta T cells. Science. 1994;266:1253–5. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- 4.Ferrick DA, Schrenzel MD, Mulvania T, Hsieh B, Ferlin WG, Lepper H. Differential production of interferon-γ and interleukin-4 in response to Th1- and Th2-stimulating pathogens by γδ T cells in vivo. Nature (London) 1995;373:255–7. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- 5.Wen L, Barber DF, Pao W, Wong FS, Owen MJ, Hayday AC. Primary γδ T cell clones can be defined phenotypically and functionally as Th1/Th2 cells, and illustrate the association of CD4 with Th2 differentiation. J Immunol. 1998;160:1965–74. [PubMed] [Google Scholar]
- 6.Kaufmann SHE. γ/δ and other unconventional T lymphocytes: what do they see and what do they do? Proc Natl Acad Sci USA. 1996;93:2272–9. doi: 10.1073/pnas.93.6.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayday AC, Pao W, Roitt I, Delves P, editors. The Encyclopedia of Immunology. 2. London: Academic Press; 1997. T cell receptor gamma/delta; pp. 2268–78. [Google Scholar]
- 8.Goodman T, Lefrancois L. Expression of the γδ cell receptor on intestinal CD8(+) intraepithelial lymphocytes. Nature. 1988;333:855–8. doi: 10.1038/333855a0. [DOI] [PubMed] [Google Scholar]
- 9.Bucy RP, Chen CL, Cihak U, Losch U, Cooper MD. Avian T cells expressing gamma delta receptors localize in the splenic sinusoids and the intestinal epithelium. J Immunol. 1988;141:2200–5. [PubMed] [Google Scholar]
- 10.Deusch K, Pfeffer K, Reich K, Gstettenbauer M, Daum S, Lulung F, Classen M. Phenotypic and functional characterization of human TCRγδ+ intestinal epithelial lymphocytes. Curr Topics Microbiol Immunol. 1991;173:279–84. [PubMed] [Google Scholar]
- 11.Lillehoj HS, Chung HS. Postnatal development of T-lymphocyte subpopulations in the intestinal intraepithelium and lamina propria in chickens. Vet Immunol Immunopathol. 1992;3–4:347–60. doi: 10.1016/0165-2427(92)90021-h. [DOI] [PubMed] [Google Scholar]
- 12.Janeway CA, Jones B, Hayday AC. Specificity and function of cells bearing γδ T cell receptors. Immunol Today. 1988;9:73–6. doi: 10.1016/0167-5699(88)91267-4. [DOI] [PubMed] [Google Scholar]
- 13.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial γδ cells. Science. 1998;279:1737–40. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 14.Rose ME, Owen DG, Hesketh P. Susceptibility to coccidiosis: effect of strain of mouse on reproduction of Eimeria vermiformis. Parasitology. 1984;88:45–54. doi: 10.1017/s0031182000054330. [DOI] [PubMed] [Google Scholar]
- 15.Rose ME. Eimeria, Isospora and Cryptosporidium. In: Soulsby EJL, editor. Immune Responses in Parasitic Infections: Immunology, Immunopathology and Immunoprophylaxis. III. London: Balliere-Tindall; 1987. pp. 275–312. Protozoa, chapter 6. [Google Scholar]
- 16.Wakelin D, Rose ME. Immunity to coccidiosis. In: Long PL, editor. Coccidiosis of Man and Domestic Animals. Boca Raton, FL: CRC Press; 1990. pp. 281–306. chapter 14. [Google Scholar]
- 17.Roberts SJ, Smith AL, West AB, Wen L, Findly RC, Owen MJ, Hayday AC. T cell αβ+ and γδ+ deficient mice display abnormal but distinct phenotypes toward a natural, widespread infection of the intestinal epithelium. Proc Natl Acad Sci USA. 1996;93:11774–9. doi: 10.1073/pnas.93.21.11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mombaerts P, Arnoldi J, Russ F, Tonogawa S, Kaufmann SHE. Different roles of αβ and γδ T cells in immunity against an intracellular bacterial pathogen. Nature (London) 1993;365:53–6. doi: 10.1038/365053a0. [DOI] [PubMed] [Google Scholar]
- 19.Tsuji M, Mombaerts P, Lefrancois L, Nussensweig RS, Zavala F, Tonegawa S. γδ T cells contribute to immunity against the liver stages of malaria in αβ T-cell-deficient mice. Proc Natl Acad Sci USA. 1994;91:345–9. doi: 10.1073/pnas.91.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eichelberger M, McMickle A, Blackman M, Mombaerts P, Tonegawa S, Doherty PC. Functional analysis of the TCR alpha– beta+ cells that accumulate in the pneumonic lung of influenza virus-infected TCR-alpha–/– mice. J Immunol. 1995;154:1569–76. [PubMed] [Google Scholar]
- 21.Ladel CH, Blum C, Dreher K, Reifenburg K, Kaufmann SHE. Protective role of γδ T cells and αβ T cells in tuberculosis. Eur J Immunol. 1995;25:2877–81. doi: 10.1002/eji.1830251025. [DOI] [PubMed] [Google Scholar]
- 22.Waters WR, Harp JA. Cryptosporidium parvum infection in T-cell-receptor (TCR)-α- and TCR-δ-deficient mice. Infect Immun. 1996;64:1854–7. doi: 10.1128/iai.64.5.1854-1857.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Findly RC, Roberts SJ, Hayday AC. Dynamic response of murine gut intraepithelial T cells after infection by the coccidian parasite Eimeria. Eur J Immunol. 1993;23:2557–64. doi: 10.1002/eji.1830231027. [DOI] [PubMed] [Google Scholar]
- 24.Lillehoj HS. Analysis of Eimeria acervulina-induced changes in the intestinal T lymphocyte subpopulations in two chicken strains showing different levels of susceptibility to coccidiosis. Res Vet Sci. 1994;56:1–7. doi: 10.1016/0034-5288(94)90188-0. [DOI] [PubMed] [Google Scholar]
- 25.Pao W, Wen L, Smith AL, et al. γδ T cell help for B cells is stimulated by repeated parasitic infection. Curr Biol. 1996;6:1317–25. doi: 10.1016/s0960-9822(02)70718-5. [DOI] [PubMed] [Google Scholar]
- 26.Peng SL, Madaio M, Hayday AC, Craft J. Propagation and regulation of systemic autoimmunity by γδ cells. J Immunol. 1996;157:5689–98. [PubMed] [Google Scholar]
- 27.Szczepnik M, Anderson LR, Ushio H, Ptak W, Owen MJ, Hayday AC, Askenase PW. γδ cells from tolerised αβ TCR deficient mice inhibit contact sensitivity T cells in vivo and their interferon γ production in vitro. J Exp Med. 1996;184:2129–39. doi: 10.1084/jem.184.6.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiohara T, Moriya N, Hayakawa J, Itohara S, Ishikawa H. Resistance to cutaneous graft-vs.-host disease is not induced in T cell receptor delta gene-mutant mice. J Exp Med. 1996;183:1483–9. doi: 10.1084/jem.183.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu Y-X, Roark CE, Kelly K, Drevets D, Campbell P, O'brien R, Born W. Immune protection and control of inflammatory tissue necrosis by gamma delta T cells. J Immunol. 1994;150:550–5. [PubMed] [Google Scholar]
- 30.D'souza CD, Cooper AM, Frank AA, Mazzacacaro RJ, Bloom BR, Orme I. An anti-inflammatory role for gamma delta T lymphocytes in immunity to Mycobacterium tuberculosis. J Immunol. 1997;158:1217–21. [PubMed] [Google Scholar]
- 31.Hiromatsu K, Yoshikai Y, Matsuzaki G, Ohga S, Muramori K, Matsumoto K, Bluestone JA, Nomoto K. A protective role of γδ T cells in primary infection with Listeria monocytogenes in mice. J Exp Med. 1992;175:49–57. doi: 10.1084/jem.175.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosat J-P, MacDonald HR, Louis JA. A role for γδ T cells during experimental infection of mice with Leishmania major. J Immunol. 1993;150:550–5. [PubMed] [Google Scholar]
- 33.Perera MK, Carter R, Goonewardene R, Mendis KN. Transient increase in circulating γδ T cells during Plasmodium vivax malarial paroxysms. J Exp Med. 1994;170:311–5. doi: 10.1084/jem.179.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hisaeda H, Nagasawa H, Maeda K-I, Maekawa Y, Ishikawa H, Ito Y, Good RA, Himeno K. Gamma delta T cells play an important role in hsp65 expression and in acquiring protective immune responses against infection with Toxoplasma gondii. J Immunol. 1995;154:244–51. [PubMed] [Google Scholar]
- 35.van der Heyde H, Elloso MM, Chang W-L, Kaplan M, Manning DD, Weidanz WP. γδ T cells function in cell-mediated immunity to acute blood-stage Plasmodium chabaudi adami malaria. J Immunol. 1995;154:3985–90. [PubMed] [Google Scholar]
- 36.Langhorne J, Mombaerts P, Tonegawa S. Alpha beta and gamma delta T cells in the immune response to the erythrocytic stages of malaria in mice. Int Immunol. 1995;7:1005–11. doi: 10.1093/intimm/7.6.1005. [DOI] [PubMed] [Google Scholar]
- 37.Williams DM, Grubbs BG, Kelly K, Pack E, Rank RG. Role of gamma-delta T cells in murine Chlamydia trachomatis infection. Infect Immun. 1996;64:3916–9. doi: 10.1128/iai.64.9.3916-3919.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rose ME, Wakelin D, Joysey HS, Hesketh P. Immunity to coccidiosis: adoptive transfer in NIH mice challenged with Eimeria vermiformis. Parasite Immunol. 1988;10:59–69. doi: 10.1111/j.1365-3024.1988.tb00203.x. [DOI] [PubMed] [Google Scholar]
- 39.Weir DM. Handbook of Experimental Immunology. 4. II. Oxford: Blackwell Scientific Publications; 1986. [Google Scholar]
- 40.Rose ME, Joysey HS, Hesketh P, Grencis RK, Wakelin D. Mediation of immunity to Eimeria vermiformis in mice by L3T4+ T cells. Infect Immun. 1988;56:1760–5. doi: 10.1128/iai.56.7.1760-1765.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kohler J, Heumann D, Garotta G, Leroy D, Bailat S, Barras C, Baumgartner J-D, Glauser MP. IFNγ involvement in the severity of Gram-negative infections in mice. J Immunol. 1993;151:916–21. [PubMed] [Google Scholar]
- 42.Rose ME, Wakelin D, Hesketh P. Interferon-gamma-mediated effects upon immunity to coccidial infections in the mouse. Parasite Immunol. 1991;13:63–74. doi: 10.1111/j.1365-3024.1991.tb00263.x. [DOI] [PubMed] [Google Scholar]
- 43.Lefrancois L. Phenotypic complexity of intraepithelial lymphocytes of the small intestine. J Immunol. 1991;147:1746–51. [PubMed] [Google Scholar]
- 44.Guy-Grand D, Cerf-Bensussan N, Malissen B, Malassis-Seris M, Briottet C, Vassalli P. Two gut intraepithelial CD8+ lymphocyte populations with different T cell receptors: a role for the gut epithelium in T cell differentiation. J Exp Med. 1991;173:471–81. doi: 10.1084/jem.173.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pereira P, Gerber D, Huang SY, Tonegawa S. Ontogenic development and tissue distribution of V gamma 1-expressing gamma/delta T lymphocytes in normal mice. J Exp Med. 1995;182:1921–30. doi: 10.1084/jem.182.6.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kilshaw PJ, Murant SJ. Expression and regulation of beta 7 (beta p) integrins on mouse lymphocytes: relevance to the mucosal immune system. Eur J Immnol. 1991;21:2591–7. doi: 10.1002/eji.1830211041. [DOI] [PubMed] [Google Scholar]
- 47.Cerf-Bensussan N, Jarry A, Brousse N, Lisowska-Grospierre A, Guy-Grand D, Griscelli C. A monoclonal antibody (HML-1) defining a novel membrane molecule present on human intestinal lymphocytes. Eur J Immunol. 1987;17:1279–85. doi: 10.1002/eji.1830170910. [DOI] [PubMed] [Google Scholar]
- 48.Lefrancois L, Barrett TA, Havran WL, Puddington L. Developmental expression of the alpha IEL beta 7 integrin on T cell receptor gamma delta and T cell receptor alpha beta T cells. Eur J Immunol. 1994;24:635–40. doi: 10.1002/eji.1830240322. [DOI] [PubMed] [Google Scholar]
- 49.Schon MP, Arya A, Murphy EA, et al. Mucosal T lymphocyte numbers are selectively reduced in integrin αE (CD103)-deficient mice. J Immunol. 1999;162:6641–9. [PubMed] [Google Scholar]
- 50.Rose ME, Wakelin D, Hesketh P. Gamma interferon controls Eimeria vermiformis primary infection in BALB/c mice. Infect Immun. 1989;57:1599–603. doi: 10.1128/iai.57.5.1599-1603.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamamoto S, Russ F, Teixeira HC, Conradt P, Kaufmann SHE. Listeria monocytogenes-induced gamma-interferon secretion by intestinal intraepithelial γδ T lymphocytes. Infect Immun. 1993;61:2154–61. doi: 10.1128/iai.61.5.2154-2161.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith AL, Rose ME, Wakelin D. The role of natural killer cells in resistance to coccidiosis: investigations in a murine model. Clin Exp Immunol. 1994;97:273–9. doi: 10.1111/j.1365-2249.1994.tb06080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guy-Grand D, DiSanto JP, Henchoz P, Malassis-Seris M, Vassalli P. Small bowel enteropathy: role of intraepithelial lymphocytes and of cytokines (IL-12, IFN-γ, and TNF) in the induction of epithelial cell death and renewal. Eur J Immunol. 1998;28:730–44. doi: 10.1002/(SICI)1521-4141(199802)28:02<730::AID-IMMU730>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 54.Laky K, Lefrancois L, Lingenheld EG, et al. Enterocyte expression of IL-7 induces development of γδ T cells and Peyer's patches. J Exp Med. 2000;191:1569–80. doi: 10.1084/jem.191.9.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eichelberger M, Doherty PC. Gamma delta T cells from influenza-infected mice develop a natural killer cell phenotype following culture. Cell Immunol. 1994;159:94–102. doi: 10.1006/cimm.1994.1298. [DOI] [PubMed] [Google Scholar]
- 56.Ladel CH, Blum C, Kaufmann SHE. Control of natural killer cell-mediated resistance against the intracellular pathogen Listeria monocytogenes by γ/δ T lymphocytes. Infect Immun. 1996;64:1774–49. doi: 10.1128/iai.64.5.1744-1749.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Skeen MJ, Ziegler HK. Induction of murine peritoneal cells and their role in resistance to bacterial infection. J Exp Med. 1993;178:971–84. doi: 10.1084/jem.178.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rock EP, Sibbald PR, Davis MM, Chien YH. CDR3 length in antigen-specific immune receptors. J Exp Med. 1994;179:323–8. doi: 10.1084/jem.179.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]