Abstract
Immunization of mice with cognate cancer-derived heat-shock protein (hsp) preparations leads to protection from cancer growth. As hsp used for vaccination or therapy are derived from autologous cancers, questions of pathological autoimmunity are of immense significance for the ongoing translation of this approach to therapy of human cancer. Employing the sera of normal adult mice as the first antibody, highly sensitive immunoblotting revealed the presence of anti-hsp natural autoantibodies in healthy animals. Natural autoantibodies of the immunoglobulin D (IgD) isotype bind to gp96, whereas hsp70 was recognized by IgD and IgM autoantibodies. Neither hsp was recognized by the IgA, IgE or IgG immunoglobulins contained in the serum. The antigen–antibody recognition was titratable and dependent on the integrity of the IgD molecule. Sera from only a subset of the animals tested were found to be positive for autoantibodies against gp96 and hsp70, and individual and strain-specific variations were detected. Injection of gp96 into healthy mice did not show sustained or consistent anti-gp96 IgD antibody response, class switching, toxicity or pathological autoimmunity. IgD autoantibodies against gp96 and hsp70 were also not detected in the autoimmune lpr mice. These observations show the existence of a measured and tightly regulated natural immune response to hsp.
Introduction
Immunization of mice and rats with cancer-derived heat-shock protein (hsp)–peptide complexes leads to protective and specific immunity against the cancer from which the hsp–peptide complexes are derived.1 This phenomenon has been shown in mice1 and rats2 in a wide array of cancer types including fibrosarcoma,3 hepatocarcinoma,2 lung carcinoma, melanoma, colon carcinoma,4 squamous cell carcinoma,5 and in prophylaxis2,3 as well as in therapy of pre-existing disease.4 As hsp are purified from each rodent tumour individually, and as during translation of this approach to immunotherapy of human cancer, individual patients' cancers are being used as a source of the hsp vaccine,6–8 questions regarding the types and levels of auto-immune responses against syngeneic hsp are of importance and are examined in this study.
Natural autoantibodies to a number of self antigens such as cytoskeletal proteins, DNA, thyroglobulin and lipoprotein9–11 have been identified in healthy animals and humans.12–14 These autoantibodies have been shown to be broadly reactive,12 and the antigens recognized by them are highly conserved molecules.14 Here we report the detection of immunoglobulins, specific for the highly conserved and ubiquitously expressed hsp, in the sera of normal healthy mice. Hsp-specific antibodies have been reported previously in sera of animals and humans infected with bacteria, protozoa, and helminthic parasites.14 However, these antibodies are directed against the hsp determinants that are dissimilar between the host and infectious agents and not against the host hsp.15 In this context, our demonstration of the existence of circulating natural antibodies against self hsp in healthy animals is a true demonstration of non-pathological autoimmunity.
Materials and methods
Animals
Inbred BALB/cJ, DBA/2, C3H, C57BL/6, non-obese diabetic mice (NOD) and BALB/nu/nu mice (6–8-weeks-old) were obtained from the Jackson Laboratory (Bar Harbor, ME), C57BL/6-lpr mice were kindly provided by Dr Leonardo Aguila. All the mice were maintained in the pathogen-free barrier mouse facilities. Urine from the NOD mice was collected daily from each mouse and tested for the presence of glucose by using Diastix (reagent strips for urine analysis, Bayer, Elkart, IN).
Antibodies
Mouse sera used as first antibody in immunoblotting were prepared from total blood. Briefly, after collection, blood was allowed to clot for 30 min at room temperature, followed by an additional hour at 4°, then centrifuged at 10 000 g for 15 min. Immediately after preparation, sera were aliquoted and stored at −80°. Peroxidase-conjugated antibodies specific for each isotype were used as second antibodies. Purified goat anti-mouse α heavy chain immunoglobulin peroxidase conjugate, purified goat anti-mouse γ heavy chain immunoglobulin peroxidase conjugate and purified goat anti-mouse µ heavy chain immunoglobulin peroxidase were purchased from Sigma (St Louis, MO). Rat monoclonal antibody peroxidase conjugate (clone LO-ME-3), specific for the mouse ε heavy chain immunoglobulin and rat monoclonal antibody peroxidase conjugate (clone PP223.U), specific for mouse δ heavy chain immunoglobulin were purchased from The Binding site Ltd. (Birmingham, UK). Mouse monoclonal antibody (clone N27F3-4, specific for both constitutive hsp73 and inducible hsp72) and the rat monoclonal antibody specific for gp96 (clone 9G10) were purchased from Neomarkers (Fremont, CA).
Purification of hsp
Liver tissue (20 g) of naive BALB/cJ mice was homogenized in 40 ml hypotonic buffer (10 mm NaHCO3, 0·5 mm phenylmethylsulphonyl fluoride (PMSF), pH 7·1), and a 100 000 g supernatant obtained. It was applied to a Blue Sepharose column to remove albumin, and was used for purification of hsp70 as described by Peng et al.,16 and for purification of gp96 as described by Srivastava.3 The purified fractions were checked for purity of hsp70 and gp96 by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and immunoblotting. All proteins were quantified using the Bradford assay and bovine serum albumin (BSA) was used as the standard (BioRad, Richmond, CA).
Immunodepletion of hsp
Purified hsp70 (3·5 µg in 50 µl) was applied on anti-hsp70 microaffinity column (Affi-gel hz from Bio-Rad crosslinked with anti-hsp70 antibody specific to the inducible and constitutive hsp70), and washed with 500 µl of phosphate-buffered saline (PBS). The flow-through and the wash were concentrated to 50 µl on Centricon-10 (Amicon). Purified gp96 (3·5 µg in 50 µl) was processed similarly to hsp70 on a anti-gp96 microaffinity column. Aliquots of the sample before and after depletion were analysed by immunoblotting.
SDS–PAGE and immunoblotting
Proteins were resolved on 10% SDS–PAGE following denaturation by boiling for 5 min in SDS sample buffer. After electrophoresis, proteins were either silver stained or transferred onto polyvinyldenefluoride (PVDF) membrane (Millipore Corp., Bedford, MA) and probed with appropriate serum and antibodies as described above.16 Individual sera were analysed by immunoblotting using a multiscreening system (Bio-Rad, CA, USA). Detection was performed using ECL chemoluminesence kit from Amersham (Amersham, UK).
Results
Presence of anti-hsp antibodies in mouse sera
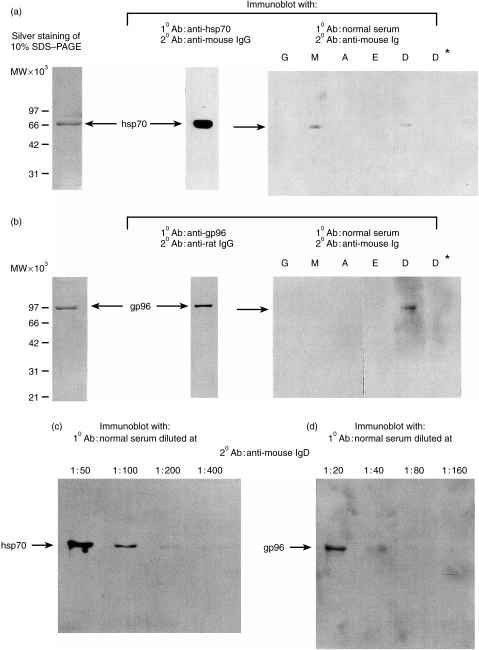
Purified preparations of mouse liver hsp70 were applied to SDS–PAGE and immunoblots prepared and probed with mouse anti-hsp70 monoclonal antibody (followed by second anti-mouse immnuoglobulin G (IgG) antibody), to establish the identity of the purified protein as hsp70. Similar blots were probed with pooled sera from four normal healthy 6–8-week-old BALB/cJ mice and further probed with second antibodies to individual immunoglobluin subclasses: IgG, IgM, IgA, IgE and IgD. The result shows the presence of anti-hsp70 antibodies of the IgD and IgM but not other classes, in normal BALB/cJ sera (Fig. 1a). As an indication of the fact that the interaction detected on the immunoblot was indeed antibody-mediated, recognition of the 70 000 MW band was found to be abrogated if the BALB/cJ sera were reduced by 2-mercaptoethanol before being used for immunoblotting (Fig. 1a, lane D and D*). The search for anti-gp96 antibodies in normal BALB/cJ sera was carried out by a similar approach and anti-gp96 antibodies of the IgD, but not other classes were detected (Fig. 1b, lane D). Probing of immunoblots of hsp70 and gp96 with serially diluted pooled normal BALB/cJ sera, followed by quantitative scanning, showed the anti-hsp70 IgD titre to be ∼1 : 100 (Fig. 1c), and anti-gp96 IgD titre to be ∼1: 30 (Fig. 1d).To determine the specificity of the anti-hsp IgD detected, ascites fluid obtained from mice injected with IgD producing plasmocytoma TEPC-1017 was used to probe the immunoblot of hsp70 and gp96. Neither were detected by this IgD antibody (data not shown).
Figure 1.
Immunodetection of (a) hsp70 and (b) gp96 by natural autoantibodies. Ten µg of hsp70 or gp96 were resolved on 10% SDS–PAGE and visualized by silver staining. The same quantity was transferred on PVDF membrane and probed with monoclonal antibodies specific for each hsp. hsp70 and gp96 were also probed with a pool of four normal sera from BALB/cJ diluted at 1 : 30, followed by secondary mouse peroxidase-conjugated antibodies, specific for each of the five isotypes. Normal sera in lane D* were treated with 70 mm 2-mercaptoethanol (1 µl of 14 m 2-mercaptoethanol in 200 µl of serum) immediately before incubation, then re-probed with the anti-IgD peroxidase antibody. Titration of sera for detection of (c) hsp70, and (d) gp96. hsp70 and gp96 were immunoblotted with increasing dilution of normal sera used as first antibody, and anti-mouse IgD peroxidase used as secondary antibody. The anti-gp96 and anti-hsp70 reactivities reached saturation at 1 : 20 and 1 : 50, respectively. Immunoblots with lower dilutions are not shown.
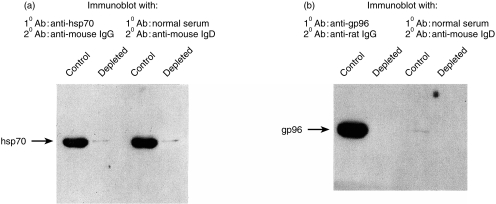
In order to confirm that the 70 000 MW and the 96 000 MW bands detected by immunoblotting with normal sera are indeed hsp70 and gp96, respectively, and not contaminating proteins of indistinguishable sizes, the purified preparations were depleted of hsp70 or gp96 using specific affinity columns and the unbound materials probed with the antibodies. Figure 2(a) shows that the starting purified 70 000 MW material is strongly positive for hsp70, as judged by its reactivity with monoclonal anti-mouse hsp70 antibody and is depleted of it almost entirely after passage through an immunoaffinity column. When these blots are probed with the normal BALB/cJ sera, it is observed that while the starting material is strongly positive for hsp70, no anti-hsp70 IgD reactivity is detected in the immunodepleted material. Anti-hsp70 IgM reactivity of normal sera is also lost after immunodepletion (data not shown). Similar studies were carried out with gp96 and it is observed that while the starting material is strongly positive for gp96, gp96-depleted preparations are not detected by anti-gp96 IgD reactivity of normal sera (Fig. 2b).
Figure 2.
Affinity depletion of preparations containing (a) hsp70 and (b) gp96. High-pressure liquid chromatography fractions, positive for hsp70 after purification were applied on an anti-hsp70 immuno-affinity agarose column. The unbound material (depleted) as well as the hsp70 purification before depletion (control) were immunoblotted for the presence of hsp70 with a monoclonal anti-hsp70 antibody or with a pool of four normal sera diluted 1 : 50 followed by the mouse anti-IgD peroxidase. The sample containing gp96 was depleted following the same procedure than for hsp70, and blotted with anti-gp96 monoclonal antibody or with a pool of sera from three normal mice diluted at 1 : 30 followed by the mouse anti-IgD peroxidase.
Individual-specific and strain-specific variation in levels of natural autoantibody to hsp70 and gp96
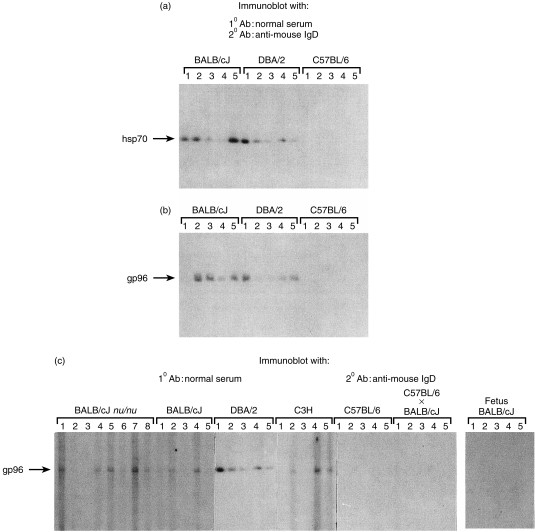
Sera of individual mice (as opposed to pooled sera, see Figs 1 and 2) were tested for autoreactivity to hsp70 and gp96, and a remarkable range of reactivities was observed, among individual mice of the same inbred strain, and also among different strains. Thus, while most individual mice of BALB/cJ or DBA/2 strains showed anti-hsp70 IgD titres close to the values from pooled sera, as discussed earlier, one of five BALB/cJ mice and two of five DBA/2 mice had barely detectable levels of anti-hsp70 IgD antibodies. Surprisingly, none of the five C57BL/6 mice tested, showed any serum anti-hsp70 IgD reactivity at all (Fig. 3).
Figure 3.
Individual variation in the presence of IgD natural autoantibodies against hsp. (a) hsp70, or (b) gp96 were immunoblotted and probed with normal sera for the presence of IgD autoantibodies as described in Fig. 1. Solutions used as first antibody were derived from individual sera, collected from three different strains of mice. The same sera was used for detection of hsp70 (dilution 1 : 50) and gp96 (dilution 1 : 30). (c) Strain variations in the presence of anti-gp96 IgD autoantibodies. Gp96 was immunoblotted as described in Fig. 1. The first antibody solutions used were derived from individual sera, collected from six strains of mice, and in 15–21-day-old BALB/cJ fetuses. Sera were diluted 1 : 30.
Analysis of anti-gp96 IgD levels in the same normal mouse sera as used above, showed that while three of five BALB/cJ mice tested had significant reactivity, two of five mice had none. Only one of five DBA/2 sera tested showed anti-gp96 IgD reactivity. Similar to the observations with anti-hsp70 reactivity, none of the five C57BL/6 mice showed no anti-gp96 IgD reactivity. No correlation between the anti-hsp70 and anti-gp96 reactivities in individual mice was evident: thus, BALB/cJ mouse no. 1 and DBA/2 mice nos 2 and 4 had strong anti-hsp70 reactivity, but no anti-gp96 reactivity, while the anti-hsp70 and anti-gp96 reactivities of BALB/cJ mouse no. 2 and DBA/2 mouse no. 5 are quite comparable (Fig. 3a, b).
The anti-gp96 IgD reactivity was examined in more detail. Sera from nu/nu and C3H mice were examined and found to show a similar variation in anti-gp96 IgD levels as the BALB/cJ mice. Further, in light of the complete absence of anti-gp96 IgD in sera of C57BL/6 mice, sera of F1 hybrids of C57BL/6 and BALB/cJ mice were tested for anti-gp96 IgD. No anti-gp96 antibodies were detected in the five of five F1 mice tested, indicating that the trait for absence of anti-gp96 IgD in C57BL/6 mice was a dominant one, suggesting a regulatory mechanism for maintenance of anti-gp96 IgD levels (Fig. 3c).
Sera from 15–20-day-old BALB/cJ fetuses were tested for anti-gp96 antibody levels. None of the mice tested (five of five) showed reactivity, even as the adult BALB/cJ mice show significant anti-gp96 reactivity (Fig. 3c). This observation suggests a developmental regulation of the appearance of anti-gp96 IgD in sera, and a mechanism for exclusion of maternal anti-gp96 IgD from the fetal circulation. However, to formally validate this statement kinetics of the appearance of anti-gp96 IgD should be performed during the embryonic development and the early neonatal period of the mice.
Immunization with gp96 does not lead to elevation of anti-gp96 immunoglobulin levels or exacerbation of autoimmune diabetes
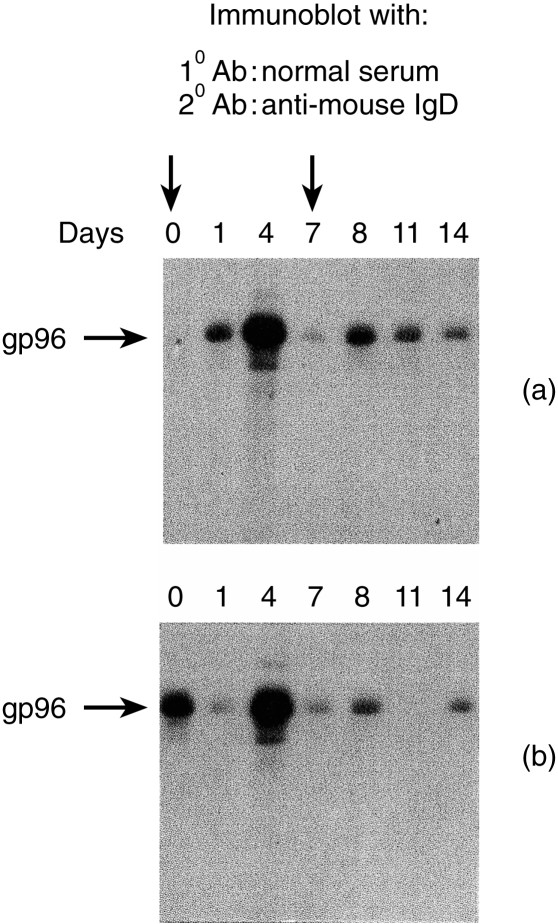
Mice were immunized subcutaneously with 50 µg BALB/cJ liver-derived gp96 in PBS at day 0 and day 7 and mice were bled on days 0,1,4,7,8,11 and 14. The sera were tested for anti-gp96 IgD levels. While some fluctuations in the antibody levels were observed, apparently in response to vaccination, no consistent and sustained elevation in the levels of anti-gp96 IgD was detected (Fig. 4). The changes observed varied from mouse to mouse and no consistent pattern was detected. Sera were also tested for conversion of anti-gp96 IgD to IgM and IgG; no such conversions were detected in an analysis of over 100 mice examined individually after weekly injection of 20–50 µg of gp96 (data not shown).
Figure 4.
(a) Lack of persistent anti-gp96 IgD autoantibody after immunization. Mice were immunized at day 0 and 7 subcutaneously with 50 µg of purified gp96. Sera were collected successively in the same animal at days 0, 1, 4, 7, 8, 11, 14 and tested for the presence of anti-gp96 IgD autoantibody.
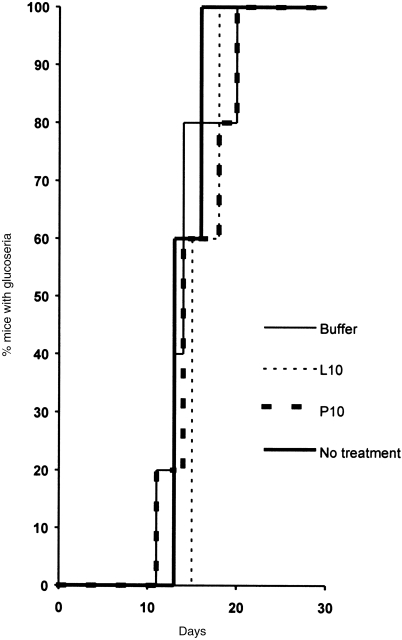
We also investigated the possibility that vaccination of NOD mice with gp96 derived from pancreas, may accelerate the onset of autoimmune diabetes. NOD mice spontaneously develop autoimmune diabetes by 8–10 weeks of age. NOD mice were unimmunized or immunized twice, 1 week apart, with 10 µg per injection of either pancreas-derived gp96, liver-derived gp96, or buffer and were monitored for glucose level in the urine. Immunization was found to have no consequence on the kinetics of appearance and progression of the disease (Fig. 5).
Figure 5.
Lack of consequence on the development of autoimmune diabetes after immunization with gp96. NOD mice of 6 weeks of age were left unimmunized or were immunized subcutaneously at days 0 and 7 with 10 µg of pancreas-derived gp96 (P10), 10 µg of liver-derived gp96 (L10), buffer. Urine sample was collected daily from each mouse and tested for the presence of glucose that is more than 100 mg/dl.
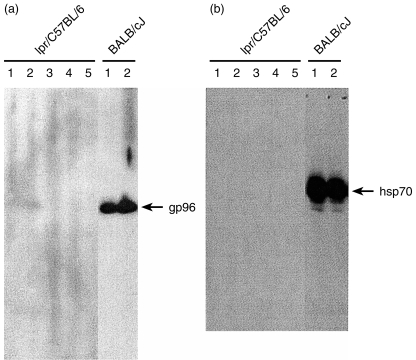
We also tested for the presence of anti-hsp70 and anti-gp96 IgD autoantibodies in 16-week-old lpr mice, another animal model for autoimmune predisposition. At this age all the mice had developed a lymphoproliterative autoimmune disease macroscopically visible in the spleen.17 While sera from individual BALB/cJ mice were positive no IgD autoantibodies were detected against hsp70 (Fig. 6a) and gp96 (Fig. 6b) in the sera of lpr mice.
Figure 6.
Lack of anti-gp96 and anti-hsp70 IgD autoantibody in autoimmune lpr mice. Individual sera were obtained from four male and and one female 16-week-old C57BL/6-lpr mice. Sera were diluted 1 : 30 as first antibody against (a) gp96 and (b) hsp70, anti mouse IgD peroxidase used as secondary antibody.
Discussion
We report here the presence of natural autoantibodies against two constitutively expressed heat-shock proteins, hsp70 and gp96, in the sera of normal healthy unimmunized adult mice. The autoantibodies are of the IgD and the IgM isotypes: no anti-hsp antibodies of IgA, IgE, or IgG isotypes were found in mouse sera. The antibodies were detected in sera of BALB/cJ, C3H-HeJ and nu/nu mice, but not in C57BL/6 mice. This observation is consistent with previous reports that show generalized diminished secretion of IgD antibodies in C57BL/6 mice, for reasons as yet unknown.18 The observation that the sera of F1 (C57BL/6 × BALB/cJ) mice do not have serum IgD antibodies to gp96 or hsp70 while the BALB/cJ mice do, indicates that the mechanism that inhibits secretion of IgD specific for hsp in C57BL/6 is a dominant one.
The presence of antibodies to hsp in normal serum may be attributed to two mutually non-exclusive reasons. They may represent a natural, pre-existing autoimmune response, or they may be an indicator of a previous encounter of the mice with infectious agents, wherein the anti-hsp response may have been elicited by the hsp of the alien invaders, as reported previously.12 In the case of antibodies to the hsp gp96, the response is clearly an autoimmune response: gp96 is expressed exclusively in the endoplasmic reticulum of vertebrates and is not found in any micro-organisms.15 The serum IgD autoantibodies against gp96 are therefore directed against a self antigen, and do not reflect previous microbial encounters. A similar argument cannot be made for hsp70, which is expressed in bacteria and mammals and is conserved across the phylogenetic ladder. Our observations do not distinguish between the possibilities that the serum antibodies to hsp70 are natural autoantibodies or that their presence is a consequence of previous exposure of mice to infectious agents.
Physiological roles of natural autoantibodies to hsp should be considered. These antibodies may be elicited initially as a consequence of cell death such as occurs in the thymus during normal development, or as a result of enhanced cell surface expression of hsp during stress such as in hypoxia, glucose starvation or malignancy.20,21 They may later serve to provide a low level of protection against a broad array of micro-organisms because of the homology between their hsp and the mammalian hsp. Conversely, the observation that vaccination with hsp preparations does not lead to an enhancement of the natural antibody titre (Fig. 4) suggests that the antibody response to hsp is tightly regulated and that a pre-existing response may serve to down-regulate stronger pathological autoimmune responses to such hsp.
As stated in the Introduction, the presence of natural auto-antibodies to hsp70 and gp96 has implications for cancer immunotherapy.6 We have reported previously that vaccination of mice or rats with hsp purified from their tumours renders the animals resistant to challenges with live cells of the tumours from which hsp are purified, but not to other tumours. Such an immune response is not directed against the hsp but against peptides chaperoned by them.22 A key point that arises in such vaccinations is the danger of autoimmunity elicited against the hsp themselves, especially in the case of pre-existing autoantibodies against the hsp. The studies reported here show that vaccination with hsp under the conditions employed here (which are practically identical to the conditions used for vaccination against cancers and infectious diseases) does not lead to potentiation of the autoantibody response against hsp70 or gp96. This is consistent with our long-term experience with several hundred hsp-immunized mice and rats, where high titred antibodies to either hsp were never detected (2 and unpublished observations).
Heat-shock proteins are increasingly emerging at the centre of a new immunological paradigm.15,19,23,24 Our observations here represent the first report of a naturally existing autoimmune response against hsp, under healthy conditions. Given the fact that hsp are among the most highly conserved molecules in living species, the existence of a basal level of autoimmune response against them, is counter-intuitive, and suggestive of a crucial role for them in health and disease.
Acknowledgments
We gratefully acknowledge Chi Truong for her technical assistance. We thank Nathalie Blachère and Daniel Levey for their criticisms and helpful discussions. This work was supported by the NIH grants CA64394 and CA44786 and a research agreement with Antigenics, Inc. in which one of us (P.K.S) has a significant financial interest. Antoine Ménoret was supported partially by the French Association pour la Recherche sur le Cancer (ARC).
Glossary
Abbreviations
- hsp
heat-shock protein
References
- 1.Srivastava PK. Peptide-binding heat shock proteins in the endoplasmic reticulum: role in immune response to cancer and in antigen presentation. Adv Cancer Res. 1993;62:153. doi: 10.1016/s0065-230x(08)60318-8. [DOI] [PubMed] [Google Scholar]
- 2.Srivastava PK, Das MR. The serologically unique cell surface antigen of Zajdela ascitic hepatoma is also its tumor-associated transplantation antigen. Int J Cancer. 1984;33:417. doi: 10.1002/ijc.2910330321. [DOI] [PubMed] [Google Scholar]
- 3.Srivastava PK, Deleo AB, Old LJ. Tumor rejection antigens of chemically induced sarcomas of inbred mice. Proc Natl Acad Sci USA. 1986;83:3407. doi: 10.1073/pnas.83.10.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamura Y, Peng P, Liu K, Daou M, Srivastava PK. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278:117. doi: 10.1126/science.278.5335.117. [DOI] [PubMed] [Google Scholar]
- 5.Janetzki S, Blachere NE, Srivastava PK. Generation of tumor-specific cytotoxic lymphocytes and memory T cells by immunization with tumor-derived heat shock protein gp96. J Immunotherapy. 1998;21:296. doi: 10.1097/00002371-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Janetzki S, Palla D, Rosenhauer V, Locks H, Lewis JJ, Srivastava PK. Immunization of cancer patients with autologous cancer-derived heat shock protein gp96 preparations: A pilot study. Int J Cancer. 2000;88:232–8. doi: 10.1002/1097-0215(20001015)88:2<232::aid-ijc14>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 7.Amato RJ, Wood I, Savary C, Wood C, Hawkins E, Reitsma D, Srivastava P. American Society of Clinical Oncology meeting. Cambridge, MA: Medical Support Systems; 2000. Patients with renal cell carcinoma (RCC) using autologous tumor-derived heat shock protein-peptide complex (HSPPC-96) with or without interleukin-2 (IL-2) [Google Scholar]
- 8.Lewis J, Janestzki Livingston S, Desantis PO, et al. American Society of Clinical Oncology meeting. Cambridge, MA: Medical Support Systems; 1999. Pilot trial of vaccination with autologous tumor-derived gp96 heat shock protein-peptide complex (HSPPC-96) in patient with resected pancreatic adenocarcinoma. (Abstract 1687) [Google Scholar]
- 9.Dighiero G, Guilbert B, Avrameas S. Naturally occuring antibodies against nine common antigens in human sera-II. high incidence of monoclonal Ig exhibing antibody activity against actin and tubulin and sharing antibody specificities with natural antibodies. J Immunol. 1982;128:2788. [PubMed] [Google Scholar]
- 10.Poncet P, Matthes T, Billecocq A, Dighiero G. Immunochemical studies of polyspecific natural autoantibodies: charge, lipid reactivity, Fab′2 fragments activity and complement fixation. Mol Immunol. 1988;25:981. doi: 10.1016/0161-5890(88)90004-1. [DOI] [PubMed] [Google Scholar]
- 11.Imai H, Suzuki S, Uchida K, Kikuchi K, Sugiyama H, Kohno H, Udema M, Inoue K. Natural autoantibody against apolipoprotein A-1. J Immunol. 1994;153:2290. [PubMed] [Google Scholar]
- 12.Avrameas S, Dighiero G, Lymberi P, Guilbert B. Studies on natural antibodies and autoantibodies. Ann Rev Immunol. 1983;134D:103. [PubMed] [Google Scholar]
- 13.Casali P, Notkins AL. CD5+ B lymphocytes, polyreactive antibodies and the human B-cell repertoire. Immunol Today. 1989;10:364. doi: 10.1016/0167-5699(89)90268-5. [DOI] [PubMed] [Google Scholar]
- 14.Cohen IR, Young DB. Autoimmunity, microbial immunity and the immunological homoculus. Immunol Today. 1991;12:105. doi: 10.1016/0167-5699(91)90093-9. [DOI] [PubMed] [Google Scholar]
- 15.Srivastava PK. Heat shock proteins in immune response to cancer: the fourth paradigm. Experientia. 1994;50:1054. doi: 10.1007/BF01923461. [DOI] [PubMed] [Google Scholar]
- 16.Peng P, Ménoret A, Srivastava PK. Purification of immunogenic heat shock protein 70-peptide complexes by ADP-affinity chromatography. J Immunol Methods. 1997;204:13. doi: 10.1016/s0022-1759(97)00017-3. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T, Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the fas ligand. Cell. 1994;76:969. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 18.Finkelman FD, Woods VL, Berning A, Scher I. Demonstration of mouse serum IgD. J Immunol. 1979;123:1253. [PubMed] [Google Scholar]
- 19.Srivastava PK, Menoret A, Basu S, Binder JR, McQuade KL. Heat shock protein come of age: primitive functions acquire new roles in an adaptive world. Immunity. 1998;8:657. doi: 10.1016/s1074-7613(00)80570-1. [DOI] [PubMed] [Google Scholar]
- 20.Multhoff G, Botzler C, Jennen L, Schmidf J, Ellwart J, Issels RD. Heat shock protein 72 on tumor cells. A recognition structure for natural killer cells. J Immunol. 1997;158:4341. [PubMed] [Google Scholar]
- 21.Altmeyer A, Maki RG, Feldweg AM, Heike M, Protopopov VP, Masur SK, Srivastava PK. Tumor-specific cell surface expression of the KDEL containing, endoplasmic reticular heat shock protein gp96. Int J Cancer. 1996;69:340. doi: 10.1002/(SICI)1097-0215(19960822)69:4<340::AID-IJC18>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 22.Udono H, Srivastava. PK. Heat shock protein 70-associated peptides elicit specific cancer immunity. J Exp Med. 1993;178:1391. doi: 10.1084/jem.178.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ménoret A, Party Y, Burg C, Le Pendu J. Co-segregation of tumor immunogenicity with expression of inducible but not constitutive hsp70 in rat colon carcinomas. J Immunol. 1995;155:740. [PubMed] [Google Scholar]
- 24.Ménoret A, Chandawarkar RY. Heat shock protein-based anticancer immunotherapy, an idea whose time has come. Semin Oncol. 1998;25:654. [PubMed] [Google Scholar]